Abstract
Despite significant advances in medical technology and pharmacology, cardiovascular disease (CVD) remains a major contributor to health care expenses and the leading cause of death in the United States. Patients with established CVD and their health care providers are challenged with achieving cardiovascular risk reduction to decrease the likelihood of recurrent cardiovascular events. This “secondary prevention” can be achieved, in part, through adherence to prescribed pharmacotherapies that favorably modify major coronary risk factors (ie, hypertension, hypercholesterolemia, diabetes, and obesity). However, lifestyle modification can also be helpful in this regard, providing independent and additive benefits to the associated reductions in cardiovascular morbidity and mortality. Accordingly, physicians and other health care providers should routinely counsel their coronary patients to engage in structured exercise and increased lifestyle physical activity, consume a heart-healthy diet, quit smoking and avoid secondhand smoke, and purposefully address psychosocial stressors that may elevate cardiovascular risk. These lifestyle interventions, either as an adjunct to medication therapy or independently in those patients where medications may be poorly tolerated, cost prohibitive, or ineffective, can significantly decrease cardiovascular mortality and the risk of recurrent cardiac events.
Keywords: secondary prevention, risk factor reduction, cardiovascular mortality, lifestyle modification
“. . . adjunctive lifestyle modification in the setting of established CVD is arguably of equal importance in reducing the risk of recurrent cardiovascular events.”
Despite a 31% decline in cardiovascular disease (CVD) death rates from 2000 to 2010,1 heart disease remains the leading cause of death in the United States, followed by cancer, respiratory disease, accidents, and stroke.2 In 2010, US$193.4 billion was spent on direct medical costs associated with stroke and heart disease, excluding associated nursing home care expenses.1 For those experiencing nonfatal cardiovascular events, coronary revascularization procedures and/or new cardiac diagnoses, patients and their health care providers are challenged with the ongoing management and associated economic burden of their disease.
Although scientific evidence supports guidelines-based pharmacotherapies for achieving cardiovascular risk reduction, adjunctive lifestyle modification in the setting of established CVD is arguably of equal importance in reducing the risk of recurrent cardiovascular events. Contemporary guidelines for coronary patients from the American Heart Association (AHA) and the American College of Cardiology Foundation (ACCF) identify “lifestyle modification” as a Class 1B recommendation for blood pressure control, physical activity, and lipid/lipoprotein, weight and diabetes management.3 Accordingly, post–myocardial infarction (MI) patients who reported adherence to just 3 healthy lifestyle habits at 30 days post–hospital discharge—smoking cessation, regular exercise, and healthy eating—demonstrated a 3.8-fold decreased risk of death, re-infarction, and stroke after 6 months as compared with those who adhered to none of these behaviors.4
Reported data regarding adherence to healthy lifestyle choices in secondary prevention, however, are discouraging. In an economically diverse cohort of 7519 individuals with self-reported coronary heart disease or stroke histories, only 4.3% reported compliance with smoking cessation, a healthy diet, and high levels of regular physical activity, whereas 14.3% reported nonadherence to these lifestyle behaviors.5 Similarly, a considerable percentage of patients receiving percutaneous coronary intervention (PCI) for stable angina do not achieve lifestyle and risk factor goals at 1-year postprocedure, leaving them at increased risk for recurrent events.6 Thus, patients with CVD should be counseled to engage in purposeful lifestyle modification, including regular exercise and physical activity, smoking cessation and avoiding secondhand smoke, heart-healthy nutrition, and addressing adverse psychosocial modulators of behavior change, such as depression or anxiety, to optimize outcomes in secondary prevention.
The Role of Pharmacotherapy in Secondary Prevention
Adherence to prescribed pharmacotherapies in patients with CVD is a proven strategy in reducing the risk of recurrent cardiovascular events. Contemporary AHA/ACCF guidelines recommend beta-blockers, angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin II receptor blockers (ARB), statins, and antithrombotic therapy in all post-MI patients.3,7 Table 1 summarizes the indications, mechanisms of action, and cautions/side effects for each.3,7,8 Collectively, these medications, particularly when used in combination, are independently and strongly associated with lower 6-month mortality in patients with acute coronary syndromes as compared with patients in whom these medications are omitted.9
Table 1.
Indications, Mechanisms of Action, and Cautions and Side Effects for Major Recommended Medication Classes After Acute Myocardial Infarction.a,b
| Medication Class | Indications in CVD | Mechanism of Action | Cautions/Side Effects |
|---|---|---|---|
| Beta-blocker |
|
|
|
| Statin |
|
|
|
| ACE-I |
|
|
|
| Antiplatelet therapy |
|
|
|
Abbreviations: CVD, cardiovascular disease; ACS, acute coronary syndrome; AMI, acute myocardial infarction; HF, heart failure; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; LDL-C, low-density lipoprotein cholesterol; total-C, total cholesterol; Apo-B, apolipoprotein-B; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; ACE-I, angiotensin-converting enzyme inhibitor; STEMI, ST-elevation myocardial infarction; LVEF, left ventricular ejection fraction; ARB, angiotensin-II receptor blocker; MI, myocardial infarction; ACE, angiotensin-converting enzyme); P2Y12, purinergic receptor P2Y, G-protein coupled, 12; PCI, percutaneous coronary intervention.
Each medication class contains agents which may differ in pharmacology, indications for approved use, and specific adverse effects. The table above represents a general summary of the medication class. For individual drug information, refer to specific drug references.
Table adapted from:
O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362-e425.
Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458-2473.
Ciccone CD. Pharmacology in Rehabilitation. 4th ed. Philadelphia, PA: F.A. Davis Company; 2007: 281-284, 292, 293, 297-299, 353, 354, 358-360.
Certainly, the efficacy of any medication is largely dependent on appropriate dosing and patient compliance. Arnold et al10 found that although 87% of post-MI patients were prescribed a beta blocker, statin, and ACE-I or ARB at hospital discharge, only 1 in 3 were taking goal doses of all three medication classes. At 12 months postdischarge, goal doses of these medications were achieved in only 12%, 26%, and 32% of eligible patients, respectively. Using the Duke Databank for Cardiovascular Disease, researchers reported a “consistent use” rate of only 21% for combined adherence to aspirin, beta-blocker, and statin therapy.11 Moreover, a recent report of adherence to secondary prevention pharmacotherapies in 7955 post-MI patients found that almost one-third had stopped taking at least 1 prescribed cardioprotective mediation at the 6-month follow-up.12 Although cardioprotective medications favorably affect morbidity and mortality, suboptimal treatment or nonadherence are associated with a broad range of adverse outcomes in patients with coronary artery disease.13 These findings suggest that medication dosing and nonadherence should be targets for physician-directed quality improvement interventions to maximize outcomes of patients with coronary disease.
Exercise, Fitness, and Physical Activity in Secondary Prevention
Cardiac medications play an integral role in optimizing secondary prevention outcomes; however, adopting healthier lifestyle behaviors are complementary in this regard. As with beta-blocker, statin, ACE-I/ARB, and anticoagulant therapy, exercise-based cardiac rehabilitation (CR) is considered a Class 1A recommendation, whereas physical activity is listed as a Class 1B intervention.3 Structured aerobic exercise, increased lifestyle physical activity, or both, are associated with an improved coronary risk factor profile, including decreased platelet aggregation, resting systolic and diastolic blood pressure, and intra-abdominal and total body fat, improved insulin sensitivity and blood lipid profiles, and enhanced cardiorespiratory fitness.14 One widely cited meta-analysis of 48 trials including 8940 patients reported that exercise-based CR was associated with reduced cardiovascular and all-cause mortality rates of 20% and 26%, respectively, as compared with usual care.15 Greater reductions in total cholesterol, triglyceride levels, systolic blood pressure, and self-reported smoking rates were also observed. Moreover, the health benefits derived from CR appear to be largely maintained, at least over a 1-year follow-up.16
Others have demonstrated similar findings. Iestra et al17 estimated regular physical activity to be associated with a 25% reduction in mortality risk for CVD patients in their review of 3 meta-analyses, 10 randomized controlled trials, and 9 cohort studies. Separate analyses have reported comparable or lesser mortality benefits in older coronary patients18 and those with systolic heart failure,19 respectively. Furthermore, an analysis comparing exercise-based programming per se with more comprehensive CR programs found that exercise-only programs were associated with a reduction in all-cause mortality of ~25%.20
Improved cardiorespiratory fitness (CRF), expressed as peak metabolic equivalents (METs; 1 MET = 3.5 mL O2/kg/min), can be achieved through progressive moderate-to-vigorous training. As with structured exercise and increased lifestyle physical activity, CRF has been consistently associated with reduced all-cause and cardiovascular mortality, as well as a lower risk for recurrent MI and coronary revascularization in secondary prevention.21-23 Reported reductions in all-cause mortality per 1-MET increase in CRF have ranged from 8% to 14% in non-CR settings,21-23 to as high as 31% to 45% in selected CR cohorts.24-27
Exercise and CRF Versus Pharmacotherapy
Studies comparing the independent and/or additive effects of pharmacotherapy versus increased CRF or physical activity in patients with CVD are limited. The survival benefit conferred by low-dose aspirin, statins, beta-blockers, and ACE-I therapy following acute MI is comparable to the reported reduction in mortality associated with a 1-MET improvement in CRF.28 Welty et al29 evaluated the impact of superimposed exercise on the Therapeutic Lifestyle Changes diet in 27 patients with established CVD. Fifty-nine percent of participants were taking lipid-lowering drugs at intake, with no change in their medications during the 6-month study. Adding just 30 minutes of daily exercise to their treatment regimen allowed 89% of participants to achieve a low-density lipoprotein cholesterol <130 mg/dL, without lowering the cardioprotective high-density lipoprotein cholesterol subfraction.
