Abstract
Background: Hypertension (HTN) in people with diabetes doubles the risk of cardiovascular disease. Prior patient activation studies largely show improved communication but little impact on behavior or health outcomes. We sought to 1) assess the impact of Office-Based Guidelines Applied to Practice (Office-GAP) Program on blood pressure (BP) control; 2) determine the rate and predictors of BP control in patients with HTN and/or diabetes mellitus (DM) in federally qualified health centers. Methods: Sample: Patients with coronary heart disease (CHD) and/or DM with history of HTN; analyzed patients with DM and HTN compared to HTN without DM. Intervention: Office-GAP included physician training, patient activation, and an Office-GAP decision checklist. Two-site intervention/control design; data collection at baseline and after 3, 6, and 12 months. Logistic regression with propensity scoring assessed impact on BP control over time. Results: Of 243 patients, HTN was present in 75% at baseline; 32% had BP controlled. Consistent trend showed Office-GAP slightly improved the rate of BP control across time, while the control arm showed a nonsignificant decrease in the rate of BP control across time, compared to baseline. BP improved at 6 months at the intervention site compared to control site (odds ratio = 2.92; 95% confidence interval = 1.11–7.69). Conclusion: BP control was better at the intervention site compared to the control site at 6 months. Office-GAP shows promise to implement guidelines-based patient-centered care that improves BP.
Keywords: patient activation, shared decision-making, low income, minority, federally qualified health center, diabetes mellitus, hypertension
Hypertension (HTN) is the major risk factor for cardiovascular diseases and stroke, which affect one in every three American (29%) adults.1 Only about half (52%) of these people have their blood pressure (BP) under control.1 In 2013, the age-adjusted HTN-related death rate increased to 23%.2 It is estimated that 9.3% of the US population has diabetes.3 The prevalence of HTN among people with diabetes is very high, and its presence doubles the risk of cardiovascular diseases.4 HTN substantially contributes to morbidity and mortality by increasing the risk of both macrovascular and microvascular complications, including stroke, coronary heart disease (CHD), cardiomyopathy, peripheral vascular disease (PVD), retinopathy, nephropathy, and neuropathy.5–7 Yearly, HTN costs the nation $46 billion in health care services, medications to treat high blood pressure (BP), and missed days of work.8 It is estimated that approximately 41% of US adults will have HTN by 2030, which is almost a fivefold increase from 2013 estimates.9 The recent Systolic Blood Pressure Intervention Trial results established new importance to aggressive BP control, since intensive management of high BP, below a commonly recommended BP target, significantly reduced rates of cardiovascular disease and lowered risk of death in a group of adults 50 years and older with high BP. However, the rates of serious adverse events of hypotension, syncope, electrolyte abnormalities, and acute kidney injury or failure were higher in the intensive-treatment group than in the standard-treatment group.10
Studies show that HTN is poorly controlled in patients with diabetes as compared to patients without diabetes.11–13 A recently published systematic review and meta-analysis of large-scale randomized clinical trials demonstrated that effective BP lowering in patients with type 2 diabetes is associated with improved mortality and reduces the risk of micro- and macrovascular complications.14 There is evidence of racial/ethnic and socioeconomic disparities in the prevalence and control of HTN15,16 that have not been fully explored. Most of the studies of HTN control have focused on the nationally representative general population. Whether these rates of BP control apply to low-income patients is unclear.
Over 24 million Americans receive health care from federally qualified health centers (FQHCs) according to the National Association of Community Health Centers Fact Sheet (March 2016).17 These FQHCs provide preventive and primary health care services to patients who live in medically underserved areas, have low income, and who would otherwise have difficulty in securing access to health care.18–23 Only a few studies have focused on degree of and factors affecting BP control in FQHCs.24
Patients who demonstrate active involvement in their health care decisions experience a positive impact on their health outcomes.25–28 The Affordable Care Act identifies patient engagement and shared decision making (SDM) as integral components of successful health system reform and recognizes them as critical to the success of accountable care organizations and patient-centered medical homes.29 SDM implementation has been limited, in part, by the lack of physician uptake. In an effort to close the disparity gap in cardiovascular care, we refined our previously described SDM intervention to produce a simple, parsimonious program, “The Office-Guidelines Applied to Practice” (Office-GAP) program of patient activation/engagement in FQHCs. Office-GAP study consisted of patients with CHD and/or diabetes mellitus (DM). The objective of the study was to assess the impact of the Office-GAP intervention on BP control among low-income patients with a history of HTN, in FQHCs.
