Abstract
Introduction: Physicians vary widely in how they treat some health conditions, despite strong evidence favoring certain treatments over others. We examined physicians’ perspectives on factors that support or hinder evidence-based decisions and the implications for delivery systems, payers, and policymakers. Methods: We used Choosing Wisely® recommendations to create four clinical vignettes for common types of decisions. We conducted semi-structured interviews with 36 specialists to identify factors that support or hinder evidence-based decisions. We examined these factors using a conceptual framework that includes six levels: patients, physicians, practice sites, organizations, networks and hospital affiliations, and the local market. In this model, population characteristics and payer and regulatory factors interact to influence decisions. Results: Patient openness to behavior modification and expectations, facilitated and hindered physicians in making evidence-based recommendations. Physicians’ communication skills were the most commonly mentioned facilitator. Practice site, organization, and hospital system barriers included measures of emergency department throughput, the order in which test options are listed in electronic health records (EHR), lack of relevant decision support in EHRs, and payment incentives that maximize billing and encourage procedures rather than medical management or counseling patients on behavior change. Factors from different levels interacted to undermine evidence-based care. Most physicians received billing feedback, but quality metrics on evidence-based service use were nonexistent for the four decisions in this study. Conclusions and Implications: Additional research and quality improvement may help to modify delivery systems to overcome barriers at multiple levels. Enhancing provider communication skills, improving decision support in EHRs, modifying workflows, and refining the design and interpretation of some quality metrics would help, particularly if combined with concurrent payment reform to realign financial incentives across stakeholders.
Keywords: decision making, evidence-based care, guidelines, health care delivery system, attitudes
Research has shown widespread unwarranted variation in the way health conditions are treated across physician specialties, clinical roles, and practice settings.1,2 In looking for the causes of this variation, researchers have generally focused on geographic factors, the distribution of providers and hospitals, and financial incentives.1–4 But there has been less examination of how factors from various levels of the health care delivery system promote or impede evidence-based recommendations by physicians at the point of care.3,4 To help address this gap in the literature, we examined physicians’ perceptions of factors that promote or hinder their use of evidence across multiple levels of the health system.
In this qualitative study, we created four clinical cases based on Choosing Wisely® recommendations5 to identify factors that promote or impede the use of evidence. These cases address common types of point of care decisions: 1) diagnostic testing for a new problem (use of ultrasound before considering a computed tomography (CT) scan for suspected appendicitis in children), 2) diagnostic testing for an ongoing health concern (asymptomatic patients with coronary artery disease [CAD]), 3) treatment and intervention decisions (first-line treatment for patients with intermittent claudication), and 4) monitoring response to treatment (titrating long-term acid suppression therapy to the lowest effective dose for patients with gastroesophageal reflux disease [GERD]). See Table 1 and the Online Appendix.
Table 1.
Topics for This Study Which Were Endorsed by Evidence-Based Guidelines From Specialty Societies and Included in Choosing Wisely Initiative
| Domain of Care | Type of Decision | Evidence-Based Recommendation |
|---|---|---|
| General surgery—Suspected appendicitis | Diagnostic testing for new patient problem | Consider an ultrasound before recommending a computed tomography scan for the evaluation of suspected appendicitis in children. Available from: http://www.choosingwisely.org/clinician-lists/american-college-surgeons-computed-tomography-to-evaluate-appendicitis-in-children/ |
| Cardiology—Asymptomatic patient with coronary artery disease | Diagnostic testing for ongoing health concern | Do not perform annual stress cardiac imaging or advanced noninvasive imaging as part of routine follow-up in asymptomatic patients. Available from: http://www.choosingwisely.org/clinician-lists/american-college-cardiology-annual-stress-cardiac-imaging/ |
| Vascular surgery—Leg pain from intermittent claudication | Treatment or intervention | Do not use interventions such as surgical bypass, angiogram, angioplasty, or stent as a first line of treatment. Available from: http://www.choosingwisely.org/clinician-lists/society-vascular-surgery-interventions-as-first-line-treatment-for-intermittent-claudication/ |
| Gastroenterology—Adjusting gastroesophageal reflux disease medication | Monitoring response to treatment | Titrate long-term acid suppression therapy to the lowest effective dose needed to achieve therapeutic goals for patients with gastroesophageal reflux disease. Available from: http://www.choosingwisely.org/clinician-lists/american-gastroenterological-association-treating-gerd/ |
Note: Full case descriptions are included in the Online Appendix.
There is limited prior literature on the barriers to and facilitators of the four clinical decisions examined in this study. For these four topics, prior work has shown that the availability of ultrasound affected imaging decisions for suspected pediatric appendicitis.6 Physician ownership of imaging equipment was associated with more aggressive testing for monitoring in asymptomatic patients with CAD.7 There has been a tremendous increase and variation in lower extremity vascular interventions over the past decade for patients with intermittent claudication,8–10 but we have little direct evidence about how physicians make intervention decisions, although payment incentives might play a role.8,11 Providers may not wish to spend their own or their staff’s time on medication titration or may feel like it is the responsibility of another physician.12
Several reviews also focused on how physicians’ knowledge, attitudes, cognitive processes, and self-efficacy affect their use of evidence-based guidelines.13–16 But other factors—those related to the patient, the practice site, the organization, networks and hospital affiliations, and the local health care market—can also affect physicians’ use of evidence. Efforts to better understand and promote evidence-based decision making at the point of care should consider all types of factors.3,17
After examining physicians’ perceptions of these factors, we identified potential implications of our findings for future research and quality improvement efforts to improve delivery systems, payment systems, and policies. A companion paper (Stepanczuk C, Williams N, Morrison K, Kemmerer C, Rich E, unpublished data) summarizes our findings from patient focus groups on patients’ decision making in the context of the same four cases.