To clarify the combined effects of statin treatment and CRF on all-cause mortality, Kokkinos et al30 followed 10 043 military veterans with dyslipidemia over a median follow-up of 10.0 years. Although only 42% of participants had a history of CVD, both statin therapy and higher CRF levels were associated with reduced all-cause mortality, independent of other clinical characteristics. Furthermore, in participants not taking statins, achieving a CRF >9 METs conferred a reduced mortality risk comparable to (or even slightly lower than) those on statin therapy with only “moderate” or “fit” exercise capacities (5.1-9.0 METs). Thus, higher levels of CRF and statin therapy, especially in combination, can profoundly increase survival in patients with known lipid abnormalities. Figure 1 conveys the impact of regular exercise on selected cardiovascular risk factors and other outcome modulators as compared with statin therapy in the secondary prevention of coronary disease.31
Figure 1.
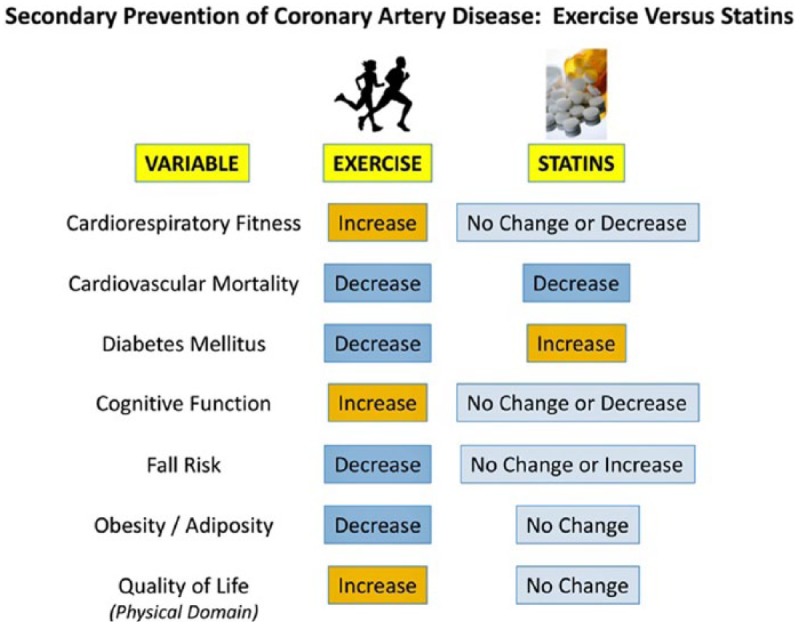
Comparison of the effects of structured exercise and statins on varied risk factors and health outcome modulators in the prevention of recurrent cardiovascular events. Although both regular exercise and statins appear to confer substantial decreases in cardiovascular mortality, the superiority of exercise over statins is apparent when other variables are considered.
Lifestyle interventions, including regular physical activity, may also be highly effective in pre-diabetes management. In a cohort of 3234 participants with elevated fasting plasma glucose concentrations, those randomized to a lifestyle modification program with the goals of ≥150 minutes of physical activity per week and a reduction in body weight of ≥7% demonstrated a 58% reduction in the incidence of diabetes over a 2.8-year follow-up, as compared with placebo controls.32 Conversely, participants randomized to metformin therapy reduced the risk of developing diabetes by only 31%, further highlighting the comparable or greater impact of lifestyle change versus drug therapy.
Considerations for Exercise in Secondary Prevention
Despite overwhelming evidence for its effectiveness in primary and secondary prevention, adherence to evidence-based structured exercise and physical activity recommendations remains suboptimal.5,33 In 2014, only 49.2% of adults reported activity levels that met contemporary recommendations,33 and nearly 30% of adults reported no leisure-time aerobic activity.1 Moreover, sedentary lifestyle behaviors (ie, prolonged sitting) are associated with metabolic derangements and other adverse health outcomes, including increased all-cause and cardiovascular mortality, independent of leisure-time physical activity.34
Because patient adherence to physical activity regimens and exercise-based CR is strongly related to the fervor of physician endorsement,35 coronary patients should be regularly counseled to engage in moderate intensity aerobic exercise for 30 to 60 minutes, ≥5 days per week, complemented by increased lifestyle physical activity.3 When encouraging patients to adopt such lifestyle changes, motivational interviewing techniques, physician-patient collaborative communication, and varied fitness-based technologies (ie, pedometers, accelerometers, heart rate monitors, software-based applications) may be helpful in promoting behavior change. For example, pedometer usage is associated with improved indices of cardiovascular health, including increased physical activity levels, decreased body mass index, and reduced blood pressure.36
Smoking Cessation in Secondary Prevention
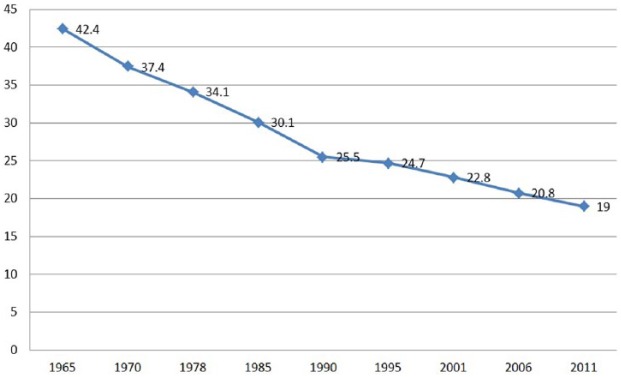
Smoking is a major preventable contributor to disease and disability, associated health care expenses, and premature death worldwide. Smoking and exposure to secondhand smoke significantly increase the risk of CVD and stroke, some cancers, premature delivery and other birth complications, respiratory illnesses including chronic obstructive pulmonary disease, fertility problems, and bone degeneration, among others.37,38 Recent data from the US Surgeon General indicates that ≥42 million American adults (almost 1 in 5) are current cigarette smokers, accounting for 480 000 deaths each year.37,39 Numerous public awareness campaigns over the past several decades have emphasized the broad health risks associated with cigarette smoking, likely orchestrating a steady decline in incidence rates (Figure 2).40
Figure 2.
Percentage of US adults who smoke cigarettes, trended from 1965 to 2011.
Smoking and exposure to secondhand smoke undoubtedly contribute to initial and recurrent cardiovascular events. Acute exposure to smoke triggers a cascade of adverse cardiovascular responses, including enhanced hypercoagulability, reduced oxygen delivery to the tissues, increased inflammation, and coronary vasoconstriction. Unfortunately, recent data suggest that approximately one-third of smokers continue to smoke after an acute MI, despite the known health consequences.4 One systematic review of 20 studies found a 36% reduction in mortality risk for coronary patients who successfully quit smoking, as compared with those who continued to smoke.41 Other reports substantiate that the risk for recurrent cardiovascular events significantly increases for active smokers following an incident MI, whereas quitters achieve a risk equal to nonsmokers 3 years after cessation.42 The risk for sudden cardiac death is also higher in coronary patients who continue to smoke.43 Compared with the salutary impact of moderate- and high-dose statin therapy in the IDEAL44 and TNT trials,45 smoking cessation was associated with more than double the reduction in absolute death rates, further supporting the need for smoking cessation interventions in secondary prevention.46
Although the morbidity and mortality benefits of smoking cessation are compelling, quitting can be challenging for many coronary patients. In particular, younger individuals, nonparticipants in CR, patients who are severely depressed, long-time cigarette smokers, and those regularly exposed to environmental smoke, report a lower readiness for smoking cessation.47 Conversely, Snaterse et al48 found that post-MI patients who quit smoking immediately after their acute cardiac event were more likely to maintain abstinence at 1-year follow-up, without relapse prevention support. Another review concluded that smoking “reduction” may also be an effective tool; however, measurable health benefits accrue only if cessation is ultimately achieved.49 Thus, encouraging patients to quit following an acute cardiac event, in addition to identifying and remediating barriers that may adversely affect motivation, should be addressed.
Approaches to Smoking Cessation
Smoking cessation interventions in secondary prevention may include both pharmacologic and behavioral approaches. In a review of clinical trials examining 6- and 12-month abstinence rates among cardiac and noncardiac populations, all smoking cessation aids demonstrated modest benefits as compared with placebo.50 Several smoking cessation medications are currently available, including nicotine inhalers, patches, and gum, antidepressants such as bupropion, and nicotinic receptor partial agonists (varenicline). Although the effectiveness of bupropion in primary prevention settings51 and in patients with stable CVD52 has been demonstrated, similar data in patients after an acute MI are lacking.53 Varenicline may be effective if initiated in-hospital following an acute cardiac event; however, additional studies are needed to substantiate the safety of this approach.54
To clarify the overall safety of pharmacologic smoking cessation therapy, a meta-analysis of 63 randomized controlled trials, 8 of which included CVD patient populations, evaluated adverse responses to nicotine replacement therapy, varenicline, and bupropion interventions.55 No increase in cardiovascular event risk was observed with bupropion or varenicline, whereas a heightened risk of less serious events (ie, tachycardia) was noted with nicotine replacement. Investigators also examined major adverse cardiovascular events, demonstrating modest protective effects with bupropion and no clear evidence of harm with nicotine replacement or varenicline. Authors concluded that pharmacologic smoking cessation interventions were unlikely to increase the risk of serious cardiovascular events.
