Abstract
Lumbar spinal stenosis (LSS) is an increasingly prevalent condition that has major health and economic implications. While there are many options for the treatment of LSS, exercise is widely considered a first-line intervention as it is associated with reduced complications and cost as compared to more invasive options. Currently, it is not clear if exercise is an effective approach to managing pain and perceived disability in patients with symptomatic LSS. Therefore, the purpose of this systematic review is to evaluate the published literature that has investigated exercise as a primary intervention for LSS. A search was conducted in electronic databases including PubMed, PEDro, SPORTDiscus, CINAHL, and AMED using the key words lumbar spinal stenosis, exercise, physical therapy, rehabilitation, and conservative treatment. Inclusion criteria consisted of published randomized controlled trials written in English that included exercise as the primary treatment in at least one of the groups, and had reported measures of pain and disability clearly stated. The search identified 310 studies of which 5 met all the inclusion parameters. Exercise appears to be an efficacious intervention for pain, disability, analgesic intake, depression, anger, and mood disturbance among patients with LSS. Further research is needed to determine which type of exercise is the most effective in managing symptoms associated with lumbar spinal stenosis.
Keywords: Lumbar, Stenosis, Exercise, Conservative Care
‘The most common symptoms of LSS are low back pain, neurogenic claudication, and radiculopathy. Causes of LSS can be congenital or acquired, with the latter being more common.’
Lumbar spinal stenosis (LSS) is characterized by narrowing of spaces in the spinal canal or lateral foramina1 as well as reduced blood flow around the spinal nerve roots.2 Narrowing of these regions, depending on the degree, may cause pressure on the thecal sac and nerves resulting in pain and an associated decline in function. Due to the sensitivity of the spinal nerves, LSS can lead to neurological deficits and functional disabilities.3 The most common symptoms of LSS are low back pain, neurogenic claudication, and radiculopathy. Causes of LSS can be congenital or acquired, with the latter being more common.1
The incidence of LSS increases with age and this condition is becoming more prevalent with the reported increase in life expectancy.4 Patients with LSS increased (94 000 to 102 000) from 2004 to 2009 according to an analysis of national data in the United States by Bae et al.5 A study by Kalichman et al6 found a 3-fold increase in the prevalence of low back pain (LBP) due to LSS. This LBP results in a decrease of the quality of life for those with LSS.4 Unfortunately, LSS is not only common but very expensive. Overall, costs for low back pain have been estimated between $50 and $100 billion per year.7 Although the contribution of LSS to this number is not specifically defined, the combined expenses from pharmaceutics, outpatient procedures, and inpatient procedures for the treatment of LSS are likely to be substantial.
There are many medical treatments available for LSS, ranging from conservative to invasive. Surgery is a common treatment and includes decompressive procedures performed with or without spinal fusion. Epidural steroid injections (ESIs) are also a medical intervention that patients with LSS can receive. This type of medical intervention is a less costly option at the early stages of LSS; however, injections are often repeated in order to maintain reduced pain levels over time.8 With the average time frame of pain relief being only 3 weeks,9 this can lead to a multitude of injections and many repeated hospital visits. Park et al10 reported that ESIs were effective for short-term relief only. Another study by Radcliff et al11 showed an increase in dural tear rates for patients with injections, which would cause potential increased length of stay and cost. The literature reports mixed results on the effectiveness of injections for patients with LSS, which combined with adverse effects such as dural puncture, increased blood glucose, and the need for many to discontinue routine anticoagulant, use make it a less than desirable option.
Many different kinds of pharmacological therapies and alternative substances have been studied to determine their effect on LSS. In a single-blind study performed by Sahin et al,12 calcitonin in combination with physical therapy treatment (including an exercise program) demonstrated no significant differences when compared to physical therapy treatment and exercise alone. Gabapentin, on the other hand, was found to decrease pain and increase daily functioning in 78 patients described as having intense pain due to LSS.13 Gabapentin was given orally for 3 months to the experimental group, and when assessed, walking distance increased while pain decreased in these patients. Prostaglandin E1 derivatives, another substance tested for effectiveness in controlling pain associated with LSS, such as limoprost, are seen to increase physical functioning as well as decreasing pain and increase overall satisfaction.2,14 Limoprost, along with other prostaglandin E1 derivatives, increase blood flow to the area of pain, which ultimately leads to temporary pain relief.
While these pharmacological treatments may be effective in managing LSS, other conservative treatments may benefit patients as well. One type of conservative treatment is therapeutic exercise, which includes aerobic activity, flexibility and mobility training, muscle strengthening, and a combination of these 3 categories. Some examples of aerobic exercise are low-intensity cycling15 and treadmill exercise with body weight support. An exercise program involving a treadmill with body weight support has been shown to decrease pain and increase functioning in patients with LSS.16 The movement orientation of exercise can also have an effect on LSS treatment. For example, a study by Weiner et al17 reported that treatment that included flexion biased movements was shown to increase walking time and distance before symptom onset in those with LSS. This apparatus is meant to allow the researcher or therapist to position the patient in the most comfortable position while walking on the treadmill. With flexion and mobility training, a decrease in pressure due to tight muscles and an increase in the strength of these muscles are seen, resulting in a decrease in pain in the area of focus.
