Abstract
This study used conditional accuracy functions (CAF), a method computing the mean accuracy of multiple reaction time (RT) ranges, to investigate the association between aerobic fitness and the utilization of cognitive control strategy during preadolescence. Thirty-eight higher- and lower-fit children were grouped according to their cardiorespiratory capacity (VO2max) and completed a modified flanker task. Seventeen young adults were recruited as a reference group of maturation. The results showed that higher-fit children exhibited an adult-like performance pattern, and demonstrated increased overall response accuracy compared to lower-fit children, with a disproportionally larger increase in individual responses when the time allowed for discriminative processing was constrained. These findings suggest that aerobic fitness is associated with enhanced cognitive control and development of a more proactive control strategy during flanker task in preadolescent children.
Keywords: cardiorespiratory capacity, cognition, flanker task, conditional accuracy functions
With the increasing development and accessibility of technology, sedentary lifestyles have progressively prevailed (Vaynman & Gomez-Pinilla, 2006), leading to a secular trend of physical inactivity (Nelson, Neumark-Stzainer, Hannan, Sirard, & Story, 2006) as well as decline in aerobic fitness, particularly during preadolescence (Tomkinson, Leger, Olds, & Cazorla, 2003). Such findings are especially concerning given that higher aerobic fitness in children has been associated with beneficial structural and functional changes in the brain (Khan & Hillman, 2014), as well as better cognitive function and academic achievement (Hillman, Erickson, & Kramer, 2008). Accordingly, a growing body of research has investigated the relation of juvenile aerobic fitness to various aspects of cognition over the past decade (Chaddock, Pontefex, Hillman, & Kramer, 2011; Khan & Hillman, 2014).
Among the various domains of cognition, cognitive control (also known as executive control or executive function) is one of the most investigated areas in the study of aerobic fitness and cognition during preadolescence (Khan & Hillman, 2014). Cognitive control refers to a subset of top-down mental processes, which implement goal-directed behavior involving inhibition, working memory, and cognitive flexibility (Diamond, 2013). The Eriksen flanker task (Eriksen & Eriksen, 1974), a paradigm that imposes variable amounts of cognitive demand through the manipulation of the congruency of target-noise arrays, has been increasingly utilized to study cognitive control (Etnier & Chang, 2009). Several cross-sectional studies using flanker tasks have indicated that higher-fit (HF) children exhibit superior task performance relative to their lower-fit (LF) counterparts (Hillman et al., 2009; Scudder et al., 2014), with disproportionally larger performance increases in task conditions requiring greater amounts of cognitive control (Chaddock et al., 2012; Pontifex et al., 2011; Voss et al., 2011). Furthermore, HF children exhibit a pattern of performance more closely related to young adults (YA) (Voss et al., 2011). Furthermore, enhanced cognitive control, as indexed by the flanker task, was associated with improved aerobic fitness in children who participated in a 9-month physical activity program, with performance comparable to that seen in young adults. Further, no such changes were observed for those children assigned to a wait-list control group for the same passage of time (Chaddock-Heyman et al., 2013). Collectively, these findings suggest that aerobic fitness may be positively associated with cognitive control in a manner that optimizes cognitive development.
One possible explanation for this beneficial relationship is that fitness level is associated with the adoption of different cognitive control strategies (Pontifex et al., 2011). In a review of the dual cognitive control framework (Braver, 2012), proactive control was described as a process of ‘early selection’ in which behavior is directed by top-down control that actively maintains goal-relevant information from the time of intention formation until the goal is satisfied. This sustained control allows for continuous adjustments and biased anticipation toward task-relevant information, which optimizes the completion of a goal prior to the occurrence of imperative events. However, such active maintenance is effortful and associated with greater metabolic demands reflected by extended activation of the lateral prefrontal cortex (PFC; Cohen et al., 1997). On the other hand, reactive control can be conceptualized as a ‘late correction’ process in which top-down control is transiently engaged in response to environmental change. As a consequence, the goal-relevant information decays quickly and is reactivated each time as needed. Although this ‘just-in-time’ feature of the reactive mechanism leaves cognitive control relatively unbiased, the activation of goal-relevant information has to be repeatedly accessed in response to cognitively demanding events (Braver, 2012). This bottom-up feature of reactive control has been associated with transient activation in the lateral PFC (Braver, 2012), and wider brain regions, particularly in the anterior cingulate cortex (ACC; Botvinick et al., 2001). Given that the ACC is thought to be a possible generator of error-related negativity (ERN; Herrman et al., 2004), which is neuroelectric activity reflecting action monitoring (Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001), ERN may provide information regarding the extent of involvement in reactive control.
Pontifex et al. (2011) examined the ERN during a modified flanker task manipulating stimulus-response compatibility in preadolescent children. Findings revealed that HF children maintained performance accuracy across two compatibility conditions along with increased ERN amplitude for incompatible relative to compatible stimulus-response conditions, suggesting an up-regulation of reactive control in response to increased cognitive demand. In contrast, although LF children exhibited larger ERN amplitude than HF children for compatible stimulus-response condition, they failed to maintain performance and did not modulate ERN amplitude as cognitive demands increased, suggesting a general reliance on reactive control regardless of cognitive demands. The findings were interpreted to suggest differential utilization of proactive and reactive strategies between HF and LF children, such that HF children adopted a flexible strategy to fine-tune the involvement of reactive control in response to variable cognitive demand while this flexible adjustment failed in LF children. Although these findings provided a novel theoretical view of the beneficial relation of aerobic fitness to cognition, more empirical evidence from a variety of methodologies are needed to corroborate the association between childhood fitness and cognitive control strategy.
The relation of aerobic fitness to proactive and reactive control during a flanker task may be further explored through the examination of conditional accuracy functions (CAF); a method that computes the mean accuracy of multiple RT ranges (i.e. bins), rather than the distribution of RTs for error-free responses. Lappin and Disch (1972a; 1972b) demonstrated that CAF is a valid measure of the rate of increase in discrimination accuracy as a function of RT, reflecting the rate of perceptual information gain along with increased time for discriminative processing. Further, according to the dual-route models of information processing (Ridderinkhof, 2002), the flanker interference effect (the difference between congruent and incongruent trials) is the result of competition between a direct route of response activation based on dominant flanking noise and a deliberate response decision process signaled by the central target. This conflict can be resolved through a top-down controlled selective suppression of initial direct response activation. CAF is thought to be a useful method to quantify the temporal course of an individuals’ proficiency of selective response suppression (Wylie, Ridderinkhof, Bashore, & van den Wildenberg, 2010; Wylie et al., 2009). Moreover, according to the two-process model of visual attention, individuals equally allocate visual attention to all regions of the visual field in a diffuse model while concentrate visual attention in a select area of interest in a focus model (Jonides, 1983). Thus, it has been suggested that there may be an attentional transition in which individuals begin attending to a stimulus field with diffuse attention, which progressively becomes focused over time, filtering out noise irrelevant to the target (Eriksen & St. James, 1986; Eriksen & Yeh, 1985). Given that it takes time for attention to become focused, CAF was suggested to reflect the temporal course of attentional transition when shifting from a diffuse to a focus state (Heitz & Engle, 2007). Additionally, CAF allows for an asymptotic analysis, which obtains the asymptotic rate of increase in response accuracy along with RT. The identification of this asymptote was suggested to reflect the time point at which individuals reach a focused attentional state for successfully filtering flanking noise and locating the central target (Heitz & Engle, 2007).