Electronic cigarettes (or e-cigarettes), which emit nicotine-containing vapors into the lungs, have been suggested as a healthier smoking alternative and cessation aid. However, e-cigarettes remain largely unregulated, resulting in inconsistent product engineering and potential health risks associated with their use. A recent scientific review including both population studies and clinical trials concluded insufficient evidence exists to support e-cigarettes as a tool for smoking cessation.56 Moreover, no long-term studies evaluating safety are available because of e-cigarettes’ limited time on the market, leaving many important questions unanswered. Thus, providers should exercise caution when suggesting e-cigarettes as a quitting aid or smoking alternative, and strongly urge patients who pursue this option to avoid long-term use.
Psychosocial interventions are also useful in helping coronary patients reduce and quit smoking, including individual and group behavioral therapeutic approaches, self-help materials focused on smoking cessation or risk factor modification, physician and nursing advice/counseling, proactive outreach, telephone support, or combinations thereof. A systematic review of 40 randomized controlled trials using behavioral approaches, self-help materials, and telephone support in coronary patients found an overall positive and comparable effect on abstinence at both 6 and 12 months, although selected cohorts were poorly represented in the study populations (ie, women, younger patients, non-MI diagnoses).57 More aggressive and longer duration interventions were associated with increased quit rates; however, the 7 trials that assessed longer-term impact found no benefit at 5 years. Proactive outreach, where smokers are purposefully identified and offered evidence-based smoking cessation interventions, has also shown promise. In a study of 6400 veterans who were identified as active smokers, including a substantial cohort with known CVD (~27%), those randomized to proactive care demonstrated higher smoking abstinence at 1-year versus a usual care control group.58
Contemporary technology is increasingly utilized in health care, and smoking cessation interventions are no exception. Electronic aids, such as Internet sites, computer programs, and text message initiatives are increasingly employed to assist individuals quit smoking. Chen et al59 reported that electronic aids increased the likelihood of smoking cessation as compared with generic self-help materials or no intervention. Although the effect was modest, it appeared to be cost-effective.
To clarify the independent or additive benefits of pharmacologic and psychosocial cessation interventions, Stead and Lancaster60 conducted a large-scale analysis of 41 randomized or quasi-randomized controlled trials, including more than 15 000 participants. Combined pharmacotherapy and behavioral treatment was superior to usual care, less intensive behavioral support, or brief advice (risk ratio: 1.82), especially when analyzing studies that recruited participants from healthcare versus community-based settings (risk ratio: 2.06). In a study that combined intensive smoking cessation counseling with pharmacotherapy in hospitalized acute-MI patients, quit rates, all-cause mortality, and hospital readmissions were all positively affected as compared with a usual care group.61 In another systematic review, Rigotti et al62 found that smoking cessation interventions initiated during hospitalization for acute coronary syndrome (ACS), when sustained for ≥1 month post–hospital discharge, were associated with increased abstinence rates. Hospitalized patients who received nicotine replacement therapy demonstrated higher quit rates over counseling alone, whereas bupropion and varenicline administration had no additive effects.
Nutrition in Secondary Prevention
Numerous studies now suggest that dietary practices are strongly associated with the development of CVD and its associated sequelae. As demonstrated by the ONTARGET and TRANSCEND trials of high-risk patients with existing CVD or diabetes who were on drug therapies for secondary prevention, a healthy diet rich in whole grains, nuts, fish, fruits, and vegetables was associated with an ~20% reduction in the risk of recurrent cardiovascular events as compared with a high-fat, high-cholesterol diet.63 Similarly, in a recent study that evaluated post-MI patients from prospective cohorts of the Nurses’ Health Study and the Health Professional Follow-Up Study, a higher quality diet was associated with a lower risk of all-cause mortality.64
Dietary Patterns and Pharmacotherapy in Primary and Secondary Prevention
The role of nutrition in the development and manifestation of CVD is multifaceted and likely involves progressive increases in inflammatory markers and conventional coronary risk factors. Historically, research on diet and CVD focused on individual dietary components such as total and saturated fat and cholesterol, and food groups, including fruits, vegetables, and fish. More recently, studies have emphasized the influence of dietary patterns, including the Dietary Approaches to Stop Hypertension (DASH) or the Mediterranean Diet (MD), as discussed below.
The DASH diet, consisting primarily of fruits, vegetables, fish, lean meat, and low-fat dairy, is naturally low in sodium, cholesterol, and fat. This dietary pattern significantly decreases blood pressure,65,66 enhances the impact of antihypertensive medications,67 and reduces the risk of developing CVD68 and its associated morbidity68 and mortality.69,70 Of note, many investigations provided DASH-specific food products to study participants. Although rigorous dietary control likely augmented study validity, this may limit the generalizability of the results to “real world” situations.71,72 Despite an abundance of primary prevention studies in apparently healthy and “at-risk” subjects, few data are available regarding the effect of the DASH diet in secondary prevention.
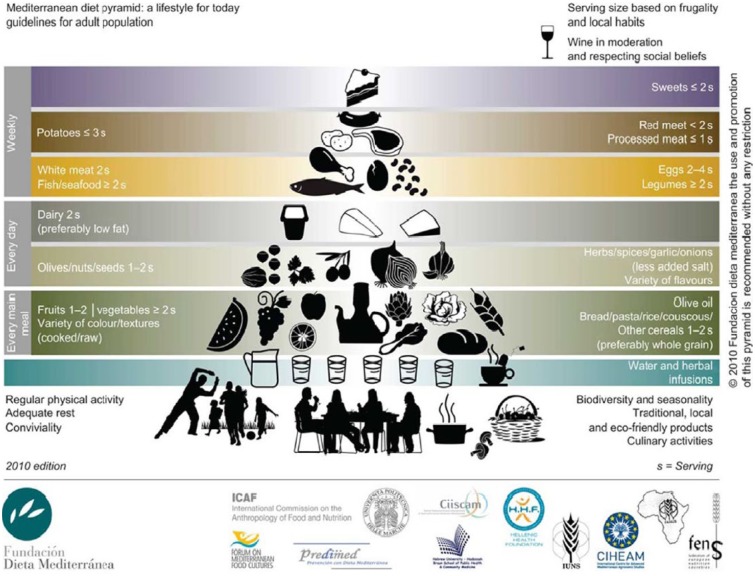
An alternative dietary approach, the MD, is characterized by a high consumption of fruits, vegetables, whole grains, marine protein, a moderate intake of olive oil and red wine, and limited meat, dairy, sweets, and processed foods (Figure 3).73,74 Literature suggests that the MD has a favorable but modest impact on blood pressure75,76 and blood lipids,76,77 whereas its effect on weight management remains unclear. A meta-analysis comparing the MD to low-fat diets found that subjects randomized to the former had greater reductions in body weight after 2 years as compared to those consuming the latter.76 Conversely, a large cohort study of apparently healthy young-to-middle-aged women failed to demonstrate decreases in body weight or waist circumference over a 12-year follow-up, even with high adherence to the MD.78
Figure 3.
The Mediterranean diet pyramid.
Additionally, research suggests that the MD may reduce the severity and incidence of coronary artery disease (CAD) and its associated inflammatory indices. The ATTICA study, including more than 3000 apparently healthy Greek men and women, demonstrated that higher adherence to a MD was independently associated with reduced inflammatory biomarkers such as C-reactive protein (CRP), interleukin-6, and white blood cell count, regardless of weight loss.79 Substantiating these findings, Nordmann et al76 reported improvements in coronary risk factors with adherence to the MD, including reduced CRP. Among persons at high cardiovascular risk, a MD supplemented with extra-virgin olive oil or nuts reduced the incidence of major cardiovascular events by ~30%.80 Similarly, the ATTICA study found that adherence to the MD reduced the risk of CVD by nearly 30% in patients without established CVD, independent of statin use.81
To evaluate the effects of the MD in secondary prevention, investigators have examined its impact on the severity of CAD and the incidence of recurrent cardiovascular events following an acute MI. Akgüllü et al82 reported a negative correlation between adherence to the MD and severity of CAD in patients with established heart disease, possibly due to the associated anti-inflammatory effects. In a single-blinded, randomized study comparing the MD with a standard cardiac diet, post-MI subjects randomized to a MD demonstrated lower rates of re-infarction and cardiac death at both the 27-month83 and 48-month84 follow-ups. Moreover, Iestra et al85 noted improved prognosis for post-MI patients who maintained at least 3 of 4 health behaviors: compliance with a MD, moderate alcohol consumption, nonsmoking behavior, and/or regular moderate to vigorous physical activity.
Others have investigated the impact of a MD on endothelial function. In 131 patients with established CVD and hyperlipidemia, those randomized to a MD (n = 68) demonstrated significant improvements in brachial artery flow–mediated vasodilatation after 1 year as compared with a usual-care control group (n = 63).86 Participants in both groups were prescribed the same statin type, dosage and frequency at baseline, highlighting the independent and additive benefits of the MD for improving endothelial function.
