Abstract
Clozapine remains the definitive gold standard for treatment-resistant schizophrenia despite limitations in use because of hematological abnormalities. Neutropenia or leukopenia are often treated with interruption of clozapine treatment, frequently resulting in clinical decompensation, hospitalization, increased burden to patient care, and increased risk of suicide. Colony-stimulating factors, including granulocyte colony-stimulating factors and granulocyte-macrophage colony-stimulating factors, are cytokines that stimulate proliferation and differentiation of myeloid precursor cells. Their use in the prevention and treatment of clozapine-associated neutropenia presents an alternative to clozapine discontinuation in certain cases. We present a case report of successful periodic granulocyte-macrophage colony-stimulating factor use with clozapine in a patient with treatment-resistant schizophrenia, as well as discussion of a practical approach to patients with possible clozapine-induced neutropenia or leukopenia.
Keywords: clozapine-induced neutropenia, clozapine-induced blood dyscrasias, schizophrenia, granulocyte-macrophage colony-stimulating factors, granulocyte colony-stimulating factors, clozapine-induced leukopenia
Background
Although clozapine remains the recommended medication for treatment-resistant schizophrenia based on efficacy,1,2 its use is often limited for multiple reasons, including its association with hematological abnormalities.3 Neutropenia and leukopenia are often treated with immediate interruption of clozapine treatment. However, sudden discontinuation of clozapine frequently results in various deleterious effects, which may include clinical decompensation, hospitalization, an increased burden to patient care, and increased risk of suicide.4
The Clozapine Risk Evaluation and Mitigation Strategy Program was updated in September 2015 with modification of monitoring requirements for an individual's absolute neutrophil count (ANC).5 The ANC thresholds for interruptions in clozapine treatment were lowered to <1000/μL in the general population and <500/μL in individuals with benign ethnic neutropenia. The update also allows for rechallenge of treatment, even in the setting of moderate or severe neutropenia, if the benefit of psychiatric treatment with clozapine outweighs the risk of recurrent neutropenia. Nevertheless, literature to guide an appropriate treatment approach in severe neutropenia remains sparse. Colony-stimulating factors (CSFs), including granulocyte colony-stimulating factors (G-CSFs) and granulocyte-macrophage colony-stimulating factors (GM-CSFs), are cytokines that stimulate proliferation and differentiation of myeloid precursor cells, and their use in the prevention and treatment of clozapine-associated neutropenia has been reported.6-13
More recently, 2 systematic reviews were published by Lally and colleagues10,11 analyzing the use of these cytokines as adjunct treatment with clozapine-induced neutropenia or leukopenia. One review focused on the acute treatment of clozapine-induced severe neutropenia, and the other examined the use of G-CSF either on a routine schedule or as required (based on degree of neutropenia) to support clozapine rechallenge following an episode of neutropenia requiring clozapine discontinuation. Of note, a majority of the literature focused on the use of G-CSF (filgrastim); whereas only 3 of the case reports utilized GM-CSF (sargramostim).12-14 The current literature concludes that based on available data, it is not yet possible to routinely recommend the use of CSFs for clozapine-induced neutropenia or leukopenia, warranting additional reports and analysis. Here, we describe a patient with clozapine-associated leukopenia and neutropenia who has been successfully maintained on clozapine plus intermittent GM-CSF.
Case Report
Mr B is a 55-year-old white male with hypertension, hyperlipidemia, type II diabetes mellitus, and chronic paranoid schizophrenia diagnosed at the age of 21 years. After multiple hospitalizations and failed antipsychotic regimens, significant clinical improvement was achieved with clozapine as he gained functional independence without hospitalization. However, after over 20 years of clozapine treatment, he reportedly developed leukopenia and clozapine was withdrawn. Despite asenapine initiation, the patient rapidly decompensated and inpatient hospitalization was required. Clozapine rechallenge was approved, but after 4 days of treatment, he again developed leukopenia (white blood cell [WBC] 3300/μL, ANC 1500/μL). Clozapine was discontinued and replaced by olanzapine. Over the next 2 years, Mr B was admitted to the acute psychiatric unit 17 times and underwent 7 independent medication trials, including olanzapine, risperidone, paliperidone long-acting injectable, quetiapine, ziprasidone, iloperidone, and asenapine.