Studies using CAF to analyze performance on a flanker task reported lower response accuracy for fast responses relative to slow responses, particularly for incongruent trial types (Coles, Gratton, Bashore, Eriksen, & Donchin, 1985; Gratton, Coles, & Donchin, 1992; Gratton, Coles, Sirevaag, Eriksen, & Donchin, 1988). This could be the result of the limited time for discriminative processing such that attention was too diffuse to filter the irrelevant flanking noise and that selective suppression was insufficiently activated to inhibit the direct response activation signaled by flanking noise. However, accuracy of slow responses was nearly perfect regardless of the existence of flanking noise because sufficient time for processing allowed an individual to narrow their focus to the central target and successfully activate selective suppression for inhibiting the direct response activation signaled by flanking noise (Coles et al., 1985; Gratton et al, 1992; Gratton et al., 1988). These features make CAF suitable to examine whether time allowed for attentional transition and activation of selective suppression modulates the effect of Parkinson’s disease, working memory capacity, or speed-accuracy tradeoff on the performance of a flanker task (Heitz & Engle, 2007; Wylie et al., 2010).
Specifically, Heitz and Engle (2007) found that a low working memory capacity group achieved asymptotic performance at individual responses within longer RT ranges than a high working memory capacity group. Given that working memory capacity relates to the active maintenance of goal-relevant information and has been associated with the activation of proactive control (Braver, 2012; Kane & Enger, 2002), it is plausible that a low working memory capacity group might be more reliant upon reactive control and thus the reactivation of task-relevant information costs more time to reach the focused phase, resulting in a failure to achieve asymptotic performance as early as a high working memory capacity group. These findings have indirectly suggested that cognitive control strategy may be measurable through CAF and asymptotic analyses. Given that it has been suggested that HF children adopt more of a proactive control strategy and LF children adopt more of a reactive control strategy during a flanker task (Pontifex et al., 2011), we speculated that the difference in CAF and asymptotic measures between HF and LF children may be attributed to cognitive control strategy. Within this context, it is reasonable to speculate that proactive control adopted by HF children is likely to be favorable for task performance, particularly when the time allowed for discriminative processing is constrained (i.e. individual fast RT ranges).
Accordingly, the purpose of the present study was to examine the association between aerobic fitness and cognitive control using CAF and asymptotic analyses during a flanker task to access cognitive control strategies employed by preadolescent children. Replicating prior work (Hillman et al., 2008; 2009; 2014), we hypothesized that aerobic fitness would be positively associated with cognitive control, as reflected by increased task performance for HF relative to LF children. Further, this association was hypothesized to be disproportionally larger when the time allowed for discriminative processing is constrained, as reflected by a gradual increase of performance for HF relative for LF children as RT decreased. It was hypothesized that aerobic fitness would be associated with a faster rate of attentional constraint, as reflected by a faster asymptote for HF than LF children. The hypothesized results from CAF and asymptotic analyses were speculated to be indicative of HF children adopting more of a proactive control strategy and LF children adopting more of a reactive control strategy. A college-aged YA sample was included to serve as a reference point for mature or optimal cognitive control in order to perform a secondary analysis examining the association between aerobic fitness and the development of cognitive control (Chaddock-Heyman et al., 2013). It was hypothesized that HF children would exhibit a similar pattern of performance in CAF and asymptotic analyses as YA, and that both groups would exhibit superior performance compared to LF children. Such findings would provide further evidence regarding a beneficial association between aerobic fitness and the developmental changes in cognitive control strategies during the preadolescence.
Method
Participants
Preadolescent children aged 8–10 years from the East-Central Illinois region were recruited specifically to participate in an ongoing clinical trial (Hillman et al., 2014, ClinicalTrials.gov number, NCT01334359). The data for the present investigation were derived from baseline measures. Participants and their guardian completed informed assent and consent approved by the Institutional Review Board of the University of Illinois at Urbana-Champaign. Prior to testing, guardians completed a health history and demographics questionnaire and other documentation indicating that their child was prepubescent (i.e., a score ≤ 2 on a 5 point scale based on the modified Tanner Staging System, Taylor et al., 2001) and free of neurological diseases, attention deficit hyperactivity disorder (ADHD Rating Scale IV; DuPaul, Power, Anastopoulos, & Reid, 1998), and physical disabilities that may be exacerbated by exercise (Physical Activity Readiness Questionnaire [PAR-Q]; Thomas et al., 1992). Socioeconomic status (SES) was calculated using a trichotomous index based on: (1) highest level of education obtained by the mother and father, (2) number of parents who worked full time, and (3) participation in a free or reduced-price lunch program at school (Birnbaum et al., 2002). Household income was acquired from an 11-point scale, with 1 reflecting income < 10,000 and 11 reflecting household income > 100,000. All participants were administered the Kaufman Brief Intelligence Test (K-BIT; Kaufman & Kaufman, 1990) to assess intelligent quotient (IQ), followed by the measurement of height, weight, and maximal oxygen consumption (VO2max) to assess body mass index (BMI), and cardiorespiratory capacity, respectively. Participants were eligible for inclusion in the investigation if (1) VO2max fell above 60th or below 30th percentile (Shvartz & Reibold, 1990), (2) body mass index (BMI) fell above 5th and below 85th percentile (Kuczmarski et al., 2000), and (3) IQ > 80. A total of thirty-eight healthy-weighted preadolescent children were included, with 19 (12 females) participants categorized as HF and 19 participants (8 females) categorized as LF. Since HF children are rare, therefore LF participants were matched on demographics to avoid confounding effects from these variables due to group differences. In addition, seventeen young adults (YA; 11 females) aged 18–24 years were recruited from the University of Illinois at Urbana-Champaign. The YA sample was tested using a similar protocol, without the collection of SES and pubertal timing. Demographic data for all participants are provided in Table 1.
Table 1.