Therapeutic exercise may address strength or flexibility deficits targeting the lower extremities, abdominal and back musculature, which can lead to increased mobility and decreased pressure on the structures associated with LSS.15,18 A balance of select strengthening and flexibility may facilitate an optimal approach for rehabilitation. For example, flexibility training of select muscle groups such as the hip flexors and strengthening of the abdominal musculature may lead to better position of the lumbopelvic complex and decreased pressure on the posterior element of the spine.19
Combinations of interventions are often used simultaneously. For example, exercise treatments are prescribed with a combination of medications, corticosteroid injections, and even electrotherapy.20 In a randomized control study done by Goren et al,15 a combination of stretching and strengthening exercises for lumbar, abdominal, and leg muscles showed a decrease in pain and disability as reported using the Oswestry Disability Index (ODI). Also, exercises combined with physical modalities such as ultrasound were considered to be effective in decreasing pain and analgesic use.15 Another randomized control study by Whitman et al21 showed the group treated with a combination of physical therapy treatment, flexion exercises, and walking on a treadmill recovered faster than the group that included a flexion exercise, body weight–supported treadmill walking, and ultrasound. Both the body weight–supported treadmill and the basic treadmill were flat. The study included 58 patients over a 6-week period after which the patients were rated on criteria including perceived recovery, pain, and other health care resources. These patients were followed-up after 1 year, and 62% recovered in the physical training, exercise, and walking group compared to only 41% of the flexion exercise, treadmill walking, and ultrasound group. These outcomes were based on questionnaires with an end result of a numerical global rating of change scale. This illustrated that individuals can benefit from physical therapy treatment in reduction of low back pain. Individual, one-on-one therapy that includes a combination of spine mobilization and stabilization exercises is another exercise combination seen to be effective in a study done by Lewis et al.22
Nonoperative treatment involving exercise is favorable as the patient is not exposed to a hospital, has no recovery time, complications, or blood loss.23 It is also less costly as surgery expenses have been shown to vary between $23 000 and $27 000 for LSS.23 The possibility of reoperation may also result in higher costs for patients. Of particular concern is that certain surgical techniques for LSS have been shown to have reoperation rates of up to 23%.24 Atlas et al25 found that surgical pain relief decreases over time while nonsurgical treatment improvement stays constant in patients with LSS. While this is an interesting finding, it certainly does not represent the status of those individuals with severe neurological signs.
Currently, it is not clear if exercise can be considered a viable treatment for LSS. In patients with LSS, most cases are long-lasting, not life-threatening, and the majority do not have symptoms that worsen over time due to compensatory postural changes.18 Because there are few trials that examine the effectiveness of exercise, there is little agreement on the effects of exercise in those with LSS. While surgery does improve LSS symptoms, patients with more mild symptoms may benefit from conservative care. Therefore, a systematic review of the literature is necessary to determine if exercise is indeed efficacious for the treatment of LSS.
Methods
Data Sources and Searches
A computerized database search was performed on PubMed (181 studies), PEDro (18 studies), SPORTDiscus (24 studies), CINAHL (70 studies), and AMED (17 studies) up to March 27, 2014. Key words used in the search included exercise, lumbar spinal stenosis, physical therapy, rehabilitation, and conservative treatment.
Study Selection Criteria
To be included in the analysis, a study must have met the following criteria: a randomized controlled trial, peer reviewed, in the English language, used exercise as the primary treatment in one of the experimental groups, and included outcome measures for pain and disability. The search used electronic databases and resulted in 310 studies for potential inclusion. Of these studies, 15 were considered possible candidates for this review based on superficial information such as the title and abstract. After careful reading of each full-text study, 10 were excluded because treatment was not specifically for improvement of symptoms associated with LSS or exercise was not the experimental variables.
Quality Assessment
The quality of the selected studies was assessed using the PEDro Scale, which is regarded as one of the most widely used assessment tools in physical therapy–related research. In a study done by Maher et al,26 Kappa (κ) values for individuals using the PEDro scale varied between 0.36 and 0.80 while κ values for a consensus varied between 0.5 and 0.79. Maher et al26 then concluded that the conclusion of item reliability ratings were “fair” to “substantial” while the total reliability ratings were “substantial” to “good.”
Results
Included Studies
After all studies were vetted and deemed to meet the inclusion criteria, 5 studies were selected for this systematic review (Figure 1).
Figure 1.
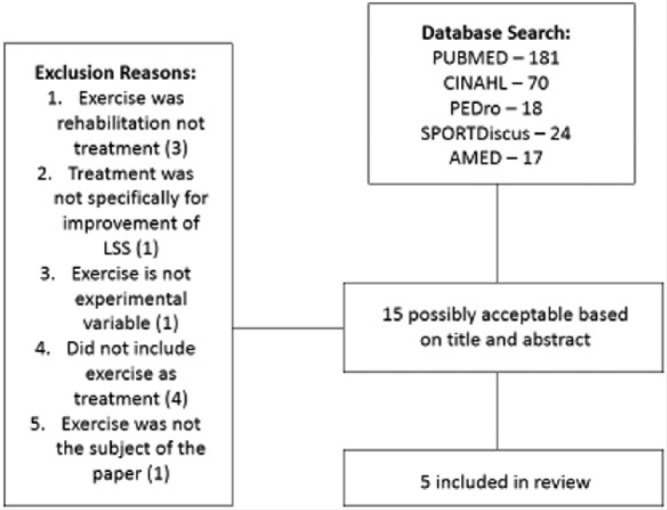
Search Strategy for Selected Studies.
Methodological Quality
The mean PEDro score of the 5 studies selected was 6.2 (out of 10) with a range between 2 and 8 (Table 1). All the studies included blind subjects except the study done by Sculco et al.27 Also, all studies used random assignment with the study done by Sculco et al27 using a matched stratified design to accomplish this.
Table 1.
