Abstract
Objective
To determine whether fragment removal on in vitro fertilization (IVF) day 2 improved the subsequent development and pregnancy outcomes of fragmented embryos compared to similar-grade embryos without fragment removal.
Methods
This study was a retrospective analysis involving 191 IVF cycles in which all embryos had over 10% fragmentation (grade 3 or 4) on day 2 of the IVF-embryo transfer cycle from March 2015 to December 2017. IVF cycles were divided into the fragment removal group (n=87) and the no fragment removal group (n=104) as a control cohort. Before fragment removal, embryos with fragmentation on day 2 were incubated in Ca2+- and Mg2+-free biopsy medium under paraffin oil for 30 minutes. Microsurgical fragment removal was performed with later-assisted hatching and a handmade suction micropipette that had an outer diameter of 30 µm.
Results
There were no significant differences in the characteristics of the patients between the control and the fragment removal groups. After fragment removal and subsequent in vitro culture for 24 hours, the number of blastomeres (7.1±1.7 vs. 6.9±1.6) was comparable between the transferred embryos in the two groups, but the morphological grade of the embryos in the fragment removal group (1.9±0.7) was significantly higher than that of the control group (3.1±0.5, p<0.01). The clinical pregnancy (43.7%) and implantation rates (25.8%) in the fragment removal group were significantly higher than those in the control group (28.8% and 14.0%, respectively; p<0.05).
Conclusion
Early fragment removal on day 2 significantly improved the subsequent development and pregnancy outcomes of fragmented embryos.
Keywords: Day 2 embryos, Embryo fragmentation, Embryo grade, Fragment removal, Pregnancy outcome
Introduction
Fragmentation of embryos is often observed during embryo culture in in vitro fertilization (IVF)-embryo transfer (ET) cycles. Embryo fragmentation is associated with a range of factors, including inadequate culture conditions, poor quality of the ovum and spermatozoon [1], increased maternal age [2,3], chromosomal abnormalities [4], abnormal cell cycle [5], apoptosis [6], and oxidative stress in embryos [7]. The presence of fragmentation limits the subsequent development of embryos [8,9,10,11] due to the loss of cytoplasmic mitochondria, mRNA, and regulatory proteins, which are essential for cell division, as well as physical interruption of the gap junctions in blastomeres, which interferes with the cell-cell interactions required for cleavage and compaction [2,12,13,14,15]. Cellular fragmentation can induce programmed cell death or apoptosis in blastomeres [6]. These fragments have been shown to secrete harmful substances that adversely affect the surrounding healthy cells, resulting in impaired embryo development [12], decreased implantation potential and pregnancy rate, and an increased abortion rate [2,8,9,10,11,14,15,16]. Furthermore, excessive fragments may induce arrest of embryo development, apoptosis and necrosis of blastomeres, and abnormal embryo development, compaction, and blastocyst formation [6,17].
It has been suggested that pregnancy rates increase when high-quality embryos displaying no irregular cells and no fragmentation are transferred [2]. It has also been demonstrated that heavily fragmented embryos did not implant [18]. Other investigators have reported that transferring embryos with large fragments led to significantly lower implantation and pregnancy rates than when embryos with minor fragmentation were transferred [9,19].
It has been theorized that removing fragments from a fragmented embryo can improve cell division and implantation by preventing the release of harmful substances and apoptosis. In fact, many authors have reported that fragment removal was beneficial for the subsequent development and implantation of fragmented embryos. It was reported that microsurgical fragment removal significantly altered the developmental process of some embryos and improved the implantation potential of fragmented embryos [3,9]. In another study, fragment removal had a positive effect on embryo development and good-quality blastocyst development [20], and ET after fragment removal resulted in clinical outcomes comparable to those observed when high-quality embryos without fragments were transferred [3]. It has been suggested that fragment removal is an effective way to restore the spatial relationship of cell-cell contact disturbed by fragments and to prevent secondary degeneration of adjacent cells caused by debris [9].