Methods
Data for this study came from 36 semistructured interviews of physicians from four specialties whose professional societies had developed the relevant Choosing Wisely® (CW) recommendations (i.e., cardiology, gastroenterology, general surgery, vascular surgery).5
To recruit physicians for interviews, we collaborated with the American College of Physicians (ACP) to identify practicing cardiologists and gastroenterologists and the American College of Surgeons (ACS) to identify practicing general and vascular surgeons. The ACP and ACS used their membership rosters to identify a sample within each specialty group that had 5 or more years of clinical practice experience, and that held no leadership roles (to avoid skewing the sample toward those working in academic medical centers or to those actively involved in guideline creation efforts). The ACP and ACS sent these samples a letter via email, which explained that the study was about “physicians’ decision making at the point of care” and included a request for volunteers for a onetime phone interview. Using the lists of volunteers from ACP and ACS, which included specialists’ geographic location, practice size, and ownership type, we then purposively selected from this list to capture a diverse representation of physicians’ practice characteristics within each specialty.
We designed four case scenarios—narrative descriptions of hypothetical patient cases—based on the CW topics (see the Online Appendix). Cases or “vignettes” are commonly used in research to elicit clinician experiences and perceptions, improving the validity of the data because of the uniformity of the case presented.18 After reading each physician the relevant case, we asked, “What would you do next for this patient?” and probed on the perceived barriers and facilitators to making the evidence-based recommendation. We then asked about barriers and facilitators to the alternative course of action. Finally, we asked what proportion of their income was dependent on quality measures, and what those measures were. We asked the same questions across specialties; only the clinical cases differed. Interviewees were not informed that the case involved a CW recommendation. Interviews lasted 30 to 45 minutes, and they were conducted by two physician researchers.
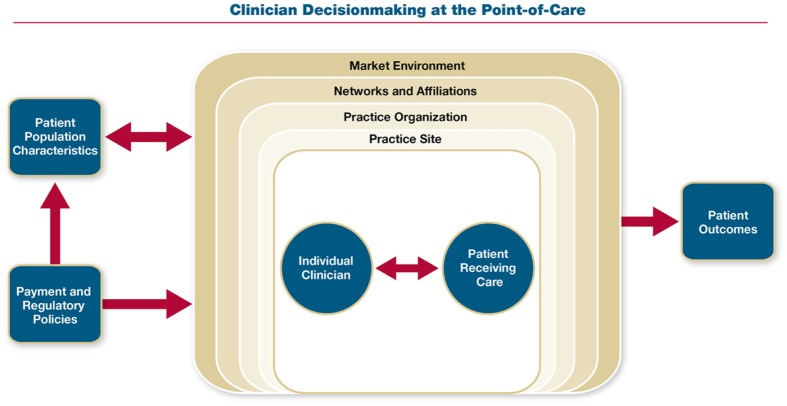
All interviews were transcribed verbatim by two trained research analysts. Interview transcripts were reviewed in their entirety by the interviewers for accuracy and to gain overall context. Codes and their definitions were developed a priori based in part on an underlying conceptual framework19 (Figure 1) that recognizes the patient-clinician interaction as the core of clinical decision making. It then theorizes that physician decision making is influenced by factors at six levels: the patient, physician, practice site, practice organization (the entity employing the physician, in the case of independent practices this is the physicians themselves), networks/hospital affiliations/other affiliations, and market environment. These levels can interact with each other and be influenced by other factors, such as patient population characteristics, payment incentives, and regulatory policies. Two coders (a senior clinician interviewer and a research analyst) analyzed the first four interview transcripts (one per specialty) separately and compared coding. Through an iterative process, they, along with the other senior researcher, came to a consensus on refinement of a priori codes and their definitions and rules for applying codes. Codes were applied to all the transcripts by the lead research analyst using ATLAS.ti20 and then were reviewed by both of the senior clinician researchers for agreement. Analysis included identifying barriers and facilitators to evidence-based decision making within each level of the framework.
Figure 1.
Conceptual framework. “Practice site” refers to the physical location at which a physician cares for a patient (e.g., for the suspected appendicitis case, this is the emergency department; for other cases, this is the office practice). “Practice organization” refers to the entity that employs the physician. For an independent physician practice, the practice site may be the same as the practice organization (e.g., small, physician-owned group).
The participating providers were given verbal and written information about the study. Verbal informed consent from the providers was electronically documented due to the busy clinical environment.