Considerations for Nutrition in Secondary Prevention
As demonstrated by the nurse-coordinated EUROACTION trial, lifestyle choices that include heart-healthy nutrition can significantly decrease cardiovascular risk.87 This family-centered primary and secondary intervention trial for those with or at high risk for CVD demonstrated reduced blood pressure and central obesity measurements after lifestyle modification, including healthier food choices and increased physical activity. Over the course of 1 year, these benefits were achieved with fewer medications, except in very high-risk individuals, as compared with the usual care group. In a more recent report, researchers determined that lifestyle counseling that included cardioprotective dietary modifications was effective in reducing cardiac events and increasing the likelihood of positive lifestyle changes among post–coronary artery bypass graft and valvular surgery patients.88
Increasing evidence suggests that a heart-healthy diet reduces cardiovascular risk not only by favorably modifying lipids,89,90 blood pressure, body weight, and insulin resistance, but by reducing inflammation as well.90,91 Independent of statins or weight loss, therapeutic lifestyle changes such as those promulgated by comprehensive CR programs result in substantial reductions in CRP, similar in magnitude to statin therapy.92 Thus, in the secondary prevention of CVD, it is imperative to address dietary habits soon after hospital discharge, ideally in an exercise-based outpatient CR program. Over time, the initial fear of recurrent cardiac events may dissipate, and the motivation, commitment, and likelihood of healthy dietary choices may diminish, particularly in those patients with inadequate or inaccurate recall of information, poor self-control, and limited psychosocial support.93
Psychosocial Considerations in Secondary Prevention
Numerous behavior patterns, psychological disorders, personality traits, and social factors, either singly or collectively, may increase the risk of initial and recurrent cardiovascular events. Included among these harbingers of CVD are depression, anxiety, hostility, anger, and social isolation.94,95 Given the number and complexity of psychosocial modulators influencing heart health, discussion will focus on the impact of depression on CVD, along with the nonpharmacologic and lifestyle interventions that have shown promise in secondary prevention.
Depression and Cardiovascular Disease
An estimated 31% to 45% of all coronary patients suffer from mild-to-moderate clinically significant depression,96 and an additional ~20% present with a major depressive disorder (MDD).97 Comparing these rates with the general population, 1 national community sample reported the prevalence of current (30-day) MDD at 4.9%, and a lifetime risk of 17.1%.98 Moreover, the prevalence of depression in secondary prevention may be underreported, as these patients are less likely to receive a depression diagnosis than their apparently healthy counterparts.99
Although not currently listed among the conventional risk factors for heart disease, a recent scientific statement by the AHA recommends that depression should be elevated to risk factor status in patients with ACS because of its profound impact on prognosis.100 This statement closely followed an earlier AHA Science Advisory recommending depression screening for all patients with heart disease.101 The 2-step AHA screening process for depression using the Patient Health Questionnaire (PHQ)-2 and PHQ-9 surveys is valid, reliable, and feasible,102 as well as highly specific for depression in identifying those patients at elevated risk for recurrent cardiac events and poorer outcomes.103 Depression screening may occur in primary care, cardiology or exercise-based CR settings, so that patients are accurately identified and treated.
Indeed, depression is an independent and powerful predictor of short- and long-term outcomes in patients with CVD.104-107 Studies have consistently demonstrated that clinical depression is associated with an approximate 2-fold increased risk of mortality in cardiac populations,106 and more specifically, a 2.0 to 2.5 fold increased risk of recurrent cardiovascular events and mortality in post-MI patients.108 Milani and Lavie109 reported an even higher mortality risk, that is, a 4-fold increase, for depressed coronary patients as compared with their nondepressed counterparts. Multiple studies have also shown that postbypass patients with depression have an increased risk of recurrent cardiac events110,111 and cardiovascular mortality.110-112
Behavioral, Biological, and Personality Mechanisms in Depression and Cardiovascular Disease
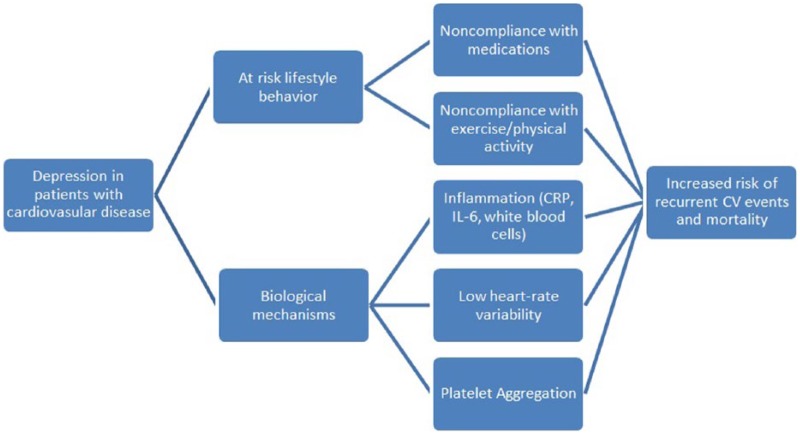
The potential mechanisms linking depression and recurrent cardiovascular events are multi-factorial and complex (Figure 4).113 Compliance with lifestyle behavior modification, such as adherence to prescribed medications, attendance at CR, and participation in regular exercise, is markedly reduced in cardiac patients with depression.114-116 Depressed patients with CVD are at twice the risk of noncompliance to prescribed therapies, as compared with their nondepressed counterparts.117,118 Depression is also a potential barrier to enrollment in comprehensive CR and exercise participation, both of which reduce the risk of recurrent cardiovascular events. Kronish et al114 found that persistent depression at 3 months post-ACS was significantly associated with lack of participation in regular exercise and enrollment in CR. Thus, the relationship between CVD and depression could, in part, be due to the fact that depressed patients are more likely to decline, avoid, or be nonadherent to prescribed therapies, resulting in poorer outcomes.
Figure 4.
Negative impact of depression on lifestyle behaviors and plausible, associated adverse biological mechanisms, leading to increased recurrent cardiovascular events and heightened mortality.
Biological mechanisms such as inflammation, increased platelet activity and aggregation, and autonomic nervous system dysfunction may also contribute to the increased risk of CVD and recurrent cardiovascular events.113 Kop et al119 reported that inflammation, evidenced by biomarkers CRP, interleukin-6, fibrinogen, and white blood cell count, accounted for 12.7% of the effects of depression on cardiovascular mortality. Moreover, in 2 studies evaluating the relation of inflammation to depression and incident CVD,120,121 the association between depression and CVD was modestly linked to inflammatory biomarkers.
The autonomic nervous system may also bridge the association between depression and inflammation in CVD. Increased sympathetic and decreased parasympathetic activity confers reduced heart-rate variability (HRV), which is associated with heightened mortality in post-MI and heart failure patients.122,123 In a study of 72 outpatients with stable CVD (40 meeting major depression criteria, and 32 nondepressed), an inverse relation was observed between HRV and depression; that is, the greater the depression, the lower the HRV.124 Others have shown the relation between HRV and inflammatory biomarkers was strongest in stable coronary patients with elevated depressive symptoms, even after controlling for potential confounders, including beta-blocker therapy.125
Several smaller studies have linked elevated platelet activation levels and depression with increased adverse outcomes.126-129 In contrast, the Heart and Soul study found that stable coronary patients with major depression exhibited no difference in platelet activation levels as compared with their nondepressed counterparts.130 Though it appears that antidepressant drug therapy may reduce platelet activation,128,131-133 it remains unclear whether this favorably affects cardiovascular morbidity and mortality.133
Psychosocial Predictors of Cardiovascular Risk
Personality traits such as anxiety, anger, and hostility are potential outcome modulators in patients with CVD. Nakamura et al134 reported that although depression was superior to anxiety for predicting adverse cardiac outcomes in hospitalized patients with CVD, anger was associated with more favorable outcomes. In a prospective study of >400 patients referred for PCI, high pre-procedure anxiety levels were associated with lower mortality and a reduced risk of major cardiovascular events.135 Most studies, however, indicate a higher risk of CVD, recurrent cardiovascular events, and/or poorer outcomes in patients with high anxiety, hostility, and/or anger.136-140
A limited social support network also predicts adverse outcomes in secondary prevention, and is comparable to traditional cardiac risk factors (i.e., hypertension, smoking) as a prognostic indicator.141 Previous studies suggest an increased risk of cardiovascular events142 and associated mortality143 in patients with established CVD having low levels of social support. Barth et al144 found that low functional support (perceived benefit of the relationship components) was associated with higher cardiovascular mortality in patients with coronary disease, whereas the impact of low structural support (frequency of contact in one’s social network) on survival was unclear. Accordingly, secondary prevention programs should include efforts to evaluate and address deficiencies in patient social support.
Pharmacotherapy, Lifestyle Interventions, and Considerations
Historically, the gold standard for the treatment of MDD has included prescribed antidepressant medications and psychotherapy; however, only 55% of clinically depressed patients seek treatment.145 Accordingly, research has increasingly focused on the effectiveness of lifestyle interventions, most notably, structured exercise and physical activity, as a complementary or alternative therapeutic option.146-149 Blumenthal et al149 evaluated 156 older men and women with diagnosed depression, randomizing them to one of 3 groups: aerobic exercise, medication treatment with sertraline, or combined exercise/antidepressant therapy. Although patients in the medication-only group showed earlier improvements in depressive symptoms, all groups demonstrated comparable and statistically significant decreases in depression scores over the 16-week study period. Accordingly, exercise was equally effective in reducing depressive symptoms in this older cohort, a promising finding for patients who may be resistant to traditional antidepressant treatments.