Previous studies have only investigated the effects of fragment removal in IVF day 3 embryos although the fragmentation of embryos first occurs on day 2. In fact, to date, no studies have focused on fragment removal on IVF day 2. The aim of this study was to determine whether fragment removal on day 2 improved the subsequent development and pregnancy outcomes of fragmented embryos compared to similar-grade embryos without fragment removal.
Methods
1. Study subjects
The present study was approved by the Institutional Review Board of Mamapapa and Baby Clinic (Mamapapa & Baby IRB 2015-01-2), and was conducted from March 2015 to December 2017. The present study is a retrospective analysis involving 191 IVF cycles in which all embryos had over 10% fragmentation and were grade 3, 4, or 5 on day 2 during an IVF-ET cycle at Mamapapa and Baby Clinic. Eight-seven cycles were performed in day 3 embryos that were transferred after fragment removal on day 2 (experimental group with fragment removal). In the remaining 104 cycles, fragmented embryos were transferred on day 3 transfer without prior fragment removal (control group without fragment removal). Patients' data were collected, including age, endometrial thickness, anti-Müllerian hormone (AMH) level, number of retrieved oocytes, oocyte fertilization rate, number of transferred embryos, and basal hormone levels. We also analyzed clinical outcomes, including rates of pregnancy, implantation, and spontaneous abortion.
2. Ovarian stimulation and oocyte aspiration
Controlled stimulation during IVF was performed with a mild stimulation protocol using a combination of a gonadotropin-releasing hormone (GnRH) antagonist and gonadotropins [21]. Patients received 150 IU of recombinant follicle-stimulating hormone (Gonal-F; Merck Serono, Rome, Italy) alone as a daily injection from cycle day 3 until the day when human chorionic gonadotropin (hCG) was administered, or with an additional combination of human menopausal gonadotropin (IVF-M; LG Lifesciences, Iksan, Korea) according to age, weight, ovarian reserve, and preresponse to controlled ovarian hyperstimulation. The GnRH antagonist (cetrorelix, 0.25 mg; Cetrotide, Merck Serono) was initiated on the day when the leading follicle reached a diameter of 14 mm. Ovarian follicular development was monitored by transvaginal ultrasonography. When two or more follicles were ≥18 mm in maximum diameter, as detected by sonography, 10,000 IU of hCG (Pregnyl; NV Organon, Oss, The Netherlands) was administered. Oocyte retrieval was performed using 20-gauge ovum aspiration needles (Cook Medical, Queensland, Australia) under standard transvaginal ultrasound guidance 35–36 hours after hCG administration.
3. Embryo culture and grade
Commercial IVF culture medium (SAGE 1-Step; Origio, Malov, Denmark) was employed in IVF and embryo culture. Fertilized oocytes were individually cultured in 15-µL drops of culture medium, at 37℃ under an atmosphere of 6% CO2 and 5% O2. All embryos were evaluated according to the number and shape of blastomeres and degree of fragmentation by modifying the Veeck classification [15]. The characteristics of the morphological grades were as follows: grade 1 (GI), no fragmentation with equal-sized blastomeres; grade 2 (GII), <10% fragmentation with equal-sized blastomeres or no fragmentation with unequal-sized blastomeres; grade 3 (GIII), 10%≤ fragmentation <25%; grade 4 (GIV), 25%≤ fragmentation <50%; and grade 5 (GV), ≥50% fragmentation [22]. Fragment removal was performed in GIII–GV embryos with fragmentation on day 2, and the subsequent development of the embryos was evaluated the next day.