Mean (SD) values for participant demographics
| Measure | LF | HF | YA |
|---|---|---|---|
| N | 19 (12 females) | 19 (8 females) | 17 (16 females) |
| Age (years) | 8.8 (0.5)a | 8.8 (0.7)a | 19.9 (1.2)b |
| K-BIT composite (IQ) | 113.3 (7.8)a | 115.6 (14.6)a | 103.2 (4.9)b |
| Pubertal timing | 1.3 (0.4)a | 1.3(0.4)a | - |
| Socioeconomic status (SES) | 2.1 (0.8)a | 2.4 (0.8)a | - |
| Household income | 7.5 (2.9)a | 8.3 (3.1)a | 7.9 (3.0)a |
| VO2 max percentile | 18.1 (6.6)a | 73.4 (7.4)b | 64.6 (31.4)b |
| BMI (kg/cm2) percentile | 46.7 (17.3)a | 42.0 (17.6)a | 37.9 (13.5)a |
Note: Values that share a common superscript are not significantly different at p < .05.
Procedure
Participants were instructed to avoid vigorous activity and caffeinated drinks at least 6 hours, and alcoholic beverages at least 24 hours, prior to the first visit to the laboratory. On the first day of testing, participants completed informed assent and K-BIT with a trained experimenter while guardians completed informed consent, health history, demographic questionnaire, ADHD Rating Scale, a modified Tanner stage system questionnaire, and PAR-Q. Participants were then escorted to a separate room to perform the VO2max assessment. After height and weight were measured, participants were fitted with a Polar heart rate monitor and completed a VO2max test. On the second day of testing, participants were seated in a sound attenuated chamber and performed a computerized cognitive battery.
Cardiorespiratory Fitness Assessment
Maximal oxygen consumption (VO2max) was measured using a computerized indirect calorimetry system (ParvoMedics True Max 2400) with averages for oxygen uptake (VO2) and respiratory exchange ratio (RER; VCO2/VO2) assessed every 20s. A modified Balke protocol (American College of Sports Medicine [ACSM], 2014) used a motor-driven treadmill at a constant speed with a 2.5% grade increase every 2 min until volitional exhaustion. Compared to other aggressive protocols (i.e. Bruce protocol), the modified Balke protocol is one of the most prevalent protocols that requires smaller work increments and shorter duration of each successive stage, therefore the possibility of early termination due to boredom or fatigue may be reduced in children (Paridon et al., 2006). A Polar heart rate monitor (Polar WearLink +31, Polar Electro, Finland) measured heart rate throughout the test, and ratings of perceived exertion (RPE) were assessed every 2 min with the children’s OMNI scale (Utter et al., 2002). Experienced experimenters from our research team administered the test, each of whom received training according to the ACSM’s guideline (ACSM, 2014). Relative peak oxygen consumption was expressed in ml/kg/min and was based upon maximal effort as evidenced by: (1) a plateau in oxygen consumption corresponding to an increase of less than 2 ml/kg/min despite an increase in workload, (2) a peak heart rate ≥ 185 bpm (ACSM, 2006) and a heart rate plateau (Freedson & Goodman, 1993); (3) RER ≥ 1.0 (Bar-Or, 1983); and/or (4) ratings on the children’s OMNI scale of perceived exertion ≥ 8 (Utter et al., 2002). The relative peak oxygen consumption for YA participants was determined by: (1) a plateau in oxygen consumption resulting in an increase of less than 2 ml/kg/min with an increase in workload, (2) a peak heart rate ≥ 85% of age-predicted HRmax (220 - age); (3) RER ≥ 1.1; and/or (4) ratings on perceived exertion ≥ 17 (Borg, 1970). The fitness data will be presented as age- and gender-adjusted percentile (Shvartz & Reibold, 1990).
Cognitive task
A modified flanker task (Chaddock et al., 2012; Pontifex et al., 2011) was employed for child participants in which five 3-cm tall yellow fish were presented focally on a blue background of a computer screen at a distance of ~1m using Neuroscan Stim2 software (Compumedics, Charlotte, NC). Participants were instructed to respond using a response pad as quickly and accurately as possible with a thumb press according to the directionality of the centrally presented target fish amid either congruous (swimming in the same direction) or incongruous (swimming in the opposite direction) flanking fish. Response compatibility was manipulated by the directionality of the stimulus-response mapping, with the compatible condition requiring a response consonant with the direction of the middle fish, and the incompatible condition requiring a response in the opposite direction of the middle fish. The stimuli were presented for 200 ms with a fixed inter-trial interval of 1700 ms. Two blocks of 75 trials were performed for the compatible condition followed by another two blocks for the incompatible condition. Forty practice trials were provided prior to each condition. A differently modified flanker task was used for YA participants who were asked to perform two blocks of 80 trials for each compatibility condition. That is, white arrows (instead of fish) were presented on a black background with a 100 ms stimulus presentation rate with a variable inter-stimulus interval (1000, 1200, and 1400ms). Mean response accuracy and RT of each task condition were used as dependent variables for analyses.
Conditional accuracy function (CAF)
Prior research has shown that CAF has good discriminative validity for detecting the rate of gaining perceptual information along with increased time for discriminative processing, regardless of stimulus-response probability (Lappin & Disch, 1972a). Other studies also suggested that CAF is a useful tool for measuring the temporal dynamic of selective suppression (Ridderinkhof, 2002; Wylie et al., 2010; Wylie et al., 2009) and attentional transition (Heitz & Engle, 2007). CAF has been used to investigate the cognitive processing during interference tasks such as flanker task (Coles et al., 1985; Gratton et al, 1992; Gratton et al., 1988; Heitz & Engle, 2007; Wylie et al., 2009). In the present study, CAF was obtained by creating 10 bins for each participant, with the first bin computing the average accuracy and latency of response trials that fell below 10th percentile of individual RT distribution, the second bin computing the average accuracy and latency of response trials that fell below 20th percentile and above 10th percentile of individual RT distribution, and the remaining bins being created in the same manner. Based on these measures, trials without response (i.e., omission errors) were excluded from computing CAF (Heitz & Engle, 2007), generating 9 ± 0.8% of each individual data in each bin. The numbers of response included in CAF analyses were different between groups, F(2,52) = 10.45, p < .001, η2p = .29, with YA (300 ± 24) had more responses than LF (262 ± 26) and HF (273 ± 25), t’s(34) ≥ 3.25, p’s ≤ .003, while no differences were observed between the two child groups, t(36) = 1.40, p = .170. The analysis of asymptotic performance was performed for obtaining the asymptotic rate of increase of response accuracy along with RT. The asymptotic rate was defined by identifying the fastest bin, which did not differ from the bin of peak accuracy (Heitz & Engle, 2007).