Methods
Design
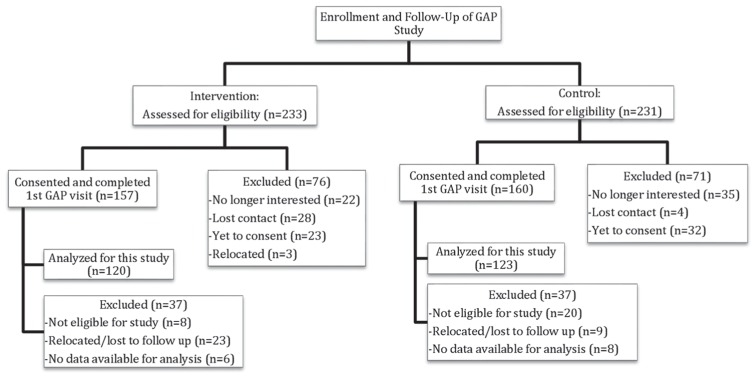
The Office-GAP study was a quasi-experimental, two-center study designed to improve collaboration between patients and providers and to improve outcomes for low-income populations in outpatient clinical settings. The study was conducted in two designated FQHCs (intervention/control) in Mid-Michigan. The centers were allocated as intervention and control by tossing a coin. The Michigan State University Institutional Review Board approved the study. Eligible patients were adults aged 18 or older, who could provide informed consent. We used International Classification of Disease (ICD)-9 codes to identify patients with either a diagnosis of CHD and/or DM. We excluded patients with cognitive impairment, dementia, and psychosis. The baseline Office-GAP program enrolled 243 patients from October 2010 to March 2014 from the two study sites (Figure 1). For our analysis of BP control, we identified patients with HTN (n = 182).
Figure 1.
Flow diagram of the study. In the intervention site: Of the 157 subjects who completed the first GAP visit (group visit), 121 subjects completed the second GAP visit (first follow-up). Of those, 105 completed the third GAP visit (second follow-up) and the entire study. In the control site: Of the 160 subjects who completed the first GAP visit (group visit), 128 subjects completed the second GAP visit (first follow- up). Of those, 116 completed the third GAP visit (second follow-up) and the entire study.
HTN and goal BP were defined based on the Seventh Report of Joint National Committee30 as systolic BP >140 mmHg (>130 mmHg in DM patients) and diastolic BP >90 mmHg (>80 mmHg in DM patients). Patient race was determined by self-report. The main outcome measure was change in proportion of patients that have reached their BP goal, by chart review for the study patients, and the secondary outcome was predictors of BP control.
In the intervention center, we have two internists (MDs) and one family physician (MDs) and two nurse practitioners (NPs). In the control center, we have two internists (MDs) and two NPs but no family physician. The two FQHCs centers were reasonably comparable in size.
Intervention
The Office-GAP intervention included three elements: 1) Physician training for patient activation/engagement/SDM in one 60- to 90-minute session was offered at four different times to accommodate staff schedules. Training included a review of CHD secondary prevention guidelines, patient-centered interviewing method and SDM,31 goal setting, and role-plays to model office visit skills. Participating physicians and practice staff were surveyed to evaluate the training session at the end of the training. 2) Patient activation/engagement intervention was offered in single 90- to 120-minute group visits conducted by the research assistant and the principal investigator (AO) with four to six patients at each session. Training included principles of SDM, patient communication skills related to engagement, activation, empowerment parallel to the provider intervention skill training,31,32 and review of decision support tools. Decision support tools included the Office-GAP checklist tool (Figure 2) and the American Diabetes Association (ADA) Booklet Living with Diabetes. A physician (AO) with a research assistant reviewed the ADA Booklet Living with Diabetes to set goals and discuss the purpose and side effects of cardiac medications with the patients. 3) The Office-GAP checklist was used in the primary care encounter at two regularly scheduled visits at 3 and 6 months. The Office-GAP tools were based on Guidelines of the American Heart Association/American College of Cardiology (AHA/ACC) on secondary prevention of heart disease and those of the ADA. They were used to stimulate SDM by providing a systematic list of interventions for the patient and provider to review together and to negotiate agreement on medication use and lifestyle changes. After the Office-GAP encounter, a signed copy of the checklist was provided to the patients, and another copy was kept in the medical record. Data collection for all enrolled patients was performed from October 2010 to March 2014. A 12-month chart abstraction follow-up was done to assess the BP control after the end of the 6-month intervention. The purpose was to assess the sustainability of the intervention effects for the patients in the two arms of the study.