Studies Involving Exercise Treatment for Lumbar Spinal Stenosis.
| Study | Population Examined; Main Inclusion Criteria | Method of Group Assignment | Experimental Group Description | Control Group Description | Main Outcome Measures | PEDro Score |
|---|---|---|---|---|---|---|
| Pua et al16 | 68 patients; ≥50 years old, history of LBP, BMI <38 kg/m2, evidence of LSS on MRI, no mental impairments, no LSS surgery, no neoplastic conditions, no severe osteoporosis, no vascular or pulmonary disease | Random assignment | Treadmill with body weight support + exercise program | Cycling + exercise program | ODI, Roland-Morris Disability Questionnaire, 100-mm VAS, Patient Perceived Benefit | 8/10 |
| Goren et al15 | 54 patients; MRI-proven LSS, back and/or leg pain, standing or walking pain, over 18 years old, symptoms lasting over 3 months, neurogenic claudication within 15 minutes on treadmill | Random assignment | Ultrasound + exercise/sham ultrasound + exercise | No exercise + no ultrasound | Pain, disability, functional capacity, consumption of analgesic, ODI, Wilcoxon’s rank test and disability index | 7/10 |
| Koc et al29 | 33 patients; diagnosed with LSS, no heart disease, no past spinal surgery, no vertebral fracture, no progressive neurologic deficit, no cauda equine | Random assignment | Physical therapy treatment/epidural steroid injections | No treatment | FFD, treadmill walk test, sit-to-stand test, weight carrying test, Roland-Morris Disability Index, Nottingham Health Profile | 6/10 |
| Whitman et al21 | 58 patients; LSS diagnosis, pain in lumbopelvic region and lower extremities, over 50 years old, patient rating of sitting being more comfortable than standing or walking, no previous LSS surgery, no artery disease | Random assignment | Manual physical therapy treatment, body-supported treadmill walking, exercise | Lumbar flexion exercises, treadmill walking program, subtherapeutic ultrasound | Global rating of change scale, ODI, Numerical Pain Rating Scale, Satisfaction Pain Rating Scale, treadmill test | 8/10 |
| Sculco et al27 | 34 patients; 25-65 years old, not involved in exercise routine, good health, no medical or psychological issues that could affect aerobic exercise, willing to follow exercise intervention, no acute low back pain, no surgical intervention for LBP in the last 6 months | Matched stratified | Aerobic exercise | No exercise | Profile of Mood States, Brief Pain Inventory, medical office visits for pain/symptoms, physical therapy treatment referrals, prescription of pain medication, work status | 2/10 |
Abbreviations: LBP, low back pain; BMI, body mass index; LSS, lumbar spinal stenosis; MRI, magnetic resonance imaging; ODI, Oswestry Disability Index; VAS, Visual Analog Scale; FFD, Finger Floor Distance.
Study Characteristics
In the study done by Pua et al,16 68 patients were split between 2 types of exercise groups. The first group jogged on a treadmill using body weight support as a means of decreasing axial load and the second group cycled. Both groups participated in a flexion exercise program that consisted of 3 flexion exercises that aimed to improve circulation in the spinal region and to help with range of motion of the spine. Pain and disability outcomes, based on the ODI and the Roland-Morris Disability Questionnaire, improved in both groups and overall outcomes were similar, suggesting the flexion exercises and any type of non-extension-based aerobic activity is efficacious (see Table 2).
Table 2.
Interventions Reported by Pua et al.16
| Treadmill Group | Cycling Group |
|---|---|
| Walked at their own pace, using the Biodex unweighting system, for the first 2 weeks followed by encouragement to walk more rapidly during the remaining 4 weeks | Training on an upright bike, cycled at their own pace (50-60 rpm) for the first 2 weeks followed by encouragement to cycle more rapidly during the remaining 4 weeks |
| Both groups were treated 2 times per week for a maximum of 30 minutes or until limited by participant tolerance | Participants remained in a flexed posture and did not extend the lumbar region during exercise |
| Both groups were treated 2 times per week for a maximum of 30 minutes or until limited by participant tolerance | |
| Oswestry Disability Index, Mean (SD) | Oswestry Disability Index, Mean (SD) |
| Baseline: 33.0 (15.8) | Baseline: 31.8 (14.1) |
| 3 weeks: 29.3 (16.5) | 3 weeks: 25.2 (14.7) |
| 6 weeks: 25.9 (16.7) | 6 weeks: 23.0 (14.2) |
In a study by Goren et al,15 54 patients were randomized into an ultrasound plus exercise group, a sham ultrasound plus exercise group, and a control group that neither received ultrasound nor participated in exercise. The 2 exercise groups participated in the same exercise program, which included flexibility exercises (stretching hamstrings, quadriceps, and lumbar paraspinal muscles), strengthening exercises (abdominals), and concluded with low-intensity cycling. While there was no statistical difference between the 2 exercise groups, there were significant differences between the exercise groups and the control group. According to a study by Kim et al,28 flexion exercises are shown to increase the central canal space that results in increased spinal mobility and increased abdominal strength. The significant differences were evidenced by lower scores on the ODI and lower reported pain in the leg(s). The result of this study suggest that exercise may be effective in decreasing disability and pain in LSS patients and the effect of therapeutic ultrasound is questionable (see Table 3).
Table 3.