Results
Participants
Of the 36 physicians interviewed, just over half worked in independent physician-owned practices; the others were employed by larger health systems. There was good variation in practice characteristics among participants; this helped capture a range of perspectives (Table 2).
Table 2.
Participant and Practice Characteristics
| n | |
|---|---|
| Participants | 36 |
| Gastroenterologists | 9 |
| Cardiologists | 9 |
| General surgeons | 9 |
| Vascular surgeons | 9 |
| Practice type | 36 |
| Independent, physician-owned practice | 20 |
| Hospital or health system–owned practice | 13 |
| Academic medical center | 3 |
| Practice size, no. of physicians at the practice physical site | |
| 1–2 | 9 |
| 3–15 | 7 |
| 16–50 | 5 |
| 51–100 | 2 |
| >100 | 13 |
| US region | |
| Northeast | 11 |
| West | 3 |
| Midwest | 7 |
| South | 15 |
Patient-Level Factors
The factors that physicians felt influenced evidence-based clinician decision making fell into four thematic areas (Table 3), three for which we provide illustrative examples.
Table 3.
Common Themes That Affected Decision Making From Physician Interviews, Organized by Level of the Conceptual Framework
| Health Care System Level | Factors Affecting Decision Making |
|---|---|
| Patient | • Openness to behavior change and treatment recommendations |
| • Patient expectations | |
| • Socioeconomic status | |
| • Ability to pay/insurance coverage | |
| Physician | • Skills and competencies (e.g., communication) |
| • Attitudes, professionalism, and knowledge of evidence | |
| • Training | |
| • Prior clinical experience | |
| • Discomfort with uncertainty | |
| • Perceived personal incentives | |
| • Malpractice concerns | |
| Practice site | • Electronic health records (present day) |
| • Internal practice’s guidelines | |
| • Peers’ standard of care | |
| • Care processes and workflow | |
| • Workload and perceived time | |
| • Resources at the practice site | |
| Practice organization | • Financial incentives |
| • Quality metrics (e.g., throughput, emergency department wait-times) | |
| • Feedback on resource use | |
| • Contractual arrangements | |
| • Culture and leadership | |
| Networks, hospital and other affiliations | • Referring provider expectations |
| • Affiliated hospital influences | |
| • Arrangements with diagnostic testing facility or surgery center | |
| • Availability of consultative support | |
| Market environment | • Local standard of care |
| • Competition | |
| • Resources (e.g., qualified ultrasonographers) |
Patient openness to behavior modification and medical management was frequently mentioned as a facilitator to evidence-based decisions for both the GERD and intermittent claudication cases. These patients were reportedly more likely to experience symptom relief and avoid unnecessary procedures.
Patient expectations played a minor role in the asymptomatic CAD case, but some physicians noted that a few patients, generally those of higher socioeconomic status, request aggressive testing. Patient expectations—often influenced by referring physicians—could pose a challenge in the vascular surgery case. One vascular surgeon said, “Frequently, I’ll get a patient like this from a doctor who has told him, ‘You need to go get this [intermittent claudication] taken care of, you might lose your leg.’ And that scares them.”
Ability to pay and insurance coverage both promoted or impeded evidence-based decisions. Physicians noted that the cost of an over-the-counter lower-dose proton pump inhibitor (PPI) was a barrier to titration for some patients. As one gastroenterologist said, “It’s not covered.” However, some surgeons and cardiologists cited generous insurance—covering imaging and procedures regardless of evidence—as a barrier to evidence-based care in these cases.
Physician-Level Factors
These factors fell into seven categories (Table 3).
Physician skills and competencies, particularly the ability to communicate evidence to patients and referring providers in the context of patients’ values and preferences, facilitated evidence-based care for all four cases. Communication skills were noted to also foster trust between patient and physician in cases where no prior relationship existed. As a general surgeon noted, “This is where working with the family is so important. That they understand what you’re thinking is, and you have a good rapport with them, and you understand what they want and how they feel.”
Vascular surgeons noted the importance of communicating in a way that acknowledged patients’ concerns, made them feel heard, and set up manageable steps to change behavior. One surgeon noted how he would avoid a patient’s push for an immediate procedure,
I can say, “Continue to stay off cigarettes for four months and exercise [with this plan we’ve set up]. Then come back and see me, and we can talk.” Statistically speaking, he’s going to be able to walk twice as far in four months, and he probably won’t want anything done at that time.
Physician attitudes, professionalism, and knowledge of the evidence also played a role. Most general surgeons felt no need for a CT scan, and some expressed concern about lifetime radiation exposure. However, they noted that emergency department (ED) physicians typically ordered scans before calling the general surgeon. Among the third of gastroenterologists interviewed who would not attempt titration, there was a strong feeling that the long-term benefits of PPIs outweighed their risks. As one said, “This is a low-stakes challenge and it’s not worth it for me to go out on a limb.” Colleagues’ understanding of evidence and guidelines was also noted to be important. Representing the sentiment of nearly all the vascular surgeons, one noted, “Interventional cardiologists are performing these procedures more and more in my community. Less and less do I see this patient.”