Because of persistent, worsening symptoms of paranoia, psychosis and hyperreligiosity, retrial of clozapine was deemed necessary. Based on published case reports and case series utilizing lithium in attempts to prevent clozapine-induced leukopenia/neutropenia, pretreatment assistance from lithium was recommended. On Day 1, lithium was initiated at 450 mg at bedtime (WBC 4500/μL, ANC 2600/μL). On Day 8, lithium was increased to 900 mg at bedtime based on a subtherapeutic serum lithium level (0.4 mEq/L). By Day 30, the patient had developed neutrophilia and leukocytosis (WBC 14 180/μL, ANC 11 480/μL) in preparation for the clozapine rechallenge. Clozapine was initiated at 25 mg at bedtime and titrated over 13 days to 100 mg every morning and 200 mg at bedtime. A serum lithium level was rechecked to ensure no toxic levels had developed following clozapine addition, which was therapeutic at 0.9 mEq/L. Mr B's symptoms had improved and he was discharged 27 days following clozapine reinitiation on clozapine 100 mg every morning and 200 mg at bedtime and lithium 900 mg at bedtime.
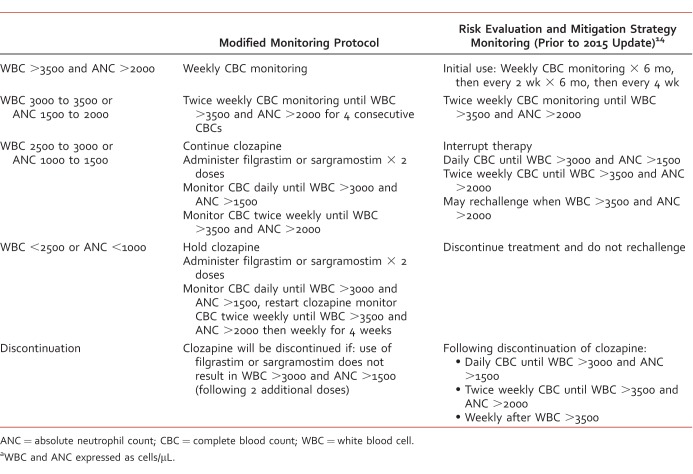
Approximately 2 months later, moderate leukopenia and mild neutropenia recurred with WBC 2530/μL and ANC 1230/μL; lithium was discontinued, clozapine was held, and hematology was consulted. Review of medications revealed no other likely culprits. A bone marrow biopsy was performed with inconclusive results; however, the report stated that “the presence of adequate myeloid precursors within the bone marrow aspirate smears disfavors clozapine-induced agranulocytosis.” Vitamin B12, folate, and copper levels were normal, ruling out nutritional deficiencies. A nuclear medicine liver spleen scan was performed and revealed findings compatible with hypersplenism. Based on patient's history of success with clozapine use, augmentation with CSF therapy was recommended. Per hematology, Mr B was initially treated with 5 doses of G-CSF (filgrastim 300 mcg/mL subcutaneously daily), his WBC and ANC recovered (WBC 9530/μL, ANC 6760/μL) within 4 days, and clozapine was restarted. A modified monitoring protocol (Table 1) was approved (by an interdisciplinary team involving consultation from the Veteran Affairs National Clozapine Registry, psychiatry, hematology, and pharmacy) that used G-CSF as needed without clozapine interruption.
TABLE 1: .