Figure 2.
Office-GAP checklist.
Statistical Analysis
Descriptive statistics were expressed as mean ± standard deviation for baseline continuous variables and as absolute frequency and percentage for categorical variables. Basic inferential analyses using t tests and chi-square tests were conducted to examine differences in baseline characteristics between the Office-GAP intervention and the control groups. For small sample sizes, we used the Fisher’s exact test and the Monte-Carlo test as appropriate.
We conducted primary analysis to evaluate the impact of the Office-GAP intervention on BP control and the secondary analysis to determine the predictors of BP control. Because of the longitudinal nature of the study, correlated data analyses using a generalized estimating equations (GEE) model33 were conducted to describe the profile of BP control across time, taking into account potential predictors. The basic starting model imposed no linear structure of the time effects on the log-odds of BP control, rather treated time as a categorical variable.
The predictors of BP control were then determined by examining their associations with the log-odds of BP control at each visit in the longitudinal data analyses. The effects of the Office-GAP intervention compared to the control were assessed using interactions between the intervention group indicator and time. Specifically, the odds ratios of BP control between follow-up and baseline visits (measuring the longitudinal rate of changes) were used to compare the Office-GAP intervention and the control group. For the latter analyses, the propensity score (PS)34 balancing strategy method was adopted to balance the two intervention arms with respect to baseline characteristics. The propensity scores (probability of being in the Office-GAP intervention arm) for each study participant were estimated using a logistic regression model with potential confounders. Variables included in this propensity scoring model include age, body mass index (BMI), gender, race (Black, White, Asian, other), smoking status, hyperlipidemia, depression, asthma, stroke, congestive heart failure, cancer, PVD, CHD, Charlson index, cardiology visit, and immigration status, type of insurance (Medicare, Medicaid, Ingham health plan, other health insurance, no insurance).
Three sensitivity analyses were conducted. First, a regression (covariance) adjustment treating the propensity scores as covariate in the longitudinal data analyses was considered. Second, a stratification (subclassification) consisting of grouping patients into strata defined in quintiles of propensity scores was also considered. Finally, a third approach based on weighing each patient in the GEE model (by 1/PS for the GAP arm and 1/[1 − PS] for the control arm) was used to control for systematic differences between the two comparison arms. Because all these adjusted longitudinal data analyses gave consistent results, only results generated from the inverse PS weight approach are reported. A P value of <0.05 is considered statistically significant in all analyses. All analyses were performed using SAS version 9.3 (SAS Institute).
Results
A total of 243 patients who met the inclusion criteria were enrolled in the Office-GAP program. Overall, the mean age was 55 ± 11 years. Approximately 57% were females. The proportion of Blacks and Whites was the same (38%) and constituted the majority of the patients in our study (Asian = 13%; Hispanic = 8.4%; other race = 2%). The majority of patients were nonimmigrants (74.5%). Medicaid insurance was 41.2%; 40% had local outpatient coverage for low-income patients (Ingham Health Plan; 40%); Medicare was 25.2%; other insurance was 14.7%. History of DM was present in 88.8% of the patients; 74.9% had HTN, 57.6% had depression, and 16% had CHD. Approximately 51% of the patients were moderately and 22.3% were severely ill based on the Charlson Index, and 35% were current smokers.
Supplemental Table S1 shows the comparison of baseline characteristics of the intervention and control groups. Black patients were the majority (51.3%) in the intervention group and White patients (42.2%) in the control group. Hispanics were more common (10.3%) in the intervention group, whereas Asians (23%) were more frequently observed race in the control group. There were more immigrants in the control group (41.7%) compared to the intervention group (8.1%). Medicaid (47.9%) and other types of insurance (20.7%) were more common in the control group. Depression was more prevalent in the intervention group (48.3%) compared to the control group (30.1%). There was a decrease in the attendance at the follow-up visits in both groups with no difference in dropouts between groups. Similar differences in baseline characteristics were observed in the subgroup of patients with a history of HTN (Table 1) at both the intervention and control sites.
