Abstract
We reviewed lifestyle factors that influence serum uric acid levels and risk of gout flare, and how to improve their deleterious effects. Since obesity increases uric acid and weight gain increases gout risk, weight reduction by daily exercise and limiting intake of excess calories is recommended. However, strenuous exercise, which causes adenine nucleotide degradation; starvation, which decreases uric acid excretion; and dehydration may raise the level of uric acid in serum and trigger gout. Increased intake of purine-rich foods, such as meat and seafood, raise the level of uric acid in serum and is associated with increased risk of gout, whereas dairy products, especially low-fat types, are associated with a lower risk of gout. Also, heavy alcohol drinking raises the uric acid level and increases the risk of gout through adenine nucleotide degradation and lactate production. Sweet fruits and soft drinks containing fructose should be moderated, since fructose may raise uric acid and increase gout risk through uric acid production and/or decreased excretion. On the other hand, the Mediterranean diet is recommended for gout patients, since it may also help prevent hyperuricemia. Furthermore, coffee and vitamin C supplementation could be considered as preventive measures, as those can lower serum uric acid levels as well as the risk of gout.
Keywords: uric acid, lifestyle, nutrition, obesity, exercise
‘There is no doubt that lifestyle is also a key contributing factor for the development of hyperuricemia and gout . . .’
A strong genetic link exists (ABCG2 dysfunction) in the development of hyperuricemia as compared to environmental risk factors such as obesity, ageing, and heavy alcohol drinking.1 Moreover, with the advent of new hypouricemic agents, it has become easier to control serum uric acid concentration. Nevertheless, there is no doubt that lifestyle is also a key contributing factor for the development of hyperuricemia and gout, and the importance of nonpharmacological treatment by changing lifestyle factors such as diet, weight control, and adequate hydration is important from the viewpoint of economic issues and possible adverse effects of uric acid–lowering agents. This review focuses on recent studies that advocate favorable lifestyle habits to reduce the risk of gout and/or hyperuricemia, including weight control, appropriate diet, and moderation of alcoholic beverage and soft drink consumption.
Body Weight and Exercise
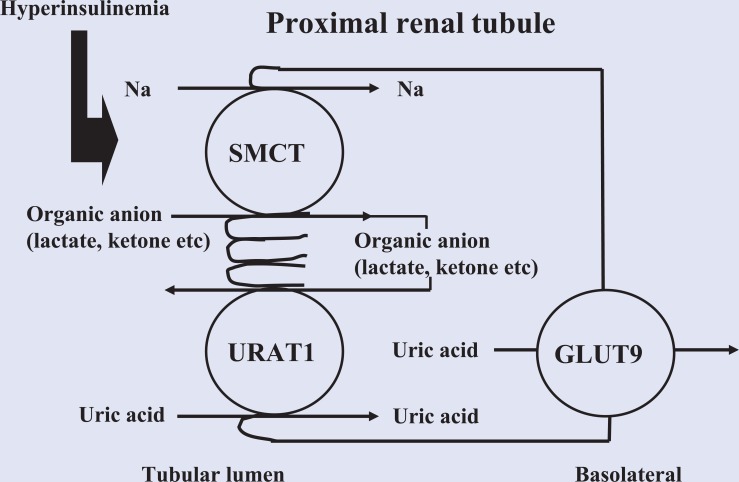
Relationships among serum uric acid level, hyperuricemia, obesity, and metabolic syndrome are well established. In cross-sectional analyses, higher serum uric acid concentration was found to be positively correlated with body mass index (BMI)2 and metabolic syndrome.3-5 It is possible to reduce serum uric acid level and the risk of gout by reducing body weight through dietary modifications and exercise. With this method, gradual weight loss is more beneficial as compared to a drastic reduction, since abrupt weight loss causes ketosis, which promotes uric acid reabsorption via urate transporter 1 (URAT1), a member of the organic acid transporter family, resulting in increased uric acid in serum (Figure 1).
Figure 1.
Increased Uric Acid Influx Induced by Hyperinsulinemia.
Insulin resistance/hyperinsulinemia increases reabsorption of sodium and monocarboxylic acid via SMCT1 with uric acid influx in exchange for monocarboxylic acid excretion via URAT1.
Yamashita et al investigated 27 overweight subjects who underwent gradual reduction of total calorie intake (1500 to 800 kcal/day) and exercise therapy (walking, riding bicycle, ergometer) and found that the uric acid clearance/creatinine clearance ratio gradually increased to near normal, while the level of uric acid in serum was reduced during the course of weight reduction.6 In another study, Scott and Sturge reported that 24-hour uric acid excretion was significantly reduced by weight loss without any change in uric acid clearance.7 Therefore, it is reasonable to assume that serum uric acid level change induced by weight reduction is ascribable to correction of both underexcretion and overproduction of uric acid associated with obesity. Those studies also suggested that hyperuricemia associated with obesity can be treated with appropriate weight reduction, without the need for drug therapy.
