Abstract
The purpose of this review is to provide health practitioners and physicians the most current state of the research on sarcopenia, its consequences, and to offer a summary of consensus guidelines for identification based on the most recent and compelling investigations and analyses. To accomplish this, the causes and consequences of sarcopenia will be described, and definitions and screening methods are updated. Importantly, interventional recommendations for sarcopenia will be discussed with a special emphasis on the effects of resistance training on sarcopenia-related outcomes. Furthermore, due to the increasing usage of hormone treatment as a strategy to combat sarcopenia, special consideration on the effects of hormone changes with aging and as interventions will be briefly reviewed.
Keywords: aging, muscle, strength, function
‘Sarcopenia is defined in the literal sense from the Greek for “poverty of the flesh” and is the natural, aging-related process of the loss of LM [lean mass]’
The aging process in the growing population of older adults is accompanied by an increase in chronic health conditions and a high prevalence of functional limitations and disability associated with declines in lean mass (LM; ie, sarcopenia) and strength.1 Healthy People 2020 estimates that more than 37 million older adults are expected to be managing at least one chronic disease or condition by the year 2030.2 The economic burden associated with disability in older adults remains high3 despite small annual declines in chronic disability attributed to improved overall health. Nevertheless, health care costs associated with sarcopenia-related disability in the year 2000 were estimated to be $12.6 billion for older men and $29.5 billion for older women.4 Moreover, given the rapid increase in the older adult population over the past 15 years, these estimates are now likely to be far higher.
Lifestyle changes that are associated with aging include a reduction in physical activity levels,5 which is related to a multitude of complex aging-related changes that affect muscle and physical functioning in older adults.6,7 As a result, determining who is at risk and what the best interventions are to improve LM and function and/or to prevent LM loss and disability is a critical public health objective.8,9 Accordingly, there has been a rapid growth in the scientific literature over the past several years investigating sarcopenia and related health outcomes. For example, as of February 2015, a PubMed search using the term “sarcopenia” yielded >2800 results, with >2000 of these published in the past 5 years. However, the most valid methods to identify sarcopenic patients and to determine recommendations for interventions that best reduce declines in physical functioning remain elusive. Therefore, recent research has focused on currently unanswered questions of how to recognize and treat patients living with sarcopenia, and excellent systematic and scholarly reviews of the sarcopenia literature have been recently performed.1,10-15
What Is Sarcopenia, and Why Is It Important to Clinicians?
Sarcopenia is defined in the literal sense from the Greek for “poverty of the flesh” and is the natural, aging-related process of the loss of LM. Skeletal muscle undergoes numerous changes during adult aging including fiber atrophy, loss of type II muscle fibers, clustering of type I fibers, a reduction in fast motor units, fatty infiltration, and neuromuscular changes that all may contribute to the process of sarcopenia.6,16 Thus, sarcopenia is a multifactorial geriatric syndrome17 and was first mentioned in the literature as a condition by Rosenberg18 over 25 years ago. The loss of LM is considered a universal hallmark of the aging process, especially later in life, and sarcopenia is viewed as a chronic state that is distinct from other conditions of LM loss such as microgravity exposure, wasting, cachexia, and other chronic conditions or muscle diseases.15 Sarcopenia is closely associated with reductions in muscle strength and power,19,20 physical function,21-23 and increased risk of disability in older adults.8
Estimates of sarcopenia prevalence vary,24,25 and the national estimates have historically been hampered by unclear definitions or cut-points due to a lack of strong clinical end-points. A recent meta-analysis suggests13 that among a host of different age groups and sarcopenia identification techniques sarcopenia prevalence can be anywhere from 1% to 29% in community-dwelling older adults, 14% to 33% in older adults in long-term care facilities, and ~10% in acute care facilities. Irrespective of the wide ranging estimates, sarcopenia and associated symptoms are considered major public health problems given their strong associations with numerous outcomes including reductions in strength,26 physical functioning,27 increased hospitalization risks,28 postoperative complications,29 and mortality.30 Consequently, clinicians and researchers often refer to sarcopenia more as a process rather than a specific clinical diagnosis, and attempts to define sarcopenia typically employ appendicular LM (ALM) cut-points, either compared to younger adult reference groups31 or as the lowest percentile of an older cohort.27 Importantly, for the purposes of the current review, it is essential to note at this time that the use of LM and ALM refers specifically to muscle mass and does not include bone mass.
Challenges to Diagnosing Sarcopenia
There have been numerous attempts to identify a viable algorithm to classify sarcopenic individuals, and the major obstacle to such a universally accepted definition has been the lack of strong clinical end-points and clinically independent predictors of sarcopenia (eg, the use of fracture risk for osteoporosis).14 Some have made a convincing arguments that sarcopenia, often used as a broad term for decreased muscle function associated with LM losses, should become a specific diagnosis that should apply only to LM losses with age, and that dynapenia should be used when describing the age-related loss of muscle strength.32 The powerful relationship between LM and muscle function previously hypothesized by investigators has been reexamined and are now considered interrelated but separate conditions as the notion of “sarcopenia with impaired mobility”15 is a more apt classification. Nevertheless, sarcopenia seems to be the most recognized term and remains the primary focus of recent investigations, although the model for using measures of physical and muscular function as primary criteria for sarcopenia identification in an individual has gained considerable traction.
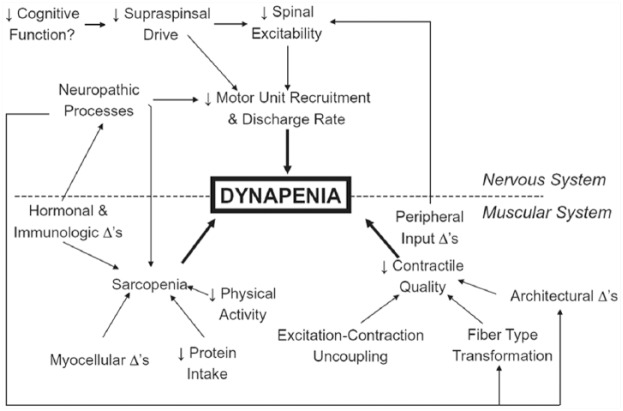
Arguably, the most commonly employed method for sarcopenia determination has been the use of the skeletal muscle index (SMI), which is equal to the quotient of a person’s ALM divided by his/her height-squared (ALM/height2). The advantage of this method is that it considers the height of the individual as taller people tend to have more LM.33 Indeed, the highly recognized New Mexico Elders Aging Process Study used the SMI with sex-specific standard deviation cut-points for comparison to a younger reference population from the Rosetta Study.31 However, there were 2 major obstacles that prevented this from becoming the accepted definition. First, the SMI did not account for body fat mass and seemed to only classify people with low body mass index (BMI) as sarcopenic. For example, when the SMI method was applied to the Health, Aging and Body Composition (Health ABC) cohort, a longitudinal study of 3075 community-dwelling older men and women, no obese participant was classified as sarcopenic.27 It is now well documented that body fat mass can adversely affect mobility,34 and because the SMI resembles the definition for BMI, it excludes some older adults with relatively low LM and high fat mass from being classified as sarcopenic. A second major limitation to the SMI method is the lack of physical function measurement as part of the definition. While LM and strength are correlated,26 there are a host of other factors that influence muscle function that cannot be ignored (Figure 1).
Figure 1.
A Working Model by Clark and Manini7 of the Hypothesized Etiologic Factors That Are Responsible for the Development of Dynapenia in Older Adults.
Used with permission from the authors.
In response to the SMI misidentifying some older individuals as normal who may have “sarcopenic obesity,” alternative methods were developed to better categorize persons who have relatively poor physical function and mobility due to low LM relative to body fat mass. Furthermore, although consensus guidelines vary in terms of cut-points for sarcopenia screening, there now seems to be some agreement that the key elements for defining sarcopenia are (a) poor physical functioning, (b) muscle weakness, and (c) low LM. Accordingly, these criteria should be examined together further to determine an individual’s sarcopenic status and also additional health-related factors to consider when evaluating treatment options.