Modified monitoring protocola
Six weeks following initial G-CSF treatment, leukopenia and neutropenia (WBC 2590/μL, ANC 980/μL) recurred, prompting interruption of clozapine and initiation of G-CSF (filgrastim 300 mcg/mL × 2 days). Because of formulary considerations, filgrastim was replaced with sargramostim. Following 2 doses, WBC and ANC had recovered (3710/μL and 1980/μL, respectively) and clozapine was reintroduced. Three years have passed since initiation of CSF therapy and the modified monitoring protocol. Mr B's symptoms of schizophrenia have remained relatively well-controlled since the introduction of CSF therapy, as clozapine use has been uninterrupted. Although periodic psychiatric hospitalizations have been required, 8 of 10 were related to CSF administration because of required daily complete blood count monitoring and only 2 were because of clinical decompensation. He received 2 doses of sargramostim during each of the 8 admissions, totaling 16 doses. He continues to have WBC and ANC monitored weekly through outpatient psychiatry and has not required inpatient admission in over a year.
Discussion: Responding to Clozapine-Induced Neutropenia and Leukopenia
When treating a patient with possible clozapine-induced neutropenia or leukopenia, a systematic approach is crucial. Evaluation of neutropenia should include prompt assessment for medical emergencies, a thorough history and physical exam, and a complete medication review. While clozapine could be the causative agent, it is imperative that alternative contributors, including disease states and medications (Table 2), are explored.
TABLE 2:
Select disease state and medication causes of neutropenia15-17

After alternative causes have been excluded, morning pseudoneutropenia should be considered.18 Morning pseudoneutropenia is a diurnal variation in the ANC in which transient neutropenia occurs during the morning hours. Although this variation can occur independently of clozapine therapy, it has been reported repeatedly throughout the literature in the setting of antipsychotic use.18-23 A study by McKee et al19 evaluated the impact of changing the timing of WBC/ANC blood draws in 10 clozapine recipients. The results demonstrated a marginally significant increase in WBC (mean increase = 667/μL, P = .07) and a statistically significant increase in ANC (mean increase = 1130/μL, P = .003) when switching blood draws from 0630 to 0830. Several case reports have been published with similar increases when comparing morning and afternoon blood samples.22,23 Experts have hypothesized that clozapine may amplify the circadian variations in circulating neutrophils by affecting the endogenous production of hematopoietic cytokines.20 In patients without a history of clozapine-induced neutropenia or leukopenia, if early morning blood samples reveal decrease in WBC count or ANC, an afternoon blood sample to rule out diurnal variation may be warranted. Recognition of the transient nature of neutropenia may allow for therapy continuation in cases of morning pseudoneutropenia.
Although lithium pretreatment was used in the above case to prevent recurrence of leukopenia and neutropenia, its use remains controversial. Lithium is known to increase the WBC count and ANC, possibly through increased granulocyte production and enhanced cortisol secretion; however, its mechanism is not completely understood and remains poorly quantified.24 Despite this, use of lithium in clozapine-induced neutropenia has been suggested because of the limited options in these patients and fear of decompensation should clozapine be discontinued.25-27 While there are several reports of successful long-term adjunctive lithium treatment, substantial concerns regarding safety remain. A large case analysis by Kanaan and Kerwin27 suggests a protective effect of lithium; however, the authors conclude that its ability to protect against genuine clozapine-induced neutropenia was unlikely. One significant issue facing those that choose to rechallenge with adjunctive lithium is that there is no clear association between the lithium dose/serum level and blood cell counts, making appropriate prescribing difficult. Kanaan and Kerwin suggest titration of lithium to a plasma level >0.4 mEq/L with subsequent initiation of clozapine when WBC count is within normal range; however, the clinical basis of this guidance is limited. In addition, use of lithium for this off-label use warrants close monitoring because of its increased side effect burden, including risk of fatal toxicity, long-term renal injury, and hypothyroidism.