4. Fragment removal
Fragment removal was mainly performed in GIII embryos (10%< degree of fragmentation <25%) on day 2 because fragment removal in GIV or GV embryos was not effective in improving their subsequent development due to the lethal extent of fragmentation. Preparation for fragment removal was performed by attaching an aspirator tube for calibrated microcapillary pipettes (A5177; Sigma-Aldrich, St. Louis, MO, USA) to a mouthpiece after attaching a micropipette with a 30-µM outer diameter to the pipette holder. We made a laser hole in the zona pellucida and used a hand-made micropipette with a mouthpiece to suction out the fragments from around and between the blastomeres without damaging the blastomeres. Before fragment removal, the fragmented embryos were incubated in Ca2+- and Mg2+-free biopsy medium (Biopsy Media, Origio) under paraffin oil (Ovoil; Vitrolife, Göteborg, Sweden) for 30 minutes. Micromanipulation was performed in prewarmed petri dishes (351006; Corning, New York, NY, USA) in 30-µL droplets consisting of biopsy medium. All micromanipulation procedures were performed on the heated stage of an inverted microscope (Nikon, Tokyo, Japan) using hydraulic manipulators (Narishige, Tokyo, Japan). After complete fragment removal, the embryos were washed with culture medium and then incubated for an additional 24 hours before embryo selection for transfer. Only one or two embryos were transferred on day 3 in order to maximize the pregnancy rate and to minimize multiple pregnancies based on the patient's age and embryo quality.
5. Embryo scoring for transfer
The embryos were scored at the following three time points after fertilization: two-cell cleavage (26 hours), day 2 (40–41 hours), and day 3 (64–65 hours). The total summed score of the highest-quality embryo was 12 points, if the embryo showed two-cell cleavage (+2 points), morphological grade 1 on day 2 (+5 points), and morphological grade 1 on day 3 (+5 points). The score was dependent on the morphological grade and cleavage speed of embryos. The morphological grades of embryos were used to score them from grade 1 to 5 according to the number and shape of blastomeres and degree of fragmentation: grade 1 (5 points), grade 2 (4 points), grade 3 (3 points), grade 4 (2 points), and grade 5 (1 point). In addition, when the embryos showed a slow developmental speed, 1 point was subtracted from the grade (−1). We selected the embryos that exhibited the highest total summed score for transfer [22].
6. Morphological changes in embryos and clinical outcomes
Morphological changes in the embryos after fragment removal were measured by embryo grade and developmental stage. We compared patient characteristics and morphological changes in the embryos between the fragment removal group and the control cohort. We also compared the morphological changes of fragmented embryos after fragment removal and subsequent culture according to the embryo grade of the fragmented embryos. We analyzed the rates of pregnancy, implantation, and abortion after ET in the two groups.
7. Statistical analysis
The values in the Tables are presented as mean±standard deviation or number (%). The Student t-test was employed to analyze differences in age, basal hormone levels, and the number of oocytes retrieved between the fragment removal cohort and the control group. Differences in clinical outcomes between the two groups were analyzed by the chi-square test, and p-values <0.05 were considered to indicate statistical significance.
Results
1. Patient characteristics in the control and fragment removal groups
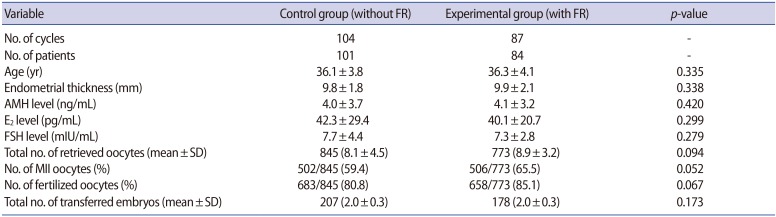
The characteristics of the patients are summarized in Table 1. There were no significant differences between the control and the experimental groups in the age of the patients (36.1±3.8 years vs. 36.3±4.1 years), endometrial thickness (9.8±1.8 mm vs. 9.9±2.1 mm), AMH levels (4.0±3.7 ng/mL vs. 4.1±3.2 ng/mL), or the number of retrieved oocytes (8.1±4.5 vs. 8.9±3.2). In addition, the oocyte fertilization rate (80.8%) and number of transferred embryos (2.0±0.3) in the control group were not different from those in the experimental group (85.1% and 2.0±0.3, respectively).
Table 1. Basal characteristics of patients in the IVF-ET program in the control and FR groups.