When asked which guidelines and information sources they use, physicians most frequently cited their specialty’s peer-reviewed journals and their specialty organizations as information sources, but few were aware that their societies presented guidelines specific to the decisions presented in this study. Skepticism of guidelines was also a factor. As one gastroenterologist said,
I’m not a big believer in the long-term consequences [of PPIs]. [In the studies I’ve read,] I thought yes, the people had problems, but this was a population study; people who take PPIs, in general, tend to be sicker than people who don’t.
The nature of one’s training could be a barrier or facilitator. An experienced general surgeon felt, “In the last decade, there was a huge shift [in training] from a clinical approach to the problem to a more diagnostic testing approach.” A cardiologist noted, “One’s training affects how one practices. Those trained [to take a more] conservative approach generally practice more conservatively.” Participants also noted the geographic influence of training programs on local markets, because trainees often settle in areas where they completed training.
Prior clinical experiences both promoted and impeded evidence-based decision making. All vascular surgeons indicated that their prior experience suggested procedural intervention for this case would only increase the risk of future limb loss. On the other hand, a few general surgeons would do a CT scan based on prior experience; as one noted, “I’ve been misled by ultrasounds, but I’ve never been misled by a CT.”
Discomfort with uncertainty was noted particularly in the appendicitis case. Some surgeons felt that their colleagues default to using CT due to discomfort with the clinical exam, and intolerance for even minimal uncertainty. Some surgeons noted a lack of understanding at the hospital leadership level of risks and benefits of CTs,
I’ve been doing this for more than 30 years. The current structure [is] such that if you don’t do a CT scan, you operate, and the appendix turns out to be normal, it will be extremely hard. Right away, you’ll [get] a letter from the CMO . . . asking why you didn’t do a CT scan.
Perceived personal incentives, including patient satisfaction measures, were important. Almost all physicians received reports, and several faced incentives, related to general patient satisfaction measures. Most said that their scores on patient satisfaction did not affect their point-of-care decisions, but noted that they have seen their peers’ decisions affected by the scores. Two physicians noted that giving patients “more is better” if they wanted to boost their satisfaction ratings. As a cardiologist said, “I certainly have some people who are insistent, and there are rare cases where I think it’s easier to satisfy them, especially these days when we’re being scored by patient satisfaction; it’s actually in our compensation model.”
Cardiologists and vascular surgeons felt that fee-for-service payment rates—and related “productivity” measures—increased use of procedures and advanced imaging. General surgeons noted they did not face personal financial incentives for imaging choice in the ED. Some gastroenterologists noted that time spent discussing PPI titration with GERD patients could instead be spent doing procedures on other patients.
The influence of pharmaceutical manufacturers on gastroenterologists to promote medications, including PPIs, was also mentioned. As a gastroenterologist noted, “[Drug manufacturers] present GI docs as opinion leaders on PPIs,” and clinical research in office settings “becomes a money maker for a lot of offices.”
Malpractice concerns had both positive and negative impacts on evidence-based decision making. Ordering a CT scan for the appendicitis case was seen by physicians as a self-preservation technique. Vascular surgeons, on the other hand, felt that avoiding early procedures decreased malpractice risk from a patient losing a leg prematurely. Gastroenterologists and cardiologists were less concerned about malpractice claims for these cases. One cardiologist said, “Good practice is what protects you in lawsuits . . . not over testing. . . . You should do what any reasonable individual would do.”
Practice Site
These themes fell into six categories.
Some participants expressed frustration that current electronic health records (EHRs) do not facilitate evidence-based treatment for these clinical decisions. For example, none of the physicians had clinical decision support (CDS) in their current EHRs relevant to these common scenarios. A few noted that the complex EHR interface, along with visit time pressures, could encourage ordering more expensive tests. As one cardiologist noted, “I wouldn’t be surprised if the complexity of using that [EHR] drives people to make one selection over another. . . . Everyone feels time-pressure, no matter what kind of practice you’re in.” He also noted that the nuclear test is the first option listed in the EHR, “Our system is set up. . . . The default that everybody uses is nuclear.” Aspects of EHRs were also facilitators. One gastroenterologist noted that access to the patient’s record across their system was a facilitator to PPI titration because he could see what the patient’s primary care physician had ordered or discussed with the patient.
Only two participants’ practices had internal practice guidelines that supported evidence-based decision making for these CW topics. And one of these noted that while “the guidelines are there. We can’t make physicians follow them.”
Peers’ standard of care could promote or impede evidence-based decisions. Some physicians were in practices where colleagues were “on the same page” about these decisions. But others noted that some partners or peers would suggest the more aggressive path. They noted that some physicians were less able to resist pressures from CEOs and from “productivity” incentives and that this altered their practice’s standard of care.
Care processes and workflow were a barrier for the suspected appendicitis and GERD cases. All general surgeons said that typically, the patient receives a CT scan based on an ED physician’s order before the general surgeon is even called. One said, “In the real world, [that patient gets] a CAT scan as soon as he gets through the [ED] door.” Surgeons noted that getting a CT (at their hospitals) was faster than an ultrasound, and thus enhanced ED throughput. Several noted that practice site workflow pressures combine with training, malpractice fears, and cultural shifts toward more testing. Some gastroenterologists noted that nurses typically handle refill requests that occur by phone, so the physician might not be triggered to think about PPI titration.