Statistical analyses
All statistical analyses were conducted using a significance level of alpha = .05. Demographic data for HF, LF, and YA were analyzed using one-way ANOVAs. Since SES and pubertal timing were not collected for YA, these two factors were analyzed using independent t-tests between the child groups. Analyses of mean accuracy and latency of CAF were conducted separately using 2 (Group: HF, LF) x 2 (Compatibility: compatible, incompatible) x 2 (Congruency: congruent, incongruent) x 10 (Bin: less than 10th percentile, 10–20th percentile, 20–30th percentile, 30–40th percentile, 40–50th percentile, 50–60th percentile, 60–70th percentile, 70–80th percentile, 80–90th percentile, above the 90th percentile) repeated ANOVAs. Analyses with three or more within-subject levels employed the Greenhouse-Geisser statistic with subsidiary univariate ANOVAs and least significant difference (LSD) for post hoc comparisons. Instead of post hoc comparisons examining any significant Bin effects using 55 paired t-tests, asymptotic analyses aimed at identifying asymptotic performance were conducted using planned, paired-samples t-tests to compare the bin of peak accuracy to the remaining bins. The fastest bin, which did not exhibit statistical difference of accuracy from the bin of peak accuracy, was identified as the bin of asymptote (Heitz & Engle, 2007). Only significant effects involved a Group x Bin interaction were subjected to asymptotic analyses. Planned independent t-tests were conducted to determine whether the accuracy at the bin of the fastest responses differed from chance performance (50%). For the secondary analyses, similar dependent variables were analyzed using repeated measures ANOVAs, with a Group factor including HF, LF, and YA. Because the purpose of the secondary analyses was to investigate the age-related difference for CAF measures, only significant effects involved Group x Bin interaction were reported.
Given that commission errors were included but omission errors were excluded from CAF analyses, general analyses were also conducted using 3 (Group: HF, LF, YA) × 2 (Compatibility: compatible, incompatible) × 2 (Congruency: congruent, incongruent) repeated ANOVAs for overall response accuracy and error-free RT, respectively.
Results
Demographics
As expected, analyses revealed a significant difference of VO2max percentile between the three groups, F(2, 52) = 49.21, p < .001, η2p = 0.65, such that LF exhibited lower VO2max percentile compared to HF, t(36) = 24.24, p < .001, d = 8.08, and YA groups, t(34) = 5.99, p < .001, d = 2.05. (see Table 1). However, no differences were observed between HF and YA groups, t(34) = 1.12, p = .276, d = 0.38. Significant effects were also revealed for Age and IQ, F’s(2, 52) ≥ 6.93, p’s ≤ .002, η2p ≥ 0.21, with YA group being older and exhibiting lower IQ than both child groups, t’s(34) ≥ 3.50, p’s ≤ .002, d’s ≥ 1.20. Additionally, no significant difference in Age and IQ were observed between HF and LF children, t’s(36) ≤ 1.15, p’s ≥ .260, d’s ≤ 0.38. No difference in household income and BMI percentile was observed between the three groups, F’s(2, 52) ≤ 1.31, p’s ≥ .278, η2p ≥ 0.05. Independent t-tests revealed no significant differences for SES and pubertal timing between HF and LF groups, t’s(34) ≤ 1.06, p’s ≥ .297, d’s ≤ 0.36.
General analyses of response accuracy
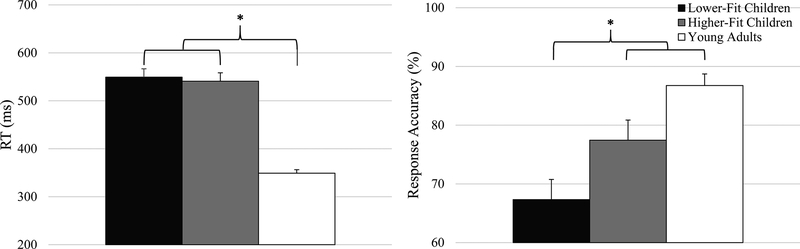
Analysis revealed a main effect of Group, F(2, 52) = 13.00, p < .001, η2p = 0.33. Post hoc tests showed reduced accuracy for LF (67.0 ± 3.3%) relative to both HF (80.1 ± 2.7%), t(36) = 3.07, p = .004, d = 1.02, and YA (86.5 ± 1.9%) groups, t(34) = 5.09, p < .001, d = 1.75 (see Figure 1). Although the data trended toward significance, no difference was observed between HF and YA groups, t(34) = 1.90, p = .066, d = 0.65. In addition, a Congruency effect was observed, F(1, 53) = 50.72, p < .001, η2p = 0.49, which was superseded by an interaction of Compatibility x Congruency, F(1, 53) = 5.93, p = .018, η2p = 0.10. Decomposition of the interaction revealed a congruency effect in both compatibility conditions, with increased accuracy for congruent relative to incongruent trials, t’s(54) ≥ 4.10, p’s < .001, d’s ≥ 1.12. This congruency effect was larger for the compatible relative for incompatible condition, t(54) = 2.30, p = .025, d = 0.62.
Figure 1.
Mean response accuracy (right) and mean reaction time (left) for each of the three groups. Vertical error bars indicate standardized error of the mean for each group.
General analyses of reaction time
Analysis revealed a main effect of Group, F(2, 52) = 47.06, p < .001, η2p = 0.64. Post hoc tests showed shorter RT for YA (344.9 ± 10.1 ms) relative to LF (545.7 ± 17.5 ms) and HF (540.4 ± 18.9 ms) groups, t’s(34) ≥ 9.114, p’s < .001, d’s ≥ 3.13, who did not differ from each other, t(36) = 0.21, p = .838, d = 0.07 (see Figure 1). This effect was superseded by an interaction of Group x Compatibility, F(2, 52) = 5.52, p = .007, η2p = 0.18. Decomposition of this interaction revealed a group effect in both compatible and incompatible conditions, F’s(2, 52) ≥ 28.15, p’s < .001, η2p ≥ 0.52. Post hoc tests revealed that YA exhibited shorter RT relative to the two child groups, with a larger decrease for incompatible, t’s(34) ≥ 9.27, p’s < .001, d’s ≥ 3.10, relative to the compatible condition, t’s(34) ≥ 7.41, p’s < .001, d’s ≥ 2.54. An interaction of Group x Congruency, F(2, 52) = 5.02, p = .010, η2p = 0.16, was also observed. Decomposition of this interaction revealed similar group effects in both congruency trials, F’s(2, 52) ≥ 38.66, p’s < .001, η2p ≥ 0.598. Post hoc tests revealed that YA exhibited shorter RT relative to the two child groups, with a larger decrease for congruent, t’s(34) ≥ 9.95, p’s < .001, d’s ≥ 3.41, relative to incongruent trials, t’s(34) ≥ 7.96, p’s < .001, d’s ≥ 2.73. In addition, main effects of Compatibility, F(1, 53) = 21.33, p < .001, η2p = 0.29, and Congruency, F(1, 53) = 114.85, p < .001, η2p = 0.69, were observed, which were superseded by an interaction of Compatibility x Congruency, F(1, 53) = 4.29, p = .043, η2p = 0.08. Decomposition of this interaction demonstrated a Congruency effect in both compatible and incompatible conditions, with shorter RT for congruent relative to incongruent trials, t’s(54) ≥ 5.15, p’s < .001, d’s ≥ 1.40. This congruency effect was marginal, with a larger trend for the compatible relative for incompatible condition, t(54) = 1.96, p = .056, d = 0.53.