Other research studies have attempted to clarify the impact of exercise as an alternative therapeutic option for treatment-resistant patients with clinical depression. Thirty-three patients taking traditional antidepressive combined therapies for 9 to 15 months, without evidence of clinical remission, were randomized to a control pharmacotherapy-only group or pharmacotherapy plus a 12-week home-based walking program.150 Although none of the participants in the control group experienced remission, 26% of those in the exercise group showed remission and 21% demonstrated improvement in depression indices and functional parameters. Thus, a simple home-based walking program as an adjunctive therapy may yield significant improvements in treatment-resistant patients with depression.
Research employing a female-only cohort also supports the notion that exercise may be an effective complementary treatment option. Carneiro et al148 reported that a small cohort of women randomized to an antidepressant/exercise group reduced anxiety (P = .025), stress (P = .012), and self-reported depressive symptoms (P = .031), as compared with an antidepressant-only control group. Moreover, women in the treatment group also demonstrated increased performance on varied functional assessments, highlighting the additional benefits accrued by participating in regular exercise.
Although woefully underutilized, comprehensive CR may be effective in reducing the adverse impact of psychosocial risk factors on recurrent cardiovascular morbidity and mortality largely via the associated counseling, social support and exercise interventions. Yohannes et al151 found that a brief, 6-week CR program improved quality of life, physical activity status, anxiety and depression, benefits that persisted at 12-month follow-up. While women may have higher reported levels of depression at program entry, both men and women experience a reduction in depressive symptoms after CR.152 And in a systematic review, Whalley et al153 found that although psychological interventions in CR did not appear to further reduce the incidence of MI or all-cause mortality, they favorably modified depression, anxiety, and cardiac mortality independent of other components of CR, such as structured exercise.
Notably, some antidepressants may negatively influence selected cardiovascular risk factors, including hypercholesterolemia, diabetes, and long-term weight management.154-156 To clarify the impact of antidepressant therapy on health outcomes, Gordon et al157 evaluated cardiovascular risk factor improvements among nondepressed and depressed medicated and nonmedicated coronary patients after CR. All cohorts demonstrated comparable improvements in blood pressure, weight, fasting glucose, body mass index, and cholesterol levels, highlighting the efficacy of exercise-based programming irrespective of antidepressant treatment.
Studies that specifically address the associations among depression, lifestyle interventions and prescribed pharmacotherapies in patients with established CVD are limited. Compared with usual care, coronary patients in the UPBEAT trial who exercised 3 times per week for 30 to 45 minutes per session achieved greater reductions in depressive symptoms.158 Demonstrated improvements were similar to, if not greater than, those achieved with antidepressants (sertraline) in those with diagnosed MDD. Despite limited evidence in secondary prevention, the range of cohorts where exercise has favorably affected depression as an adjunct to antidepressants (ie, older participants, women) is encouraging. Moreover, patients with and without CVD who engage in regular exercise demonstrate acute and chronic physiological responses that may combat depression, including reduced inflammation, improved metabolic parameters, and augmented release of mood-elevating hormones.
Like other bona fide treatments for depression, the efficacy of exercise and/or CR may be attenuated if patient compliance is suboptimal. Research consistently cites long-term exercise compliance as a significant challenge.159 Thus, when suggesting or recommending exercise to depressed patients, physicians and health care providers should clearly communicate the potential benefits of exercise, consider incentivizing strategies that may optimize adherence, and provide appropriate resources and support (ie, referral to a comprehensive CR program) for initiating and maintaining a regular exercise program. One investigation reported a 73% lower mortality rate in depressed patients who completed CR versus their nonadherent counterparts.109 Accordingly, empowering patients with individualized education, counseling, and support is critical in optimizing treatment for depression and achieving desirable improvements in cardiovascular outcomes.
Conclusion
Physicians and other health care providers have a unique opportunity to provide comprehensive secondary prevention interventions to their patients with established CVD. Accordingly, recommendations should extend beyond prescribed cardioprotective pharmacotherapies to include lifestyle modifications that facilitate cardiovascular risk reduction. Contemporary guidelines for coronary and other atherosclerotic disease patients include lifestyle modification strategies in addition to pharmacotherapy3,7 that when incorporated into clinical practice result in decreased 30-day and 1-year mortality.160 Lifestyle changes, including regular exercise, following a heart-healthy diet, quitting smoking and avoiding secondhand smoke, and addressing depression and other psychosocial modulators of behavior, provide independent and additive benefits to patients with CVD. Thus, physicians and paramedical professionals should purposefully educate their patients regarding evidence-based lifestyle changes, discuss implementation strategies, identify perceived barriers to behavior change, provide access to resources and support, engage in goal setting, and revisit mutually agreed–upon goals and actions on an ongoing basis, to further optimize secondary prevention.
The proven, salutary impact of comprehensive lifestyle modification has been overlooked and underemphasized as a first-line approach to stabilize overt CVD. Effective pharmacotherapies and coronary revascularization should be complemented by enhanced efforts to identify and favorably modify conventional risk factors and their underlying causes. More than a decade ago, Wald and Law161 proposed a cardioprotective polypill as a population strategy to combat CVD, combining the incremental benefits of combination drug therapies (ie, aspirin, statins, beta-blockers, ACE-I) in a single pill. Numerous promising preliminary studies162 as well as pharmaceutical interests and consumer demand (ie, convenience) suggest that a cardioprotective polypill may soon be commercially available. We recommend that treatment with the pill be accompanied by the following “User Directions”:
Take medication each day in the prescribed dosage, followed or preceded by ≥30 minutes of moderate-to-vigorous physical activity, in combination with a heart-healthy diet, weight management, the cessation of cigarette smoking and the avoidance of secondhand smoke, and interventions to address psychosocial risk factors, if appropriate.163
Acknowledgments
The authors would like to acknowledge Brenda White for her comprehensive editorial support.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. LCWK9. Deaths, percent of total deaths, and death rates for the 15 leading causes of death: United States and each state, 2013. 2014. http://www.cdc.gov/nchs/data/dvs/LCWK9_2013.pdf. Accessed February 3, 2016.
- 3. Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458-2473. [DOI] [PubMed] [Google Scholar]
- 4. Chow CK, Jolly S, Rao-Melacini P, Fox KA, Anand SS, Yusuf S. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation. 2010;121:750-758. [DOI] [PubMed] [Google Scholar]
- 5. Teo K, Lear S, Islam S, et al. Prevalence of a healthy lifestyle among individuals with cardiovascular disease in high-, middle- and low-income countries: the Prospective Urban Rural Epidemiology (PURE) study. JAMA. 2013;309:1613-1621. [DOI] [PubMed] [Google Scholar]
- 6. Khattab AA, Knecht M, Meier B, et al. Persistence of uncontrolled cardiovascular risk factors in patients treated with percutaneous interventions for stable coronary artery disease not receiving cardiac rehabilitation. Eur J Prev Cardiol. 2013;20:743-749. [DOI] [PubMed] [Google Scholar]
- 7. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362-e425. Erratum in: Circulation 2013;128(25): e481. [DOI] [PubMed] [Google Scholar]
- 8. Ciccone CD. Pharmacology in Rehabilitation. 4th ed. Philadelphia, PA: F. A. Davis Company; 2007:281-284, 292,, 293, 297,-299, 353,, 354, 358-360. [Google Scholar]
- 9. Mukherjee D, Fang J, Chetcuti S, Moscucci M, Kline-Rogers E, Eagle KA. Impact of combination evidence-based medical therapy on mortality in patients with acute coronary syndromes. Circulation. 2004;109:745-749. [DOI] [PubMed] [Google Scholar]
- 10. Arnold SV, Spertus JA, Masoudi FA, et al. Beyond medication prescription as performance measures: optimal secondary prevention medication dosing after AMI. J Am Coll Cardiol. 2013;62:1791-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113:203-212. [DOI] [PubMed] [Google Scholar]