Workload and perceived lack of time, exacerbated by measures of productivity, often seemed to make ordering a test easier than working with patients on behavioral modification and medication adherence. One gastroenterologist noted shrinking time for patient discussions and education. Another said, “That period of time [for patient education] gets shorter and shorter.” A cardiologist noted, “When you’re working for a hospital, they tell you, ‘Look, you’ve got to see so many patients in an hour.’”
Resources (lack of availability of qualified ultrasonographers) arose at both the practice and market levels (see market factors) and was often mentioned as a barrier to evidence-based care in the appendicitis case.
Practice Organization
These themes fell into five categories.
Organizational financial incentives created barriers to physicians’ recommending evidence-based care for their patients. A cardiologist in an academic medical center reflected that patients are “driven through” the expensive equipment, both to justify the expense and to generate enough procedures to train fellows. Some hospital employed physicians felt pressure to do more tests/procedures. A cardiologist noted,
The hospital is making tons of money on nuclear imaging. . . . The machine is right outside [our office suite]. The medical director or the COO comes and asks me how I’m doing, but what he’s trying to find out is how many tests I have ordered.
Several physicians noted unintended consequences of their organizations’ quality metrics. While none of the participants faced performance assessment and quality metrics on whether their decisions and diagnostic test ordering was evidence-based, several surgeons noted that hospital quality metrics on ED wait-times had the unintended effect of increased use of CT scans. A surgeon noted, “Measures of hospital ED wait-times are influencing the ED docs to do knee-jerk CAT scans before they’ve even examined the patients.”
General surgeons and gastroenterologists generally did not receive feedback on resource use for the topics in our CW cases. What feedback they did report urged “productivity” (i.e., billable services) rather than evidence-based care. Contractual arrangements for various hospital-employed physicians in vascular surgery and cardiology emphasized volume-based incentives as well.
Participants had mixed experiences on the role of culture and leadership for these clinical decisions. Capturing the sentiment of most, a cardiologist in a system-owned practice said that within his system, it is “part of the environment” to receive signals promoting more imaging. A vascular surgeon noted that, despite his efforts, the hospital system is not open to vascular surgeons’ input on disseminating practice guidelines for intermittent claudication.
Network and Hospital Affiliations–Level Themes
These themes fell into four categories.
Referring provider expectations were most frequently mentioned by vascular surgeons. Echoing the comments of others, a vascular surgeon reported:
I routinely will have referrals from interventional cardiologists in particular who do diagnostic angiograms, find asymptomatic lesions, evaluate them for peripheral vascular procedures, and either define that they can’t do them or don’t think they would be favorable, and refer them to me specifically for bypass.
Some felt affiliated hospital influences encouraged aggressive interventions. A vascular surgeon in a small independent practice described “incredible” pressure from his affiliated hospital to treat these cases more aggressively. He said, “The hospital caters to the cardiology department. They are the largest producers in the hospital.”
Cardiologists and vascular surgeons noted that hospitals’ or practices’ ownership or arrangements with diagnostic testing facilities or surgery centers increased use of advanced imaging, angiograms, and angioplasties. Scheduling these procedures was described as “the easiest thing in the world.”
Participants felt that the availability of consultative support was good within their practices and among their peers. Academic medical centers generally provided avenues for discussion of evidence-based care, including guidelines. There were a few exceptions. A general surgeon employed by a for-profit hospital noted with regret that there is no incentive for people to get together and question whether CT scans are appropriate. A vascular surgeon in an independent practice lamented that “decision-making is individual.”
Market-Level Themes
These themes fell into three categories.
Most general surgeons felt that local standards of care encouraged ED physicians to order CT scans, except in children’s hospitals which had better access to ultrasonographers and more awareness of lifetime radiation exposure risk. Two gastroenterologists in medium-sized independent practices remarked that titrating PPIs was not the local area standard of care.
Cardiologists noted local market differences in nuclear imaging rates of patients with asymptomatic CAD; most could also cite a private cardiology group or health system in their community that tested more aggressively than guidelines recommend. Several vascular surgeons noted that as interventional cardiologists and radiologists sometimes enter “the realm of peripheral vascular disease,” they may influence local treatment patterns.
Market competition was primarily an issue for cardiologists and vascular surgeons in these cases. A few cardiologists felt that high competition in their markets influences more aggressive treatment and testing decisions.
Resources, including a dearth of qualified ultrasonographers in some markets and readily available CT scanners “25 feet from the ED,” increased use of CT as the first-line test for suspected appendicitis. Capturing a prevalent sentiment, a general surgeon noted, “To do a good ultrasound, you need a good ultrasonographer. To do a great CAT scan, you don’t need a great anything.”