Mean accuracy of CAF
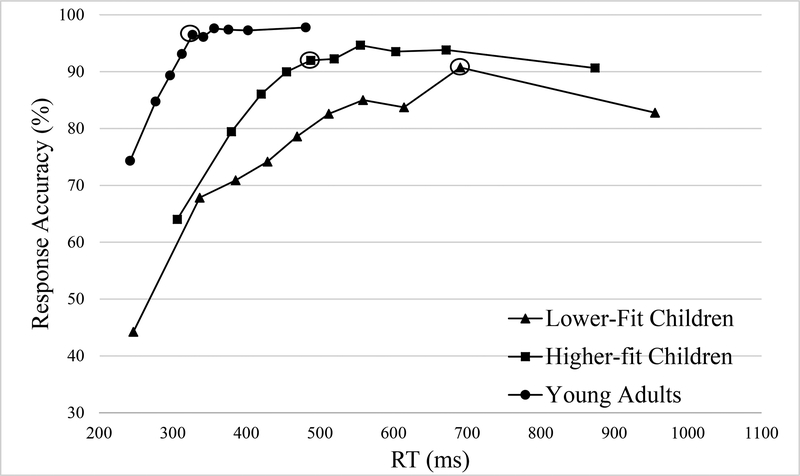
The omnibus analyses revealed main effects of Congruency, F(1, 36) = 21.72, p < .001, η2p = 0.38, and Bin, F(9, 28) = 63.67, p < .001, η2p = 0.64, and Group, F(1, 36) = 11.84, p < .001, η2p = 0.25, with HF exhibited greater response accuracy than LF group. These main effects were superseded by 2-way interactions of Group x Bin, F(9, 28) = 2.78, p = .022, η2p = 0.07, Compatibility x Bin, F(9, 28) = 2.22, p = .043, η2p = 0.06, and Congruency x Bin, F(9, 28) = 2.36, p < .030, η2p = 0.06. Decomposition of the Group x Bin interaction revealed significantly higher accuracy for HF relative for LF group across all bins, t’s(36) ≥ 2.18, p’s ≤ .036, d’s ≥ 0.73, with the exception of the bin representing data between 80–90th percentile, t(36) = 1.28, p = .212, d’s = 0.43 (see Figure 2). Decomposition of the Compatibility x Bin interaction revealed no compatibility effect across all bins, t’s(37) ≤ 1.81, p’s ≥ .079, d’s ≤ 0.60, with the exception of the bins representing data between the 10–20th and 30–40th percentile, with incompatible condition exhibited higher accuracy than compatible condition, t’s(37) ≥ 2.53, p’s ≤ .016, d’s ≥ 0.83. Decomposition of the Congruency x Bin interaction revealed no congruency effect at bins representing data between the 30–50th and above 80th percentile, t’s(37) ≤ 1.76, p’s ≥ .088, d’s ≤ 0.58. However, a significant congruency effect was observed at bins representing data between the 50–80th and below 30th percentile, t’s(37) ≥ 2.05, p’s ≤ .047, d’s ≥ 0.67, with the congruent condition exhibiting higher accuracy at bins representing data below the 30th percentile while lower accuracy at bins above the 80th percentile relative to the incongruent condition.
Figure 2.
Conditional accuracy functions collapsing congruent and incongruent trials for the three groups. Circled data points indicate the bin of asymptotic performance.
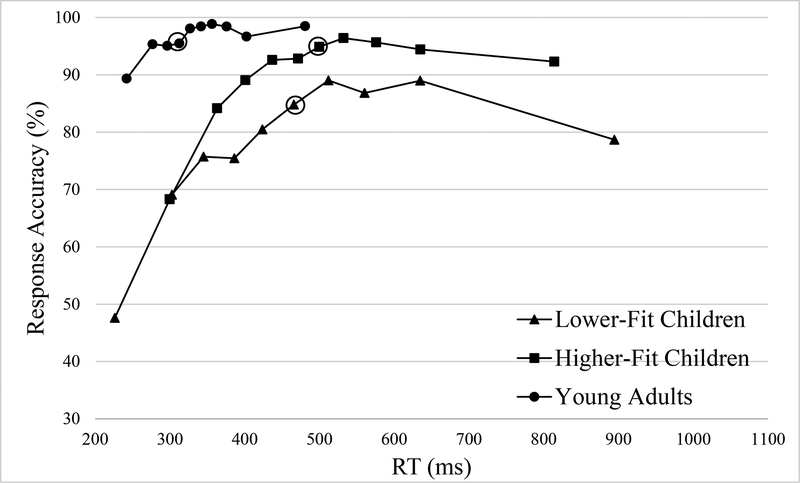
The secondary CAF analysis including YA revealed the main effect of Group, F(2, 52) = 16.25, p < .001, η2p = 0.39. Post hoc tests showed reduced accuracy for LF (67.0 ± 3.3%) relative to both HF (80.1 ± 2.7%), t(36) = 3.07, p = .004, d = 1.02, and YA (86.5 ± 1.9%) groups, t(34) = 5.09, p < .001, d = 1.75. Although the data trended toward significance, no difference was observed between HF and YA groups, t(34) = 1.90, p = .066, d = 0.65. This main effect was superseded by 2-way interactions of Group x Bin, F(18, 36) = 3.09, p = .001, η2p = 0.11, and a 3-way interaction of Group x Congruency x Bin, F(18, 36) = 3.05, p = .001, η2p = 0.11. Decomposition of the 3-way interaction revealed that the Group x Bin interaction was marginal for incongruent trials, F(18, 36) = 1.65, p = .086, η2p = 0.06, and significant for congruent trials, F(18, 36) = 5.00, p < .001, η2p = 0.16. Decomposition of the Group x Bin interaction for congruent trials revealed a significant Group effect in all bins for congruent trials, F’s(2, 52) ≥ 4.52, p’s ≤ .016, η2p ≥ 0.15, (see Figure 3). Post hoc comparisons between the two child groups demonstrated lower accuracy for LF relative to HF group across all bins, t’s(36) ≥ 2.30, p’s ≤ .027, d’s ≥ 0.77, with the exception of the bins representing data between the 80–90th percentile, t(36) = 1.82, p = .078, d’s = 0.61. Post hoc comparisons between LF and YA groups revealed reduced accuracy for LF group at all bins, t’s(34) ≥ 2.84, p’s ≤ .009, d’s ≥ 0.97. Post hoc comparisons between HF and YA groups showed significant effects at bins representing data below 20th, between the 40–50th, and above 90th percentile, t’s(34) ≥ 2.26, p’s ≤ .033, d ≥ 0.78, with HF exhibited lower accuracy than YA group, t’s(34) ≥ 2.89, p’s ≤ .008, d’s ≥ 0.99.