Functional Outcomes
There are several simple, valid, and reliable ways to measure physical function in older adults involving tests for balance, chair rising (standing from a seated position), and walking (eg, Short Physical Performance Battery).35 Most functional tests include some measure of gait speed and typically employ the 6-minute walk, 400-meter walk, and/or timed up-and-go tests.36 The importance of gait speed for daily activities and inclusion in the definition of sarcopenia cannot be understated as gait speed is associated with a myriad of health outcomes including overall physical functioning, executive function,37 and mortality risk.38 In addition, gait speed is one of the defining criteria for frailty,39 which is a broader health concept that may include sarcopenia and related conditions.17 For example, walking speed predicts survival and increases in gait speed as little as 0.1 m/s significantly improve survival likelihood.38 Additionally, to emphasize this importance, some have suggested that gait speed should be assessed clinically with breathing, temperature, heart rate, pain, and color and become the “sixth vital sign.”40 Appropriately, a gait speed cut-point of <0.8 m/s is now used as part of the differential diagnosis for sarcopenia in at least 2 novel definitions.14,17
Muscle Function
Muscle strength is considered a component of physical fitness and muscle weakness is associated with poor physical functioning41,42 and an increased risk for metabolic disease,43 hospitalization,28 and mortality30 in older adults. Peak strength typically plateaus sometime in the 30s followed by a steady decline thereafter, and the age-associated loss of muscle strength that is not caused by neurologic or muscular disease is referred to as dynapenia.19,44 Nevertheless, like sarcopenia, dynapenia is a complex process and appears to increase in severity during the seventh decade.12,31 In addition, the direct effects of sarcopenia on functional outcomes may be best illustrated by the dramatic 30% declines in competitive international weightlifting records observed in men between 30 and 60 years45 (Figure 2). Furthermore, this relationship seems to be associated with a stepwise strength decrease beginning in the fourth decade, which continues into the seventh decade at which point women appear to retain only ~30% of the strength formerly possessed in their 30s45 (Figure 2).
Figure 2.
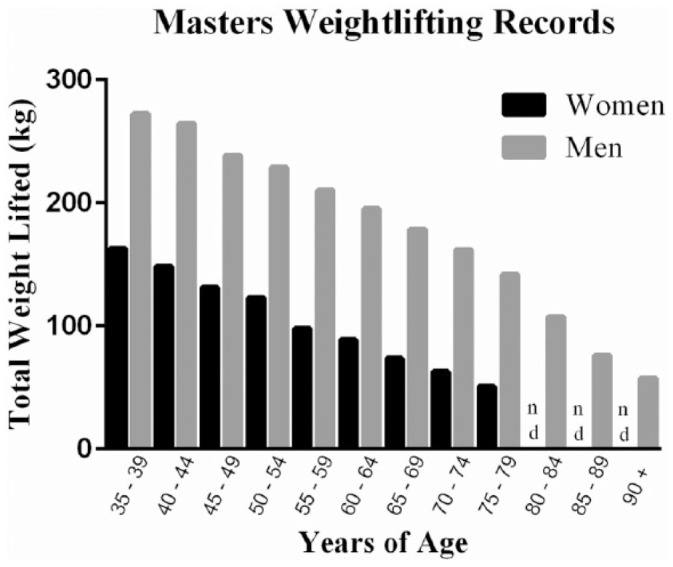
Average National and International Masters Level Men’s and Women’s Combined Total Weightlifting Records Are Presented From Competition Results of the USA National, Pan American, and American Masters Competition Weightlifting Events Occurring Between August 1, 2014 and February 20, 2015.
Adapted from the United States Masters Program weightlifting records available at www.mastersweightlifting.org.45
Recent findings from the Health ABC study26 confirm that over a 5-year period muscle strength declines at a much faster rate than muscle cross-sectional area (CSA), suggesting reduction in muscle quality (force production per unit area of LM) (Figure 3). In a 5-year study of 1678 participants, men and women exhibited decreases in knee extensor muscle torque by 16.1% and 13.4%, respectively. These losses amount to an annualized decrease in knee torque that is 2 to 5 times greater than the decreases in leg muscle CSA. Interestingly, although some participants gained weight and increased muscle CSA over the 5-year period, torque loss was similar to overall group change, despite increases in contractile tissue. These greater age-related decrements in strength compared to muscle CSA indicate a loss in muscle quality, further illustrating that LM is not the sole determinant of muscle strength or function in older adults.26
Figure 3.
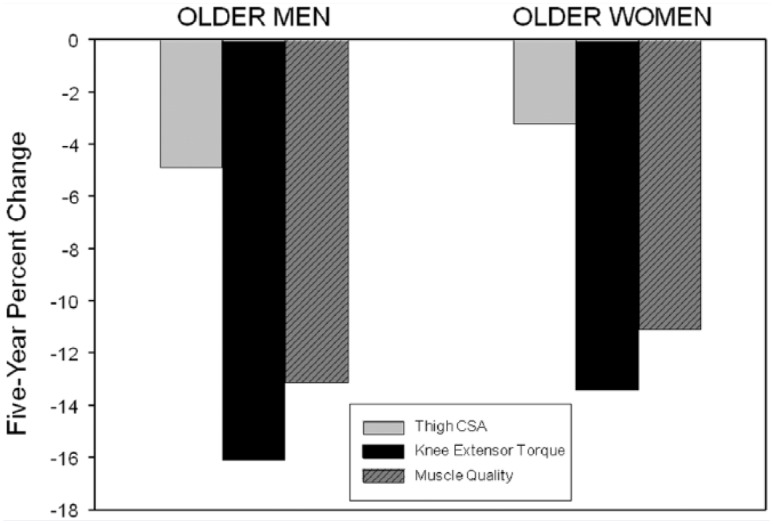
Five-Year Percentage Changes in Mid-Thigh Muscle Cross-Sectional Area (CSA), Knee Extensor Torque, and Muscle Quality (Torque Divided by CSA) in 1678 men and women from the Health, Aging and Body Composition Study.
Adapted with permission from Delmonico et al.26
In addition, it should be noted that muscle power is a measure of the rate of performing mechanical work and explains variance in physical function in older adults better than measures of strength alone.46,47 Power has been shown to decrease roughly 3% to 4% faster than strength,19,48,49 with aging and power declines earlier in women when compared to men.19 Furthermore, muscle power in older adults is associated with worsened muscle structure, function, tendon characteristics, and increased sarcopenia in specific muscle groups.50 In fact, some age comparisons19 suggest that a decrease of >50% in muscle power occurs between the ages of 20 and 80 years without substantial differences in muscle CSA, which further suggests that muscle power declines are a consequence of causes other than LM.
In most studies, muscle strength is more strongly associated with low physical function compared to LM in older adults, which further supports the evidence for uncoupling of muscle strength and LM with age (ie, declines in muscle quality). Based on their findings, Manini and Clark7 have further suggested that screening for dynapenia is a more important clinical outcome rather than, or at least in addition to, LM (Figure 1). Despite the mounting evidence sarcopenia is still considered a more commonly recognized condition and the introduction of a new diagnosis (ie, dynapenia) might result in misunderstandings for clinicians and patients.17
Lean Mass
The definition of sarcopenia began as a description of LM losses, and LM continues to be at least partly responsible for decreased strength and function in older adults. Therefore, it is essential for evidence-based reasons and consistency purposes to include LM in an operational definition of sarcopenia. In community-dwelling men and women in their 70s, low muscle CSA is related with lower physical function.51 Longitudinal data from the Health ABC Study26 also suggest that men and women in their 70s experience 5-year mid-thigh LM losses of ~5% in men and ~3% in women and a dramatic increases of intermuscular fat infiltration (~49% in men, ~29% in women), indicating specific changes in regional body composition with aging. Moreover, low-thigh muscle CSA increases the risk of major disability by ~40%.41
Body fat mass is a strong predictor of physical function, particularly in women who seem to be more susceptible to sarcopenia and its consequences. Indeed, Riebe et al34 observed in the Study of Exercise and Nutrition in Older Rhode Islanders investigation that obese older women have lower physical function scores, determined by gait speed, compared with obese older men and therefore are at increased risk for future mobility impairment. Likewise, older women have reduced physical function compared with men, which is explained primarily by higher fat mass.52 Furthermore, higher body fat mass and/or higher fat mass to LM ratios may lead to biomechanical disadvantages in women and increased effort with movement. Accordingly, data seem to support the inclusion of fat mass measurement when evaluating individuals for sarcopenia.