Discussion
This study identified physicians’ experiences with factors from multiple levels of the health care system that impeded or promoted evidence-based decision making. Patient-level factors were identified as facilitators (openness to behavior change), barriers (patient expectations for more aggressive testing), or both (insurance coverage of services and ability to pay). At the physician level, factors were mixed; communication skills were clearly facilitators, compensation models were often barriers, and current patient satisfaction measures were largely perceived as barriers. Practice site–level factors also differed: several appeared to act as both barriers and facilitators (EHRs, internal guidelines, peer standards of care), but several were barriers (the lack of CDS, care processes and workflows, workload and perceived lack of time and resources). And factors at almost all of the remaining levels (practice organization, networks and hospital affiliations, and market environment) were seen as barriers: organizations’ emphasis on billable activities, a lack of feedback to physicians on whether tests and interventions were consistent with current evidence, referring provider expectations, hospitals’ or practices’ ownership or other arrangements with diagnostic testing facilities and surgery centers, local standards of care, market competition and available resources.
Barriers from multiple levels of the health care system also combine to exacerbate challenges to evidence-based decision making. For example, patient expectations combine with financial incentives for physicians and the unintended consequences of particular quality metrics (e.g., patient satisfaction and ED wait-times), reportedly leading to more aggressive diagnostic tests and procedures. Misaligned incentives can subvert health care systems’ support for evidence-based decisions by physicians, including inadequate access to resources like ultrasound, insufficient or inappropriate CDS tools in EHRs, and a lack of support for specialists to gather to discuss or consult one another on evidence-based decisions.
Implications for Practices and Hospital Systems
These findings have potential implications for future research and quality improvement efforts to modify health care delivery systems (Table 4). Addressing the individual and combined effect of diverse challenges to evidence-based decision making likely require multifaceted approaches ranging from strengthening communication skills and EHR supports to altering the design of clinical workflows and of some performance metrics.
Table 4.
Examples of Potential Implications for the Delivery System, Payers, and Policy
| Health Care delivery systems |
| Communication skills |
| • Increase clinician support to engage in training on effective communication about evidence with patients at the point of care.20 |
| Electronic health records |
| • Build more Clinical Decision Support into electronic
health records (EHRs) for these types of clinical decisions
and prescription and imaging ordering systems.
• Present diagnostic tests/imaging on the screen of the EHR in a way that does not promote non–evidence-based services (e.g., do not list nuclear studies before a standard electrocardiogram). • Prior to completing a referral request in an EHR, for certain types of referrals (e.g., for a patient to see an interventional cardiologist regarding stent placement), include an option for the referring physician in the EHR to see evidence-based information about indications. |
| Workflows |
| • Alter care processes in the emergency department (ED) so
that they do not inadvertently encourage non–evidence-based
testing (e.g., computed tomography as first-line test in
children with suspected appendicitis). • Encourage providers to establish care coordination agreements or care compacts (e.g., among primary care physicians, cardiologists, interventional cardiologists, and vascular surgeons to address knowledge gaps, appropriate referrals, respective responsibilities). |
| Refine performance metrics to avoid unintended consequences |
| • Refine ED wait-time metrics to be less blunt instruments.
• Consider exceptions or allowances in ED throughput measures for certain types of clinical scenarios. • Refine patient satisfaction measures (and their interpretation) to avoid perverse incentives for providers to recommend non–evidence-based care to boost their satisfaction scores. |
| Payers and policy makers |
| • Reduce payment incentives that impede evidence-based care.
• Collaborate with providers to support data aggregation to provide feedback about judicious and effective use of services. • Appeal to clinicians’ sense of professionalism through supports for evidence-based recommendations at the point of care. |
In our study, physicians commonly cited communication skills as a facilitator in helping patients follow an evidence-based course of treatment, but some feel time pressures prevent them from discussing evidence-based treatments and therapies with their patients. Prior work has also shown that physicians perceive that payment policies do not reward their taking time to discuss issues like behavioral modification or medication adjustment.12 There are, however, tools to help clinicians communicate more efficiently and effectively with patients at the point of care. The CW Initiative, for example, has free online training modules for physicians, as well as resources for patients.21
Physicians desired more user-friendly EHRs, and they noted that in some cases, better CDS could facilitate evidence-based decision making. CDS has helped with identifying medications that are promising targets for deprescribing.22,23 A recent review on diagnostic imaging found that EHR-integrated CDS can improve appropriate use, but cautioned that more data are needed on potential harms.24 EHR vendors could work with clinicians and neutral scientific organizations to build and test CDS for these CW recommendations into EHRs.
Organizations should not structure their EHR interface to promote ordering the most expensive tests. EHRs can also be used to provide more informative real-time feedback to physicians regarding their use of evidence-based services in cases like these.
To improve the efficiency of EHR documentation in discussions with patients, and to allay clinicians’ concerns about malpractice, language from neutral scientific organizations could be used by clinicians, modified as needed for individual patients, to help document why a particular test or procedure is not indicated.
Overcoming some barriers may require modification to team workflows. While our study focused on physician perspectives, physicians work as part of a large team with other physicians, nurse practitioners, nurses, physician assistants, medical assistants, and technicians playing key roles. Since nurses typically handle patients’ refill requests, for example, they might work with the patient and clinician to raise the issue of PPI titration in patients with well-controlled GERD.