Figure 3.
Conditional accuracy functions for congruent trials for the three groups. Circled data points indicate the bin of asymptotic performance.
Mean latencies of CAF
The omnibus analyses revealed main effects of Compatibility, F(1, 36) = 23.11, p < .001, η2p = 0.39, and Bin, F(9, 28) = 442.08, p < .001, η2p = 0.93, which were superseded by 2-way interactions of Group x Bin, F(9, 28) = 5.31, p = .016, η2p = 0.13 and Compatibility x Bin, F(9, 28) = 5.07, p = .017, η2p = 0.12. Decomposition of the Group x Bin interaction revealed no difference between HF and LF groups across all bins, t’s(36) ≤ 1.78, p’s ≥ .083, d’s ≤ 0.59, with the exception of the bin below the 10th percentile, t(36) = 2.57, p = .014, d’s = 0.86, with LF exhibited shorter response latency than HF group. Decomposition of the Compatibility x Bin interaction revealed compatibility effect across all bins, t’s(37) ≥ 2.14, p’s ≤ .039, d’s ≥ 0.70, with the incompatible exhibited longer response latency than compatible condition (see Figure 2). A main effect of Congruency was also revealed, F(1, 36) = 16.42, p < .001, η2p = 0.31, with congruent exhibited shorter RT than incongruent trials.
The secondary analyses including YA revealed the main effects of Group, F(2, 52) = 34.82, p < .001, η2p = .57, with YA exhibited shorter RT (344.9 ± 10.1 ms) relative for LF (545.7 ± 17.5 ms) and HF (540.4 ± 18.9 ms) groups, t’s(34) ≥ 9.114, p’s < .001, d’s ≥ 3.13, who did not differ from each other, t(36) = 0.21, p = .838, d = 0.07. This main effect was superseded by a Group x Bin interaction, F(18, 36) = 40.96, p < .001, η2p = 0.61, demonstrated a significant group effect at all bins, F’s(2, 52) ≥ 6.20, p’s ≤ .004, η2p ≥ 0.20 (see Figure 2). Post hoc comparisons between the two child groups demonstrated a non-significant effect across all bins, t’s(36) ≤ 1.78, p’s ≥ .083, d’s ≤ 0.59, with the exception of the bin representing data below the 10th percentile in which LF exhibited shorter mean latency (246 ± 81ms) than HF group (306 ± 60ms), t(36) = 2.57, p = .014, d = 0.86. Post hoc comparisons between the LF and YA groups demonstrated a non-significant effect for the bin representing data below the 10th percentile, t(34) = 0.22, p = .827, d = 0.08, and significant differences at all other bins, t’s(34) ≥ 2.93, p’s ≤ .007, d’s ≥ 1.00, with LF exhibiting longer mean latency relative to YA group. Post hoc comparisons between HF and YA demonstrated a significant effect across all bins, t’s(34) ≥ 4.03, p’s < .001, d’s ≥ 1.38, with HF exhibiting longer mean latency relative to YA group.
Asymptotic analyses
The asymptotic analyses were conducted separately for LF and HF children collapsing compatibility and congruency because there was no any fitness effects associated these task conditions for response accuracy. As shown in Figure 2, LF children achieved both peak accuracy and asymptotic performance at the bin representing data between the 80–90th (70–80th percentile vs 80–90th percentile, t(18) = 2.42, p = .026, d = 1.14), followed by a decreased accuracy at bin representing data above 90th (80–90th percentile vs above 90th percentile, t(18) = 4.05, p < .001, d = 1.91. HF children achieved peak accuracy at the bin representing data between the 60–70th percentile and reached asymptotic performance at the bin representing data between the 40–50th percentile, (40–50th percentile vs 60–70th percentile, t(18) = 1.38, p = .184, d = 0.65. Planned t tests comparing accuracy at the bin representing data below 10th with chance revealed that HF children exhibited greater accuracy than chance, t(18) = 3.95, p = .001, d = 1.86, while LF children exhibited no different from chance, t(18) = 1.17, p = .259, d = 0.55.