Weight reduction caused by daily exercise and adequate food portion size can help reduce the risk of gout, insulin resistance, and associated comorbidities, as well as serum uric acid level. Dessein et al noted the beneficial effects of weight reduction in a number of gout attacks, as well as serum level of uric acid and lipid profiles.8 In their subjects, 16 weeks of diet consisting of 1600 kcal/day resulted in weight loss of 7.7 ± 5.4 kg (P = .002) and decreased the frequency of monthly gout attacks from 2.1 ± 0.8 to 0.6 ± 0.7 (P = .002), while the level of uric acid in serum was decreased from 0.57 ± 0.10 to 0.47 ± 0.09 mmol/L (P = .001). More recently, Tsunoda et al treated overweight hypertensive patients with a low-energy diet (3360 kJ/day for 3 weeks) and found that serum uric acid was decreased (0.4 ± 0.2 mg/dL, P < .05) along with improvement in insulin resistance.9
In an epidemiological study with a longer duration, relationships among obesity, weight change, and risk of gout were investigated over a 12-year period. When compared with males with a BMI ranging from 21 to 22.9, the multivariate relative risks of gout were 1.95 for males with a BMI of 25 to 29.9, 2.33 for males with a BMI of 30 to 34.9, and 2.97 for males with a BMI of 35 or more. Moreover, as compared with males who had maintained their weight (±2.3 kg) since the age of 21 years, the multivariate relative risk of gout for those who had gained 13.5 kg or more since the age of 21 years was 1.99. In contrast, the multivariate relative risk for males who had lost 4.5 kg or more since the study baseline values were obtained was 0.61 (95% confidence interval = 0.40-0.92). Therefore, it is considered that greater adiposity and weight gain are strong risk factors for gout in males, while weight loss is protective against gout risk.10
Insulin resistance and hyperinsulinemia caused by visceral fat obesity increases reabsorption of sodium and monocarboxylic acid via sodium and monocarboxylic acid cotransporter (SMCT) with uric acid influx in exchange of monocarboxylic acid excretion via URAT1, resulting in increased uric acid in serum (Figure 1). Weight reduction improves insulin resistance, and thus abrogates increased uric acid reabsorption in proximal renal tubules. On the other hand, it is has been speculated that hyperinsulinemia interferes with glyceraldehyde-3-phosphate dehydrogenase (GA3PDH) activity, thus promoting glycolytic intermediates toward ribose-5-phosphate and phosphoribosyl-pyrophosphate, with overproduction of uric acid following when there is diminished GA3PDH activity.11
To reduce body weight, it is important to burn more calories by exercise and restrict calorie input, which consequently leads to a reduction in serum uric acid concentration. However, strenuous muscle exercise increases uric acid in serum, whereas moderate exercise does not have such an effect.12 Exercise (VO2max 70%) increases adenine nucleotide degradation and lactic acid production, and also induces noradrenaline release, resulting in increases in the plasma concentration and urinary excretion of oxypurines (hypoxanthine, xanthine), and plasma concentration of uric acid, as well as decreases in the urinary excretion of uric acid, along with fractional excretion of uric acid and xanthine.13 Yamanaka et al showed that muscle exercise not exceeding the anaerobic threshold does not cause adenine nucleotide degradation; thus, aerobic exercise is expected to be beneficial for patients with gout and/or hyperuricemia.14 Whether aerobic exercise itself decreases serum uric acid and gout risk is uncertain, though it has been suggested that moderate-intensity physical activity is associated with lower uric acid concentration in obese individuals.15 In addition, physically active males as compared with sedentary males seem to have a lower risk of gout.16
Dehydration and Rehydration
Sauna bathing has been shown to increase the plasma concentrations of uric acid and oxypurines (hypoxanthine, xanthine), while it decreased urinary and fractional excretion of uric acid, suggesting a relationship between dehydration and enhanced purine degradation and decreased urinary excretion of uric acid, leading to an increase in plasma concentration of uric acid.17 Exercise-induced profuse sweating reduces urinary uric acid excretion and leads to increased serum uric acid concentration; thus, it is recommended to drink plenty of water to avoid an increase in serum uric acid level after exercise that produces heavy sweating.18
Thus, dehydration may be a risk factor for a gouty attack by raising the serum uric acid level. The ability for uric acid excretion is proportional to urine flow.19 Gout patients are also encouraged to drink plenty of fluids, as results of an Internet-based case-crossover study suggested that adequate water consumption in the 24-hour period prior to a gout flare is associated with a significant decrease in recurrent gout attacks (reduction of 46% with water consumption ≥1920 mL).20 In addition, urine alkalization by eating nutritionally well-designed food, such as vegetables and dairy products, is important and effective for promoting uric acid excretion.21
Dietary Factors
Recently conducted prospective epidemiological and open-labeled dietary studies have provided novel insight into the roles of dietary factors, discretionary items, and various supplements in development and/or prevention of hyperuricemia and gout. Some foods can lead to increased risk, while others can lead to decreased risk of their development. Furthermore, it has been reported that individuals with hyperuricemia tend to have a poor diet, such as higher alcohol consumption and lower vegetable and dairy product intake.22
Purine-Rich Foods
Since uric acid is an end-product of purine metabolism in humans, it is reasonable to suggest that excessive ingestion of purine-rich foods causes an increase in serum uric acid. In fact, ingestion of RNA (corresponding to 225 mg purine/kg of body weight) increased plasma uric acid by 0.74 mg/dL, indicating that excessive consumption of purine increases plasma uric acid concentration.23 However, not all purine-containing foods have the same effect on serum uric acid level and gout risk.
The Third National Health and Nutrition Examination Survey (1988-1994), which investigated the relationship between intake of purine-rich foods and serum level of uric acid, indicated that higher levels of meat and seafood consumption were associated with higher levels of uric acid in serum.24 In addition, a large-scale study involving more than 45 000 males over a period of 12 years (The Health Professionals Follow-Up Study) found that such increased consumption was also associated with a higher risk of gout, while a moderate intake of purine-rich vegetables (peas, beans, lentils, asparagus, spinach, mushrooms) was not associated with that increased risk.25 Moreover, intake of purine-rich vegetables was not associated with plasma uric acid level in a population-based case–control study conducted in Scotland (1999-2006).26 The reason why purine-rich vegetables do not increase uric acid in serum or gout risk is uncertain, though fiber contained in such vegetables may be related, since increased fiber intake was reported to be significantly associated with a decreased risk of hyperuricemia,27 probably by inhibiting purine or adenine absorption in the digestive system.28
The relationship between purine intake and risk of recurrent gout attacks was also investigated using an Internet-based questionnaire study. When compared with the lowest quintile of total purine intake over a 2-day period, the odds ratios of recurrent gout attacks were 1.17, 1.38, 2.21, and 4.76, respectively, with each increasing quintile, suggesting that acute purine intake increases the risk of recurrent gout attacks. The authors recommended that gout patients should avoid or reduce the amount of purine-rich foods intake.29
A purine-rich diet for 1 to 2 weeks produces only a small transient rise in serum uric acid level of 1 to 2 mg/dL, while an isocaloric low-purine diet for 7 to 10 days will slightly reduce serum uric acid by about 1 to 2 mg/dL.30,31 suggesting that strict restriction of consumption of purine is not practical or effective for uric acid control. Accordingly, adequate calorie intake and weight management with moderation of meat and seafood in the diet has been recently recommended for gout/hyperuricemia subjects.