Refinement of some performance metrics would be helpful. Many physicians noted the problems with current measures of physician productivity for these cases. In addition, more nuanced use and interpretation of quality metrics like patient satisfaction, ED throughput, and patient wait-times might also facilitate more evidence-based decision-making.
For each of these four clinical scenarios, there are strategies available to practices and hospitals to facilitate more evidence-based recommendations. EDs, for example, could provide ready access to qualified ultrasonographers (in areas where the population size supports having this resource). Similarly, providers and systems could support better patient education at the point of care.25,26
Decisions for some imaging studies or procedures go beyond the specialty traditionally associated with it in CW recommendations (e.g., primary care physicians and gastroenterologists typically manage GERD). Providers could be encouraged to participate in delivery system, local, or state learning collaboratives27,28 and/or establish care coordination agreements among relevant clinicians and practices to address knowledge gaps, appropriate referrals, and clinician roles.29,30
Implications for Payers and Policy Makers
Putting some of these delivery system modifications in place would be more feasible if accompanied by changes in payment incentives and policies to reduce barriers.19 Additional research could examine opportunities for payers and purchasers to adjust the nature and terms of payment to support physicians and hospitals offering more evidence-based recommendations to patients. Seemingly simple payment reforms, however, may not increase evidence-based recommendations because of the interplay of barriers at different levels and the potential for unintended consequences. In the appendicitis case, for example, a payment change directed at surgeons would be unlikely to improve use of ultrasound given the importance of timely availability of the technology and the role of ED physicians in test ordering. Even in the CAD and claudication cases, where various adjustments in physician payment for these services are possible (e.g., fee-for-service revisions, bundled payment, P4P), effective payment policy would require careful redesign.
More feasible strategies might include payers and policy makers offering resources or preferential payments to support changes in the practice setting or delivery organization to reduce current barriers to evidence-based care. For example, payers and policy makers could enhance supports for clinicians to improve their communication skills in discussing evidence with patients and referring clinicians, as well as to modify clinical workflows and EHRs to support clinical decision making. Removing current payment incentives that pose barriers to evidence-based care might promote a reorientation of professional culture and organizational leadership toward doing what is best for patients. Modifying financial incentives and some performance measures for health systems may be important in supporting the professional time and other resources needed to engage with patients and deliver evidence-based care. Combined efforts of provider and payer organizations with local or regional data aggregators to develop databases with clinically meaningful data also has potential to provide feedback to clinicians and organizations on the judicious use of services (particularly where evidence does not support such interventions).
Limitations
While this qualitative study provides nuanced information on physicians’ decisions in common cases, we must be cautious in generalizing our findings to other specialties or cases. Titrating PPIs, nonetheless, is relevant for primary care physicians, as well as for gastroenterologists, and diagnostic testing for suspected appendicitis applies to emergency room physicians, as well as to general surgeons. Furthermore, these cases were chosen as exemplars of the types of decisions faced by many physicians working in diverse clinical settings. Because the goal of this study was to explore barriers and facilitators to evidence-based recommendations, we necessarily limited the number of comorbidities and other clinically relevant circumstances reflected in each case scenario. Other important patient contexts (e.g., time of day of presentation, social supports, etc.) are also important factors in clinical decisions, and were often mentioned by interviewees.
In sum, barriers at multiple levels combine to exacerbate challenges to providing evidence-based care; solutions to overcoming them need to recognize the multiple levels of the health care system from which they arise. One cannot possibly micromanage the tens of thousands of clinical decisions that physicians and patients make every day. Rather, the removal or minimization of barriers and the creation of effective methods for implementation of evidence could help place the decision-making power where it should rest, between well-informed patients and their clinicians.
Supplementary Material
Acknowledgments
We are grateful to Patrick Alguire, Arlene Weissman, and Alicia Ludwig of the American College of Physicians (ACP) and Frank Opelka and Molly Peltzman of the American College of Surgeons (ACS) for their help in setting up a random selection of their physician members in each of the relevant specialties. We would also like to thank our technical expert panel, which advised us on various phases of this project: Patrick Alguire, Michael Barry, Robert Berenson, Darren DeWalt, Natasha Gajewski, Sanne Magnan, Tara Montgomery, Frank Opelka, William Rich III, Lew Sandy, and Daniel Wolfson. We are also grateful to the reviewers for their valuable feedback on an earlier version of this article. We were advised that human subjects review was not necessary for this study, and a post-study examination of the protocol by an institutional review board concurred that the methods would be exempt from institutional review board review.
Footnotes
The authors declare that they do not have any conflicts of interest.
This study was funded by the Robert Wood Johnson Foundation. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
The online appendix for this article is available on the Medical Decision Making Policy & Practice Web site at http://journals.sagepub.com/doi/suppl/10.1177/2381468316660375.