- 12. Mathews R, Wang TY, Honeycutt E, et al. Persistence with secondary prevention medications after acute myocardial infarction: insights from the TRANSLATE-ACS study. Am Heart J. 2015;170:62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155:772-779. [DOI] [PubMed] [Google Scholar]
- 14. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2009:9-10. [Google Scholar]
- 15. Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682-692. [DOI] [PubMed] [Google Scholar]
- 16. Gupta R, Sanderson BK, Bittner V. Outcomes at one-year follow-up of women and men with coronary artery disease discharged from cardiac rehabilitation: what benefits are maintained? J Cardiopulm Rehab Prev. 2007;27:11-18. [DOI] [PubMed] [Google Scholar]
- 17. Iestra JA, Kromhout D, van Der Schouw YT, Grobbee DE, Boshuizen HC, van Staveren WA. Effect size estimates of lifestyle and dietary changes on all-cause mortality in coronary artery disease patients: a systematic review. Circulation. 2005;112:924-934. [DOI] [PubMed] [Google Scholar]
- 18. Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009;54:25-33. [DOI] [PubMed] [Google Scholar]
- 19. O’Connor CM, Whellon DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown A, Taylor R, Noorani H, Stone J, Skidney B. Exercise-Based Cardiac Rehabilitation Programs for Coronary Artery Disease: A Systematic Clinical and Economic Review. Ottawa, Ontario, Canada: Canadian Coordinating Office for Health Technology Assessment (CCOHTA); 2003. [Google Scholar]
- 21. Hung RK, Al-Mallah MH, McEvoy JW, et al. Prognostic value of exercise capacity in patients with coronary artery disease: the FIT (Henry Ford ExercIse Testing) project. Mayo Clin Proc. 2014;89:1644-1654. [DOI] [PubMed] [Google Scholar]
- 22. Dorn J, Naughton J, Imamura D, Trevisan M. Results of a multicenter randomized trial of exercise and long-term survival in myocardial infarction patients: the National Exercise and Heart Disease Project (NEHDP). Circulation. 1999;100:1764-1769. [DOI] [PubMed] [Google Scholar]
- 23. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793-801. [DOI] [PubMed] [Google Scholar]
- 24. Kavanagh T, Mertens DJ, Hamm LF, et al. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J Am Coll Cardiol. 2003;42:2139-2143. [DOI] [PubMed] [Google Scholar]
- 25. Keteyian SJ, Brawner CA, Savage PD, et al. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J. 2008;156:292-300. [DOI] [PubMed] [Google Scholar]
- 26. Goel K, Thomas RJ, Squires RW, et al. Combined effect of cardiorespiratory fitness and adiposity on mortality in patients with coronary artery disease. Am Heart J. 2011;161:590-597. [DOI] [PubMed] [Google Scholar]
- 27. Kavanagh T, Mertens DJ, Hamm LF, et al. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation. 2002;106:666-671. [DOI] [PubMed] [Google Scholar]
- 28. Boden WE, Franklin BA, Wenger NK. Physical activity and structured exercise for patients with stable ischemic heart disease. JAMA. 2013;309:143-144. [DOI] [PubMed] [Google Scholar]
- 29. Welty FK, Stuart E, O’Meara M, Huddleston J. Effect of addition of exercise to therapeutic lifestyle changes diet in enabling women and men with coronary heart disease to reach Adult Treatment Panel III low-density lipoprotein cholesterol goal without lowering high-density lipoprotein cholesterol. Am J Cardiol. 2002;89:1201-1204. [DOI] [PubMed] [Google Scholar]
- 30. Kokkinos PF, Faselis C, Myers J, Panagiotakos D, Doumas M. Interactive effects of fitness and statin treatment on mortality risk in veterans with dyslipidaemia: a cohort study. Lancet. 2013;381:394-399. [DOI] [PubMed] [Google Scholar]
- 31. Franklin BA, Lavie CJ. Impact of statins on physical activity and fitness: ally or adversary? Mayo Clin Proc. 2015;90:1314-1319. [DOI] [PubMed] [Google Scholar]
- 32. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Centers for Disease Control and Prevention. Exercise or Physical Activity. NCHS FastStats Web site. http://www.cdc.gov/nchs/fastats/exercise.htm. Accessed September 16, 2015.
- 34. Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162:123-132. [DOI] [PubMed] [Google Scholar]
- 35. Grace SL, Gravely-Witte S, Brual J, et al. Contribution of patient and physician factors to cardiac rehabilitation enrollment: a prospective multilevel study. Eur J Cardiovasc Prev Rehabil. 2008;15:548-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systemic review. JAMA. 2007;298:2296-2304. [DOI] [PubMed] [Google Scholar]
- 37. US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report From the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 38. U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: What It Means to You: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- 39. Centers for Disease Control and Prevention. Current cigarette smoking among adults—United States, 2005-2013. Morb Mortal Wkly Rep. 2014;63:1108-1112. [PMC free article] [PubMed] [Google Scholar]
- 40. Centers for Disease Control and Prevention. Trends in Current Cigarette Smoking Among High School Students and Adults, United States, 1965-2011. http://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/. Accessed February 2, 2016.
- 41. Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290:86-97. [DOI] [PubMed] [Google Scholar]
- 42. Rea TD, Heckbert SR, Kaplan RC, Smith NL, Lemaitre RN, Psaty BM. Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med. 2002;137:494-500. [DOI] [PubMed] [Google Scholar]
- 43. Peters RW, Brooks MM, Todd L, Liebson PR, Wilhelmsen L. Smoking cessation and arrhythmic death: the CAST experience. The Cardiac Arrhythmia Suppression Trial (CAST) investigators. J Am Coll Cardiol. 1995;25:1287-1292. [DOI] [PubMed] [Google Scholar]
- 44. Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437-2445. [DOI] [PubMed] [Google Scholar]
- 45. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425-1435. [DOI] [PubMed] [Google Scholar]
- 46. Frey P, Waters DD, DeMicco DA, et al. Impact of smoking on cardiovascular events in patients with coronary disease receiving contemporary medical therapy (from the Treating to New Targets [TNT] and the Incremental Decrease in End Points Through Aggressive Lipid Lowering [IDEAL] trials). Am J Cardiol. 2011;107:145-150. [DOI] [PubMed] [Google Scholar]
- 47. Prugger C, Wellmann J, Heidrich J, et al. Readiness for smoking cessation in coronary heart disease patients across Europe: results from the EUROASPIRE III survey. Eur J Prev Cardiol. 2015;22:1212-1219. [DOI] [PubMed] [Google Scholar]
- 48. Snaterse M, Scholte Op, Reimer WJ, Dobber J, et al. Smoking cessation after an acute coronary syndrome: immediate quitters are successful quitters. Neth Heart J. 2015;23:600-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Begh R, Lindson-Hawley N, Aveyard P. Does reduced smoking if you can’t stop make any difference? BMC Med. 2015;13:257. doi: 10.1186/s12916-015-0505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ludvig J, Miner B, Eisenberg MJ. Smoking cessation in patients with coronary artery disease. Am Heart J. 2005;149:565-572. [DOI] [PubMed] [Google Scholar]
- 51. Fossati R, Apolone G, Negri E, et al. A double-blind, placebo-controlled, randomized trial of bupropion for smoking cessation in primary care. Arch Intern Med. 2007;167:1791-1797. [DOI] [PubMed] [Google Scholar]
- 52. Tonstad S, Farsang C, Klaene G, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicenter, randomised study. Eur Heart J. 2003;24:946-955. [DOI] [PubMed] [Google Scholar]
- 53. Eisenberg MJ, Grandi SM, Gervais A, et al. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: a randomized, placebo-controlled trial. J Am Coll Cardiol. 2013;61:524-532. [DOI] [PubMed] [Google Scholar]
- 54. Eisenberg MJ, Windle SB, Roy N, et al. Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation. 2015;133:21-30. [DOI] [PubMed] [Google Scholar]
- 55. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129:28-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barth J, Jacob T, Daha I, Critchley J. Psychosocial interventions for smoking cessation in patients with coronary heart disease. Cochrane Database Syst Rev. 2015;7:CD006886. doi: 10.1002/14651858.CD006886.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fu SS, van Ryn M, Sherman SE, et al. Proactive tobacco treatment and population-level cessation: a pragmatic randomized clinical trial. JAMA Intern Med. 2014;174:671-677. [DOI] [PubMed] [Google Scholar]
- 59. Chen YF, Madan J, Welton N, et al. Effectiveness and cost-effectiveness of computer and other electronic aids for smoking cessation: a systematic review and network meta-analysis. Health Technol Assess. 2012;16:1-205. [DOI] [PubMed] [Google Scholar]
- 60. Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2012;10:CDC008286. [DOI] [PubMed] [Google Scholar]
- 61. Mohiuddin SM, Mooss AN, Hunter CB, Grollmes TL, Cloutier DA, Hillerman DE. Intensive smoking cessation intervention reduces mortality in high-risk smokers with cardiovascular disease. Chest. 2007;131:446-452. [DOI] [PubMed] [Google Scholar]
- 62. Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012;5:CD001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dehghan M, Mente A, Teo KK, et al. Relationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31 546 high-risk individuals from 40 countries. Circulation. 2012;126:2705-2712. [DOI] [PubMed] [Google Scholar]
- 64. Li S, Chiuve SE, Flint A, et al. Better diet quality and decreased mortality among myocardial infarction survivors. JAMA Intern Med. 2013;173:1808-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH Sodium Collaborative Research Group. N Engl J Med. 2001;344:3-10. [DOI] [PubMed] [Google Scholar]