Fructose
Fructose, found in fruits and vegetables, is a simple sugar that provides fuel for the human body, and it is the only sugar that raises the level of uric acid in serum. Consumption of sugars, such as table sugar and high-fructose corn syrup, has dramatically risen in recent decades and correlates closely with increased incidence of obesity, metabolic syndrome, diabetes, and hyperuricemia/gout. Consumption of sugar-sweetened beverages, a major source of fructose, raises the serum uric acid level and is associated with increased risk of gout. The increased prevalence of gout is probably secondary to the recent rise in fructose consumption as well as the increase in obesity, though it remains a significant contributing factor.32
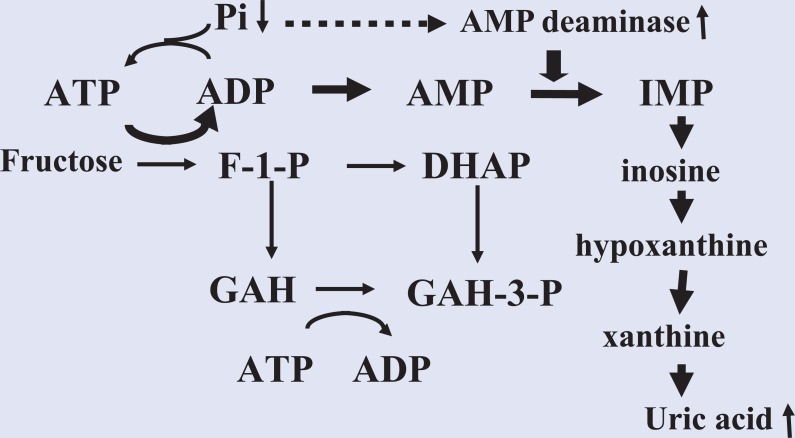
As for the mechanism of fructose-induced production of uric acid, when fructose is metabolized to fructose-1-phosphate by fructokinase in the liver, adenosine triphosphate (ATP) and phosphate are consumed. Depletion of phosphate leads to decreased ATP recycling and activates adenosine monophosphate (AMP) deaminase, which is suppressed by phosphate, then subsequently increases AMP degradation to inosine monophosphate (IMP), inosine, hypoxanthine, xanthine, and finally uric acid (Figure 2). Thus, elevated levels of uric acid induced by high fructose consumption are likely associated with depletion of ATP in the liver.33 In contrast, an experimental high-fructose diet given for 4 to 6 days led to lower urinary uric acid clearance and fractional uric acid excretion as compared with a low-fructose diet, suggesting that decreased urinary uric acid excretion may contribute to fructose-induced hyperuricemia.34 Also, 10% fructose given intravenously (0.5 g/kg/h) for 2 hours increased blood lactate concentration, which may be attributable to decreased uric acid excretion via URAT1.35 Furthermore, it has been reported that excessive consumption of a high-fructose diet is associated with insulin resistance,36 and may cause decreased uric acid excretion and/or increased uric acid production. However, the effect of consumed fructose on uric acid has yet to be clarified.
Figure 2.
Increased Adenosine Nucleotide Degradation by Fructose.
Fructose is metabolized to fructose-1-phosphate by fructokinase in the liver, which then consumes ATP and phosphate. Depletion of phosphate induces decreased ATP recycling and activates AMP deaminase, which is suppressed by phosphate, then subsequently increases AMP degradation to IMP, inosine, hypoxanthine, xanthine, and finally uric acid.
Findings of the Third National Health and Nutrition Examination Survey (1988-1994) suggested that sugar-sweetened soft drink consumption is associated with serum uric acid level and frequency of hyperuricemia.37 More recently, a cross-sectional study of Mexican adults reported that consumption of sweetened beverages is associated with increased risk of hyperuricemia.38 The Health Professionals Follow-up Study of 46 393 males conducted for a period of 12 years suggested that consumption of sugar-sweetened soft drinks, fructose, fructose-rich fruits, and fruit juices is associated with increased risk of gout in males, whereas that of diet soft drinks did not have an association.39 Among females, consumption of fructose-rich beverages is also associated with increased risk of gout. A prospective cohort study of 78 906 females conducted from 1984 to 2006 (Nurses’ Health Study) showed that compared with consumption of less than 1 serving per month of sugar-sweetened soda or orange juice, the multivariate relative risk of gout was 1.74 for 1 serving per day and 2.39 for 2 or more servings per day. When compared with consumption of less than 1 serving per month of orange juice, the multivariate relative risk of gout for 1 serving per day was 1.41 and that for 2 or more servings per day was 2.42 (P = .02 for trend).40
Oral administration of sucrose at 1.5 g/kg of body weight in healthy male volunteers increased the plasma concentration of uric acid by 11% (P < .01), as well as oxypurines (hypoxanthine and xanthine), suggesting that enhanced purine degradation by sucrose plays a major role in the increased plasma concentration of uric acid.41 It has been reported that higher-than-normal daily intake of fructose may lead to hyperuricemia.42-45 Two weeks of acute fructose diet raised uric acid concentration in serum compared to glucose diet, irrespective of isocaloric or hypercaloric status.46 On the other hand, it has been reported that 10-week moderate47 to high consumption of fructose (50 g) did not induce hyperuricemia.48 Furthermore, meta-analysis of 21 trials investigating the effects of fructose intake on serum uric acid suggested that isocaloric fructose in exchange for other sources of carbohydrate did not raise uric acid concentration in serum, while hypercaloric fructose intake raised uric acid concentration in serum.49 Therefore, larger and long-term studies under free-living conditions as well as interventional studies to reduce fructose intake are awaited to assess the effect of fructose on uric acid in daily life independent of excess energy.