As demonstrated in the case above, use of CSFs may be an appropriate treatment option in some cases. This is supported by a recent systematic review, in which 75% of patients continued clozapine with the use of either regular prophylactic (70% success rate) or as-required administration (89% success rate) of G-CSF.10 However, several considerations are warranted prior to pursuing this treatment. Most importantly, there is a lack of conclusive evidence regarding the use of CSFs for this indication. As demonstrated in several case reports, if a patient encounters agranulocytosis whereby antibody crosslinking and hapten-carriers target neutrophils for cell-mediated or apoptotic death, CSFs will likely be unsuccessful.28,29
If a trial of CSF therapy is deemed appropriate, an appropriate dosing and monitoring strategy must be established. As in Mr B's case, the clozapine registry will allow special protocols with consent from hematology/oncology at the facility. Several dosing strategies have been employed throughout the literature, including both maintenance prophylaxis or as-required administration. Prophylactic dosing is typically administered weekly or twice weekly, with an average weekly dose of filgrastim 399 mcg (125 mcg to 900 mcg).10 Dosing of sargramostim has not been well-defined. In the case documented above, hematology recommended sargramostim 250 mcg to 500 mcg intramuscularly daily for 2 days as required per modified protocol.
Filgrastim (G-CSF) is derived from bacterial cells, whereas sargramostim (GM-CSF) is derived from yeast cells. Whereas bone pain is the most commonly reported adverse event with filgrastim, fever is the most common with sargramostim. Injection site reactions as well as exacerbation of preexisting inflammatory conditions have similar frequencies between the two. Comparative safety and efficacy data of G-CSF versus GM-CSF are limited and conflicting, and the long-term consequences of maintenance G-CSF or GM-CSF use in the absence of a primary hematological problem are unclear.30-33 For this reason, choice of one agent over the other is generally based on the system's formulary preference.
Although there are many limitations to the use of CSFs, benefit versus risk must be considered on an individual basis. In the case of Mr B, the efficacy and tolerability of clozapine was known as he had been stable on the medication for over 20 years. This led providers to conclude that the benefits of attempting use of CSFs outweighed the risks. In contrast, if neutropenia or leukopenia occurs in a patient's first few weeks of clozapine exposure, the conversation and resolution may be very different.
Conclusion
The case report presented above demonstrates that the use of GM-CSF may be a valid alternative to clozapine discontinuation secondary to neutropenia in patients with severe treatment-resistant schizophrenia and prior clinical stability on clozapine. The discussion also provides an example of a therapeutic monitoring protocol modified for the intermittent use of G-CSF or GM-CSF (Table 1), a review of the literature surrounding adjunctive lithium, and reiteration of the importance of ruling out alternative causes, such as medications, disease states, and morning pseudoneutropenia. The use of G-CSF and GM-CSF is limited because of its high cost, potential adverse reactions, and lack of definitive evidence. A prospective, placebo-controlled trial to establish efficacy is necessary prior to conclusive recommendations. Until such a study is conducted, the available literature, including this case report, suggests that CSF therapy may be a valuable tool in the treatment of leukopenia and neutropenia in those on clozapine.