- 66. Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr. 2015;113:1-15. [DOI] [PubMed] [Google Scholar]
- 67. Conlin PR, Erlinger TP, Bohannon A, et al. The DASH diet enhances the blood pressure response to losartan in hypertensive patients. Am J Hypertens. 2003;16:337-342. [DOI] [PubMed] [Google Scholar]
- 68. Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases-incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29:611-618. [DOI] [PubMed] [Google Scholar]
- 69. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713-720. [DOI] [PubMed] [Google Scholar]
- 70. Maruthur NM, Wang NY, Appel LJ. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER trial. Circulation. 2009;119:2026-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jiang J, Liu M, Troy LM, Bangalore S, Hayes RB, Parekh N. Concordance with DASH diet and blood pressure change: results from the Framingham Offspring Study (1991-2008). J Hypertens. 2015;33:2223-2230. [DOI] [PubMed] [Google Scholar]
- 72. Stradling C, Hamid M, Taheri S, Thomas GN. A review of dietary influences on cardiovascular health: part 2: dietary patterns. Cardiovasc Hematol Disord Drug Targets. 2014;14:50-63. [DOI] [PubMed] [Google Scholar]
- 73. Huhn S, Kharabian Masouleh S, Stumvoll M, Stumvoll M, Villringer A, Witte AV. Components of a Mediterranean diet and their impact on cognitive functions in aging. Front Aging Neurosci. 2015;7:132. doi: 10.3389/fnagi.2015.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14:2274-2284. [DOI] [PubMed] [Google Scholar]
- 75. Nissensohn M, Román-Viñas B, Sánchez-Villegas A, Piscopo S, Serra-Majem L. The effect of the Mediterranean Diet on hypertension: a systematic review and meta-analysis. J Nutr Educ Behav. 2016;48:42-53. [DOI] [PubMed] [Google Scholar]
- 76. Nordmann AJ, Suter-Zimmermann K, Bucher HC, et al. Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am J Med. 2011;124:841-851. [DOI] [PubMed] [Google Scholar]
- 77. Mertens E, Mullie P, Deforche B, et al. Cross-sectional study on the relationship between the Mediterranean Diet Score and blood lipids. Nutr J. 2014;13:88. doi: 10.1186/1475-2891-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li Y, Roswall N, Ström P, Sandin S, Adami HO, Weiderpass E. Mediterranean and Nordic diet scores and long-term changes in body weight and waist circumference: results from a large cohort study. Br J Nutr. 2015;114:2093-2102. [DOI] [PubMed] [Google Scholar]
- 79. Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: the ATTICA study. J Am Coll Cardiol. 2004;44:152-158. [DOI] [PubMed] [Google Scholar]
- 80. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279-1290. [DOI] [PubMed] [Google Scholar]
- 81. Panagiotakos DB, Georgousopoulou EN, Georgiopoulos GA, et al. Adherence to Mediterranean diet offers an additive protection over the use of statin therapy: results from the ATTICA study (2002-2012). Curr Vasc Pharmacol. 2015;13:778-787. [DOI] [PubMed] [Google Scholar]
- 82. Akgüllü Ç, Sırıken F, Eryılmaz U, et al. The relation between compliance to the Mediterranean diet and the extensiveness of coronary artery disease. Turk Kardiyol Dern Ars. 2015;43:340-349. [DOI] [PubMed] [Google Scholar]
- 83. de Lorgeril M, Renaud S, Mamelle N, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343:1454-1459. [DOI] [PubMed] [Google Scholar]
- 84. de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779-785. [DOI] [PubMed] [Google Scholar]
- 85. Iestra J, Knoops K, Kromhout D, de Groot L, Grobbee D, van Staveren W. Lifestyle, Mediterranean diet and survival in European post–myocardial infarction patients. Eur J Cardiovasc Prev Rehabil. 2006;13:894-900. [DOI] [PubMed] [Google Scholar]
- 86. Søndergaard E, Møller JE, Egstrup K. Effect of dietary intervention and lipid-lowering treatment on brachial vasoreactivity in patients with ischemic heart disease and hypercholesterolemia. Am Heart J. 2003;145:903. [DOI] [PubMed] [Google Scholar]
- 87. Wood DA, Kotseva K, Connolly S, et al. Nurse-coordinated multidisciplinary, family-based cardiovascular disease prevention programme (EUROACTION) for patients with coronary heart disease and asymptomatic individuals at high risk of cardiovascular disease: a paired, cluster-randomised controlled trial. Lancet. 2008;371:1999-2012. [DOI] [PubMed] [Google Scholar]
- 88. Kadda O, Kotanidou A, Manginas A, Stavridis G, Nanas S, Panagiotakos DB. Lifestyle intervention and one-year prognosis of patients following open heart surgery: a randomised clinical trial. J Clin Nurs. 2015;24:1611-1621. [DOI] [PubMed] [Google Scholar]
- 89. Iestra JA, Kromhout D, van der Schouw YT, Grobbee DE, Boshuizen HC, van Staveren WA. Effect size estimates of lifestyle and dietary changes on all-cause mortality in coronary artery disease patients: a systematic review. Circulation. 2005;112:924-934. [DOI] [PubMed] [Google Scholar]
- 90. Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685. [DOI] [PubMed] [Google Scholar]
- 91. Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569-2578. [DOI] [PubMed] [Google Scholar]
- 92. Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43:1056-1061. [DOI] [PubMed] [Google Scholar]
- 93. Doyle B, Fitzsimons D, McKeown P, McAloon T. Understanding dietary decision-making in patients attending a secondary prevention clinic following myocardial infarction. J Clin Nurs. 2012;21:32-41. [DOI] [PubMed] [Google Scholar]
- 94. Franklin BA. Impact of psychosocial risk factors on the heart: changing paradigms and perceptions. Phys Sports Med. 2009;37:1-3. [DOI] [PubMed] [Google Scholar]
- 95. Shibeshi WA, Young-Xu Y, Blatt CM. Anxiety worsens prognosis in patients with coronary artery disease. J Am Coll Cardiol. 2007;49:2021-2027. [DOI] [PubMed] [Google Scholar]
- 96. Celano CM, Huffman JC. Depression and cardiac disease: a review. Cardiol Rev. 2011;19:130-142. [DOI] [PubMed] [Google Scholar]
- 97. Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121:S20-S27. [DOI] [PubMed] [Google Scholar]
- 98. Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151:979-986. [DOI] [PubMed] [Google Scholar]
- 99. Jiang W, Krishnan RR, O’Connor CM. Depression and heart disease: evidence of a link, and its therapeutic implications. CNS Drugs. 2002;16:111-127. [DOI] [PubMed] [Google Scholar]
- 100. Lichtman JH, Froelicher ES, Blumenthal JA, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129:1350-1369. [DOI] [PubMed] [Google Scholar]
- 101. Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768-1775. [DOI] [PubMed] [Google Scholar]
- 102. Celano CM, Suarez L, Mastromauro C, Januzzi JL, Huffman JC. Feasibility and utility of screening for depression and anxiety disorders in patients with cardiovascular disease. Circ Cardiovasc Qual Outcomes. 2013;6:498-504. [DOI] [PubMed] [Google Scholar]
- 103. Elderon L, Smolderen KG, Na B, Whooley MA. Accuracy and prognostic value of American Heart Association–recommended depression screening in patients with coronary heart disease: data from the Heart and Soul Study. Circ Cardiovasc Qual Outcomes. 2011;4:533-540. [DOI] [PubMed] [Google Scholar]
- 104. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Borowicz L, Jr, Royall R, Grega M, Selnes O, Lyketsos C, McKhann G. Depression and cardiac morbidity 5 years after coronary artery bypass surgery. Psychosomatics. 2002;43:464-471. [DOI] [PubMed] [Google Scholar]
- 106. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802-813. [DOI] [PubMed] [Google Scholar]
- 107. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305-315. [DOI] [PubMed] [Google Scholar]
- 108. van Melle J, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66:814-822. [DOI] [PubMed] [Google Scholar]
- 109. Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med. 2007;120:799-806. [DOI] [PubMed] [Google Scholar]
- 110. Connerney I, Shapiro PA, McLaughlin JS, Bagiella E, Sloan RP. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet. 2001;358:1766-1771. [DOI] [PubMed] [Google Scholar]
- 111. Protogerou C, Fleeman N, Dwan K, Richardson M, Dundar Y, Hagger MS. Moderators of the effect of psychological interventions on depression and anxiety in cardiac surgery patients: a systematic review and meta-analysis. Behav Res Ther. 2015;73:151-164. [DOI] [PubMed] [Google Scholar]
- 112. Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003;362:604-609. [DOI] [PubMed] [Google Scholar]
- 113. Huffmann JC, Celano CM, Beach SR, Motiwala SR, Januzzi JL. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925. doi: 10.1155/2013/695925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kronish IM, Rieckmann N, Halm EA, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med. 2006;21:1178-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bautista LE, Vera-Cala LM, Colombo C, Smith P. Symptoms of depression and anxiety and adherence to antihypertensive medication. Am J Hypertens. 2012;25:505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Murphy BM, Grande MR, Navaratnam HS, et al. Are poor health behaviours in anxious and depressed cardiac patients explained by sociodemographic factors? Eur J Prev Cardiol. 2013;20:995-1003. [DOI] [PubMed] [Google Scholar]
- 117. Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med. 2005;165:2508-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101-2107. [DOI] [PubMed] [Google Scholar]