Dairy Products
Dairy products are recognized as important dietary factors for reducing serum uric acid and the risk of gout development. According to epidemiological studies by Choi and colleagues, higher levels of dairy consumption were associated with lower serum levels of uric acid and gout risk.24,25 Although the underlying mechanism is not clear from these studies, acute ingestion of milk corresponding to 80 grams of protein led to a decrease in serum uric acid concentration by approximately 10%, while that of the soy control increased the serum uric acid concentration by 10%.50 Kurajoh et al also found that milk ingestion at 15 mL/kg of body weight increased the urinary and fractional clearance of uric acid excretion.51 In addition, administration of 80 grams of isolated dairy proteins, casein, and lactalbumin significantly reduced serum uric acid concentrations over a 3-hour period, while that of soy protein increased uric acid in serum. The reduction in serum uric acid concentration was also associated with an increase in clearance of uric acid.52 Thus, the uricosuric effects of casein and lactalbumin and low purine content in milk seem to contribute to an acute uric acid–lowering effect. Additionally, orotic acid, which is present in milk, decreases the reabsorption of uric acid and promotes its excretion by the kidneys.53
Dalbeth et al identified the anti-inflammatory properties of glycomacropeptide (GMP) and G600 milk fat product in models of acute gout. They also found that gout patients who drank enriched skim milk (GMP, G600) had a much greater reduction in gout flare-ups and greater pain improvement.54
Cherries
As a complementary and alternative medical therapy, cherries have been used for gout for decades, largely based on anecdotal evidence. The first study of correlation between cherries and gout appeared in medical literature in 1950.55 Blau suffered from gout and noticed that gout pain subsided after eating some black cherries. Other investigators also began exploring the phenomenon, and additional studies found that cherries and cherry juice contain antioxidants and anti-inflammatory agents that are effective for alleviating pain associated with gouty arthritis. A study of 633 patients with confirmed gout for 1 year found that those who consumed cherries (1/2 cup serving or equivalent to 10-12 cherries) or a cherry-based extract for 2 days had their chance of a subsequent gout attack reduced by 35%.56 In another study, 18 healthy adults who ate 280 grams of Bing cherries each day for 1 month had a significant reduction in blood levels of substances associated with inflammation and immune cell activity (C-reactive protein, nitric oxide, RANTES); thus, the anti-inflammatory effects of substances in cherries may be beneficial for the management and prevention of gout flare.57 Ingestion of cherry juice concentrate also reduced the incidence of flare-ups in gout patients, though no significant change in serum urate level was found. These results suggested an anti-inflammatory action of cherry juice.58
High intake of fruits such as cherries and of vegetable protein may reduce serum uric acid levels. Ten healthy females who ate 2 servings of Bing cherries had plasma uric acid decreased by 15% at 5 hours after consumption, as compared with the preconsumption baseline (P < .05) along with an increase in urinary uric acid excretion, supporting the anecdotal anti-gout reputation of cherries.59 When 10 participants (38.1 ± 12.5 years old; BMI 32.2 ± 4.6; 5 obese, 5 overweight) consumed 8 oz/day of 100% tart cherry juice for 4 weeks, 70% (7/10) reduced serum uric acid levels.60
Mediterranean Diet
The Mediterranean diet is a traditional dietary pattern that includes a proportionally high consumption of unrefined cereals, legumes, fruits and vegetables, and olive oil, with moderate consumption of fish, dairy products such as cheese and yogurt, and wine, and low consumption of meat, meat products, and sweets. It is often cited as beneficial for obesity, diabetes, and coronary heart disease.
In a prospective cross-sectional analysis of 4449 elderly participants with high cardiovascular risk, an inverse association was observed between increasing level of adherence to the Mediterranean diet and decreasing incidence of hyperuricemia (P < .001).61 Also, Kontogianni et al used MedDiet scoring to explore potential associations between adherence to the Mediterranean diet and serum uric acid levels in the ATTICA study.62 They found that MedDiet scores were inversely associated with serum urate level independent of sex, weight, hypertension, abnormal glucose metabolism, and alcohol or coffee intake, supporting a potential role of the diet for prevention and treatment of hyperuricemia. Moreover, in elderly individuals without known cardiovascular disease, linear regression analysis revealed that MedDiet scores were inversely associated with uric acid level,63 though the effect of the Mediterranean diet on the incidence of gout has not been reported.
Soy Foods
Although short-term experiments have shown that soy protein consumption increases serum uric acid concentration,50,52 the amounts used in those studies were much greater than that routinely consumed in an entire day.
In a review by Messina et al, epidemiological data indicate that soy food intake is not associated with hyperuricemia, while clinical studies also indicate that such intake does not markedly affect serum uric acid levels.64 Furthermore, Villegas et al conducted a cross-sectional study of 3978 middle-aged Chinese men in Shanghai and found an inverse association between soy food consumption and hyperuricemia (odds ratios = 1.00, 0.92, 0.86, 0.85, and 0.80 for quintiles of intake, P for trend = .07).65
Discretionary Items and Supplements
Alcohol
The effect of ethanol on uric acid metabolism was reviewed in detail by Yamamoto et al.66
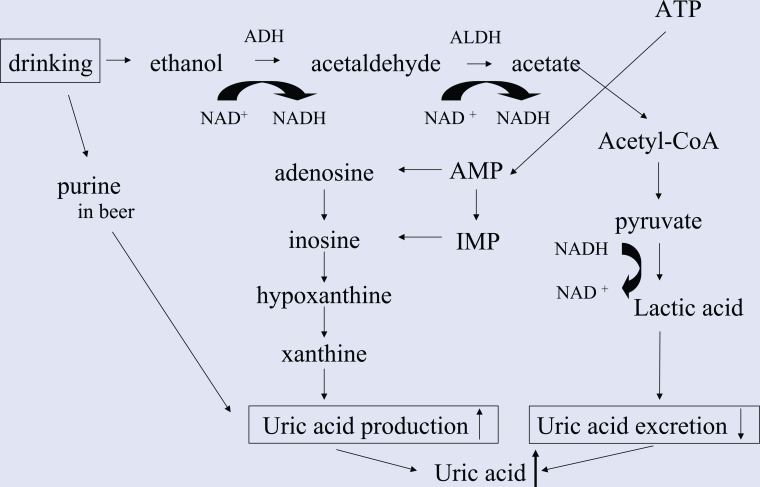
Ethanol increases uric acid production resulting from acetate metabolism and enhanced adenosine nucleotide turnover,67,68 and increases lactic acid level in blood, leading to increased serum uric acid in serum (Figure 3). Purines contained in beer also contribute to an increase in plasma uric acid.69
Figure 3.
Mechanism of Increased Uric Acid by Alcoholic Beverage Consumption.
Ethanol increases uric acid production resulting from acetate metabolism and enhanced adenosine nucleotide turnover,59,60 and increases lactic acid level in blood, leading to increased uric acid in serum.