Interventions Reported by Goren et al.15
| Control | Exercise + Ultrasound | Exercise + Sham Ultrasound |
|---|---|---|
| No treatment | Both experimental groups participated in exercise 5 days per week for 3 weeks | Both experimental groups participated in exercise 5 days per week for 3 weeks |
| All groups did not take NSAIDs or muscle relaxant drugs | Flexibility | Flexibility |
| • Stretching of iliopsoas, quadriceps, hamstrings, and paraspinal muscles in the lumbar region | • Stretching of iliopsoas, quadriceps, hamstrings, and paraspinal muscles in the lumbar region | |
| Strengthening | Strengthening | |
| • Abdominals and posterior pelvic tilt lasting 20 minutes | • Abdominals and posterior pelvic tilt lasting 20 minutes | |
| Cycling | Cycling | |
| • Low intensity | • Low intensity | |
| • 5 minutes of warm up and cool down following exercise | • 5 minutes of warm up and cool down following exercise | |
| • 15 minutes of cycling | • 15 minutes of cycling | |
| 10 minutes of ultrasound on paravertebral area of lumbar spine | Sham ultrasound with acoustic gel was used | |
| All groups did not take NSAIDs or muscle relaxant drugs | All groups did not take NSAIDs or muscle relaxant drugs | |
|
| ||
| Oswestry Disability Index, Mean (SD) | Oswestry Disability Index, Mean (SD) | Oswestry Disability Index, Mean (SD) |
|
| ||
| Baseline: 32.30 (9.60) | Baseline: 25.46 (7.70) | Baseline: 26.90 (10.19) |
| Posttreatment: 28.60 (9.20) | Posttreatment: 21.50 (9.30) | Posttreatment: 19.10 (8.00) |
|
| ||
| Visual Analog Scale, Mean (SD) | Visual Analog Scale, Mean (SD) | Visual Analog Scale, Mean (SD) |
|
| ||
| Back pain | Back pain | Back pain |
| Baseline: 5.26 (3.36) | Baseline: 5.53 (1.96) | Baseline: 6.20 (2.60) |
| Posttreatment: 5.66 (2.90) | Posttreatment: 3.33 (2.79) | Posttreatment: 4.26 (3.26) |
| Leg pain | Leg pain | Leg pain |
| Baseline: 6.60 (2.80) | Baseline: 5.80 (2.90) | Baseline: 6.33 (3.33) |
| Posttreatment: 7.13 (3.04) | Posttreatment: 4.33 (2.99) | Posttreatment: 3.86 (3.02) |
Abbreviations: SD, standard deviation; NSAID, nonsteroidal anti-inflammatory drug.
Koc et al29 randomly allocated 33 patients into 3 groups (physical therapy treatment only, epidural steroid injection only, and no treatment/control) to investigate the effectiveness of physical therapy treatment and corticosteroid injections on pain and disability in people with LSS. Evaluations were performed after 2 weeks, 1 month, 3 months, and 6 months for multiple parameters including VAS (visual analog scale), FFD (finger floor distance), a treadmill walk test, a sit-to-stand test, and a weight-carrying test. After analyzing the results, no significant difference was found between the physical therapy treatment only group and the ESI only group. As stated by Genevay et al,30 both the treatment groups had decreased pain and improved function measures; however, the only significant difference found was within the ESI group at the 2-week measurement. In this measurement, there was a significant improvement in the VAS score (pain intensity) of the group receiving corticosteroid injections only compared to the control group (see Table 4).
Table 4.
Interventions Reported by Koc et al.29
| Inpatient Physical Therapy Treatment | Lumbar Epidural Steroid Injections | Control Group |
|---|---|---|
| • Ultrasound for 10 minutes | • Used intralaminar method | No treatment |
| • 20 minutes of hot pack | • 3 mL of contrast followed by 10 mL of combination of triamcinolone (60 mg), 0.5% bupivacaine hydrochloride (15 mg), and normal saline (5.5 mL) | |
| • 20 minutes of TENS to lumbar region of back | ||
| • Conservative program given 5 days per week for 2 weeks | ||
|
| ||
| Nottingham Health Profile; Changes in Median Scores | Nottingham Health Profile; Changes in Median Scores | Nottingham Health Profile; Changes in Median Scores |
|
| ||
| Pain (VAS) | Pain (VAS) | Pain (VAS) |
| Baseline: 54.1 | Baseline: 56.3 | Baseline: 58.6 |
| 2-week follow-up: 19.4 | 2-week follow-up: 7.3 | 2-week follow-up: 33.0 |
| 1-month follow-up: 31.2 | 1-month follow-up: 36.2 | 1-month follow-up: 20.1 |
| 3-month follow-up: 18.2 | 3-month follow-up: 20.5 | 3-month follow-up: 27.7 |
| 6-month follow-up: 23.2 | 6-month follow-up: 23.0 | 6-month follow-up: 20.1 |
| Physical mobility | Physical mobility | Physical mobility |
| Baseline: 41.8 | Baseline: 41.8 | Baseline: 41.8 |
| 2-week follow-up: 31.2 | 2-week follow-up: 21.9 | 2-week follow-up: 31.2 |
| 1-month follow-up: 37.2 | 1-month follow-up: 31.9 | 1-month follow-up: 20.5 |
| 3-month follow-up: 32.5 | 3-month follow-up: 31.2 | 3-month follow-up: 31.0 |
| 6-month follow-up: 37.1 | 6-month follow-up: 31.2 | 6-month follow-up: 20.5 |
| Energy | Energy | Energy |
| Baseline: 88.0 | Baseline: 100 | Baseline: 63.2 |
| 2-week follow-up: 30.4 | 2-week follow-up: 60.8 | 2-week follow-up: 63.2 |