Table 1.
Characteristics for Patients With Hypertension From the Intervention and the Control Centers
| Intervention (n =
95) |
Control (n=87) |
P Value | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Age (years) | 94 | 59.3 | 10.3 | 86 | 57.4 | 10.7 | 0.24 |
| BMI | 91 | 33.2 | 8.3 | 81 | 33.1 | 8.4 | 0.98 |
| n | % | n | % | ||||
| Gender | 95 | 87 | 0.71 | ||||
| Females | 55 | 57.9 | 48 | 55.2 | |||
| Race | 93 | 86 | 0.0003 | ||||
| White | 31 | 33.3 | 39 | 45.4 | |||
| Black | 48 | 51.6 | 23 | 26.7 | |||
| Asian | 3 | 3.2 | 16 | 18.6 | |||
| Hispanic | 9 | 9.7 | 5 | 5.8 | |||
| Others | 2 | 2.2 | 3 | 3.5 | |||
| Smokers | 95 | 33 | 34.7 | 87 | 27 | 31.0 | 0.60 |
| Immigrant | 89 | 85 | |||||
| Nonimmigrant | 84 | 94.4 | 54 | 63.5 | <0.0001 | ||
| Immigrant | 5 | 5.6 | 31 | 36.5 | |||
| Insurance (multiple-choice) | 93 | 86 | |||||
| Medicaid | 33 | 35.5 | 41 | 47.7 | 0.10 | ||
| Medicare | 29 | 31.2 | 17 | 19.8 | 0.08 | ||
| Ingham Health Plan | 41 | 44.1 | 34 | 39.5 | 0.54 | ||
| Others | 8 | 8.6 | 15 | 17.4 | 0.12 | ||
| Medical history | 95 | 87 | |||||
| High cholesterol | 61 | 64.2 | 51 | 58.6 | 0.44 | ||
| Depression | 50 | 52.6 | 26 | 29.9 | 0.0019 | ||
| Asthma | 14 | 14.7 | 10 | 11.5 | 0.52 | ||
| Stroke | 6 | 6.3 | 8 | 9.2 | 0.58 | ||
| Congestive heart failure | 6 | 6.3 | 5 | 5.8 | 1.0 | ||
| Cancer | 10 | 10.5 | 10 | 11.5 | 0.83 | ||
| PVD | 7 | 7.4 | 5 | 5.8 | 0.77 | ||
| CAD | 21 | 22.1 | 15 | 17.2 | 0.41 | ||
| Diabetes mellitus | 94 | 86 | 91.5 | 87 | 73 | 83.9 | 0.12 |
| Charlson Index | 95 | 87 | 0.64 | ||||
| Mildly ill (1 ≤ CCI ≤ 2) | 13 | 13.7 | 15 | 17.2 | |||
| Moderately ill (3 ≤ CCI ≤ 4) | 54 | 56.8 | 51 | 58.6 | |||
| Severely ill (5 ≤ CCI) | 28 | 29.5 | 21 | 24.1 | |||
| Medication use (eligible) | |||||||
| Aspirin/Plavix | 91 | 62 | 68.1 | 85 | 31 | 36.5 | <0.0001 |
| Beta-blockers | 92 | 38 | 41.3 | 85 | 38 | 44.7 | 0.65 |
| ACEI/ARB | 90 | 62 | 68.9 | 85 | 49 | 57.7 | 0.12 |
| Statins | 94 | 62 | 66 | 85 | 50 | 58.8 | 0.32 |
Note: BMI = body mass index; PVD = peripheral vascular disease; CAD = coronary artery disease; CCI = Charlson Comorbidity Index; ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker. Bold P values are significant.