Measuring Lean Mass
Computed tomography (CT) and magnetic resonance imaging (MRI)53 are best for measuring ALM, but these techniques are expensive, are typically restricted to well-funded research studies17 or clinics, and include exposure to radiation (CT). Bioelectrical impedance analysis (BIA) is an alternative and inexpensive technique for measuring total body LM, and hand-held and bathroom scale–type devices are easy to use and common. Subsequently, total body LM measured via BIA is employed frequently in research and has been included in some sarcopenia definitions that utilize total body LM.17 Unfortunately, inexpensive BIA devices are unable to identify specific regional sites (ie, ALM). Newly developed, advanced, multifrequency BIA devices can measure ALM,54 but the higher costs and the relatively low prevalence may limit their utility. Consequently, dual energy X-ray absorptiometry (DXA) continues to be accepted as the clinical “gold standard” for body composition assessment. Bone density in the aging population is routinely examined using DXA,15 and increased use in both clinical and research settings has resulted in regional LM estimates for increased numbers of older adults. However, more work is necessary to improve LM databases for specific gender age ranges and racial/ethnic groups.14
Moving Toward a Sarcopenia Definition Consensus
Without a universally accepted definition for sarcopenia, most studies could not effectively screen participants for sarcopenia and/or used definitions based on preference and available measures for LM, and thus interpreting or comparing results has been a challenge. Accordingly, several “working groups” have convened to establish consensus and progress has been made toward a definition of sarcopenia. The most prominent statements have been from the European Working Group on Sarcopenia in Older People (EWGSOP), the International Working Group (IWG), and the Foundation for the National Institutes of Health Sarcopenia Project (FNIHSP).
EWGSOP Guidelines
The EWGSOP developed a classification method to identify individuals based on clinical symptoms associated with sarcopenia rather than LM alone17 (Table 1). Moreover, given the known and unknown causes of sarcopenia, the EGWSOP determined that sarcopenia should be described as “primary,” the natural multifactorial age-related decline in LM, or “secondary” due to one or more identifiable causes such as chronic inactivity, other diseases, or specific dietary factors.17 Thus, the EWGSOP’s algorithm for screening for sarcopenia includes gait speed and grip strength as primary criteria, and LM is only considered in the presence of low gait or grip scores.17 A classification system to grade sarcopenia for tracking those with varying degrees of sarcopenia was also developed. Essentially, this novel classification system recognizes that primary sarcopenia typically develops over a long period and therefore EWGSOP established sarcopenia stages17 (ie, presarcopenia, sarcopenia, and severe sarcopenia), similar to those adopted for osteoporosis55 or hypertension,56 with presarcopenia defined as the presence of low LM only.
Table 1.
The European Working Group on Sarcopenia in Older People Sarcopenia (EWGSOP) and International Working Group on Sarcopenia (IWG) Screening Guidelines for Sarcopenia and Severe Sarcopeniaa.
| Presarcopenia | EWGSOP | IWG |
|---|---|---|
| Lean mass, ALM/height2 | ||
| Men | <7.23 kg/m2 | N/A |
| Women | <5.67 kg/m2 | N/A |
| Sarcopenia | ||
| Gait speed | <0.8 m/s | <1.0 m/s |
| Muscle strengthb | OR | |
| Men | <30 kg | N/Ac |
| Women | <20 kg | N/Ac |
| Lean mass, ALM/height2 | AND | |
| Men | <7.23 kg/m2 | <7.23 kg/m2 |
| Women | <5.67 kg/m2 | <5.67 kg/m2 |
| Severe sarcopenia | ||
| Gait speed | <0.8 m/s | N/A |
| Muscle strengtha | AND | |
| Men | <30 kg | N/A |
| Women | <20 kg | N/A |
| Lean mass, ALM/height2 | AND | |
| Men | <7.23 kg/m2 | N/A |
| Women | <5.67 kg/m2 | N/A |
IWG Guidelines
Concurrently, the IWG, a team of scientists and geriatricians from both academia and the private sector, proposed a somewhat similar set of criteria15 (Table 1). While the IWG similarly included gait speed, cut-points were less conservative (<1.0 m/s) than EWGSOP guidelines. The IWG did not specify a measure or cut-point for muscle strength, but they suggest that the ability to rise from a chair unassisted should be used as an indicator of lower-body physical function. Importantly, this test assesses lower body function adjusted automatically for total body mass and, in our opinion, was a strong choice. The need to screen for LM using the IWG system is predicated on a mobility deficiency and suggests that the sex-specific SMI cut-points be used.15 Although these (or similar) LM cut-points have been used in other sarcopenia definitions,27,31 there seems to be no recommendation for adjustments for fat mass or BMI, and therefore the use of LM-only cut-points may diminish the ability to identify those with sarcopenic obesity.27
The definitions of sarcopenia developed by the EWGSOP and IWG employ a combination of mobility and strength with LM, but the use of absolute sex-specific LM cut-points that do not account for fat mass, which has considerable interindividual variability and is a major predictor of function in sarcopenic individuals,27,34,58 may not be optimal.
How Do These New Definitions Compare?
The EGWSOP and IWG guidelines for sarcopenia identification were not tested, per se, at the time of their adoption, and analyses have been conducted post hoc to examine their clinical validity.
Alley et al59 examined the combined study pool and suggested a clinical “weak” cut-point of <26 kg in men and <16 kg for women, as these cut-points are associated with a ~7.6- and ~4.4-fold greater risk of mobility impairment, respectively, when compared to normal strength men and women. Additionally, they established an “intermediate” range for grip strength of 26 to 32 kg and 16 to 20 kg for men and women that were associated with a ~3.6- and ~2.4-fold increased mobility risk, respectively. Together, a much higher percentage of women (18%) in this cohort were identified as “weak” compared with men (5%), which reinforces previous findings that women may be more susceptible to dynapenia. Grip strength measures were further adjusted for BMI, and in women seemed to be a better predictor of mobility impairment compared with absolute strength. However, these differences were not substantial enough to recommend the further use of BMI adjusted values.
In a follow-up study, Cawthon et al60 established cut-points for low LM that correspond to weakness. Low ALM levels in men were found to be <19.75 kg and <15.02 kg in women, and were associated with an increased risk of weakness when compared with normal men (odds ratio [OR] = 6.9, 95% confidence interval [CI] = 5.4, 8.9) and women (OR = 3.6, 95% CI = 2.9, 4.3). Furthermore, ALM was an independent predictor of mobility in men with a 2- to 4.5-fold greater risk of mobility limitation in men with low ALM, but only muscle strength independently predicted gait speed in women. In addition to absolute LM cut-points, an adjustment for BMI was made to the ALM cut-points (ie, ALM/BMI; “ALMBMI”), and these low ALMBMI cut-points (men = 0.789, women = 0.512) corresponded to a higher risk of weakness in men (OR = 4.3, 95% CI = 3.4, 5.5) and women (OR = 2.2, 95% CI = 1.8, 2.8) compared with their normal-strength counterparts. Not surprisingly, using this metric, the prevalence of weakness in women at this cut-point was 31% while only ~12% in men. Interestingly, those women whose ALMBMI was ≥0.512 (ie, higher LM) there was a strong influence of BMI as the prevalence of weakness in women who had a BMI of <23.7 kg/m2 was only 12% while the weakness prevalence rose to over 24% in women with a BMI >23.7 kg/m2. These findings support previous study evidence58 suggesting that body fat mass mediates the relationship between LM, strength, and mobility in older women.60 Finally, these data are supported by other FNIHSP analyses61 that show that these sex-specific cut-points for grip strength and ALMBMI, but not absolute ALM, are independent predictors of incident mobility impairment in men and women.
The FNIHSP recommends that individuals should be identified using either “weak with low LM” or “weak and slow with low LM” rather than using the term sarcopenia. The overall FNIHSP recommendations for cut-points in identifying sarcopenia in older adults are shown in Table 2. The FNIHSP concurs with the EWGSOP and IWG on the use of <0.8 m/s for a gait speed cut-point as this is an easy measure to obtain in most settings and is a strong predictor of major health outcomes.38
Table 2.
The Foundation for the National Institutes of Health Sarcopenia Project Recommended Modalities and Cut-Points for Identifying Functional Limitation, Muscle Weakness, and Low Lean Mass in Older Men and Womena.
| Cut-Point for Men | Cut-Point for Women | |
|---|---|---|
| Weakness with low lean mass | ||
| Gait speed | — | — |
| Muscle strength: grip strength | <26 kg | <16 kg |
| Lean mass: ALMBMI | <0.789 | <0.512 |
| Weakness and slowness with low lean mass | ||
| Gait speed | <0.8 m/s | <0.8 m/s |
| Weakness | <26 kg | <16 kg |
| Lean mass: ALMBMI | <0.789 | <0.512 |
Abbreviations: ALM, appendicular lean mass; BMI, body mass index; ALMBMI, ALM divided by BMI.
Adapted from Studenski et al.14
What Can Be Done to Improve Lean Mass and Function in Sarcopenic Adults?