| 1-month follow-up: 24.0 | 1-month follow-up: 100 | 1-month follow-up: 60.8 |
| 3-month follow-up: 30.4 | 3-month follow-up: 62.0 | 3-month follow-up: 100 |
| 6-month follow-up: 48.8 | 6-month follow-up: 81.6 | 6-month follow-up: 63.2 |
| Sleep | Sleep | Sleep |
| Baseline: 55.9 | Baseline: 58.0 | Baseline: 55.9 |
| 2-week follow-up: 31.8 | 2-week follow-up: 26.2 | 2-week follow-up: 12.5 |
| 1-month follow-up: 12.5 | 1-month follow-up: 44.7 | 1-month follow-up: 12.5 |
| 3-month follow-up: 12.5 | 3-month follow-up: 14.3 | 3-month follow-up: 28.6 |
| 6-month follow-up: 12.5 | 6-month follow-up: 25.5 | 6-month follow-up: 28.6 |
| Social isolation | Social isolation | Social isolation |
| Baseline: 28.9 | Baseline: 41.7 | Baseline: 0.0 |
| 2-week follow-up: 18.0 | 2-week follow-up: 22.0 | 2-week follow-up: 0.0 |
| 1-month follow-up: 18.9 | 1-month follow-up: 22.0 | 1-month follow-up: 0.0 |
| 3-month follow-up: 11.0 | 3-month follow-up: 32.0 | 3-month follow-up: 0.0 |
| 6-month follow-up: 0.0 | 6-month follow-up: 32.3 | 6-month follow-up: 0.0 |
| Emotional reactions | Emotional reactions | Emotional reactions |
| Baseline: 33.0 | Baseline: 45.0 | Baseline: 23.7 |
| 2-week follow-up: 17.1 | 2-week follow-up: 13.3 | 2-week follow-up: 0.0 |
| 1-month follow-up: 15.1 | 1-month follow-up: 46.1 | 1-month follow-up: 9.7 |
| 3-month follow-up: 0.0 | 3-month follow-up: 41.4 | 3-month follow-up: 9.7 |
| 6-month follow-up: 6.9 | 6-month follow-up: 27.5 | 6-month follow-up: 0.0 |
Abbreviations: TENS, transcutaneous electrical nerve stimulation; VAS, Visual Analog Score.
In a 6-week experiment done by Whitman et al,21 58 patients were randomly assigned to either an exercise and walking combination group or an exercise, walking, and manual physical therapy treatment combination group. Exercises were focused on flexion of the spine. A body weight–supported treadmill was used for the walking program. Body-supported treadmill exercise has been shown by Joffe et al31 to be more effective than unaided treadmill exercise because the unloading of the spine results in less pain and discomfort. Participants walked for a maximum of 45 minutes depending on their pain tolerance. The manual physical therapy intervention focused on techniques for the lumbar spine, hips, and legs. After 1 year, the exercise, walking, and manual physical therapy treatment combination group improved greater (62% vs 41%) than the exercise and walking combination group based on perceived recovery (see Table 5).
Table 5.
Interventions Reported by Whitman et al.21
| Flexion Exercise + Walking | Manual Physical Therapy Treatment + Exercise + Walking |
|---|---|
| • 10 minutes ultrasound | Therapy |
| • Flexion exercises (knee-to-chest exercises, level treadmill where participants were advised to stop when they felt enough pain to make them stop normally) | • Spine (lumbar and thoracic) |
| • Treadmill lasted up to 45 minutes with participants stopping once they reached their pain threshold | • Pelvis |
| • All patients kept taking medications they were using for LSS | • Lower extremities |
| • No steroid injections were performed 6 weeks before treatment | • Thrust and nonthrust manipulations |
| • Stretching and strengthening | |
| • Exercises regarding mobility = 3 sets of 30 seconds | |
| • Exercises regarding stretching = 3 sets of 30 seconds | |
| • Used a body weight–supported treadmill program | |
| • All patients kept taking medications they were using for LSS | |
| • No steroid injections were performed 6 weeks before treatment | |
|
| |
| Patient Global Rating Scale (based on perceived recovery and the threshold for this being a +3 or greater on a scale from −7 to 7); % | Patient Global Rating Scale (based on perceived recovery and the threshold for this being a +3 or greater on a scale from −7 to 7); % |
|
| |
| 6-week follow-up: 41 | 6-week follow-up: 79 |
| 1-year follow-up: 41 | 1-year follow-up: 62 |
Abbreviation: LSS, lumbar spinal stenosis.
In a study done by Sculco et al,27 34 patients were randomly assigned to either an aerobic exercise group or a control group that performed no exercise. Participants in the aerobic exercise group were given a 2.5-month home exercise program that consisted of cycling or walking for 4 days per week. After 10 weeks, the aerobic exercise group reported a decrease in depression, anger, and mood disturbance. Bjornsdottir et al32 demonstrated that mental health issues such as depression increase in patients with chronic pain because of the loss of everyday function and discomfort associated with the condition. Participants completed follow-up measures after 2.5 years, and the aerobic exercise group had fewer analgesic prescriptions, fewer referrals to physical therapy treatment, and an increased work status compared to the no exercise control group (see Table 6).
Table 6.