Out of 176 patients with a history of HTN, only 56 (31.8%) had their BP controlled at baseline. Among these 56 patients, 45 (29.2%) were the patients with DM and 16 (59.2%) were without DM. Our study revealed that hypertensive patients with diabetes were less likely to have their BP controlled (odds ratio [OR] = 0.38; 95% confidence interval [CI] = 0.17–0.84; P = 0.015) compared to nondiabetic patients. In a longitudinal logistic regression model, we did not find any significant predictors of BP control except Black race. Blacks have much lower rates of BP control at baseline compared to Whites (OR = 0.56; 95% CI = 0.33–0.96, P = 0.035).
In an unadjusted analysis, we found GAP had an effect on BP control across all the three time points (Figure 3). Albeit nonsignificant, compared to baseline and taking into account important confounders the Office-GAP slightly improved the rate of BP control at 3 months (OR = 1.23; 95% CI = 0.66–2.30) and at 6 months (OR = 1.22; 95% CI = 0.68–2.19) but not at 12 months (OR = 0.84; 95% CI = 0.47–1.49). In contrast, the control arm showed a nonsignificant decrease in the rate of BP control at 3 months (OR = 0.62; 95% CI = 0.30–1.31), at 6 months (OR = 0.42; 95% CI = 0.19–0.90) and at 12 months (OR = 0.35; 95% CI = 0.11–1.18), compared to baseline (Table 2). The rate of BP control was higher in the Office-GAP site at 6 month compared to the control site (ratio of the OR = 2.92; 95% CI = 1.11–7.69; P = 0.030).
Figure 3.
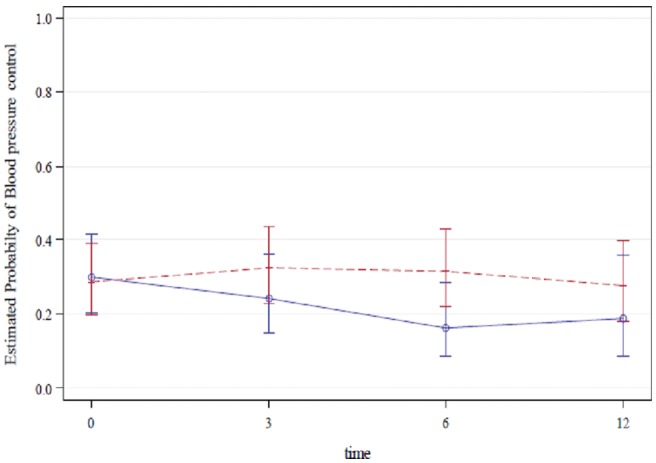
Unadjusted probabilities of blood pressure controlled for GAP intervention group (Red) and control (blue) group, at baseline (0 month), at 3 months, at 6 months, and at 12 months (standard errors take into account the within-subject association).
Table 2.
Adjusted Odds Ratiosa, 95% Confidence Intervals (in Brackets) and P Values for Blood Pressure Control at Follow-Up Visits Compared to Baseline Among Hypertensive Patients
| GAP | Control | Ratio of ORs: GAP/Control | |
|---|---|---|---|
| 3 Months | 1.23 [0.66, 2.30], 0.51 | 0.62 [0.30, 1.31], 0.21 | 1.97 [0.75, 5.20], 0.17 |
| 6 Months | 1.22 [0.68, 2.19], 0.51 | 0.42 [0.19, 0.90], 0.026 | 2.92 [1.11, 7.69], 0.030 |
| 12 Months | 0.84 [0.47, 1.49], 0.55 | 0.35 [0.11, 1.18], 0.09 | 2.38 [0.63, 9.04], 0.20 |
The odds ratios (ORs) are adjusted for the potential confounders in the propensity scores.
The one-page Office-GAP checklist was reported to be simple and easy to use by the patients and providers in the study.
Discussion
We found that the prevalence of coexisting HTN in patients with history of DM, attending the FQHCs (75%), was slightly higher than the national prevalence (71%) reported by National Center for Chronic Disease Prevention and Health Promotion (2009–2012).3 The rate of BP control at baseline in our study was 32%, which is substantially lower than the rate of BP control reported by the most recent National Health and Nutrition Examination Survey, which showed BP control in hypertensive patients of nearly 50% (2007–2008). This is a substantial improvement in the general population when compared with 27.3% in the prior cycle (1988–1994).3
The Office-GAP improved BP control from baseline compared to control; the improvement was significant at 6 months (one of the endpoints of the intervention). This improvement in BP control suggests the importance of patient activation/engagement, SDM, and provider use of evidence-based guidelines and decision support tools in clinical practice. The decline from 6 months to 12 months suggests that sustained intervention or reinforcement may be required to produce sustained behavior change.