The effects of ethanol on serum uric acid and gout risk differ depending on the type of alcoholic beverage. In the Third National Health and Nutrition Examination Survey (1988-1994), the relationships of beer, liquor, and wine consumption with serum uric acid level were studied using data obtained from 14 809 participants (6932 males, 7877 females; ≥20 years old), which showed that the levels of uric acid in serum increased with increasing beer or liquor intake, but not with increasing wine intake, suggesting that the effects of individual alcoholic beverages on uric acid vary according to type of alcoholic beverage.70 However, all alcohol beverages, even wine, are associated with increased risk of recurrent gout attacks. Therefore, gout patients should limit alcohol intake of all types to reduce the risk of a recurrent attacks.71
According to Nishimura et al, drinking 0.5 g ethanol/kg of body weight increased serum uric acid levels in regular drinkers by 0.8 ± 0.4 mg/dL, whereas no such increase was seen in non- or occasional drinkers.72
Moreover, beer ingestion for a period of 1 month increased serum and urinary uric acid concentrations,73 suggesting that a daily drinking habit was also related to increased serum uric acid. Therefore, it is important to pay attention to these factors, including ingested ethanol volume and type of alcoholic beverage, to prevent and treat ethanol-induced hyperuricemia.
Coffee
Coffee consumption has been found to be associated with lowered serum uric acid level and hyperuricemia frequency.74,75 Kiyohara et al performed a cross-sectional examination of the relationship of coffee consumption with serum uric acid concentration in 2240 male Self-Defense Force officials in Japan who received a preretirement health examination between 1993 and 1994, and found a clear inverse relationship.75 Choi et al also showed that the level of uric acid in blood was significantly decreased with increasing coffee but not tea intake. On the other hand, there was no association seen between total caffeine intake from beverages and uric acid levels.74 They also prospectively examined the relationship between coffee consumption and gout risk in 45 869 males over a period of 12 years and reported that the risk of gout was decreased to 0.41 in those who consumed 6 or more cups of coffee each day as compared to subjects who did not drink coffee, and suggested that long-term coffee consumption is associated with a lower risk of gout incidence.76 However, coffee contains moderate to high amount of oxalate. Therefore, individuals possessing renal stone and/or past stone formers may well limit coffee intake.
The mechanisms related to how coffee lowers uric acid in serum and decreases gout risk is not clear from those observational studies, though chlorogenic acid, a polyphenol abundant in coffee, may play an important role. Chlorogenic acid has been shown to have antioxidant properties and inhibit the activity of xanthine oxidase, though those were much weaker as compared with allopurinol (IC50 of chlorogenic acid against xanthine oxidase as compared to allopurinol: 26.37 ± 2.83 µM vs 1.47 ± 0.32 µM77; 126.28 ± 6.92 µM vs 11.00 ± 6.39 µM78), which may contribute to the uric acid–lowering effect and anti-gout property of coffee. Epidemiologic and experimental data have also suggested that chlorogenic acid prevents diet-induced insulin resistance.79,80 Thus, chlorogenic acid may reduce uric acid in serum by alleviating insulin resistance.
Vitamin C
The Health Professional Follow-up Study that includes 1387 males examined serum uric acid levels according to total vitamin C intake and found a decreasing trend with increased intake (6.4, 6.1, 6.0, 5.7, and 5.7 mg/dL with <90, 90-249, 250-499, 500-999, and ≥1000 mg/day, respectively; P for trend < .001), with greater vitamin C intake associated with lower prevalence of hyperuricemia.81 It has also been suggested that vitamin C may lower the level of uric acid in serum by increasing the excretion of uric acid in urine,82 probably due to competitive inhibition of an anion exchange transport system in the renal proximal tubules.83 It is also possible that vitamin C increases glomerular filtration rate,84,85 thereby increasing uric acid excretion.
A randomized control study indicated that supplementation with 500 mg/day of vitamin C for 2 months reduces serum uric acid by increasing the estimated glomerular filtration rate.86 Moreover, a meta-analysis of 13 randomized controlled trials showed that vitamin C supplementation significantly lowered serum uric acid level.87 Choi et al prospectively examined the relationship between vitamin C intake and risk of incidence of gout in 46 994 males with no history of gout from 1986 through 2006, and they found that subjects with higher vitamin C intake had lower risk. In men with supplemental vitamin C intake of 1500 mg/day or greater, the multivariate relative risk of gout was 0.55 as compared with those with vitamin C intake less than 250 mg/day.88 The exact anti-gout mechanism of vitamin C is unknown, though its antioxidative property has been widely reported.89 Although these results are attractive, the upper limit of vitamin C ingestion per day has been stated by the US Food and Drug Administration to be below 2.0 g/day in adults (age >19 years), as gastrointestinal effects such as diarrhea have been reported with doses over 1000 mg/day. In addition, it was recently reported that vitamin C supplementation had an anti-gout effect but did not reduce serum uric acid by a clinically significant degree in patients with established gout.90
Stress
The relationship between stress and uric acid has recently attracted attention, as it was found that uric acid in serum is temporarily elevated by various kinds of daily emotional stress.91 In the study, elevated levels of uric acid in serum were suppressed by administering diazepam, suggesting that uric acid level rose as a result of stress.91