Interventions Reported by Sculco et al.27
| Aerobic Exercise | Control |
|---|---|
| • Participants took part in an at-home exercise program for 10 weeks at 4 times per week | No treatment |
| • Week 1 = 20 minutes of exercise | |
| • Week 2 = 30 minutes of exercise | |
| • Week 3 to Week 10 = 45 minutes of exercise | |
| • Included either cycling or walking at 60% of max heart rate | |
| • Pulse rate was self-monitored during exercise | |
|
| |
| Profile of Mood States, Mean (SD) | Profile of Mood States, Mean (SD) |
|
| |
| Depression | Depression |
| Baseline: 3.64 (4.06) | Baseline: 6.16 (8.35) |
| Week 5: 2.35 (4.12) | Week 5: 5.88 (10.17) |
| Week 10: 3.64 (5.74) | Week 10: 9.44 (12.31) |
| Fatigue | Fatigue |
| Baseline: 4.58 (4.43) | Baseline: 7.72 (6.51) |
| Week 5: 4.41 (4.89) | Week 5: 8.33 (6.31) |
| Week 10: 3.88 (4.24) | Week 10: 8.05 (7.33) |
| Anger | Anger |
| Baseline: 2.35 (2.99) | Baseline: 4.11 (5.49) |
| Week 5: 0.94 (2.19) | Week 5: 5.77 (7.10) |
| Week 10: 1.82 (3.57) | Week 10: 8.16 (11.27) |
| Confusion | Confusion |
| Baseline: 1.52 (5.02) | Baseline: 1.88 (4.43) |
| Week 5: 0.88 (2.71) | Week 5: 2.00 (3.27) |
| Week 10: 0.58 (2.59) | Week 10: 3.38 (4.91) |
| Tension | Tension |
| Baseline: 2.05 (3.63) | Baseline: 4.16 (6.80) |
| Week 5: 0.12 (4.13) | Week 5: 4.50 (6.64) |
| Week 10: 0.94 (4.94) | Week 10: 4.27 (7.02) |
| Vigor | Vigor |
| Baseline: 15.00 (6.08) | Baseline: 15.61 (5.61) |
| Week 5: 16.52 (8.46) | Week 5: 15.00 (7.00) |
| Week 10: 17.88 (7.32) | Week 10: 13.72 (6.96) |
| Total mood disturbance | Total mood disturbance |
| Baseline: • 2.11 (17.31) | Baseline: 11.22 (32.15) |
| Week 5: • 8.00 (21.69) | Week 5: 11.94 (32.94) |
| Week 10: • 9.58 (19.89) | Week 10: 19.11 (43.72) |
|
| |
| Brief Pain Inventory (Average), Mean (SD) | Brief Pain Inventory (Average), Mean (SD) |
|
| |
| Baseline: 3.70 (1.31) | Baseline: 4.27 (2.21) |
| Week 5: 3.05 (2.19) | Week 5: 4.00 (2.24) |
| Week 10: 3.23 (1.67) | Week 10: 4.05 (2.33) |
Abbreviation: SD, standard deviation.
Discussion
While these studies showed that exercise is an effective treatment for LSS, study design limitations were noted. First, the participants included in the studies were disproportionately female. The Koc et al29 and Goren et al15 studies included 56 females out of a total of 88 (63.6%) participants. This underrepresentation of males could have had an effect on the external validity of the study. According to an article published by the Centers for Disease Control and Prevention, more men than women met the set guidelines for muscle strengthening and aerobic exercise (23.4% vs 17.9%).33 In a retrospective analysis of 160 LSS patients, Kim et al34 aimed to analyze the difference in sensitivity to pain between males and females. The patients received a pain sensitivity questionnaire on their first visit to a clinic and were asked to rank their pain based on perception. This study found that, in relation to the severity of the injury, females had a significantly higher pain sensitivity than males and that this resulted in a lower quality of life.34 This study clearly illustrates that the differences in pain sensitivity found between the genders is an important consideration for studies of exercise efficacy given that gender, pain, and exercise tolerance are associated.
In the previously discussed study by Koc et al,29 the physical therapy treatment intervention was prescribed as a home program. In comparison, however, a systematic review done by Fokkenrood et al35 evaluated the effect of supervised physical therapy treatment compared to nonsupervised physical therapy treatment to determine if one was more effective.35 After reviewing 14 studies regarding supervised and nonsupervised physical therapy treatment, patients receiving supervised physical therapy treatment demonstrated significantly greater walking distance improvements compared to nonsupervised physical therapy treatment patients using a treadmill walking test.35 While nonsupervised physical therapy treatment was effective, supervision was found to be ideal in regard to a physical therapy treatment regimen with respect to walking distance. Prescribing a home physical therapy treatment program could result in many additional limitations including poor patient compliance and inaccurate documentation of the procedures.
Every study included in this systematic review had a limited sample size.15,16,21,27,29 While small sample sizes are a common and an understandable by-product of clinical patient-based research, the small sample sizes could have had an effect on the outcomes based on an inability to detect small differences. Freiman et al36 conducted a review of 71 randomized controlled trials and found that 67 could have missed a 25% increase in improvement and 50 could have missed a 50% increase due to therapy. Thus, stating that there was no difference between groups or compared to a control could have been as a result of sample size leading the study to be underpowered. This limitation should be considered in future studies so that the results are able to detect a small effect size due to similarities between interventions.
The duration of the exercises was also noted as a limitation of the studies included in this review.15,29 Koc et al29 assigned a physical therapy treatment program for 2 weeks while Goren et al15 assigned a 3-week program. In a study with 116 subjects in a rehabilitation program, Kirk-Sanchez et al37 demonstrated the effect that duration of exercise has on the level of patient mobility. Those researchers found that the duration of physical therapy treatment was positively correlated with their score on the FIM (Functional Independence Measure), which includes information on mobility throughout the process.37 Kirk-Sanchez et al37 concluded that patients who were involved in a physical therapy program longer had improved mobility when compared to those who have been treated with less time based on the FIM. This illustrates the limitation created by the use of short-term exercise programs in the experimental design of studies like this; potential positive outcomes from the exercise/physical therapy treatment group may not be detected.