Establishing successful intervention strategies that preserve and/or improve LM and function is crucial. There has been a significant effort to determine the most effective and efficacious interventions for treating sarcopenia and its associated symptoms. The primary focus of current research includes diet and dietary supplementation, hormone therapies, physical activity programs, and resistance training (RT).13 Indeed, Fragala and colleagues investigated the combined effect of vitamin D with physical activity, fish oil supplementation, DHEA, or 17-β-estradiol therapy on physical and muscle function and LM based on FNIHSP cut-points in women (n = 378) who were weak and had low LM versus women who were considered normal and reported that muscle weakness, a primary symptom of sarcopenia in the FNIHSP definition, can be improved in low-functioning older women.62 Furthermore, results suggest that weaker female participants respond more favorably to interventions with regard to strength, and interestingly, women with higher LM respond better. However, although well designed and executed, this analysis combined different intervention modes, and the individual impact of each cannot be assessed. Therefore, a brief overview of dietary/supplementation, hormonal therapies, and physical activity interventions independently is important.
Protein Intake
It has been suggested that up to 38% of adult men and 41% of adult women have dietary protein intakes below the recommended daily allowance (RDA).63 The Food and Nutrition Board of the United States National Academy of Science recommends 0.8 g protein/kg/day for all adults. This value represents a minimum amount of protein required to avoid progressive loss of LM in most healthy individuals, which may not apply to the aging population.64-66 Some investigations suggest this protein RDA may actually be insufficient to maintain LM, not to mention recover what has been lost to decades of aging.63,64,66 In addition, some research suggests that the efficiency of protein utilization is degraded with aging and results in blunted muscle protein synthesis, and decreases in dietary protein intake further facilitates sarcopenia by promoting an internal environment of net muscle protein catabolism and loss. Indeed, in older individuals who perform RT, increases in LM occur principally in those consuming 50% more protein per day than is currently recommended.64,67-69
Historically, clinicians and research investigators were hesitant to recommend high-protein diets for older adults due to concerns of the potential harmful effects on bone health and renal, neurological, and cardiovascular function.65,70 However, further investigations of high-protein diets in elderly participants suggest that many of these factors are actually improved.66,71-77 In addition, the promising observation that muscle protein synthesis increases in response to protein or amino acid ingestion78-80 in older participants similar to younger adults63,78-80 without a compensatory change in muscle protein breakdown was recently reported. Unfortunately, dietary protein intake is reduced with aging and this relationship is multifactorial and includes increased expense, satiety, dentition/chewing difficulties, and/or changes in digestion, gastric emptying, and peristaltic dysrhythmias.63 In response, amino acid supplementation to combat reduced caloric and protein intake in the aging population has been suggested. However, it appears that complex nutritional supplements are required beyond simple amino acid tablets and the development of novel easily ingestible and digestible protein supplements may be required to meet the anabolic demands of skeletal muscle for protein in the elderly. Regardless, there is currently insufficient longitudinal research to identify an ideal value for daily protein requirements or to elucidate the benefits of dietary protein on sarcopenia-related conditions.65,66,81 It should be noted that kidney function decreases with age, high protein intake is contraindicated in individuals with renal disease, and assessment of renal function is recommended for older individuals before they adopt a higher protein diet.70
In addition, there is evidence that novel dietary supplements may be efficacious in treating sarcopenia and include creatine and β-hydroxy-β-methylbutyrate (HMB).82 Creatine has been widely studied as an ergogenic aid for its muscular performance improvements,83 and there is evidence84 that creatine supplementation may have additive beneficial effects with RT for increasing LM, muscle strength and function, but not all studies agree.85,86 The effects of combining dietary HMB, arginine, and lysine was investigated in 40 older adults (age = 76 ± 1.6 years)87 and significant improvements in LM after 1 year were reported, and participants who received the treatment combination and who also had low vitamin D levels initially exhibited improved muscle strength. Additionally, HMB supplementation maintains LM in adults in their late 60s during 10 days of bed rest.88 Despite some promising results of creatine and HMB as potential treatments for low LM and strength in older adults, data are sparse and further investigation is warranted to determine the benefits of nutritional and supplemental dietary interventions for age-related losses of LM and function.
A Brief Update on Hormone Replacement Therapy
An increasing number of clinical trials have been performed evaluating the efficacy of hormones such as testosterone, growth hormone, insulin-like growth factor, dehydroepiandrosterone, estrogen, and estradiol, with conflicting results. Therefore, while there is great interest in discovering a pharmacotherapeutic treatment for sarcopenia, there has not been a consensus demonstrating the efficacy for hormone replacement therapy (HRT) for the treatment of sarcopenia as studies tend toward mixed results. Furthermore, there are indications for possible adverse clinical outcomes with HRT in older adults including diabetes and glucose intolerance,89 breast cancer,90 coronary artery disease,91 stroke,92 and all-cause mortality.93,94 A complete review of the rich literature on this topic is beyond the scope of this article, but due to the increasing usage of testosterone and estrogen replacement, we offer a brief summary of HRT as a treatment for sarcopenia.
Testosterone Replacement Therapy
Production of testosterone and serum testosterone concentrations decline as men age and it appears that androgens determine LM maintenance in older men.95-97 Indeed, hypogonadism, or the low serum concentration of testosterone, is associated with LM and strength loss.98,99 Interestingly, declines in circulating androgens seem to parallel the myofiber degradation and muscular strength declines in older men. Some reports suggest that the incidence of low total serum testosterone (<325 ng/dL) is 20% for men in their 60s, 30% for men in their 70s, and 50% for men over 80 years.100,101 It was observed in another investigation that 12.3% of men aged 40 to 70 years have a total serum testosterone of <200 ng/dL with 3 or more symptoms of hypogonadism.102 Another study suggests that the adjusted mortality risk in men over 40 years with low testosterone is increased by 88%.93 Interestingly, muscular declines also begin in the fourth decade and are associated with dramatic changes in body composition (LM declines and the mass of adipose tissue expands), which form the basis of many metabolic disorders ultimately resulting in an increased incidence of morbidity and mortality.19
Within this population, testosterone administration dose-dependently improves LM, and some studies have reported substantial increases in muscle strength98,103,104 while others have reported only modest increases.105,106 Studies reporting more substantial effects of testosterone replacement therapy (TRT) administer testosterone at higher than physiologic replacement dosages and administered via intramuscular injection.103,104 Collectively, research suggests that the beneficial effects of TRT for the treatment of sarcopenia involve multiple signal transduction pathways, and currently it seems that testosterone reverses sarcopenia in older hypogonadal men through stimulation of cellular metabolism and survival pathways together with the inhibition of muscle cell apoptosis and suppression of age-specific increases in oxidative stress.107 Clinically, Bhasin et al demonstrated that LM and strength are substantially increased by a very high dose of testosterone108 without adverse events, and combined, research suggests that modest enhancement of muscle can be expected with physiologic replacement TRT doses, whereas higher-than-replacement dosages are required for more robust improvement in musculoskeletal gains and adipose reduction109,110; however, concerns about TRT exist.
A recent meta-analysis suggests that the primary risk of adverse events in response to TRT include polycythemia, a small reduction in high-density lipoprotein cholesterol (HDL-C), and an increased urinary symptoms, benign prostatic hyperplasia, and prostate cancer.111-113 In contrast, another meta-analysis observed no significant evidence of increased prostate cancer in response to TRT.111 Nevertheless, TRT is being employed at a swiftly increasing rate in the United States, with sales in 2011 reaching $1.6 billion.114 Studies investigating the combined effects of TRT and the pharmacologic inhibition of the testosterone to dihydrotestosterone conversion have been employed to determine if benefits of TRT can be achieved without the risk of adverse prostatic events. One investigation suggested that older hypogonadal men treated with high-dose testosterone and finasteride experienced increases in physical performance and grip strength while suppressing adiponectin and lowering low-density lipoprotein cholesterol (LDL-C) without affecting HDL-C or the prostate.115,116 Moreover, Borst et al, when investigating the effects of intramuscular injection of higher than replacement dose testosterone combined with finasteride treatment for 1 year, observed significant increases in LM and muscle strength and reductions in body fat without prostate enlargement.98
Unfortunately, similar to investigations of sarcopenia, studying the health risks associated with untreated hypogonadism is often limited by study design variation and the lack of longitudinal research, randomized controlled investigations, and universally accepted diagnostic criteria.117 Nevertheless, until a scientific and clinical consensus can be reached, further investigation of the risks and/or benefits of TRT in men with hypogonadism and sarcopenia is essential.