Values are presented as mean±standard deviation (SD) unless otherwise indicated.
IVF, in vitro fertilization; ET, embryo transfer; FR, fragment removal; AMH, anti-Müllerian hormone; E2, estradiol; FSH, follicle-stimulating hormone; MII, metaphase II.
2. Beneficial effect of fragment removal on morphological grade and subsequent development of fragmented embryos
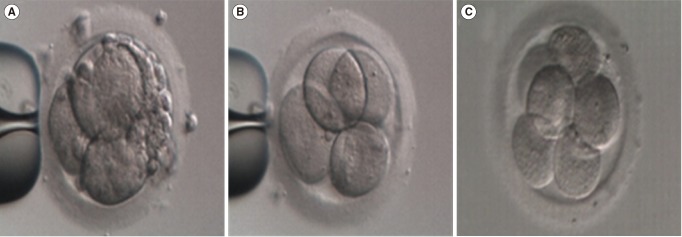
As shown in Figure 1, a significant improvement in the morphological grade of fragmented embryos was observed from GIII (Figure 1A) to GI (Figure 1B) after fragment removal. Furthermore, we found that the improvement in the morphological grade was maintained until the next day, with refragmentation occurred to a limited extent (Figure 1C).
Figure 1. Improvements in the morphological grade and subsequent development of fragmented embryos after fragment removal. (A) Day 2 fragmented embryo (grade 3, GIII) before fragment removal, (B) day 2 embryo (grade 1, GI) after fragment removal, (C) day 3 embryo (GI) cultured for 24 hours after fragment removal. Magnification, ×250.
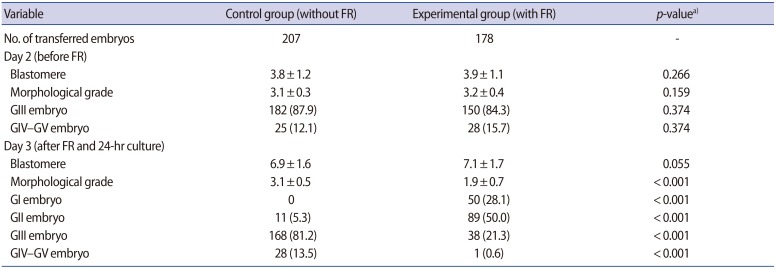
3. Developmental cell stage (number of blastomeres) and morphological grades of the embryos transferred in the fragment removal and control groups
There were no significant differences in embryo grade (3.1±0.3 vs. 3.2±0.4), number of blastomeres (3.8±1.2 vs. 3.9±1.1), or the percentage of GIII embryos (87.9% vs. 84.3%) between the control and experimental groups before fragment removal (Table 2). Although the number of blastomeres was not significantly different between the control (6.9±1.6) and experimental groups (7.1±1.7), the mean embryo morphological grade of the experimental group (1.9±0.7) had significantly improved in comparison to the control group (3.1±0.5 p<0.001) after fragment removal on day 3. In particular, the proportions of high-quality embryos (GI, 28.1%; GII, 50%) in the experimental group were significantly higher than those in the control group (GI, 0%; GII, 5.3%) on day 3. The proportion of GIII embryos (21.3%) on day 3 in the experimental group was significantly lower than that in the control group (81.2%), which means that at least some of the fragment-removed embryos regenerated new fragments, despite complete fragment removal.
Table 2. Comparison of the number of blastomeres and morphological grades of the embryos transferred in the control and FR groups before and after FR.
Values are presented as mean±standard deviation or number (%).
FR, fragment removal; GI–GV, grade 1–grade 5.
a)The p-values<0.05 were considered to indicate statistical significance.