Studies performed by Whitman et al,21 Goren et al,15 and Sculco et al27 showed a possible response or nonresponse bias because the follow-up questionnaires about symptoms and pain severity were mailed to participants. Response bias could skew the results toward a positive outcome because patients feel pressure to meet the goals of the study. Also, nonresponse bias could affect the results of the study by underrepresenting a certain group of patients. In a study performed by Sheikh and Mattingly,38 significant differences in rehabilitation outcomes were found between the 84% of participants who responded and the 16% that did not respond to the mailed questionnaire. The researchers concluded that nonresponse rates can create bias that could negatively impact the results of a study.
A high level of participant attrition in some of the studies may have also compromised the study interpretation. It is fairly common to have participants who decide not to complete or participate in every part of the study. An example is seen in the study by Whitman et al,21 in which a patient chose to not perform the treadmill test due to possible hypertension.15,16,21,27,29 In the same study, 2 patients failed to complete treadmill tests: one because of allergy symptoms and the other because of a grand mal seizure that was unrelated to the study.21 However, an intention-to-treat principle was used, which may have influenced the outcome. Raboud et al39 aimed to evaluate the effect of attrition by observing 245 patients in which only 52% completed the study. The attrition contributed to an underestimation of the treatment effect because those who did not finish the study could have showed a significant improvement compared to those who did.
Finally, the ODI differences were often not large enough to be of clinical importance. The ODI has been shown to be an effective outcome measure in the treatment of spinal issues by Fairbank and Pynsent.40 The difference between posttreatment and pretreatment for Group 1 and 2 and the control group in the study done by Goren et al15 was −3.94 ± 7.20, −7.80 ± 10.26, and −3.60 ± 11.66, respectively. However, according to a review of data done by Copay et al,41 the minimum clinically importance difference (MCID) for the ODI is 12.8 among patients receiving surgeries for back pain. According to Goren et al,15 this lack of an MCID could be due to the low number of physical therapy treatment sessions and short duration of the physical therapy treatment program in the study.
Conclusion
LSS is an increasingly prevalent condition and surgical intervention is costly. Exercise is a low-cost, noninvasive, and convenient treatment option that should be considered before surgery for patients with milder symptoms. Research has shown that exercise is effective to decrease pain level, disability, and pain medication intake as well as increase psychological stability by decreasing anger, depression, and mood disturbance.15 However, further research is needed to determine which type of exercise is the most efficacious and if exercise is superior to other treatments available for LSS.
References
- 1. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549. [DOI] [PubMed] [Google Scholar]
- 2. Ohtori S, Yamashita M, Murata Y, et al. Conservative and surgical treatment improves pain and ankle-brachial index in patients with lumbar spinal stenosis. Yonsei Med J. 2013;54:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nagler W, Hausen HS. Conservative management of lumbar spinal stenosis. Identifying patients likely to do well without surgery. Postgrad Med. 1998;103:69-71. [DOI] [PubMed] [Google Scholar]
- 4. Otani K, Kikuchi S, Yabuki S, et al. Lumbar spinal stenosis has a negative impact on quality of life compared with other comorbidities: an epidemiological cross-sectional study of 1862 community-dwelling individuals. ScientificWorldJournal. 2013;2013:590652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bae HW, Rajaee SS, Kanim LE. Nationwide trends in the surgical management of lumbar spinal stenosis. Spine. 2013;38:916-926. [DOI] [PubMed] [Google Scholar]
- 6. Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009;9:545-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frymoyer JW, Cats-Baril WL. An overview of the incidences and costs of low back pain. Orthop Clin North Am. 1991;22:263-271. [PubMed] [Google Scholar]
- 8. Udeh BL, Costandi S, Dalton JE, Ghosh R, Yousef H, Mekhail N. The 2-year cost-effectiveness of 3 options to treat lumbar spinal stenosis patients [published online January 3, 2014]. Pain Pract. doi: 10.1111/papr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manchikanti L, Candido KD, Kaye AD, et al. Randomized trial of epidural injections for spinal stenosis published in the New England Journal of Medicine: further confusion without clarification. Pain Physician. 2014;17:E475-E488. [PubMed] [Google Scholar]
- 10. Park CH, Lee SH. Correlation between severity of lumbar spinal stenosis and lumbar epidural steroid injection. Pain Med. 2014;15:556-561. [DOI] [PubMed] [Google Scholar]
- 11. Radcliff K, Hilibrand A, Lurie JD, et al. The impact of epidural steroid injections on the outcomes of patients treated for lumbar disc herniation: a subgroup analysis of the SPORT trial. J Bone Joint Surg Am. 2012;94:1353-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sahin F, Yilmaz F, Kotevoglu N, Kuran B. Efficacy of salmon calcitonin in complex regional pain syndrome (type 1) in addition to physical therapy. Clin Rheumatol. 2006;25:143-148. [DOI] [PubMed] [Google Scholar]
- 13. Kasimcan O, Kaptan H. Efficacy of gabapentin for radiculopathy caused by lumbar spinal stenosis and lumbar disk hernia. Neurol Med Chir (Tokyo). 2010;50:1070-1073. [DOI] [PubMed] [Google Scholar]