Estrogen Replacement Therapy
Women experience changes in sex hormones (ie, estrogen and progesterone) a decade or more prior to menopause, and because the life expectancy of females may typically exceed 80 years, it is reasonable to suggest that a woman will spend nearly one third of her life in a sex-hormone-deficient state.118 Initially there is a significant decline in inhibin B and an increase in follicle-stimulating hormone followed by declines in inhibin A and estrogen.119 Consequently, concentrations of estrogen in an older woman can be half that observed before menopause.119 During the transition from pre- to postmenopause and thereafter, women without estrogen replacement therapy (ERT) exhibit increased incidence of osteopenia and osteoporosis, central and visceral adiposity, insulin resistance, dyslipidemia, sarcopenia, and frailty.120 Indeed, positive relationships between LM and estrogen and/or estradiol levels have been observed in women, and low estrogen levels may be directly linked to the decrease in protein synthesis and increased prevalence of sarcopenia observed in postmenopausal women.121-124
Estrogen supplementation is considered a potential strategy to protect against these losses in LM and strength, although earlier reports of increased health risks with ERT in postmenopausal women led to a decline in ERT use.90,125 However, recent reevaluation regarding the effects of ERT indicates a general lack of risks and a number of significant health benefits from ERT use when initiated early and before the onset of menopause.126,127 Indeed, ERT has been shown to reverse both menopause-related obesity and LM loss.128 Furthermore, the increase in LM may be explained by increased muscle anabolism, and ERT may prevent sarcopenia in elderly women.128 In contrast, some studies suggest that decreases in LM and strength associated with the natural decline in estrogen after menopause is as common in women who are long-term ERT users and women who are not using ERT suggesting no beneficial effect.129 Nevertheless, there is a growing body of evidence supporting the beneficial effects of ERT for the maintenance and improvement in LM and strength.118,128,130,131 As well, the potential efficacy of ERT on skeletal muscle function and quality in combination with the additive health-related benefits associated with reduced risk of cardiovascular events, overall mortality, metabolic dysfunction, and bone should suggest strong consideration for ERT as possible treatment for sarcopenia in women.
Physical Activity
Even among active older adults there is evidence that aerobic activity may not mitigate the loss of muscle strength with aging. For example, Marcell et al132 recently observed in a longitudinal investigation of nearly 5 years that older men and women who exercised regularly (eg, jogging) showed no change in LM. However, participants lost isometric knee extensor and flexor strength at a rate of ~5% and 3.6% per year, respectively. Those data indicate an uncoupling of muscle strength and LM, indicating that even in active, middle-aged men and women there is a rapid annual loss of muscle strength. Recently, researchers from the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) Study reported133 that older adults who engaged in 12 months of physical activity exhibited decreased symptoms of frailty and preserved LM. Additionally, longitudinal data (2.6 years) from the LIFE Study show that older adults who participated in the physical activity intervention had an 18% lower incidence of major mobility disability and a 28% lower risk of persistent mobility disability compared with healthy education participants.134
A recent systematic review13 of exercise interventions that employed general physical activity or combined modality training in sarcopenic older adults found that there are no studies that specifically recruited participants based on current criteria for diagnosing sarcopenia. Moreover, the results from studies that conducted exercise programs in older adults are mixed with regard to improvements in LM, strength, and physical functioning.13 For example, a study by Rydwik et al135 found that a 12-week combined aerobic, balance, and RT intervention in older adult ≥75 years resulted in muscle strength improvement (~17%), while a nutrition counseling intervention did not. However, no improvement in gait speed was found in any group, and while RT was part of the training program, combined exercise programs cannot isolate the individual contribution of RT.
Resistance Training
Resistance training (RT) has long been known to improve skeletal muscle strength and function in older adults, and during the last 2 decades a surge in research studies exploring the potential effects of RT on sarcopenia-related outcomes has emerged. These studies, performed in clinical, laboratory,136-138 and community-based settings139 have employed a wide variety of intervention protocols, RT modalities (eg, free weights,140 machines,141 body weight,142 whole-body vibration,143 and elastic bands/tubing144,145). Importantly, RT is a commonly used modality5 due to its safety, efficacy, and of a host of additional established benefits.
A recent meta-analysis by Peterson et al12 examining the effects of RT on muscle strength reported that among 47 studies including 1079 men and women >50 years old that muscle strength increases with RT for upper and lower body exercises by 24% to 33%. A follow-up meta-analysis of RT on LM changes10 also found that LM was responsive to RT in middle-aged to older adults but not to the same extent as muscle strength. The results indicate that in 49 studies with a mean duration of ~20 weeks, adjusted LM increased by 1.1 kg, with evidence of a positive dose–response relationship as greater volume of RT elicited greater improvement. Although there was a wide range in the RT protocols used, most studies employed RT exercise programs that met or exceeded the American College of Sports Medicine’s (ACSM) recommendations for RT in older adults.10 These findings are supported by Stewart et al11 who found that in most RT studies in adults who were >75 years that LM improves (3 of 4 studies) by 1.5% to 15.6%, with one study showing a decrease of 3%. Muscle strength improved in the 3 studies with increases of ~39% to 156%, while the study that did not show improvement in the intervention group showed far less of a decline during the course of the study versus the control group.
Importantly, RT in older adults is effective in improving muscle power, a strong predictor of physical function.146-151 Recently, higher velocity RT protocols utilizing faster movement contractions have been investigated and studies suggest that muscle power improvements may be comparable to gains in maximum strength/force production after traditional lower velocity RT programs.5 There is also increasing evidence that improvements in muscle function in older adults can be accomplished using a host of training protocols at faster or slower movement velocities,152 which indicates that multiple approaches are possible for developing an exercise prescription to improve muscular fitness in older adults. Convincingly, these findings suggest that positive responses to RT for most muscle and physical function outcomes occur despite substantial variations in RT programs, populations studied, and outcomes measured. Furthermore, group means tend to show positive responses, although considerable individual responses to RT exist among older individuals due at least in part to sex141,153,154 and genetic differences.155,156 Although total genetic variation can explain a significant amount of muscle function variability,157,158 this is a complex trait and individual gene polymorphisms likely do not have a substantial impact.
Current Recommendations for Older Adults to Improve Sarcopenia-Related Health Outcomes
Physical activity is perhaps the best-known treatment for many chronic diseases,159 but older adults tend to be less active than younger adults.5 According to the Centers for Disease Control,160 recent estimates suggest only 24% of adults ≥45 years and 16% aged 65 to 74 years meet recommendations for regular RT exercise. The ACSM recommends that older adults should participate in muscle strengthening activities for the major muscle groups 2 to 3 times per week on nonconsecutive days utilizing moderate- to vigorous-intensity RT (ie, ~65% to 80% of maximal strength152) and this prescription should also include balance training exercises for general health and for improvement and maintenance of functional independence.5,161
It should be emphasized that single-set training and/or fixed volume RT programs may not be advisable as a properly periodized (ie, planned variations such as regular alterations in training loads and movement velocities) RT training program for older adults is recommended to improve sarcopenia-related conditions.10 Although the optimal periodized RT program has not been determined, there is current research exploring this topic. In an early study, Newton et al examined the effects of a 10-week periodized RT intervention that incorporated faster movement velocities in older (n =10, age = 61 years) and younger (n = 8, age = 30 years) men.162 While younger men performed better in leg power and strength, older men exhibited a similar ability to improve muscle function. Villanueva et al163 recently observed improvements in LM, stair climbing ability, and upper and lower muscle strength in older men in their late 60s after 8 weeks of periodized RT with short and long rest intervals, although there were significantly greater improvements in the shorter rest interval group. While more research, especially including women, needs to be conducted examining various periodized RT protocols, initial results indicate that older adults may respond similarly to their younger counterparts and that periodized RT programs should be strongly considered in future intervention studies targeted at reducing sarcopenia-related conditions in older adults.
Conclusions
Sarcopenia is a complex syndrome that is attributed to aging, genetic factors, hormonal shifts, diet factors, body composition, neuromuscular and skeletal muscle changes, physical activity, and other chronic disease states. While the individual contributions and interactions between these variables are still being investigated, it is now understood that the condition, as it is currently being defined, is associated with risk of disability in older adults.
It is unclear whether dynapenia or sarcopenia will be the future focus of clinical screening for at-risk patients, but it is encouraging that the current definitions of sarcopenia include measures of muscle strength and function in their algorithms. Research suggests that dynapenia and obesity may exert a stronger influence on clinical measures of function compared to low LM levels, and women may be particularly susceptible. If an older patient is not able to walk at a sufficient gait speed or rise from a chair and these symptoms are not secondary to a clearly diagnosed etiologic factor, this may be the most pressing reason to recommend an intervention strategy to reverse the age-related strength and function deficiencies based on the patient’s individual needs. Thus, health practitioners should encourage positive behavior change by promoting physical activity (especially RT) in older adults who have demonstrated deficits in gait speed or muscle strength according to the aforementioned consensus guidelines. Primary care physicians should include an evaluation of gait speed and muscle strength during routine examinations to identify those who currently are or may be at risk for sarcopenia. Moreover, preceding investigations strongly suggest that even active older adults remain at risk for age-related declines in muscle strength and functional limitations, and it is critical that older adults engage in a regular exercise program with special emphasis on improving muscle strength and physical function.