The root causes of disparities are complex in multicultural and minority populations. The linguistic and contextual barriers may preclude effective provider-patient communication.35 Low literacy may affect patients’ ability to participate in the decision making more than other groups.36 Our intervention taught and prepared patients for engagement and SDM with their providers during the clinical encounter. It used the checklist to encourage and improve systematic communication about medication use between patients and providers.
In 1999, the ESFT (Explanatory, Social Risk, Fears, Therapeutic Contracting) Model developed by Betancourt and others37,38 comprises a series of questions that allows screening for barriers to compliance such as motivations, concerns about medications, or economic struggles and illustrates strategies for interventions that might improve outcomes for all hypertensive patients. Weiner and others39 reported that the health care outcomes improve when physicians take into account individual patient’s circumstance.
Patient activation/engagement is an essential element in SDM, and provider training in patient engagement and decision support tools also aid in improving satisfaction with physician communication and confidence in the decision.40 SDM is the cornerstone of patient-centered care,41 but unfortunately only 10% of face-to-face consultations involve SDM.42,43 Our results are consistent with Cooper and others, who demonstrated that physician training in patient-centered communication and patient coaching by community health workers improves patient perceptions of engagement in care and may improve SBP among patients with uncontrolled HTN and low socioeconomic status patients.40 In this study, we tested a multilevel approach that integrates patient-centered care with the use of decision support tools. This likely improved patient adherence to medication use, which resulted in better BP control.
Several interventions such as patient decision support interventions, health risk appraisal instruments, and patient reminders have been used to explore patient engagement as a means to improved health outcomes.35,36 There is evidence that the use of one modality does not improve outcomes. In a clustered randomized controlled trial by Tinsel and others,37 the sole provider training did not improve the patients’ perceived participation. In another clustered randomized controlled trial, Thiboutot and others38 demonstrated that the use of an interactive website designed to overcome clinical inertia for HTN care did not improve BP control. Our Cochrane review of interventions for providers to deliver patient-centered care shows that studies using complex interventions focused on providers and patients with condition-specific materials generally showed benefit in health behavior and satisfaction, as well as consultation processes, with mixed effects on health status.44 It appears that the Office-GAP program is unique among SDM studies in improving BP control and being feasible in FQHCs; the intervention was consistently implemented. The Office-GAP tool was found completed in the medical record 98.7% of the time during the office visits. The patients and the providers found the checklist very simple and easy to use during patient-provider encounters. It could serve as a framework for implementing guidelines and improving patient outcomes across chronic diseases.
Limitations
There are some limitations of our study. First, only two FQHCs clinics were studied, one intervention and one control, limiting the generalizability of our findings. However, we demonstrated the feasibility of implementing patient-centered care and SDM in community centers that provide care for minority and low-income populations. Data were obtained from chart abstraction and surveys; therefore, our results for BP control, the main outcome measure, are dependent on the accuracy of medical records. However, we used trained chart abstractors to obtain all our data, and intervention and control measures shared the same measurement techniques. There were differences in the baseline characteristics of patients in the intervention and control arms; however, we used propensity scores in the multiple logistic regression analysis for matching and control for confounders. The authors are currently designing a randomized controlled trial of 12 FQHCs clinics in Michigan to further determine the efficacy and the impact of the GAP intervention on health outcomes.
Conclusions
Our data demonstrate that a majority of patients attending FQHCs in our study did not have their BP controlled at baseline. BP control among hypertensive diabetics was significantly less frequent than in nondiabetic patients. Office-GAP patients showed a higher rate of BP control than controls. The results suggest that the Office-GAP program could serve as a model for improving health outcomes for patients in outpatient clinical settings. Further studies are needed to determine the efficacy in a larger sample and the cost-effectiveness of this approach.
Supplementary Material
Acknowledgments
The authors thank Kim Eagle, MD, Professor of Medicine, University of Michigan, a consultant, to this project. We gratefully acknowledge Dr. Sugandha Lowhim, Dr. Jeffrey Meier, Dr. Priti Pathak, and patients and staff of Ingham Community Health Centers for their time and contribution to this project.