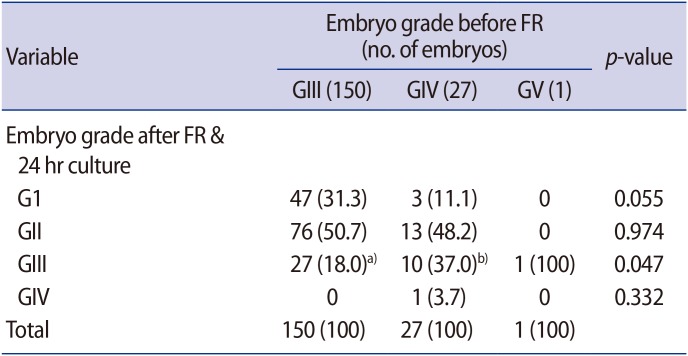
4. Regeneration of fragments in the fragment-removed embryos
We compared the regeneration of fragments in the fragment-removed embryos after fragment removal and 24 hours of in vitro culture according to the grade of the fragmented embryos (Table 3). Of the 150 GIII embryos, only 27 (18.0%) regenerated fragments and were classified as GIII on day 3. In contrast, 11 of the 27 GIV embryos (40.7%) developed into GIII and GIV embryos, with new fragmentation.
Table 3. Regeneration of new fragments in the fragment-removed embryos according to their grade before FR.
Values are presented as number (%).
FR, fragment removal; GI–GV, grade 1–grade 5.
a,b)Results are significantly different (p<0.05).
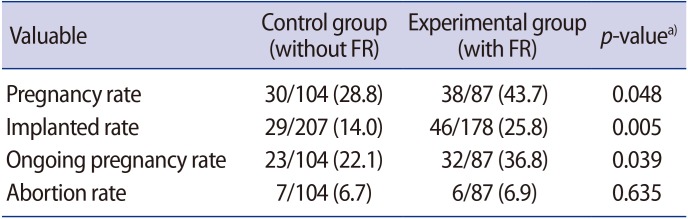
5. Clinical outcomes in the fragment removal and control groups
As shown in Table 4, the rates of clinical pregnancy (43.7%), ongoing pregnancy (36.8%), and implantation (25.8%) in the experimental group were significantly higher than the corresponding rates in the control group (28.8%, 22.1%, and 14.0%, respectively, p<0.05). However, there was no significant difference in the abortion rate between the two groups (p=0.635).
Table 4. Clinical outcomes in the control and FR groups.
Values are presented as number (%).
FR, fragment removal.
a)The p-values<0.05 were considered to indicate statistical significance.
Discussion
Embryo fragments have been reported to cause apoptosis or necrosis in blastomeres and to adversely affect the development of surrounding blastomeres by secreting harmful substances such as reactive oxygen species [6,9,12,17,20,23]. Embryo fragments first appear during the initial cleavage process of zygotes on day 2 in vitro, and the proportion of fragments gradually increases during subsequent in vitro culture. Severe fragmentation adversely affects normal blastomere development and results in low-grade embryos [12] and even arrest of embryo development [2,9,14,15,16,24].
In the present study, to confirm the detrimental effects of embryo fragments in IVF, fragment removal was performed on day 2, when fragments began to appear, and embryo development on the next day was observed (Table 2, Figure 1). As discussed above, we observed that fragment removal had a beneficial effect, as shown by the improvement in the morphological grade immediately after fragment removal on day 2 (Figure 1). This improvement in the morphological grade is consistent with the results of other researchers who studied fragment removal on day 3 [3,9,20]. We also found that a high proportion of the fragment-removed GIII embryos (82.0%) did not exhibit any new fragments (Table 3). However, a high percentage of GIV-V embryos (40.7%) showed new fragmentation after fragment removal and subsequent in vitro culture. This result is similar to that reported by other authors who performed fragment removal in day 3 embryos and observed a sustained reduction in fragmentation until day 5 [24]. In the present study, fragment removal significantly improved the morphological grade of the embryos in the fragment removal group (grade, 1.9±0.7) on day 3, in comparison to the control group (grade, 3.1±0.5). In addition, the proportion of GI and GII embryos in the fragment removal group (78.1%) was significantly higher than in the control group (5.3%).