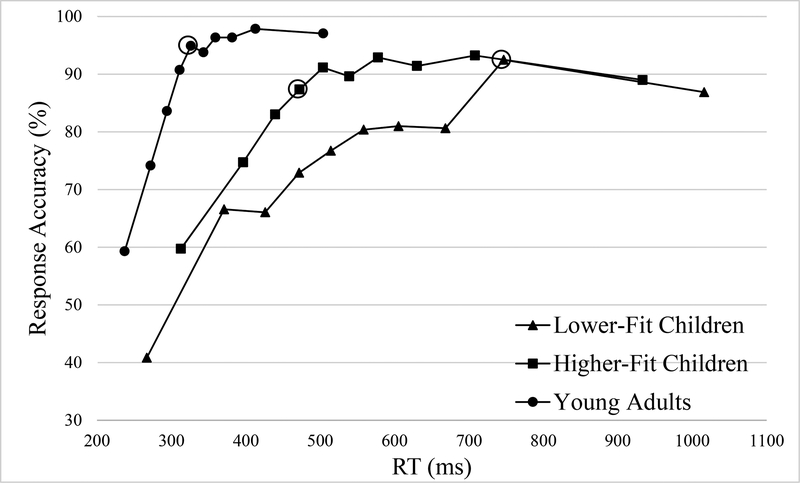
The secondary analyses of asymptotic performance including YA group were conducted separately for congruent and incongruent trials in each group because there was a significant 3-way interaction of Group x Congruency x Bin for response accuracy. All three groups achieved peak accuracy at the bin representing data between the 60–70th percentile for congruent trials (see Figure 3) and at the bin representing data between the 80–90th percentile for incongruent trials (see Figure 4). LF children reached asymptotic performance at the bin representing data between the 50–60th percentile for congruent trials (50–60th percentile vs 60–70th percentile, t(18) = 1.82, p = .085, d = 0.86), followed by decreased accuracy at the bin representing data above 90th percentile. LF children showed asymptotic performance for incongruent trials at bin representing data between 80–90th percentile (70–80th percentile vs 80–90th percentile, t(18) = 5.43, p < .001, d = 2.56). HF children reached asymptotic performance at the bin representing data between the 50–60th percentile for congruent trials, (50–60th percentile vs 60–70th percentile, t(18) = 0.84, p = .415, d = 0.40), and at the bin representing data between the 30–40th percentile for incongruent trials, (30–40th percentile vs 80–90th percentile, t(18) = 1.99, p = .062, d = 0.94). YA group reached asymptotic performance at the bin representing data between the 30–40th percentile for congruent trials (30–40th percentile vs 60–70th percentile, t(16) = 2.10, p = .052, d = 1.05), at the bin representing data between the 40–50th (40–50th percentile vs 80–90th percentile, t(16) = 1.72, p = .106, d = 0.86). The asymptote for YA group collapsing congruent and incongruent was presented because of the Group x Bin interaction for response latency (see Figure 2). YA group achieved peak performance at the bin representing data above 90th percentile and reached asymptotic performance at the bin representing data between 40–50th percentile (40–50th percentile vs above 90th percentile, t(16) = 1.35, p = .197, d = 0.68). Planned t tests revealed non-significant difference in accuracy of the bin representing data below 10th percentile in comparison with chance for LF children, t’s(18) ≤ 2.08, p’s ≥ .052, d’s ≤ 0.98. HF children exhibited higher performance accuracy than chance for both congruent and incongruent trials, t’s(18) ≥ 2.51, p’s ≤ .022, d’s ≥ 1.18. In YA group the accuracy at bin representing data below 10th percentile was greater than chance for congruent trials, t(16) = 14.86, p < .001, d = 7.43, and no different from chance for incongruent trials, t(16) = 1.65, p = .119, d = 0.83.
Figure 4.
Conditional accuracy functions for incongruent trials for the three groups. Circled data points indicate the bin of asymptotic performance.
In sum, HF and YA groups exhibited similar early asymptotic performance compared to LF children for incongruent trials. Furthermore, for congruent trials HF and YA groups exhibited increased accuracy relative for chance the fastest individual responses. However, the accuracy of the fastest individual responses was lower than chance in LF children.
Discussion
The current findings indicated that HF children exhibited higher overall task performance than LF children in tasks requiring variable amounts of cognitive control, with a disproportionally larger benefit for responses below the 80th percentile of individual RT. HF children also exhibited an earlier asymptotic performance than LF children. These findings support the hypothesis that HF children adopt a more proactive strategy, and in turn were able to constrain their attention focus and suppress noise-induced response activation during the early stage of stimulus evaluation. Moreover, a similar pattern of performance was observed between HF children and YA, who served as a reference point for mature cognitive function, suggesting that aerobic fitness may be associated with more mature-like development of cognitive ability and strategy to perform tasks requiring variable amounts of cognitive control during preadolescence.
This study replicated previous findings indicating that aerobic fitness is related to general improvements in task performance, such that HF children show higher task accuracy than LF children regardless of cognitive demand. Although the general benefits observed herein are contradictory with some prior findings, which have indicated a selective benefit in task conditions requiring greater amounts of cognitive control (Chaddock et al., 2012; Pontifex et al., 2011; Voss et al., 2011), it is consistent with other reports (Hillman et al., 2009; Scudder et al., 2014) indicating that a consensus regarding this area of study has yet to be reached. Prior research has shown that cognitive control develops throughout childhood as reflected by age-related increases in performance on a variety of cognitive control tasks (Ven Der Wildenberg & Ven Der Molen, 2004). Specifically, Ridderinkhof and van der Molen (1995) demonstrated that response accuracy during a flanker task was lower for children below 10 years of age relative to children aged 10–12 years, who did not differ from adults, suggesting a developmental asymptote of cognitive control. Accordingly, it is possible that cognitive development attenuates the relation of aerobic fitness on task conditions requiring lesser amounts of cognitive control. This notion corresponds with the extant literature suggesting general benefits to flanker task performance for children younger than 10 years (Hillman et al., 2009; Scudder et al., 2014), and may also explain selective benefits that have been observed in children older than 10 years (Chaddock et al., 2012; Pontifex et al., 2011; Voss et al., 2011). However, further examination into the modulating effect of age during preadolescence on the general versus selective relation between aerobic fitness and cognitive control is warranted.
The current results are also consonant with previous findings indicating no significant association of aerobic fitness on RT during flanker tasks in preadolescent children. Although prior study has found a negative association between aerobic fitness and RT (Scudder et al., 2014), a field assessment of aerobic fitness (PACER) and a large sample size (N = 397) were used in the analyses. Other studies utilizing VO2max and smaller sample sizes (N ≤ 48) to compare fitness groupings revealed that increased aerobic fitness is selectively associated with response accuracy but not RT (Chaddock et al., 2012; Hillman et al., 2009; Pontifex et al., 2011). Furthermore, the absence of a relationship of fitness on RT may be attributed to the stage of cognitive development during childhood. That is, children have been shown to maintain RT at the cost of response accuracy when they perform tasks requiring cognitive control (Davidson, Amso, Anderson, & Diamond, 2006). Therefore, it is likely that the relation of childhood fitness on performance during a flanker task is more likely reflected by response accuracy rather than RT.
A novel finding in this study is the association between aerobic fitness and cognitive control strategy using CAF. The investigation of performance accuracy as a function of individual RT provided a potential means to infer the temporal course of attentional transition and proficiency of selective suppression, and in turn, the adoption of proactive or reactive control. Proactive control refers to a process of ‘early selection’ in which behavior is directed by sustained active maintenance of goal-relevant information while reactive control is a process of ‘late correction’ in which behavior is the result of a transient ‘just-in-time’ activation of goal-relevant information (Braver, 2012). Given the competing information from central target and flanking noise (Coles et al., 1985; Gratton et al, 1992; Gratton et al., 1988), individuals may experience perceptual interference in the flanker task, with disproportionally larger interference during the early stage of stimulus evaluation because diffuse attentional focus and the noise-induced response activation were dominant (Heitz & Engle, 2007; Wylie et al., 2009). Such interference is reduced by adopting a proactive control strategy, particularly when the time allowed for discriminative processing is constrained. This prediction is supported by the current findings given that HF children exhibited superior response accuracy across congruent and incongruent trial types for responses at bins representing data below the 80th percentile of RT compared to LF children, who showed no such difference for the bin representing the 80–90th percentile of RT. In addition, a trend was observed for the difference in response accuracy between HF and LF groups to become larger as RT decreased (10% for bin 6–8, 10–15% for bin 2–5; 20% at bin 1; see Figure 1). Collectively, the current findings suggest that HF children were better able to narrow their attentional focus and suppress response activation signaled by flanking noise when the time allowed for discriminative processing was limited, possibly due to the adoption of a proactive control strategy, which biased attention toward the central target.