Footnotes
The abstract has been presented at the 37th Annual North American Meeting of the Society for Medical Decision Making, St. Louis, MO.
The authors declare that they do not have any conflicts of interest.
Financial support for this study was provided by the Agency for Healthcare Research and Quality (AHRQ) #1 KO8 HS018104. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Supplementary material for this article is available on the Medical Decision Making Policy & Practice Web site at http://journals.sagepub.com/doi/suppl/10.1177/2381468316656010.
References
- 1. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief. 2013;(133):1–8. [PubMed] [Google Scholar]
- 2. Kung HC, Xu J. Hypertension-related mortality in the United States, 2000–2013 (NCHS Data Brief, No. 193). Hyattsville, MD: National Center for Health Statistics; 2015. Available from: http://www.cdc.gov/nchs/data/databriefs/db193.pdf [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States. Atlanta, GA: US Department of Health and Human Services; 2014. Available from: http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf [Google Scholar]
- 4. Arauz-Pacheco C, Parrott MA, Raskin P. The treatment of hypertension in adult patients with diabetes. Diabetes Care. 2002;25(1):134–47. [DOI] [PubMed] [Google Scholar]
- 5. Parati G, Bilo G, Ochoa JE. Benefits of tight blood pressure control in diabetic patients with hypertension: importance of early and sustained implementation of effective treatment strategies. Diabetes Care. 2011;34(suppl 2):S297–S303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kengne AP, Patel A, Barzi F, et al. Systolic blood pressure, diabetes and the risk of cardiovascular diseases in the Asia-Pacific region. J Hypertens. 2007;25(6):1205–13. [DOI] [PubMed] [Google Scholar]
- 7. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321(7258):412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mozzafarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. [DOI] [PubMed] [Google Scholar]
- 9. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44. [DOI] [PubMed] [Google Scholar]
- 10. SPRINT Research Group; Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Burgos-Lunar C, del Cura-González I, Salinero-Fort MA, Gómez-Campelo P, Pérez de Isla L, Jiménez-García R. Delayed diagnosis of hypertension in diabetic patients monitored in primary care. Rev Esp Cardiol (Engl Ed). 2013;66(9):700–6. [DOI] [PubMed] [Google Scholar]
- 12. Wang JT, Vasan RS. Epidemiology of uncontrolled hypertension in the United States. Circulation. 2005;112(11):1651–62. [DOI] [PubMed] [Google Scholar]
- 13. Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Hypertension control: how well are we doing? Arch Intern Med. 2003;163(22):2705–11. [DOI] [PubMed] [Google Scholar]
- 14. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603–15. [DOI] [PubMed] [Google Scholar]
- 15. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–50. [DOI] [PubMed] [Google Scholar]
- 16. Yoon SS, Burt V, Louis T, Carroll M. Hypertension among adults in the United States, 2009–2010 (NCHS Data Brief, No. 107). Hyattsville, MD: US Department of Health and Human Services; 2012. Available from: http://www.cdc.gov/nchs/data/databriefs/db107.htm [PubMed] [Google Scholar]
- 17. National Association of Community Health Centers. Fact sheet, “America’s Health Centers”; October 2014. Available from: http://nachc.org/wp-content/uploads/2015/06/Americas-Health-Centers-March-2016.pdf.