It is generally accepted that GI and GII embryos have a higher implantation potential than GIII and GIV embryos. In fact, almost all embryos with fragments had a low grade and a reduced implantation potential [8,9,10,11,18,19,25], and the removal of fragments has been found to improve not only embryo development, but also pregnancy outcomes [2,3,9,20,26]. Moreover, transfer after fragment removal in lower-grade embryos resulted in implantation and live-birth rates comparable to those observed when GI embryos were transferred [3]. This indicates that fragment removal may ultimately improve the implantation rate of the fragment-removed embryos by improving their grade [2,13,14,15]. In the present study, we also observed beneficial effects of fragment removal on clinical outcomes (Table 3). The rates of clinical pregnancy (43.7%), ongoing pregnancy (36.8%), and implantation (25.8%) in the fragment removal group were significantly higher than in the control group (28.8%, 22.1%, and 14.0%, respectively; p<0.05). These results suggest that fragment removal is helpful in the normal development of the remaining blastomeres by eliminating the detrimental effects of fragments [18].
In addition, there was no significant difference in the spontaneous abortion rate (6.7% vs. 6.9%, p=0.635) between the two groups. This result indicates that although fragment removal can rescue an embryo with fragmentation caused by a cell cleavage disorder or transient environmental influences, it may be impossible to completely rescue fragmented embryos induced by genetic defects. Therefore, although fragment removal improved the morphological grade and implantation ability of fragmented embryos with genetic defects, it could not ensure the maintenance of ongoing pregnancies. In the present study (Table 3), fragment removal was mainly performed on day 2 in GIII embryos (10%< degree of fragmentation <25%) at the 2–4 cell stage, because fragment removal was not effective for highly fragmented embryos (GIV and GV). Excessive fragmentation of embryos induces lethal cell loss in the embryo, which is nearly impossible to rescue by fragment removal. Some authors have reported that fragment removal did not have a beneficial effect on assisted reproductive technology outcomes [27]. This contrasting result could be attributed to differences in the grade of the embryos subjected to fragment removal. Unlike the present study, previous studies performed fragment removal in embryos with 10%–50% fragmentation [27]. In contrast, in the present study, some of the GIV embryos were successfully implanted after fragment removal and transfer although the frequency of implantation was lower than that of the GIII embryos (data not shown). Therefore, we recommend transferring GIV embryos after fragment removal instead of transferring them without fragment removal if there is no other choice but to transfer GIV embryos.
Additionally, we confirmed that fragment removal on day 2 was more efficient than on day 3. In our previous study [28], we reported the rates of pregnancy (31.3%), implantation (12.3%), and ongoing pregnancy (28.1%) after the transfer of embryos that underwent fragment removal on day 3. In the present study, the corresponding rates for the embryos that underwent fragment removal on day 2 were 43.7%, 25.8%, and 36.8%, respectively. Although those differences were not statistically significant, fragment removal on day 2 showed more favorable results than day 3 fragment removal in IVF-ET cycles. This result may be due to the following advantages that contribute to the greater safety of day 2 fragment removal compared to day 3 fragment removal. First, day 2 embryos are larger and have fewer blastomeres and wide intercellular spaces. These features can reduce the time needed to remove the fragments and the exposure time outside the incubator, which may otherwise damage the embryos. Second, day 2 fragment removal eliminates the harmful substances generated by fragments as soon as possible, thereby promoting the development of normal blastomeres. Third, the cytoplasmic membrane of day 2 embryos seems to be more elastic and durable against micromanipulation for fragment removal than that of day 3 embryos. Since embryo culture medium cannot adequately supply the major constituents of cell membrane lipid proteins, the cell membranes of embryos gradually lose elasticity and become fragile as cell division continues in in vitro culture. Therefore, early fragment removal on day 2 poses less risk of mechanical damage to embryos than fragment removal on day 3. Lastly, the continuation of in vitro culture for 24 hours after fragment removal on day 2 makes it more reasonable to select and transfer the embryos that show a higher grade and developmental capacity, without fragment reoccurrence, than is the case when embryos are directly transferred immediately after fragment removal on day 3.