- 14. Matsudaira K, Seichi A, Kunogi J, et al. The efficacy of prostaglandin E1 derivative in patients with lumbar spinal stenosis. Spine. 2009;34:115-120. [DOI] [PubMed] [Google Scholar]
- 15. Goren A, Yildiz N, Topuz O, Findikoglu G, Ardic F. Efficacy of exercise and ultrasound in patients with lumbar spinal stenosis: a prospective randomized controlled trial. Clin Rehabil. 2010;24:623-631. [DOI] [PubMed] [Google Scholar]
- 16. Pua YH, Cai CC, Lim KC. Treadmill walking with body weight support is no more effective than cycling when added to an exercise program for lumbar spinal stenosis: a randomised controlled trial. Aust J Physiother. 2007;53:83-89. [DOI] [PubMed] [Google Scholar]
- 17. Weiner M, Johnson-Greene D, Tolchin R, Abratt L, Frank B. Improving treadmill performance in patients with lumbar stenosis: evaluation of a custom angle load reduction device. Am J Phys Med Rehabil. 2013;92:553-564. [DOI] [PubMed] [Google Scholar]
- 18. Mazanec DJ, Podichetty VK, Hsia A. Lumbar canal stenosis: start with nonsurgical therapy. Cleve Clin J Med. 2002;69:909-917. [DOI] [PubMed] [Google Scholar]
- 19. Sakai Y. [Locomotive syndrome and frailty. Lumbar canal stenosis as an underlying disorder in the locomotive syndrome]. Clin Calcium. 2012;22(4):59-66. [PubMed] [Google Scholar]
- 20. Jarrett MS, Orlando JF, Grimmer-Somers K. The effectiveness of land based exercise compared to decompressive surgery in the management of lumbar spinal-canal stenosis: a systematic review. BMC Musculoskelet Disord. 2012;13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whitman JM, Flynn TW, Childs JD, et al. A comparison between two physical therapy treatment programs for patients with lumbar spinal stenosis: a randomized clinical trial. Spine (Phila Pa 1976). 2006;31:2541-2549. [DOI] [PubMed] [Google Scholar]
- 22. Lewis JS, Hewitt JS, Billington L, Cole S, Byng J, Karayiannis S. A randomized clinical trial comparing two physiotherapy interventions for chronic low back pain. Spine. 2005;30:711-721. [DOI] [PubMed] [Google Scholar]
- 23. Lucio JC, Vanconia RB, Deluzio KJ, Lehmen JA, Rodgers JA, Rodgers W. Economics of less invasive spinal surgery: an analysis of hospital cost differences between open and minimally invasive instrumented spinal fusion procedures during the perioperative period. Risk Manag Healthc Policy. 2012;5:65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patil CG, Sarmiento JM, Ugiliweneza B, et al. Interspinous device versus laminectomy for lumbar spinal stenosis: a comparative effectiveness study. Spine J. 2014;14:1484-1492. [DOI] [PubMed] [Google Scholar]
- 25. Atlas SJ, Keller RB, Robson D, Deyo RA, Singer DE. Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the Maine lumbar spine study. Spine. 2000;25:556-562. [DOI] [PubMed] [Google Scholar]
- 26. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713-721. [PubMed] [Google Scholar]
- 27. Sculco AD, Paup DC, Fernhall B, Sculco MJ. Effects of aerobic exercise on low back pain patients in treatment. Spine J. 2001;1:95-101. [DOI] [PubMed] [Google Scholar]
- 28. Kim ER, Kang MH, Kim YG, Oh JS. Effects of a home exercise program on the self-report disability index and gait parameters in patients with lumbar spinal stenosis. J Phys Ther Sci. 2014;26:305-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koc Z, Ozcakir S, Sivrioglu K, Gurbet A, Kucukoglu S. Effectiveness of physical therapy and epidural steroid injections in lumbar spinal stenosis. Spine (Phila Pa 1976). 2009;34:985-989. [DOI] [PubMed] [Google Scholar]
- 30. Genevay S, Atlas SJ. Lumbar spinal stenosis. Best Pract Res Clin Rheumatol. 2010;24:253-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joffe D, Watkins M, Steiner L, Pfeifer BA. Treadmill ambulation with partial body weight support for the treatment of low back and leg pain. J Orthop Sports Phys Ther. 2002;32:202-213. [DOI] [PubMed] [Google Scholar]
- 32. Bjornsdottir S, Jonsson S, Valdimarsdottir U. Mental health indicators and quality of life among individuals with musculoskeletal chronic pain: a nationwide study in Iceland. Scand J Rheumatol. 2014;43:419-423. [DOI] [PubMed] [Google Scholar]
- 33. Adult participation in aerobic and muscle-strengthening physical activities—United States, 2011. MMWR. Morb Mortal Wkly Rep. 2013;62(17):326-330. [PMC free article] [PubMed] [Google Scholar]
- 34. Kim HJ, Suh BG, Lee DB, et al. Gender difference of symptom severity in lumbar spinal stenosis: role of pain sensitivity. Pain Physician. 2013;16:E715-E723. [PubMed] [Google Scholar]
- 35. Fokkenrood HJ, Bendermacher BL, Lauret GJ, Willigendael EM, Prins MH, Teijink JA. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev. 2013;(8):CD005263. [DOI] [PubMed] [Google Scholar]
- 36. Freiman JA, Chalmers TC, Smith H, Jr, Kuebler RR. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 “negative” trials. N Engl J Med. 1978;299:690-694. [DOI] [PubMed] [Google Scholar]
- 37. Kirk-Sanchez NJ, Roach KE. Relationship between duration of therapy services in a comprehensive rehabilitation program and mobility at discharge in patients with orthopedic problems. Phys Ther. 2001;81:888-895. [PubMed] [Google Scholar]
- 38. Sheikh K, Mattingly S. Investigating non-response bias in mail surveys. J Epidemiol Community Health. 1981;35:293-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raboud JM, Montaner JS, Thorne A, Singer J, Schechter MT. Impact of missing data due to dropouts on estimates of the treatment effect in a randomized trial of antiretroviral therapy for HIV-infected individuals. Canadian HIV Trials Network A002 Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:46-55. [DOI] [PubMed] [Google Scholar]
- 40. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25:2940-2952. [DOI] [PubMed] [Google Scholar]
- 41. Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008;8:968-974. [DOI] [PubMed] [Google Scholar]