Footnotes
Authors’ Note: Both authors contributed equally to the writing of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Healthy People 2020. Older Adults. Washington, DC: US Department of Health and Human Services; https://www.healthypeople.gov/2020/topics-objectives/topic/older-adults. Accessed February 20, 2015. [Google Scholar]
- 3. Manton KG. Recent declines in chronic disability in the elderly U.S. population: risk factors and future dynamics. Annu Rev Public Health. 2008;29:91-113. [DOI] [PubMed] [Google Scholar]
- 4. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80-85. [DOI] [PubMed] [Google Scholar]
- 5. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510-1530. [DOI] [PubMed] [Google Scholar]
- 6. Kostek MC, Delmonico MJ. Age-related changes in adult muscle morphology. Curr Aging Sci. 2011;4:221-233. [DOI] [PubMed] [Google Scholar]
- 7. Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010;13:271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond). 2005;29:1011-1029. [DOI] [PubMed] [Google Scholar]
- 9. Delmonico MJ, Lofgren IE. Resistance training during weight loss in overweight and obese older adults: what are the benefits? Am J Lifestyle Med. 2010;4:309-313. [Google Scholar]
- 10. Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc. 2011;43:249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewart VH, Saunders DH, Greig CA. Responsiveness of muscle size and strength to physical training in very elderly people: a systematic review. Scand J Med Sci Sports. 2014;24:e1-e10. [DOI] [PubMed] [Google Scholar]
- 12. Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev. 2010;9:226-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43:748-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12:249-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alchin DR. Sarcopenia: describing rather than defining a condition. J Cachexia Sarcopenia Muscle. 2014;5:265-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenberg I. Epidemiologic and methodologic problems in determining nutritional status of older persons—Proceedings of a meeting held in Albuquerque, New Mexico, October 19-21, 1988—Summary comments. Am J Clin Nutr. 1989;50:1231-1233. [PubMed] [Google Scholar]
- 19. Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997;52:B267-B276. [DOI] [PubMed] [Google Scholar]
- 20. Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851-1860. [DOI] [PubMed] [Google Scholar]
- 21. Hughes VA, Frontera WR, Wood M, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209-B217. [DOI] [PubMed] [Google Scholar]
- 22. Rantanen T, Guralnik JM, Ferrucci L, Leveille S, Fried LP. Coimpairments: strength and balance as predictors of severe walking disability. J Gerontol A Biol Sci Med Sci. 1999;54:M172-M176. [DOI] [PubMed] [Google Scholar]
- 23. Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558-560. [DOI] [PubMed] [Google Scholar]
- 24. Sturm R, Ringel JS, Andreyeva T. Increasing obesity rates and disability trends. Health Aff (Millwood). 2004;23:199-205. [DOI] [PubMed] [Google Scholar]
- 25. Manton KG, Gu XL. Changes in the prevalence of chronic disability in the United States black and nonblack population above age 65 from 1982 to 1999. Proc Natl Acad Sci U S A. 2001;98:6354-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Newman AB, Kupelian V, Visser M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602-1609. [DOI] [PubMed] [Google Scholar]
- 28. Cawthon PM, Fox KM, Gandra SR, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valero V, III, Amini N, Spolverato G, et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg. 2015;19:272-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72-77. [DOI] [PubMed] [Google Scholar]
- 31. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755-763. [DOI] [PubMed] [Google Scholar]
- 32. Clark BC, Manini TM. Sarcopenia ≠ dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829-834. [DOI] [PubMed] [Google Scholar]
- 33. Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123-136. [DOI] [PubMed] [Google Scholar]
- 34. Riebe D, Blissmer BJ, Greaney ML, Garber CE, Lees FD, Clark PG. The relationship between obesity, physical activity, and physical function in older adults. J Aging Health. 2009;21:1159-1178. [DOI] [PubMed] [Google Scholar]
- 35. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94. [DOI] [PubMed] [Google Scholar]
- 36. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314-322. [DOI] [PubMed] [Google Scholar]
- 37. McGough EL, Kelly VE, Logsdon RG, et al. Associations between physical performance and executive function in older adults with mild cognitive impairment: gait speed and the timed “up & go” test. Phys Ther. 2011;91:1198-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. [DOI] [PubMed] [Google Scholar]
- 40. Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23:314-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324-333. [DOI] [PubMed] [Google Scholar]
- 42. Yang M, Ding X, Luo L, Hao Q, Dong B. Disability associated with obesity, dynapenia and dynapenic-obesity in Chinese older adults. J Am Med Dir Assoc. 2014;15:150-156. [DOI] [PubMed] [Google Scholar]
- 43. Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813-1818. [DOI] [PubMed] [Google Scholar]
- 44. Lindle RS, Metter EJ, Lynch NA, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20-93 yr. J Appl Physiol. 1997;83:1581-1587. [DOI] [PubMed] [Google Scholar]
- 45. MastersWeightlifting.org. Masters weightlifting records. http://www.mastersweightlifting.org/records.htm. Accessed February 22, 2015.
- 46. Bean JF, Kiely DK, Herman S, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461-467. [DOI] [PubMed] [Google Scholar]
- 47. Foldvari M, Clark M, Laviolette LC, et al. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55A:M192-M199. [DOI] [PubMed] [Google Scholar]
- 48. Bassey EJ, Fiatarone MA, O’Niell EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci. 1992;82:321-327. [DOI] [PubMed] [Google Scholar]
- 49. Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65-89 years. Age Ageing. 1994;23:371-377. [DOI] [PubMed] [Google Scholar]
- 50. Runge M, Rittweger J, Russo CR, Schiessl H, Felsenberg D. Is muscle power output a key factor in the age-related decline in physical performance? A comparison of muscle cross section, chair-rising test and jumping power. Clin Physiol Funct Imaging. 2004;24:335-340. [DOI] [PubMed] [Google Scholar]
- 51. Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897-904. [DOI] [PubMed] [Google Scholar]
- 52. Tseng LA, Delmonico MJ, Visser M, et al. Body composition explains sex differential in physical performance among older adults. J Gerontol A Biol Sci Med Sci. 2014;69:93-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heymsfield SB, Wang ZM, Baumgartner RN, Ross R. Human body composition: advances in models and methods. Ann Rev Nutr. 1997;17:527-558. [DOI] [PubMed] [Google Scholar]
- 54. Anderson LJ, Erceg DN, Schroeder ET. Utility of multifrequency bioelectrical impedance compared with dual-energy x-ray absorptiometry for assessment of total and regional body composition varies between men and women. Nutr Res. 2012;32:479-485. [DOI] [PubMed] [Google Scholar]
- 55. Johnston CC., Jr Development of clinical practice guidelines for prevention and treatment of osteoporosis. Calcif Tissue Int. 1996;59(suppl 1):S30-S33. [DOI] [PubMed] [Google Scholar]
- 56. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure—the JNC 7 report. JAMA. 2003;289:2560-2572. [DOI] [PubMed] [Google Scholar]
- 57. Dam TT, Peters KW, Fragala M, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69:584-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769-774. [DOI] [PubMed] [Google Scholar]
- 59. Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cawthon PM, Peters KW, Shardell MD, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:567-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the Foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci. 2014;69:576-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fragala MS, Dam TT, Barber V, et al. Strength and function response to clinical interventions of older women categorized by weakness and low lean mass using classifications from the Foundation for the National Institute of Health sarcopenia project. J Gerontol A Biol Sci Med Sci. 2015;70:202-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87:1562S-1566S. [DOI] [PubMed] [Google Scholar]
- 64. Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56:M373-M380. [DOI] [PubMed] [Google Scholar]