In conclusion, we suggest that early fragment removal on day 2, as soon as fragments appear, is the best alternative to improve clinical outcomes in IVF patients especially those in whom pregnancy has previously failed due to the transfer of fragmented embryos.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Salumets A, Suikkari AM, Mols T, Soderstrom-Anttila V, Tuuri T. Influence of oocytes and spermatozoa on early embryonic development. Fertil Steril. 2002;78:1082–1087. doi: 10.1016/s0015-0282(02)04215-2. [DOI] [PubMed] [Google Scholar]
- 2.Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzmann J, et al. Embryo score to predict implantation after in-vitro fertilization: based on 957 single embryo transfers. Hum Reprod. 1995;10:2427–2431. doi: 10.1093/oxfordjournals.humrep.a136312. [DOI] [PubMed] [Google Scholar]
- 3.Keltz MD, Skorupski JC, Bradley K, Stein D. Predictors of embryo fragmentation and outcome after fragment removal in in vitro fertilization. Fertil Steril. 2006;86:321–324. doi: 10.1016/j.fertnstert.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 4.Phan V, Littman E, Harris D, La A. Correlation between embryo morphology and development and chromosomal complement. Asian Pac J Reprod. 2014;3:85–89. [Google Scholar]
- 5.Stensen MH, Tanbo TG, Storeng R, Abyholm T, Fedorcsak P. Fragmentation of human cleavage-stage embryos is related to the progression through meiotic and mitotic cell cycles. Fertil Steril. 2015;103:374–381. doi: 10.1016/j.fertnstert.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Jurisicova A, Varmuza S, Casper RF. Programmed cell death and human embryo fragmentation. Mol Hum Reprod. 1996;2:93–98. doi: 10.1093/molehr/2.2.93. [DOI] [PubMed] [Google Scholar]
- 7.Otasevic V, Surlan L, Vucetic M, Tulic I, Buzadzic B, Stancic A, et al. Expression patterns of mitochondrial OXPHOS components, mitofusin 1 and dynamin-related protein 1 are associated with human embryo fragmentation. Reprod Fertil Dev. 2016;28:319–327. doi: 10.1071/RD13415. [DOI] [PubMed] [Google Scholar]
- 8.Alikani M, Calderon G, Tomkin G, Garrisi J, Kokot M, Cohen J. Cleavage anomalies in early human embryos and survival after prolonged culture in-vitro. Hum Reprod. 2000;15:2634–2643. doi: 10.1093/humrep/15.12.2634. [DOI] [PubMed] [Google Scholar]
- 9.Alikani M, Cohen J, Tomkin G, Garrisi GJ, Mack C, Scott RT. Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil Steril. 1999;71:836–842. doi: 10.1016/s0015-0282(99)00092-8. [DOI] [PubMed] [Google Scholar]
- 10.Ebner T, Yaman C, Moser M, Sommergruber M, Polz W, Tews G. Embryo fragmentation in vitro and its impact on treatment and pregnancy outcome. Fertil Steril. 2001;76:281–285. doi: 10.1016/s0015-0282(01)01904-5. [DOI] [PubMed] [Google Scholar]
- 11.Van Blerkom J, Davis P, Alexander S. A microscopic and biochemical study of fragmentation phenotypes in stage-appropriate human embryos. Hum Reprod. 2001;16:719–729. doi: 10.1093/humrep/16.4.719. [DOI] [PubMed] [Google Scholar]
- 12.Antczak M, Van Blerkom J. Temporal and spatial aspects of fragmentation in early human embryos: possible effects on developmental competence and association with the differential elimination of regulatory proteins from polarized domains. Hum Reprod. 1999;14:429–447. doi: 10.1093/humrep/14.2.429. [DOI] [PubMed] [Google Scholar]
- 13.Lewin A, Schenker JG, Safran A, Zigelman N, Avrech O, Abramov Y, et al. Embryo growth rate in vitro as an indicator of embryo quality in IVF cycles. J Assist Reprod Genet. 1994;11:500–503. doi: 10.1007/BF02216029. [DOI] [PubMed] [Google Scholar]