References
- 1. Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA. 2013;310(12):1227–8. [DOI] [PubMed] [Google Scholar]
- 2. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–45. [DOI] [PubMed] [Google Scholar]
- 3. Rich EC, Lake TK, Valenzano CS, Maxfield MM. Paying the doctor: evidence-based decisions at the point-of-care and the role of fee-for-service incentives. J Comp Eff Res. 2013;2(3):235–47. [DOI] [PubMed] [Google Scholar]
- 4. Wennberg JE. Unwarranted variations in healthcare delivery: implications for academic medical centres. BMJ. 2002;325(7370):961–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choosing Wisely. Available from: http://www.choosingwisely.org/
- 6. Burr A, Renaud EJ, Manno M, et al. Glowing in the dark: time of day as a determinant of radiographic imaging in the evaluation of abdominal pain in children. J Pediatr Surg. 2011;46(1):188–91. [DOI] [PubMed] [Google Scholar]
- 7. Shah BR, Cowper PA, O’Brien SM, et al. Association between physician billing and cardiac stress testing patterns following coronary revascularization. JAMA. 2011;306(18):1993–2000. [DOI] [PubMed] [Google Scholar]
- 8. Anderson PL, Gelijns A, Moskowitz A, et al. Understanding trends in inpatient surgical volume: vascular interventions, 1980–2000. J Vasc Surg. 2004;39(6):1200–8. [DOI] [PubMed] [Google Scholar]
- 9. Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50(1):54–60. [DOI] [PubMed] [Google Scholar]
- 10. Goodney PP, Dzebisashvili N, Goodman DC, Bronner KK. Variation in the Care of Surgical Conditions: Diabetes and Peripheral Arterial Disease (Dartmouth Atlas of Health Care); 2014. Available from: http://www.dartmouthatlas.org/downloads/atlases/Surgical_Atlas_2014.pdf [PubMed]
- 11. van Zitteren M, Vriens PW, Burger DH, et al. Determinants of invasive treatment in lower extremity peripheral arterial disease. J Vasc Surg. 2014;59(2):400–8.e2. [DOI] [PubMed] [Google Scholar]
- 12. Anderson K, Stowasser D, Freeman C, Scott I. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open. 2014;4(12):e006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swennen MH, van der Heijden GJ, Boeije HR, et al. Doctors’ perceptions and use of evidence-based medicine: a systematic review and thematic synthesis of qualitative studies. Acad Med. 2013;88(9):1384–96. [DOI] [PubMed] [Google Scholar]
- 14. Godin G, Bélanger-Gravel A, Eccles M, Grimshaw J. Healthcare professionals’ intentions and behaviours: a systematic review of studies based on social cognitive theories. Implement Sci. 2008;3:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A; “Psychological Theory” Group. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care. 2005;14(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–65. [DOI] [PubMed] [Google Scholar]
- 17. Timbie JW, Fox DS, Van Busum K, Schneider EC. Five reasons that many comparative effectiveness studies fail to change patient care and clinical practice. Health Aff (Millwood). 2012;31(10):2168–75. [DOI] [PubMed] [Google Scholar]
- 18. Converse L, Barrett K, Rich E, Reschovsky J. Methods of observing variations in physicians’ decisions: the opportunities of clinical vignettes. J Gen Intern Med. 2015;30(suppl 3):S586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reschovsky JD, Rich EC, Lake TK. Factors contributing to variations in physicians’ use of evidence at the point of care: a conceptual model. J Gen Intern Med. 2015;30(suppl 3):S555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ATLAS.ti GmbH. Atlas.ti, Version 7.5.7. Berlin: ATLAS.ti GmbH; 2015. [Google Scholar]
- 21. Choosing Wisely. Physician communication modules. Available from: http://www.choosingwisely.org/resources/modules/
- 22. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bain KT, Holmes HM, Beers MH, Maio V, Handler SM, Pauker SG. Discontinuing medications: a novel approach for revising the prescribing stage of the medication-use process. J Am Geriatr Soc. 2008;56(10):1946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldzweig CL, Orshansky G, Paige NM, et al. Electronic health record-based interventions for improving appropriate diagnostic imaging: a systematic review and meta-analysis. Ann Intern Med. 2015;162(8):557–65. [DOI] [PubMed] [Google Scholar]
- 25. Contreary K, Rich E, O’Malley AS, Reschovsky J. Payer and Purchaser Options for Promoting More Evidence-based Recommendations at the Point of Care. Available from: https://www.mathematica-mpr.com/our-publications-and-findings/publications/supporting-better-physician-decisions-at-the-point-of-care-what-payers-and-purchasers-can-do.
- 26. Collins A, Stepanczuk C, Williams N, Rich E. Policy options to promote evidence-based decisions by patients. Available from: https://www.mathematica-mpr.com/our-publications-and-findings/publications/supporting-better-patient-decisions-at-the-point-of-care-what-payers-and-delivery-systems-can-do.
- 27. Centers for Medicare & Medicaid Services. Transforming Clinical Practice Initiative Awards. Available from: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-09-29.html
- 28. Washington Health Alliance. Choosing Wisely Task Force. Available from: http://wahealthalliance.org/alliance-reports-websites/choosing-wisely/washington-state-choosing-wisely-task-force/
- 29. Carrier E, Dowling MK, Pham HH. Care coordination agreements: barriers, facilitators, and lessons learned. Am J Manag Care. 2012;18(11):e398–404. [PubMed] [Google Scholar]
- 30. American College of Physicians. High value care. Available from: https://www.acponline.org/clinical-information/high-value-care
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.