Footnotes
Disclosures: The authors have no actual or potential conflicts of interest in relation to this manuscript.
References
- 1.Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry. 2001;158(4):518–26. doi: 10.1176/appi.ajp.158.4.518. 11282684 DOI: 10.1176/appi.ajp.158.4.518 PubMed PMID: PubMed PMID: 11282684. [DOI] [PubMed] [Google Scholar]
- 2.Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385–92. doi: 10.1192/bip.bp.115.177261. 27388573 DOI: 10.1192/bip.bp.115.177261 PubMed PMID: PubMed PMID: 27388573. [DOI] [PubMed] [Google Scholar]
- 3.Alvir JM, Lieberman J, Safferman A, Schwimmer J, Schaaf J. Clozapine-induced agranulocytosis -- incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162–7. doi: 10.1056/NEJM199307153290303. 8515788 DOI: 10.1056/NEJM199307153290303 PubMed PMID: PubMed PMID: 8515788. [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration [Internet] Silver Spring (MD): US Food and Drug Administration; c2015. FDA Drug Safety Communication: FDA modifies monitoring for neutropenia associated with schizophrenia medicine clozapine; approves new shared REMS program for all clozapine medicines. [updated 2016 Jan 15; cited 2018 Feb 23] Available from: http://www.fda.gov/Drugs/DrugSafety/ucm461853.htm. [Google Scholar]
- 5.Mathewson K, Lindenmayer J- Clozapine and granulocyte colony-stimulating factor. J Clin Psychopharmacol. 2007;27(6):714–5. doi: 10.1097/JCP.0b013e31815a583b. 18004146 DOI: 10.1097/JCP.0b013e31815a583b PubMed PMID: PubMed PMID: 18004146. [DOI] [PubMed] [Google Scholar]
- 6.Hägg S, Rosenius S, Spigset O. Long-term combination treatment with clozapine and filgrastim in patients with clozapine-induced agranulocytosis. Int Clin Psychopharmacol. 2003;18(3):173–4. doi: 10.1097/01.yic.0000062800.74434.6c. 12702898 DOI: 10.1097/01.yic.0000062800.74434.6c PubMed PMID: PubMed PMID: 12702898. [DOI] [PubMed] [Google Scholar]
- 7.Comacchio C, Dusi N, Lasalvia A. Successful use of single doses of granulocyte-colony stimulating factor (G-CSF) in the treatment of late-onset agranulocytosis associated with clozapine in a patient with treatment-resistant schizophrenia: a case report. J Clin Psychopharmacol. 2016;36(2):173–4. doi: 10.1097/JCP.0000000000000467. 26859277 DOI: 10.1097/JCP.0000000000000467 PubMed PMID: PubMed PMID: 26859277. [DOI] [PubMed] [Google Scholar]
- 8.Freeman GM, Jr, Martin B, Hu R. G-CSF dosing to prevent recurrent clozapine-induced agranulocytosis. Am J Psychiatry. 2016;173(6):643. doi: 10.1176/appi.ajp.2016.15101303. 27245191 DOI: 10.1176/appi.ajp.2016.15101303 PubMed PMID: PubMed PMID: 27245191. [DOI] [PubMed] [Google Scholar]
- 9.Lally J, Malik S, Whiskey E, Taylor D, Gaughran F, Krivoy A, et al. Clozapine-associated agranulocytosis treatment with granulocyte colony-stimulating factor/granulocyte-macrophage colony-stimulating factor. J Clin Psychopharmacol. 2017;37(4):441–6. doi: 10.1097/JCP.0000000000000715. 28437295 DOI: 10.1097/JCP.0000000000000715 PubMed PMID: PubMed PMID: 28437295. [DOI] [PubMed] [Google Scholar]
- 10.Lally J, Malik S, Krivoy A, Whiskey E, Taylor D, Gaughran F, et al. The use of granulocyte colony-stimulating factor in clozapine rechallenge. J Clin Psychopharmacol. 2017;37(5):600–4. doi: 10.1097/JCP.0000000000000767. 28817489 DOI: 10.1097/JCP.0000000000000767 PubMed PMID: PubMed PMID: 28817489. [DOI] [PubMed] [Google Scholar]