- 65. Wolfe RR, Miller SL. The recommended dietary allowance of protein: a misunderstood concept. JAMA. 2008;299:2891-2893. [DOI] [PubMed] [Google Scholar]
- 66. Wolfe RR, Miller SL, Miller KB. Optimal protein intake in the elderly. Clin Nutr. 2008;27:675-684. [DOI] [PubMed] [Google Scholar]
- 67. Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422-424. [DOI] [PubMed] [Google Scholar]
- 68. Castaneda C, Charnley JM, Evans WJ, Crim MC. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am J Clin Nutr. 1995;62:30-39. [DOI] [PubMed] [Google Scholar]
- 69. Castaneda C, Dolnikowski GG, Dallal GE, Evans WJ, Crim MC. Protein turnover and energy metabolism of elderly women fed a low-protein diet. Am J Clin Nutr. 1995;62:40-48. [DOI] [PubMed] [Google Scholar]
- 70. Zeller K, Whittaker E, Sullivan L, Raskin P, Jacobson HR. Effect of restricting dietary protein on the progression of renal failure in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1991;324:78-84. [DOI] [PubMed] [Google Scholar]
- 71. Martin WF, Armstrong LE, Rodriguez NR. Dietary protein intake and renal function. Nutr Metab (Lond). 2005;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Friedman AN. High-protein diets: potential effects on the kidney in renal health and disease. Am J Kidney Dis. 2004;44:950-962. [DOI] [PubMed] [Google Scholar]
- 73. Morais JA, Chevalier S, Gougeon R. Protein turnover and requirements in the healthy and frail elderly. J Nutr Health Aging. 2006;10:272-283. [PubMed] [Google Scholar]
- 74. Ferrara LA, Innelli P, Palmieri V, et al. Effects of different dietary protein intakes on body composition and vascular reactivity. Eur J Clin Nutr. 2006;60:643-649. [DOI] [PubMed] [Google Scholar]
- 75. Hu FB, Stampfer MJ, Manson JE, et al. Dietary protein and risk of ischemic heart disease in women. Am J Clin Nutr. 1999;70:221-227. [DOI] [PubMed] [Google Scholar]
- 76. Obarzanek E, Velletri PA, Cutler JA. Dietary protein and blood pressure. JAMA. 1996;275:1598-1603. [DOI] [PubMed] [Google Scholar]
- 77. Wilson MM, Purushothaman R, Morley JE. Effect of liquid dietary supplements on energy intake in the elderly. Am J Clin Nutr. 2002;75:944-947. [DOI] [PubMed] [Google Scholar]
- 78. Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86:451-456. [DOI] [PubMed] [Google Scholar]
- 79. Katsanos CS, Chinkes DL, Paddon-Jones D, Zhang XJ, Aarsland A, Wolfe RR. Whey protein ingestion in elderly persons results in greater muscle protein accrual than ingestion of its constituent essential amino acid content. Nutr Res. 2008;28:651-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215-219. [DOI] [PubMed] [Google Scholar]
- 81. Wolfe RR. Regulation of muscle protein by amino acids. J Nutr. 2002;132:3219S-3224S. [DOI] [PubMed] [Google Scholar]
- 82. Martone AM, Lattanzio F, Abbatecola AM, et al. Treating sarcopenia in older and oldest old. Curr Pharm Des. 2015;21:1715-1722. [DOI] [PubMed] [Google Scholar]
- 83. Tarnopolsky MA. Caffeine and creatine use in sport. Ann Nutr Metab. 2010;57(suppl 2):1-8. [DOI] [PubMed] [Google Scholar]
- 84. Devries MC, Phillips SM. Creatine supplementation during resistance training in older adults-a meta-analysis. Med Sci Sports Exerc. 2014;46:1194-1203. [DOI] [PubMed] [Google Scholar]
- 85. Cooke MB, Brabham B, Buford TW, et al. Creatine supplementation post-exercise does not enhance training-induced adaptations in middle to older aged males. Eur J Appl Physiol. 2014;114:1321-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Villanueva MG, He J, Schroeder ET. Periodized resistance training with and without supplementation improve body composition and performance in older men. Eur J Appl Physiol. 2014;114:891-905. [DOI] [PubMed] [Google Scholar]
- 87. Fuller JC, Jr, Baier S, Flakoll P, Nissen SL, Abumrad NN, Rathmacher JA. Vitamin D status affects strength gains in older adults supplemented with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and lysine: a cohort study. JPEN J Parenter Enteral Nutr. 2011;35:757-762. [DOI] [PubMed] [Google Scholar]
- 88. Deutz NE, Pereira SL, Hays NP, et al. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. 2013;32:704-712. [DOI] [PubMed] [Google Scholar]
- 89. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148-e304. [DOI] [PubMed] [Google Scholar]
- 90. Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419-427. [DOI] [PubMed] [Google Scholar]
- 91. Tirabassi G, Gioia A, Giovannini L, et al. Testosterone and cardiovascular risk. Intern Emerg Med. 2013;8(suppl 1):S65-S69. [DOI] [PubMed] [Google Scholar]
- 92. Shores MM, Arnold AM, Biggs ML, et al. Testosterone and dihydrotestosterone and incident ischaemic stroke in men in the Cardiovascular Health Study. Clin Endocrinol (Oxf). 2014;81:746-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166:1660-1665. [DOI] [PubMed] [Google Scholar]
- 94. Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050-2058. [DOI] [PubMed] [Google Scholar]
- 95. Compston JE. Sex steroids and bone. Physiol Rev. 2001;81:419-447. [DOI] [PubMed] [Google Scholar]
- 96. Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocr Rev. 2004;25:389-425. [DOI] [PubMed] [Google Scholar]
- 97. Beck DT, Yarrow JF, Beggs LA, et al. Influence of aromatase inhibition on the bone-protective effects of testosterone. J Bone Miner Res. 2014;29:2405-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Borst SE, Yarrow JF, Conover CF, et al. Musculoskeletal and prostate effects of combined testosterone and finasteride administration in older hypogonadal men: a randomized, controlled trial. Am J Physiol Endocrinol Metab. 2014;306:E433-E442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Petak SM, Nankin HR, Spark RF, Swerdloff RS, Rodriguez-Rigau LJ. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients—2002 update. Endocr Pract. 2002;8:440-456. [PubMed] [Google Scholar]
- 100. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724-731. [DOI] [PubMed] [Google Scholar]
- 101. Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833-876. [DOI] [PubMed] [Google Scholar]
- 102. Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004;89:5920-5926. [DOI] [PubMed] [Google Scholar]
- 103. Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678-688. [DOI] [PubMed] [Google Scholar]
- 104. Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601-E607. [DOI] [PubMed] [Google Scholar]
- 105. Nair KS, Rizza RA, O’Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647-1659. [DOI] [PubMed] [Google Scholar]
- 106. Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2839-2853. [DOI] [PubMed] [Google Scholar]
- 107. Kovacheva EL, Hikim AP, Shen R, Sinha I, Sinha-Hikim I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology. 2010;151:628-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1-7. [DOI] [PubMed] [Google Scholar]
- 109. Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63:280-293. [DOI] [PubMed] [Google Scholar]
- 110. Tracz MJ, Sideras K, Bolona ER, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91:2011-2016. [DOI] [PubMed] [Google Scholar]
- 111. Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451-1457. [DOI] [PubMed] [Google Scholar]
- 112. Fernandez-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560-2575. [DOI] [PubMed] [Google Scholar]
- 113. Haddad RM, Kennedy CC, Caples SM, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:29-39. [DOI] [PubMed] [Google Scholar]
- 114. Spitzer M, Huang G, Basaria S, Travison TG, Bhasin S. Risks and benefits of testosterone therapy in older men. Nat Rev Endocrinol. 2013;9:414-424. [DOI] [PubMed] [Google Scholar]
- 115. Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502-1510. [DOI] [PubMed] [Google Scholar]
- 116. Page ST, Herbst KL, Amory JK, et al. Testosterone administration suppresses adiponectin levels in men. J Androl. 2005;26:85-92. [PubMed] [Google Scholar]
- 117. Maggi M, Schulman C, Quinton R, Langham S, Uhl-Hochgraeber K. The burden of testosterone deficiency syndrome in adult men: economic and quality-of-life impact. J Sex Med. 2007;4(pt 1):1056-1069. [DOI] [PubMed] [Google Scholar]
- 118. Tiidus PM, Lowe DA, Brown M. Estrogen replacement and skeletal muscle: mechanisms and population health. J Appl Physiol (1985). 2013;115:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum Reprod Update. 2007;13:559-565. [DOI] [PubMed] [Google Scholar]
- 120. Maltais ML, Desroches J, Dionne IJ. Changes in muscle mass and strength after menopause. J Musculoskelet Neuronal Interact. 2009;9:186-197. [PubMed] [Google Scholar]
- 121. van Geel TA, Geusens PP, Winkens B, Sels JP, Dinant GJ. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle mass, muscle strength and bone mineral density in postmenopausal women: a cross-sectional study. Eur J Endocrinol. 2009;160:681-687. [DOI] [PubMed] [Google Scholar]