Based on these results, it is considered that gout patients should avoid stress. However, it is difficult to check the influence of only stress on uric acid with other factors excluded and it is not easy to measure stress in a quantitative manner; thus, the precise relationship between stress and uric acid in humans remains to be clarified.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Nakayama A, Matsuo H, Nakaoka H, et al. Common dysfunctional variants of ABCG2 have stronger impact on hyperuricemia progression than typical environmental risk factors. Sci Rep. 2014;4:5227. doi: 10.1038/srep05227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonora E, Targher G, Zenere MB, et al. Relationship of uric acid concentration to cardiovascular risk factors in young men. Role of obesity and central fat distribution. The Verona Young Men Atherosclerosis Risk Factors Study. Int J Obes Relat Metab Disord. 1996;20:975-980. [PubMed] [Google Scholar]
- 3. Hara S, Tsuji H, Ohmoto Y, et al. High serum uric acid level and low urine pH as predictors of metabolic syndrome: a retrospective cohort study in a Japanese urban population. Metabolism. 2012;61:281-288. [DOI] [PubMed] [Google Scholar]
- 4. Gonçalves JP, Oliveira A, Severo M, Santos AC, Lopes C. Cross-sectional and longitudinal associations between serum uric acid and metabolic syndrome. Endocrine. 2012;41:450-457. [DOI] [PubMed] [Google Scholar]
- 5. Lin WY, Liu CS, Li TC, et al. In addition to insulin resistance and obesity, hyperuricemia is strongly associated with metabolic syndrome using different definitions in Chinese populations: a population-based study (Taichung Community Health Study). Ann Rheum Dis. 2008;67:432-433. [DOI] [PubMed] [Google Scholar]
- 6. Yamashita S, Matsuzawa Y, Tokunaga K, Fujioka S, Tarui S. Studies on the impaired metabolism of uric acid in obese subjects: marked reduction of renal urate excretion and its improvement by a low-calorie diet. Int J Obes. 1986;10:255-264. [PubMed] [Google Scholar]
- 7. Scott JT, Sturge RA. The effect of weight loss on plasma and urinary uric acid and lipid levels. Adv Exp Med Biol. 1997;76B:274-277. [DOI] [PubMed] [Google Scholar]
- 8. Dessein P, Shipton E, Stanwix A, Joffe B, Ramokgadi J. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis. 2000;59:539-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsunoda S, Kamide K, Minami J, Kawano Y. Decreases in serum uric acid by amelioration of insulin resistance in overweight hypertensive patients: effect of a low-energy diet and an insulin-sensitizing agent. Am J Hypertens. 2002;15:697-701. [DOI] [PubMed] [Google Scholar]
- 10. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165:742-748. [DOI] [PubMed] [Google Scholar]
- 11. Leyva F, Wingrove CS, Godsland IF, Stevenson JC. The glycolytic pathway to coronary heart disease: a hypothesis. Metabolism. 1998;47:657-662. [DOI] [PubMed] [Google Scholar]
- 12. Green H, Fraser IG. Differential effects of exercise intensity on serum uric acid concentration. Med Sci Sports Exerc. 1988;20:55-59. [DOI] [PubMed] [Google Scholar]
- 13. Kaya M, Moriwaki Y, Ka T, et al. Plasma concentrations and urinary excretion of purine bases (uric acid, hypoxanthine, and xanthine) and oxypurinol after rigorous exercise. Metabolism. 2006;55:103-107. [DOI] [PubMed] [Google Scholar]
- 14. Yamanaka H, Kawagoe Y, Taniguchi A, et al. Accelerated purine nucleotide degradation by anaerobic but not by aerobic ergometer muscle exercise. Metabolism. 1992;41:364-369. [DOI] [PubMed] [Google Scholar]
- 15. Nishida Y, Iyadomi M, Higaki Y, Tanaka H, Hara M, Tanaka K. Influence of physical activity intensity and aerobic fitness on the anthropometric index and serum uric acid concentration in people with obesity. Intern Med. 2011;50:2121-2128. [DOI] [PubMed] [Google Scholar]
- 16. Williams PT. Effects of diet, physical activity and performance, and body weight on incident gout in ostensibly healthy, vigorously active men. Am J Clin Nutr. 2008;87:1480-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamamoto T, Moriwaki Y, Ka T, et al. Effect of sauna bathing and beer ingestion on plasma concentrations of purine bases. Metabolism. 2004;53:772-776. [DOI] [PubMed] [Google Scholar]
- 18. Huang L, Huang C, Chen M, Mao I. Effects of profuse sweating induced by exercise on urinary uric acid excretion in a hot environment. Chin J Physiol. 2010;53:254-261. [DOI] [PubMed] [Google Scholar]
- 19. Diamond HS, Lazarus R, Kaplan D, Halberstam D. Effect of urine flow rate on uric acid excretion in man. Arthritis Rheum. 1972;15:338-346. [DOI] [PubMed] [Google Scholar]
- 20. Neogi T, Chen C, Chaisson C, Hunter DJ, Zhang Y. Drinking water can reduce the risk of recurrent gout attacks. Paper presented at: ACR Annual Scientific Meeting; October 16-21, 2009; Philadelphia, PA. [Google Scholar]
- 21. Kanbara A, Hakoda M, Seyama I. Urine alkalization facilitates uric acid excretion. Nutr J. 2010;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ryu KA, Kang HH, Kim SY, et al. Comparison of nutrient intake and diet quality between hyperuricemia subjects and controls in Korea. Clin Nutr Res. 2014;43:56-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zöllner N. Influence of various purines on uric acid metabolism. Bibl Nutr Dieta. 1973;(19):34-43. [PubMed] [Google Scholar]
- 24. Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;52:283-289. [DOI] [PubMed] [Google Scholar]
- 25. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350:1093-1103. [DOI] [PubMed] [Google Scholar]
- 26. Zgaga L, Theodoratou E, Kyle J, et al. The association of dietary intake of purine-rich vegetables, sugar-sweetened beverages and dairy with plasma urate, in a cross-sectional study. PLoS One. 2012;7:e38123. doi: 10.1371/journal.pone.0038123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun SZ, Flickinger BD, Patricia S, Williamson-Hughes PS, Empie MW. Lack of association between dietary fructose and hyperuricemia risk in adults. Nutr Metab (Lond). 2010;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koguchi T, Koguchi H, Nakajima H, et al. Dietary fiber suppresses elevation of uric acid and urea nitrogen concentrations in serum of rats with renal dysfunction induced by dietary adenine. Int J Vitam Nutr Res. 2004;74:253-263. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y, Chen C, Choi H, et al. Purine-rich foods intake and recurrent gout attacks. Ann Rheum Dis. 2012;71:1448-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu TF. Milestones in the treatment of gout. Am J Med. 1974;56:676-685. [DOI] [PubMed] [Google Scholar]