- 11.Barnas C, Zwierzina H, Hummer M, Sperner-Unterweger B, Stern A, Fleischhacker W. Granulocyte-macrophage colony-stimulating factor (GM-CSF) treatment of clozapine-induced agranulocytosis: a case report. J Clin Psychiatry. 1992;53(7):245–7. PubMed PMID: 1639744. [PubMed] [Google Scholar]
- 12.Conus P, Nanzer N, Baumann P. An alternative to interruption of treatment in recurrent clozapine-induced severe neutropenia. Br J Psychiatry. 2001;179(2):180. doi: 10.1192/bjp.179.2.180. 11483490 DOI: 10.1192/bjp.179.2.180 PubMed PMID: PubMed PMID: 11483490. [DOI] [PubMed] [Google Scholar]
- 13.Patel N, Dorson P, Bettinger T. Sudden late onset of clozapine-induced agranulocytosis. Ann Pharmacother. 2002;36(6):1012–5. doi: 10.1345/aph.1A417. 12022904 DOI: 10.1345/aph.1A417 PubMed PMID: PubMed PMID: 12022904. [DOI] [PubMed] [Google Scholar]
- 14.Teva Clozapine [Internet] North Wales (PA): Teva Pharmaceuticals USA; c2017. Teva Clozapine monitoring guidelines. [updated 2017 Dec; cited 2018 Feb 23] Available from: http://www.tevaclozapine.com/documents/Clozapine_Monitoring_Guidelines.pdf. [Google Scholar]
- 15.Hashiguchi Y, Kasai M, Fukuda T, Ichimura T, Yasui T, Sumi T. Chemotherapy-induced neutropenia and febrile neutropenia in patients with gynecologic malignancy. Anti Cancer Drugs. 2015;26(10):1054–60. doi: 10.1097/CAD.0000000000000279. 26267078 PMC4588600 DOI: 10.1097/CAD.0000000000000279 PubMed PMID: 26267078 PubMed Central PMCID: PubMed PMID: 26267078 PubMed Central PMCID: PMC4588600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibáñez L, Vidal X, Ballarín E, Laporte J- Population-based drug-induced agranulocytosis. Arch Intern Med. 2005;165(8):869–74. doi: 10.1001/archinte.165.8.869. 15851637 DOI: 10.1001/archinte.165.8.869 PubMed PMID: PubMed PMID: 15851637. [DOI] [PubMed] [Google Scholar]
- 17.Gibson C, Berliner N. How we evaluate and treat neutropenia in adults. Blood. 2014;124(8):1251–8. doi: 10.1182/blood-2014-02-482612. 24869938 DOI: 10.1182/blood-2014-02-482612 PubMed PMID: PubMed PMID: 24869938. [DOI] [PubMed] [Google Scholar]
- 18.Spina S, Corrigan S. Continuing clozapine therapy despite morning pseudoneutropenia. Can J Hosp Parm. 2007;60(4):260–4. [Google Scholar]
- 19.McKee J, Wall T, Owensby J. Impact of complete blood count sampling time change on white blood cell and absolute neutrophil count values in clozapine recipients. Clin Schizophr Relat Psychoses. 2011;5(1):26–32. doi: 10.3371/CSRP.5.1.4. 21459736 DOI: 10.3371/CSRP.5.1.4 PubMed PMID: PubMed PMID: 21459736. [DOI] [PubMed] [Google Scholar]
- 20.Esposito D, Chouinard G, Hardy P, Corruble E. Successful initiation of clozapine treatment despite morning pseudoneutropenia. Int J Neuropsychopharmacol. 2006;9(4):489–91. doi: 10.1017/S146114570500605X. 16191206 DOI: 10.1017/S146114570500605X PubMed PMID: PubMed PMID: 16191206. [DOI] [PubMed] [Google Scholar]
- 21.Esposito D, Aouillé J, Rouillon F, Limosin F. Morning pseudoneutropenia during clozapine treatment. World J Biol Psychiatry. 2003;4(4):192–4. doi: 10.1080/15622970310029918. PubMed PMID: 14608591. [DOI] [PubMed] [Google Scholar]
- 22.Pinnaka S, Roberto A, Giordano A, Siller P, Lapidus K. Aripiprazole-induced transient morning pseudoneutropenia in an 11-year-old male. J Child Adolesc Psychopharmacol. 2016;26(9):858–9. doi: 10.1089/cap.2015.0128. 26397725 DOI: 10.1089/cap.2015.0128 PubMed PMID: PubMed PMID: 26397725. [DOI] [PubMed] [Google Scholar]