- 119. Kop WJ, Stein PK, Tracey RP, Barzilay JI, Schulz R, Gottdiener JS. Autonomic nervous dysfunction and inflammation contribute to the increased cardiovascular mortality risk associated with depression. Psychosom Med. 2010;72:626-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Vaccarino V, Johnson BD, Sheps DS, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50:2044-2050. [DOI] [PubMed] [Google Scholar]
- 121. Davidson KW, Schwartz JE, Kirkland SA, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. 2009;103:755-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Frenneaux MP. Autonomic changes in patients with heart failure and in post–myocardial infarction patients. Heart. 2004;90:1248-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Buccelletti E, Gilardi E, Scaini E, et al. Heart rate variability and myocardial infarction: systematic literature review and meta-analysis. Eur Rev Med Pharmacol Sci. 2009;13:229-307. [PubMed] [Google Scholar]
- 124. Stein PK, Carney RM, Freedland KE, et al. Severe depression is associated with markedly reduced heart rate variability in patients with stable coronary heart disease. J Psychosom Res. 2000;48:493-500. [DOI] [PubMed] [Google Scholar]
- 125. Frasure-Smith N, Lespérance F, Irwin MR, Talajic M, Pollock BG. The relationships among heart-rate variability, inflammatory markers and depression in coronary heart disease patients. Brain Behav Immun. 2009;23:1140-1147. [DOI] [PubMed] [Google Scholar]
- 126. Williams MS, Rogers HL, Wang NY, Ziegelstein RC. Do platelet-derived microparticles play a role in depression, inflammation, and acute coronary syndrome? Psychosomatics. 2014;55:252-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Musselman DL, Tomer A, Manatunga AK, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry. 1996;153:1313-1317. [DOI] [PubMed] [Google Scholar]
- 128. Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol. 2000;20:137-140. [DOI] [PubMed] [Google Scholar]
- 129. Kuijpers PM, Hamulyak K, Strik JJ, Wellens HJ, Honig A. Beta-thromboglobulin and platelet factor 4 levels in post–myocardial infarction patients with major depression. Psychiatr Res. 2002;109:207-210. [DOI] [PubMed] [Google Scholar]
- 130. Gehi A, Musselman D, Otte C, Bruce Royster E, Ali S, Whooley MA. Depression and platelet activation in outpatients with stable coronary heart disease: findings from the Heart and Soul Study. Psychiatry Res. 2010;175:200-204. [DOI] [PubMed] [Google Scholar]
- 131. Serebruany VL, Glassman AH, Malinin AI. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation. 2003;108:939-944. [DOI] [PubMed] [Google Scholar]
- 132. Musselman DL, Marzec UM, Manatunga A, et al. Platelet reactivity in depressed patients treated with paroxetine: preliminary findings. Arch Gen Psychiatry. 2000;57:875-882. [DOI] [PubMed] [Google Scholar]
- 133. Bruce EC, Musselman DL. Depression, alterations in platelet function, and ischemic heart disease. Psychosom Med. 2005;67(suppl 1):S34-S36. [DOI] [PubMed] [Google Scholar]
- 134. Nakamura S, Kato K, Yoshida A, et al. Prognostic value of depression, anxiety, and anger in hospitalized cardiovascular disease patients for predicting adverse cardiac outcomes. Am J Cardiol. 2013;111:1432-1436. [DOI] [PubMed] [Google Scholar]
- 135. Meyer T, Hussein S, Lange HW, Herrmann-Lingen C. Anxiety is associated with a reduction in both mortality and major adverse cardiovascular events five years after coronary stenting. Eur J Prev Cardiol. 2015;22:75-82. [DOI] [PubMed] [Google Scholar]
- 136. Thurston RC, Rewak M, Kubzansky LD. An anxious heart: anxiety and the onset of cardiovascular diseases. Prog Cardiovasc Dis. 2013;55:524-537. [DOI] [PubMed] [Google Scholar]
- 137. Suls J. Anger and the heart: perspectives on cardiac risk, mechanisms and interventions. Prog Cardiovasc Dis. 2013;55:538-547. [DOI] [PubMed] [Google Scholar]
- 138. Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. J Am Coll Cardiol. 2009;53:936-946. [DOI] [PubMed] [Google Scholar]
- 139. Haukkala A, Konttinen H, Laatikainen T, Kawachi I, Uutela A. Hostility, anger control, and anger expression as predictors of cardiovascular disease. Psychosom Med. 2010;72:556-562. [DOI] [PubMed] [Google Scholar]
- 140. Schmidt MM, Lopes RD, Newby LK, et al. Anger control and cardiovascular outcomes. Int J Cardiol. 2013;168:4338-4339. [DOI] [PubMed] [Google Scholar]
- 141. Mookadam F, Arthur HM. Social support and its relationship to morbidity and mortality after acute myocardial infarction: systematic overview. Arch Intern Med. 2004;164:1514-1518. [DOI] [PubMed] [Google Scholar]
- 142. Lett HS, Blumenthal JA, Babyak MA, Strauman TJ, Robins C, Sherwood A. Social support and coronary heart disease: epidemiologic evidence and implications for treatment. Psychosom Med. 2005;67:869-878. [DOI] [PubMed] [Google Scholar]
- 143. Brummett BH, Barefoot JC, Siegler IC, et al. Characteristics of socially isolated patients with coronary artery disease who are at elevated risk for mortality. Psychosom Med. 2001;63:267-272. [DOI] [PubMed] [Google Scholar]
- 144. Barth J, Schneider S, von Känel R. Lack of social support in the etiology and the prognosis of coronary heart disease: a systematic review and meta-analysis. Psychosom Med. 2010;72:229-238. [DOI] [PubMed] [Google Scholar]
- 145. Lepine JP, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011;7(suppl 1):3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Knapen J, Vancampfort D, Moriën Y, Marchal Y. Exercise therapy improves both mental and physical health in patients with major depression. Disabil Rehab. 2014;37:1490-1495. [DOI] [PubMed] [Google Scholar]
- 147. Silveira H, Moraes H, Oliveira N, Coutinho ES, Laks J, Deslandes A. Physical exercise and clinically depressed patients: a systematic review and meta-analysis. Neuropsychobiology. 2013;67:61-68. [DOI] [PubMed] [Google Scholar]
- 148. Carneiro LS, Fonseca AM, Vieira-Coelho MA, Mota MP, Vasconcelos-Raposo J. Effects of structured exercise and pharmacotherapy vs. pharmacotherapy for adults with depressive symptoms: a randomized clinical trial. J Psychiatr Res. 2015;71:48-55. [DOI] [PubMed] [Google Scholar]
- 149. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349-2356. [DOI] [PubMed] [Google Scholar]
- 150. Mota-Pereira J, Silverio J, Carvalho S, Ribeiro JC, Fonte D, Ramos J. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res. 2011;45:1005-1011. [DOI] [PubMed] [Google Scholar]
- 151. Yohannes AM, Doherty P, Bundy C, Yalfani A. The long-term benefits of cardiac rehabilitation on depression, anxiety, physical activity and quality of life. J Clin Nurs. 2010;19:2806-2813. [DOI] [PubMed] [Google Scholar]
- 152. Josephson EA, Casey EC, Waechter D, Rosneck J, Hughes JW. Gender and depression symptoms in cardiac rehabilitation: women initially exhibit higher depression scores but experience more improvement. J Cardiopulm Rehabil. 2006;26:160-163. [DOI] [PubMed] [Google Scholar]
- 153. Whalley B, Rees K, Davies P, et al. Psychological interventions for coronary heart disease. Cochrane Database Syst Rev. 2011;8:CD002902. doi: 10.1002/14651858.CD002902.pub3. [DOI] [PubMed] [Google Scholar]
- 154. Sussman N, Ginsberg DL, Bikoff J. Effects of nefazodone on body weight: a pooled analysis of selective serotonin reuptake inhibitor- and imipramine-controlled trials. J Clin Psychiatry. 2001;62:256-260. [PubMed] [Google Scholar]
- 155. Raeder MB, Bjelland I, Emil Vollset S, Steen VM. Obesity, dyslipidemia, and diabetes with selective serotonin reuptake inhibitors: the Hordaland Health Study. J Clin Psychiatry. 2006;67:1974-1982. [DOI] [PubMed] [Google Scholar]
- 156. Khoza S, Barner JC, Bohman TM, Rascati K, Lawson K, Wilson JP. Use of antidepressants and the risk of type 2 diabetes mellitus: a nested case-control study. Int J Clin Pharm. 2012;34:432-438. [DOI] [PubMed] [Google Scholar]
- 157. Gordon NF, Habib A, Salmon RD, et al. Effect of exercise-based cardiac rehabilitation on multiple atherosclerotic risk factors in patients taking antidepressant medication. Am J Cardiol. 2013;111:346-351. [DOI] [PubMed] [Google Scholar]
- 158. Blumenthal JA, Sherwood A, Babyak MA, et al. Exercise and pharmacological treatment of depressive symptoms in patients with coronary heart disease: results from the UPBEAT (Understanding the Prognostic Benefits of Exercise and Antidepressant Therapy) study. J Am Coll Cardiol. 2012;60:1053-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Rosenbaum S, Tiedemann A, Sherrington C, Curtis J, Ward PB. Physical activity interventions for people with mental illness: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75:964-974. [DOI] [PubMed] [Google Scholar]
- 160. Eagle KA, Montoye CK, Riba AL, et al. Guideline-based standardized care is associated with substantially lower mortality in Medicare patients with acute myocardial infarction: the American College of Cardiology’s Guidelines Applied in Practice (GAP) Projects in Michigan. J Am Coll Cardiol. 2005;46:1242-1248. [DOI] [PubMed] [Google Scholar]
- 161. Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ. 2003;326:1419-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Smith R, McCready T, Yusuf S. Combination therapy to prevent cardiovascular disease: slow progress. JAMA. 2013;309:1595-1596. [DOI] [PubMed] [Google Scholar]
- 163. Franklin BA, Kahn JK, Gordon NF, Bonow RO. A cardioprotective “polypill”? Independent and additive benefits of lifestyle modification. Am J Cardiol. 2004;94:162-166. [DOI] [PubMed] [Google Scholar]