- 18. Darnell J. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170(11):946–53. [DOI] [PubMed] [Google Scholar]
- 19. US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Primary Health Care. Health centers: America’s primary care safety net, reflections on success, 2002– 2007; June 2008. Available from: ftp://ftp.hrsa.gov/bphc/HRSA_HealthCenterProgramReport.pdf [Google Scholar]
- 20. Department of Health Policy, Washington University. Quality incentives for federally qualified health centers, rural health clinics and free clinics: a report to Congress 2012. Available from: https://www.healthit.gov/sites/default/files/pdf/quality-incentives-final-report-1-23-12.pdf
- 21. Dievler A, Giovannini T. Community health centers: promise and performance. Med Care Res Rev. 1998;55:405–31. [DOI] [PubMed] [Google Scholar]
- 22. Politzer RM, Yoon J, Shi L, Hughes RG, Regan J, Gaston MH. Inequality in America: the contribution of health centers in reducing and eliminating disparities in access to care. Med Care Res Rev. 2001;58(2):234–48. [DOI] [PubMed] [Google Scholar]
- 23. Gale J, Coburn A. The Characteristics and Roles of Rural Health Clinics in the United States: A Chartbook. Portland, ME: University of Southern Maine, Muskie School of Public Service, Maine Rural Health Research Center; 2003. [Google Scholar]
- 24. Shelley D, Tseng TY, Andrews H, et al. Predictors of blood pressure control among hypertensives in community health centers. Am J Hypertens. 2011;24(12):1318–23. [DOI] [PubMed] [Google Scholar]
- 25. Hibbard JH, Mahoney E. Toward a theory of patient and consumer activation. Patient Educ Couns. 2010;78(3):377–81. [DOI] [PubMed] [Google Scholar]
- 26. Rosenthal TC. The medical home: growing evidence to support a new approach to primary care. J Am Board Fam Med. 2008;21(5):427–40. [DOI] [PubMed] [Google Scholar]
- 27. Roumie CL, Greevy R, Wallston KA, et al. Patient centered primary care is associated with patient hypertension medication adherence. J Behav Med. 2011;34(4):244–53. [DOI] [PubMed] [Google Scholar]
- 28. Kaelber DC, Bates DW. Health information exchange and patient safety. J Biomed Inform. 2007;40(6 suppl.):S40–S45. [DOI] [PubMed] [Google Scholar]
- 29. Hibbard JH, Greene J. What the evidence shows about patient activation: Better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32(2):207–14. [DOI] [PubMed] [Google Scholar]
- 30. US Department of Health and Human Services. The seventh report of the Joint National Committee. Available from: http://www.nhlbi.nih.gov/files/docs/guidelines/express.pdf
- 31. Smith RC, Marshall-Dorsey AA, Osborn GG, et al. Evidence-based guidelines for teaching patient-centered interviewing. Patient Educ Couns. 2000;39(1):27–36. [DOI] [PubMed] [Google Scholar]
- 32. Dwamena FC, Mavis B, Holmes-Rovner M, Walsh KB, Loyson AC. Teaching medical interviewing to patients: the other side of the encounter. Patient Educ Couns. 2009;76(3):380–4. [DOI] [PubMed] [Google Scholar]
- 33. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 34. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carrillo JE, Green AR, Betancourt JR. Cross-cultural primary care: a patient-based approach. Ann Intern Med. 1999;130(10):829–34. [DOI] [PubMed] [Google Scholar]
- 36. Aboumatar HJ, Carson KA, Beach MC, Roter DL, Cooper LA. The impact of health literacy on desire for participation in healthcare, medical visit communication, and patient reported outcomes among patients with hypertension. J Gen Intern Med. 2013;28(11):1469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Betancourt JR, Carrillo JE, Green AR. Hypertension in multicultural and minority populations: linking communication to compliance. Curr Hypertens Rep. 1999;1(6):482–8. [DOI] [PubMed] [Google Scholar]
- 38. Betancourt JR. Cultural competency: providing quality care to diverse populations. Consult Pharm. 2006;21(12):988–95. [DOI] [PubMed] [Google Scholar]
- 39. Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573–9. [DOI] [PubMed] [Google Scholar]
- 40. Cooper LA, Roter DL, Carson KA, et al. A randomized trial to improve patient-centered care and hypertension control in underserved primary care patients. J Gen Intern Med. 2011;26(11):1297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barry MJ, Edgman-Levitan S. Shared decision making: the pinnacle of patient-centered care. N Engl J Med. 2012;366:780–1. [DOI] [PubMed] [Google Scholar]
- 42. Russell A, Abidi SR, Abidi SS. Shared decision making: using theories and technology to engage the patient in their health journey. Stud Health Technol Inform. 2014;205:303–7. [PubMed] [Google Scholar]
- 43. Godolphin W. Shared decision-making. Healthc Q. 2009;12 Spec No Patient:e186–90. [DOI] [PubMed] [Google Scholar]
- 44. Dwamena F, Holmes-Rovner M, Gaulden CM, et al. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database Syst Rev. 2012;(12):CD003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.