- 14.Tasdemir M, Tasdemir I, Kodama H, Fukuda J, Tanaka T. Two instead of three embryo transfer in in-vitro fertilization. Hum Reprod. 1995;10:2155–2158. doi: 10.1093/oxfordjournals.humrep.a136252. [DOI] [PubMed] [Google Scholar]
- 15.Veeck LL, Rosenwaks Z. An atlas of human gametes and conceptuses: an illustrated reference for assisted reproductive technology. New York: Parthenon; 1999. [Google Scholar]
- 16.Isik AZ, Gaglar GS, Sozen E, Tuncay G, Vicdan K. The effect of assisted hatching and defragmentation on IVF outcome in patients without good quality embryos for transfer. J Turk Soc Obstet Gynecol. 2013;10:138–142. [Google Scholar]
- 17.Chi HJ, Koo JJ, Choi SY, Jeong HJ, Roh SI. Fragmentation of embryos is associated with both necrosis and apoptosis. Fertil Steril. 2011;96:187–192. doi: 10.1016/j.fertnstert.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Staessen C, Camus M, Bollen N, Devroey P, Van Steirteghem AC. The relationship between embryo quality and the occurrence of multiple pregnancies. Fertil Steril. 1992;57:626–630. [PubMed] [Google Scholar]
- 19.Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12:1545–1549. doi: 10.1093/humrep/12.7.1545. [DOI] [PubMed] [Google Scholar]
- 20.Eftekhari-Yazdi P, Valojerdi MR, Ashtiani SK, Eslaminejad MB, Karimian L. Effect of fragment removal on blastocyst formation and quality of human embryos. Reprod Biomed Online. 2006;13:823–832. doi: 10.1016/s1472-6483(10)61031-0. [DOI] [PubMed] [Google Scholar]
- 21.Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NG, Verhoeff A, et al. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22:980–988. doi: 10.1093/humrep/del484. [DOI] [PubMed] [Google Scholar]
- 22.Chi HJ, Kim SG, Kim YY, Park JY, Yoo CS, Park IH, et al. ICSI significantly improved the pregnancy rate of patients with a high sperm DNA fragmentation index. Clin Exp Reprod Med. 2017;44:132–140. doi: 10.5653/cerm.2017.44.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998;13:998–1002. doi: 10.1093/humrep/13.4.998. [DOI] [PubMed] [Google Scholar]
- 24.Keltz M, Fritz R, Gonzales E, Ozensoy S, Skorupski J, Stein D. Defragmentation of low grade day 3 embryos resulted in sustained reduction in fragmentation, but did not improve compaction or blastulation rates. Fertil Steril. 2010;94:2406–2408. doi: 10.1016/j.fertnstert.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Bongso A, Ng SC, Lim J, Fong CY, Ratnam S. Preimplantation genetics: chromosomes of fragmented human embryos. Fertil Steril. 1991;56:66–70. doi: 10.1016/s0015-0282(16)54418-5. [DOI] [PubMed] [Google Scholar]
- 26.Halvaei I, Khalili MA, Safari S, Esfandiari N. Ongoing pregnancies following cosmetic micromanipulation of preimplantation embryos in patients with implantation failure. Case Rep Med. 2015;2015:734793. doi: 10.1155/2015/734793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halvaei I, Khalili MA, Esfandiari N, Safari S, Talebi AR, Miglietta S, et al. Ultrastructure of cytoplasmic fragments in human cleavage stage embryos. J Assist Reprod Genet. 2016;33:1677–1684. doi: 10.1007/s10815-016-0806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi HJ, Koo JJ, Lee JO, Ryu HE, Kim KR, Park C, et al. Effect of fragment removal on development of human fragmented embryos in IVF-ET program. Korean J Reprod Med. 2010;37:339–348. [Google Scholar]