- 31. Fam AG. Gout, diet and the insulin resistance syndrome. J Rheumatol. 2002;29:1350-1355. [PubMed] [Google Scholar]
- 32. Rho YH, Zhu Y, Choi HK. The epidemiology of uric acid and fructose. Semin Nephrol. 2001;31:410-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abdelmalek MF, Lazo M, Horska A, et al. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology. 2012;56:952-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lecoultre V, Egli L, Theytaz F, Despland C, Schneiter P, Tappy L. Fructose-induced hyperuricemia is associated with a decreased renal uric acid excretion in humans. Diabetes Care. 2013;36:e149-e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sahebjami H, Scalettar R. Effects of fructose infusion on lactate and uric acid metabolism. Lancet. 1971;297:366-369. [DOI] [PubMed] [Google Scholar]
- 36. Johnson RJ, Perez-Pozo SE, Sautin YY, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30:96-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59:109-116. [DOI] [PubMed] [Google Scholar]
- 38. Meneses-Leon J, Denova-Gutiérrez E, Castañón-Robles S, et al. Sweetened beverage consumption and the risk of hyperuricemia in Mexican adults: a cross-sectional study. BMC Public Health. 2014;14:445. doi: 10.1186/1471-2458-14-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336:309-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in women. JAMA. 2010;304:2270-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kobayashi T, Inokuchi T, Yamamoto A, et al. Effects of sucrose on plasma concentrations and urinary excretion of purine bases. Metabolism. 2007;56:439-443. [DOI] [PubMed] [Google Scholar]
- 42. Livesey G. Fructose ingestion: dose-dependent responses in health research. J Nutr. 2009;139:1246S-1252S. [DOI] [PubMed] [Google Scholar]
- 43. Le MT, Frye RF, Rivard CJ, et al. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism. 2012;61:641-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brown IJ, Stamler J, van Horn L, et al. International Study of Macro/Micronutrients and Blood Pressure Research Group. Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure: international study of macro/micronutrients and blood pressure. Hypertension. 2011;57:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Perez-Pozo SE, Schold J, Nakagawa T, Sánchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond). 2010;34:454-461. [DOI] [PubMed] [Google Scholar]
- 46. Johnson RJ, Stephenson MC, Crossland H, et al. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology. 2013;145:1016-1025. [DOI] [PubMed] [Google Scholar]
- 47. Livesey G, Taylor R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr. 2008;88:1419-1437. [DOI] [PubMed] [Google Scholar]
- 48. Angelopoulos TJ, Lowndes J, Sinnett S, Rippe JM. Fructose containing sugars do not raise blood pressure or uric acid at normal levels of human consumption. J Clin Hypertens (Greenwich). 2015;17:87-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang DD, Sievenpiper JL, de Souza RJ, et al. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr. 2012;142:916-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dalbeth N, Wong S, Gamble GD, et al. Acute effect of milk on serum urate concentrations: a randomised controlled crossover trial. Ann Rheum Dis. 2010;68:1677-1682. [DOI] [PubMed] [Google Scholar]
- 51. Kurajoh M, Ka T, Okuda C, et al. Effects of bovine milk ingestion on urinary excretion of uric acid. Int J Clin Pharmacol Ther. 2011;49:366-370. [DOI] [PubMed] [Google Scholar]
- 52. Garrel DR, Verdy M, PetitClerc C, Martin C, Brulé D, Hamet P. Milk- and soy-protein ingestion: acute effect on serum uric acid concentration. Am J Clin Nutr. 1991;53:665-669. [DOI] [PubMed] [Google Scholar]
- 53. Simmonds HA, Webster DR, Becroft DM, Potter CF. Purine and pyrimidine metabolism in hereditary orotic aciduria: some unexpected effects of allopurinol. Eur J Clin Invest. 1980;10:333-339. [DOI] [PubMed] [Google Scholar]
- 54. Dalbeth N, Ames R, Gamble GD, et al. Effects of skim milk powder enriched with glycomacropeptide and G600 milk fat extract on frequency of gout flares: a proof-of-concept randomised controlled trial. Ann Rheum Dis. 2012;71:929-934. [DOI] [PubMed] [Google Scholar]
- 55. Blau LW. Cherry diet control for gout and arthritis. Tex Rep Biol Med. 1950;8:309-311. [PubMed] [Google Scholar]
- 56. Zhang Y, Neogi T, Chen C, Chaisson C, Hunter DJ, Choi HK. Cherry consumption and decreased risk of recurrent gout attacks. Arthritis Rheum. 2012;64:4004-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kelley DS, Rasooly R, Jacob RA, Kader AA, Mackey BE. Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women. J Nutr. 2006;136:981-986. [DOI] [PubMed] [Google Scholar]
- 58. Schlesinger N, Rabinowitz R, Schlesinger M. Pilot studies of cherry juice concentrate for gout flare prophylaxis. J Arthritis. 2012;1:1-5. [DOI] [PubMed] [Google Scholar]
- 59. Jacob RA, Spinozzi GM, Simon VA, et al. Consumption of cherries lowers plasma urate in healthy women. J Nutr. 2003;133:1826-1829. [DOI] [PubMed] [Google Scholar]
- 60. Martin KR, Bopp J, Burrell L, Hook G. The effect of 100% tart cherry juice on serum uric acid levels, biomarkers of inflammation and cardiovascular disease risk factors. FASEB J. 2011;25(Meeting Abstract Supplement):339.2. [Google Scholar]
- 61. Guasch-Ferré M, Bulló M, Babio N, et al. Mediterranean diet and risk of hyperuricemia in elderly participants at high cardiovascular risk. J Gerontol A Biol Sci Med Sci. 2013;68:1263-1270. doi: 10.1093/gerona/glt028. [DOI] [PubMed] [Google Scholar]
- 62. Kontogianni MD, Chrysohoou C, Panagiotakos DB, et al. Adherence to the Mediterranean diet and serum uric acid: the ATTICA study. Scand J Rheumatol. 2012;41:442-449. [DOI] [PubMed] [Google Scholar]