- 23.Singh G, Kodela S. Morning pseudoneutropenia during risperidone treatment. BMJ Case Rep. 2009;2009:bcr06.2008.0288. doi: 10.1136/bcr.06.2008.0288. 21686871 PMC3029895 DOI: 10.1136/bcr.06.2008.0288 PubMed PMID: 21686871 PubMed Central PMCID: PubMed PMID: 21686871 PubMed Central PMCID: PMC3029895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suraweera C, Hanwella R, de Silva V. Use of lithium in clozapine-induced neutropenia: a case report. BMC Res Notes. 2014;7(1):635. doi: 10.1186/1756-0500-7-635. 25214394 PMC4167504 DOI: 10.1186/1756-0500-7-635 PubMed PMID: 25214394 PubMed Central PMCID: PubMed PMID: 25214394 PubMed Central PMCID: PMC4167504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghaznavi S, Nakic M, Rao P, Hu J, Brewer J, Hannestad J, et al. Rechallenging with clozapine following neutropenia: treatment options for refractory schizophrenia. Am J Psychiatry. 2008;165(7):813–8. doi: 10.1176/appi.ajp.2008.07111823. 18593787 DOI: 10.1176/appi.ajp.2008.07111823 PubMed PMID: PubMed PMID: 18593787. [DOI] [PubMed] [Google Scholar]
- 26.Small J, Klapper M, Malloy F, Steadman T. Tolerability and efficacy of clozapine combined with lithium in schizophrenia and schizoaffective disorder. J Clin Psychopharmacol. 2003;23(3):223–8. doi: 10.1097/01.jcp.0000084026.22282.5f. 12826983 DOI: 10.1097/01.jcp.0000084026.22282.5f PubMed PMID: PubMed PMID: 12826983. [DOI] [PubMed] [Google Scholar]
- 27.Kanaan R, Kerwin R. Lithium and clozapine rechallenge: a retrospective case analysis. J Clin Psychiatry. 2006;67(5):756–60. doi: 10.4088/jcp.v67n0509. PubMed PMID: 16841625. [DOI] [PubMed] [Google Scholar]
- 28.Hazewinkel AW, Bogers JPA, Giltay E. Add-on filgrastim during clozapine rechallenge unsuccessful in preventing agranulocytosis. Gen Hosp Psychiatry. 2013;35(5):576.e11–2. doi: 10.1016/j.genhosppsych.2013.01.002. 23395419 DOI: 10.1016/j.genhosppsych.2013.01.002 PubMed PMID: PubMed PMID: 23395419. [DOI] [PubMed] [Google Scholar]
- 29.Majczenko T, Stewart J. Failure of filgrastim to prevent severe clozapine-induced agranulocytosis. South Med J. 2008;101(6):639–40. doi: 10.1097/SMJ.0b013e318172f6c6. 18475227 DOI: 10.1097/SMJ.0b013e318172f6c6 PubMed PMID: PubMed PMID: 18475227. [DOI] [PubMed] [Google Scholar]
- 30.Beveridge R, Miller J, Kales A, Binder R, Robert N, Harvey J, et al. A comparison of efficacy of sargramostim (yeast-derived RhuGM-CSF) and filgrastim (bacteria-derived RhuG-CSF) in the therapeutic setting of chemotherapy-induced myelosuppression. Cancer Invest. 1998;16(6):366–73. doi: 10.3109/07357909809115775. PubMed PMID: 9679526. [DOI] [PubMed] [Google Scholar]
- 31.Stull D, Bilmes R, Kim H, Fichtl R. Comparison of sargramostim and filgrastim in the treatment of chemotherapy-induced neutropenia. Am J Health Syst Pharm. 2005;62(1):83–7. doi: 10.1093/ajhp/62.1.83. PubMed PMID: 15658078. [DOI] [PubMed] [Google Scholar]
- 32.Wong S-, Chan H. Effects of a formulary change from granulocyte colony-stimulating factor to granulocyte-macrophage colony-stimulating factor on outcomes in patients treated with myelosuppressive chemotherapy. Pharmacotherapy. 2005;25(3):372–8. doi: 10.1592/phco.25.3.372.61608. 15843284 DOI: 10.1592/phco.25.3.372.61608 PubMed PMID: PubMed PMID: 15843284. [DOI] [PubMed] [Google Scholar]
- 33.Milkovich G, Moleski R, Reitan J, Dunning D, Gibson G, Paivanas T, et al. Comparative safety of filgrastim versus sargramostim in patients receiving myelosuppressive chemotherapy. Pharmacotherapy. 2000;20(12):1432–40. doi: 10.1592/phco.20.19.1432.34861. PubMed PMID: 11130215. [DOI] [PubMed] [Google Scholar]