- 122. Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772-M777. [DOI] [PubMed] [Google Scholar]
- 123. Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care. 2003;6:295-299. [DOI] [PubMed] [Google Scholar]
- 124. Brown M. Skeletal muscle and bone: effect of sex steroids and aging. Adv Physiol Educ. 2008;32:120-126. [DOI] [PubMed] [Google Scholar]
- 125. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333. [DOI] [PubMed] [Google Scholar]
- 126. Rossouw JE. Hormones for coronary disease-full circle. Lancet. 2002;360:1996-1997. [DOI] [PubMed] [Google Scholar]
- 127. Suzuki S, Brown CM, la Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104:6013-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sorensen MB, Rosenfalck AM, Hojgaard L, Ottesen B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res. 2001;9:622-626. [DOI] [PubMed] [Google Scholar]
- 129. Kenny AM, Dawson L, Kleppinger A, Iannuzzi-Sucich M, Judge JO. Prevalence of sarcopenia and predictors of skeletal muscle mass in nonobese women who are long-term users of estrogen-replacement therapy. J Gerontol A Biol Sci Med Sci. 2003;58:M436-M440. [DOI] [PubMed] [Google Scholar]
- 130. Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939-953. [DOI] [PubMed] [Google Scholar]
- 131. Messier V, Rabasa-Lhoret R, Barbat-Artigas S, Elisha B, Karelis AD, ubertin-Leheudre M. Menopause and sarcopenia: a potential role for sex hormones. Maturitas. 2011;68:331-336. [DOI] [PubMed] [Google Scholar]
- 132. Marcell TJ, Hawkins SA, Wiswell RA. Leg strength declines with advancing age despite habitual endurance exercise in active older adults. J Strength Cond Res. 2014;28:504-513. [DOI] [PubMed] [Google Scholar]
- 133. Cesari M, Vellas B, Hsu FC, et al. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J Gerontol A Biol Sci Med Sci. 2015;70:216-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rydwik E, Lammes E, Frandin K, Akner G. Effects of a physical and nutritional intervention program for frail elderly people over age 75. A randomized controlled pilot treatment trial. Aging Clin Exp Res. 2008;20:159-170. [DOI] [PubMed] [Google Scholar]
- 136. Frontera WR, Hughes VA, Krivickas LS, Kim SK, Foldvari M, Roubenoff R. Strength training in older women: early and late changes in whole muscle and single cells. Muscle Nerve. 2003;28:601-608. [DOI] [PubMed] [Google Scholar]
- 137. Vincent KR, Braith RW, Feldman RA, et al. Resistance exercise and physical performance in adults aged 60 to 83. J Am Geriatr Soc. 2002;50:1100-1107. [DOI] [PubMed] [Google Scholar]
- 138. Henwood TR, Taaffe DR. Improved physical performance in older adults undertaking a short-term programme of high-velocity resistance training. Gerontology. 2005;51:108-115. [DOI] [PubMed] [Google Scholar]
- 139. Straight CR, Lofgren IE, Delmonico MJ. Resistance training in older adults: are community-based interventions effective for improving health outcomes? Am J Lifestyle Med. 2012;5:407-414. [Google Scholar]
- 140. Brill PA, Probst JC, Greenhouse DL, Schell B, Macera CA. Clinical feasibility of a free-weight strength-training program for older adults. J Am Board Fam Pract. 1998;11:445-451. [DOI] [PubMed] [Google Scholar]
- 141. Delmonico MJ, Kostek MC, Doldo NA, et al. Effects of moderate-velocity strength training on peak muscle power and movement velocity: do women respond differently than men? J Appl Physiol. 2005;99:1712-1718. [DOI] [PubMed] [Google Scholar]
- 142. Watanabe Y, Tanimoto M, Oba N, Sanada K, Miyachi M, Ishii N. Effect of resistance training using bodyweight in the elderly: comparison of resistance exercise movement between slow and normal speed movement [published online January 17, 2015]. Geriatr Gerontol Int. doi: 10.1111/ggi.12427. [DOI] [PubMed] [Google Scholar]
- 143. Machado A, Garcia-Lopez D, Gonzalez-Gallego J, Garatachea N. Whole-body vibration training increases muscle strength and mass in older women: a randomized-controlled trial. Scand J Med Sci Sports. 2010;20:200-207. [DOI] [PubMed] [Google Scholar]
- 144. Britt E, Hudson SM, Blampied NM. Motivational interviewing in health settings: a review. Patient Educ Couns. 2004;53:147-155. [DOI] [PubMed] [Google Scholar]
- 145. Mikesky AE, Topp R, Wigglesworth JK, Harsha DM, Edwards JE. Efficacy of a home-based training program for older adults using elastic tubing. Eur J Appl Physiol Occup Physiol. 1994;69:316-320. [DOI] [PubMed] [Google Scholar]
- 146. Bean JF, Herman S, Kiely DK, et al. Increased Velocity Exercise Specific to Task (InVEST) training: a pilot study exploring effects on leg power, balance, and mobility in community-dwelling older women. J Am Geriatr Soc. 2004;52:799-804. [DOI] [PubMed] [Google Scholar]
- 147. Earles DR, Judge JO, Gunnarsson OT. Velocity training induces power-specific adaptations in highly functioning older adults. Arch Phys Med Rehabil. 2000;82:872-878. [DOI] [PubMed] [Google Scholar]
- 148. Ferri A, Scaglioni G, Pousson M, Capodaglio P, Van Hoecke J, Narici MV. Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand. 2003;177:69-78. [DOI] [PubMed] [Google Scholar]
- 149. Fielding RA, LeBrasseur NK, Cuoco A, Bean J, Mizer K, Fiatarone Singh MA. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc. 2002;50:655-662. [DOI] [PubMed] [Google Scholar]
- 150. Hakkinen K, Kraemer WJ, Newton RU, Alen M. Changes in electromyographic activity, muscle fibre and force production characteristics during heavy resistance/power strength training in middle-aged and older men and women. Acta Physiol Scand. 2001;171:51-62. [DOI] [PubMed] [Google Scholar]
- 151. Jozsi AC, Campbell WW, Joseph L, Davey SL, Evans WJ. Changes in power with resistance training in older and younger men and women. J Gerontol A Biol Sci Med Sci. 1999;54:M591-M596. [DOI] [PubMed] [Google Scholar]
- 152. Cadore EL, Pinto RS, Bottaro M, Izquierdo M. Strength and endurance training prescription in healthy and frail elderly. Aging Dis. 2014;5:183-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tracy BL, Ivey FM, Hurlbut D, et al. Muscle quality. II. Effects of strength training in 65- to 75-yr-old men and women. J Appl Physiol. 1999;86:195-201. [DOI] [PubMed] [Google Scholar]
- 154. Walts CT, Hanson ED, Delmonico MJ, Yao L, Wang MQ, Hurley BF. Do sex or race differences influence strength training effects on muscle or fat? Med Sci Sports Exerc. 2008;40:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Delmonico MJ, Kostek MC, Doldo NA, et al. Alpha-actinin-3 (ACTN3) R577X polymorphism influences knee extensor peak power response to strength training in older men and women. J Gerontol A Biol Sci Med Sci. 2007;62:206-212. [DOI] [PubMed] [Google Scholar]
- 156. Pereira A, Costa AM, Izquierdo M, Silva AJ, Bastos E, Marques MC. ACE I/D and ACTN3 R/X polymorphisms as potential factors in modulating exercise-related phenotypes in older women in response to a muscle power training stimuli. Age (Dordr). 2013;35:1949-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Arden NK, Spector TD. Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Mineral Res. 1997;12:2076-2081. [DOI] [PubMed] [Google Scholar]
- 158. Tiainen K, Sipila S, Alen M, et al. Shared genetic and environmental effects on strength and power in older female twins. Med Sci Sports Exerc. 2005;37:72-78. [DOI] [PubMed] [Google Scholar]
- 159. Blair SN, Sallis RE, Hutber A, Archer E. Exercise therapy—the public health message. Scand J Med Sci Sports. 2012;22:e24-e28. [DOI] [PubMed] [Google Scholar]
- 160. Vezina JW, Der Ananian CA, Greenberg E, Kurka J. Sociodemographic correlates of meeting US Department of Health and Human Services muscle strengthening recommendations in middle-aged and older adults. Prev Chronic Dis. 2014;11:E162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.US Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Washington, DC: US Department of Health and Human Services; http://www.health.gov/paguidelines/pdf/paguide.pdf. Accessed April 20, 2015. [Google Scholar]
- 162. Newton RU, Hakkinen K, Hakkinen A, McCormick M, Volek J, Kraemer WJ. Mixed-methods resistance training increases power and strength of young and older men. Med Sci Sports Exerc. 2002;34:1367-1375. [DOI] [PubMed] [Google Scholar]
- 163. Villanueva MG, Lane CJ, Schroeder ET. Short rest interval lengths between sets optimally enhance body composition and performance with 8 weeks of strength resistance training in older men. Eur J Appl Physiol. 2015;115:295-308. [DOI] [PubMed] [Google Scholar]