- 63. Chrysohoou C, Skoumas J, Pitsavos C, et al. Long-term adherence to the Mediterranean diet reduces the prevalence of hyperuricaemia in elderly individuals, without known cardiovascular disease: the Ikaria study. Maturitas. 2011;70:58-64. [DOI] [PubMed] [Google Scholar]
- 64. Messina M, Messina VL, Chan P. Soyfoods, hyperuricemia and gout: a review of the epidemiologic and clinical data. Asia Pac J Clin Nutr. 2011;20:347-358. [PubMed] [Google Scholar]
- 65. Villegas R, Xiang YB, Elasy T, et al. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: the Shanghai Men’s Health Study. Nutr Metab Cardiovasc Dis. 2012;22:409-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yamamoto T, Moriwaki Y, Takahashi S. Effect of ethanol on metabolism of purine bases (hypoxanthine, xanthine, and uric acid). Clin Chim Acta. 2005;356:35-57. [DOI] [PubMed] [Google Scholar]
- 67. Faller J, Fox IH. Ethanol-induced hyperuricemia: evidence for increased urate production by activation of adenine nucleotide turnover. N Engl J Med. 1982;307:1598-1602. [DOI] [PubMed] [Google Scholar]
- 68. Puig JG, Fox IH. Ethanol-induced activation of adenine nucleotide turnover. Evidence for a role of acetate. J Clin Invest. 1984;74:936-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yamamoto T, Moriwaki Y, Ka T, et al. Effect of purine-free low-malt liquor (happo-shu) on the plasma concentrations and urinary excretion of purine bases and uridine—comparison between purine-free and regular happo-shu. Horm Metab Res. 2004;36:231-237. [DOI] [PubMed] [Google Scholar]
- 70. Choi HK, Curhan G. Beer, liquor, and wine consumption and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2004;51:1023-1029. [DOI] [PubMed] [Google Scholar]
- 71. Neogi T, Chen C, Niu J, Chaisson C, Hunter DJ, Zhang Y. Alcohol quantity and type on risk of recurrent gout attacks: an internet-based case-crossover study. Am J Med. 2014;127:311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nishimura T, Shimizu T, Mineo I, et al. Influence of daily drinking habits on ethanol-induced hyperuricemia. Metabolism. 1994;43:745-748. [DOI] [PubMed] [Google Scholar]
- 73. Moriwaki Y, Ka T, Takahashi S, Tsutsumi Z, Yamamoto T. Effect of beer ingestion on the plasma concentrations and urinary excretion of purine bases: one-month study. Nucleosides Nucleotides Nucleic Acids. 2006;25:1083-1085. [DOI] [PubMed] [Google Scholar]
- 74. Choi HK, Curhan G. Coffee, tea, and caffeine consumption and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57:816-821. [DOI] [PubMed] [Google Scholar]
- 75. Kiyohara C, Kono S, Honjo S, et al. Inverse association between coffee drinking and serum uric acid concentrations in middle-aged Japanese males. Br J Nutr. 1999;82:125-130. [PubMed] [Google Scholar]
- 76. Choi HK, Willett W, Curhan G. Coffee consumption and risk of incident gout in men—a prospective study. Arthritis Rheum. 2007;56:2049-2055. [DOI] [PubMed] [Google Scholar]
- 77. Wang SH, Chen CS, Huang SH, et al. Hydrophilic ester-bearing chlorogenic acid binds to a novel domain to inhibit xanthine oxidase. Planta Med. 2009;75:1237-1240. [DOI] [PubMed] [Google Scholar]
- 78. Chan WS, Wen PC, Chiang HC. Structure-activity relationship of caffeic acid analogues on xanthine oxidase inhibition. Anticancer Res. 1995;15:703-705. [PubMed] [Google Scholar]
- 79. Agardh EE, Carlsson S, Ahlbom A, et al. Coffee consumption, type 2 diabetes and impaired glucose tolerance in Swedish men and women. J Intern Med. 2004;255:645-652. [DOI] [PubMed] [Google Scholar]
- 80. Mubarak A, Hodgson JM, Considine MJ, Croft KD, Matthews VB. Supplementation of a high-fat diet with chlorogenic acid is associated with insulin resistance and hepatic lipid accumulation in mice. J Agric Food Chem. 2013;61:4371-4378. [DOI] [PubMed] [Google Scholar]
- 81. Gao X, Curhan G, Forman JP, Ascherio A, Choi HK. Vitamin C intake and serum uric acid concentration in men. J Rheumatol. 2008;35:1853-1858. [PMC free article] [PubMed] [Google Scholar]
- 82. Stein HB, Hasan A, Fox IH. Ascorbic acid-induced uricosuria. A consequence of megavitamin therapy. Ann Intern Med. 1976;84:385-388. [DOI] [PubMed] [Google Scholar]
- 83. Berger L, Gerson CD, Yu TF. The effect of ascorbic acid on uric acid excretion with a commentary on the renal handling of ascorbic acid. Am J Med. 1977;62:71-76. [DOI] [PubMed] [Google Scholar]
- 84. Tian N, Thrasher KD, Gundy PD, Hughson MD, Manning RD., Jr Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension. 2005;45:934-939. [DOI] [PubMed] [Google Scholar]
- 85. Schäufele TG, Schlaich MP, Delles C, Klingbeil AU, Fleischmann EH, Schmieder RE. Impaired basal NO activity in patients with glomerular disease and the influence of oxidative stress. Kidney Int. 2006;70:1177-1181. [DOI] [PubMed] [Google Scholar]
- 86. Huang HY, Appel LJ, Choi MJ, et al. The effects of vitamin C supplementation on serum concentrations of uric acid: results of a randomized controlled trial. Arthritis Rheum. 2005;52:1843-1847. [DOI] [PubMed] [Google Scholar]
- 87. Juraschek SP, Miller ER, 3rd, Gelber AC. Effect of oral vitamin C supplementation on serum uric acid: a meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken). 2011;63:1295-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Choi HK, Gao X, Curhan G. Vitamin C intake and the risk of gout in men: a prospective study. Arch Intern Med. 2009;169:502-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Padayatty SJ, Katz A, Wang Y, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18-35. [DOI] [PubMed] [Google Scholar]
- 90. Stamp LK, O’Donnell JL, Frampton C, Drake JM, Zhang M, Chapman PT. Clinically insignificant effect of supplemental vitamin C on serum urate in patients with gout; a pilot randomised controlled trial. Arthritis Rheum. 2013;65:1636-1642. [DOI] [PubMed] [Google Scholar]
- 91. Rahe RR, Rubin RT, Arther RJ. The three investigators study. Serum uric acid, cholesterol and cortisol variability during stress of everyday life. Psychosom Med. 1974;36:258-268. [DOI] [PubMed] [Google Scholar]