A similar association between cognitive control strategy and aerobic fitness was revealed from different perspective using ERN to infer the engagement of reactive control (Pontifex et al., 2011). Their findings indicated that LF children failed to maintain task performance and upregulate ERN as task difficulty increased, suggesting that LF children might adopt a more reactive strategy. Within this context, Voss et al. (2011) utilized fMRI to investigate differences in brain activation during a flanker task between task performance-matched HF and LF children. Task-related activation was observed in regions associated with cognitive control (i.e. anterior prefrontal cortex, anterior cingulate cortex, pre- and post-central gyrus, supplementary motor areas), and as the demand of cognitive control increased, LF children exhibited greater activation in these areas while HF children exhibited decreased activation. These alternate patterns of brain activation were interpreted to suggest that LF children failed to efficiently engage reactive control during increased cognitive demand, which resulted in greater activation of the network, reflecting inefficient regulation of cognitive control. In contrast, HF children were better able to upregulate proactive control as task difficulty increased, resulting in decreased activation and more efficient processing.
It is noteworthy that LF exhibited lower response accuracy than HF children at the bin representing the data above 90th percentile of RT. This observation was not unexpected as lower aerobic fitness was associated with more frequent and longer lapses of sustained attention (Pontifex, Scudder, Drollette, & Hillman, 2012). Further, given that the presence of perceptual interference (i.e., flanking stimuli) results in competition for cognitive resources regardless of compatibility with the central target, and that a randomized stimulus sequence creates uncertainty of subsequent trial type, cognitive control is activated across congruent and incongruent trials (Eriksen & Eriksen, 1974). Thus, it was not surprising that the observed pattern of response accuracy change as a function of RT was similar across congruent and incongruent trials because both trial types may be sufficiently challenging to warrant cognitive interference in preadolescent children. Moreover, the result of mean latency of CAF analyses revealed that LF children exhibited shorter RT than HF children at the bin representing data below the 10th percentile of RT, where response accuracy was lower than HF and did not differ from chance. This speed-accuracy tradeoff at the fastest bin suggests an impulsive response tendency in LF children for those very fast responses. Thus, the failure for LF children to perform as accurate as HF children for very fast responses may not only be attributed to cognitive control strategy but also the impulsive responding tendency.
In addition, results derived from asymptotic analyses provided auxiliary support for the association between aerobic fitness and cognitive control strategy. That is, HF children achieved earlier peak response accuracy and asymptotic performance compared to LF children. This suggests that the dominance of proactive control strategy may benefit the attentional transition from a diffuse to focused state to support the filtering out of flanking noise and successful target location. In contrast, a greater reliance on reactive control resulted in longer time to reach the attentional state for successful performance of the flanker task. Similar findings supporting this hypothesis were reported by Heitz and Engle (2007), such that enhanced working memory, which is important for the activation of a proactive control strategy (Braver, 2012; Kane & Enger, 2002), was associated with faster asymptote of response accuracy as a function of RT.
Given the small differences in the design of the flanker tasks between the children and YA groups, inferences should be drawn with cautious interpretation. The general analysis of response accuracy showed that HF children exhibited comparable performance to the YA group, suggesting that children with higher aerobic fitness may have cognitive control ability that is closer to the YA group (Chaddock-Heyman et al., 2013; Voss et al., 2011). In addition, the results from secondary CAF analyses showed that in congruent trials, where cognitive control demands were reduced, LF children exhibited an overall lower accuracy than YA across all bins while HF children performed as well as YA for individual responses at bins representing data between the 20–40th and between the 50–90th percentile. Such a pattern of results suggests that HF children exhibited a more adult-like performance pattern. Furthermore, LF children reached later asymptotic performance than the YA group, while HF children exhibited a similar asymptote to that of the YA group across both congruent and incongruent trial types. These findings suggest that HF children reached an effective attentional state as fast as YA, possibly due to the dominant activation of proactive control in both groups. Moreover, although LF children responded as quickly as YA at the very fast response bins, the response accuracy was below chance for both congruent and incongruent trials while the YA group only exhibited chance performance for incongruent trials. In contrast, the latency of very fast responses for HF children was slower relative to YA, but more accurate than chance regardless of trial type. These findings suggest that aerobic fitness during childhood may be associated with better control over impulsive responses. Collectively, the current findings imply that aerobic fitness may play a role in boosting age-related development of cognitive control processes and proficiency of proactive control strategy during flanker tasks.
However, limitations in the current study require further investigation to confirm the association between aerobic fitness and the development of cognitive control strategy. First, this relationship was only demonstrated using a cross-sectional design and requires further clarification as to whether changes in aerobic fitness also influence the adoption of certain strategies. In addition, given there are associations between cognitive control and sedentary behavior as well as physical activity in school-aged children (Van Der Niet et al., 2015), future research is warranted to investigate whether physical activity is also associated with the adoption of cognitive control strategy and whether physical activity has confounding/moderating effects on the relation between cardiorespiratory fitness and cognitive control strategy. Further, given that CAF and asymptote measures are not widely used indices for cognitive control strategy, additional study combining these measures with brain image data (i.e. ERN, fMRI) is needed to establish their validity. Moreover, the flanker task used in the secondary analyses for the YA group was slightly different from the one administered to the child groups, leading to possible confounding effects on the inference of cognitive development. Lastly, a difference in IQ was observed between children and YA groups, possibly due to sampling bias in our study. That is, the child subjects were recruited from a university town area, which has been found beneficial for enriching childhood experience and superior intellectual development (Chase-Lansdale, Gordon, Brooks-Gunn, & Klebanov, 1997). The origin of the YA participants was no collected and as such, they likely originate from more diverse neighborhoods.
In conclusion, the current study used CAF and asymptotic analyses to demonstrate a beneficial association between aerobic fitness and cognitive control during preadolescence, with a selectively larger benefit for individual responses when time allowed for discriminative processing is constrained. These results are thought to reflect the adoption of a more optimal cognitive control strategy. Importantly, these benefits were shown in a manner that enables children to perform comparably to a mature reference group, suggesting more mature cognitive development. As opportunities for physical activity continues to diminish, establishing the importance of aerobic fitness for the development of cognitive and brain health is crucial and will serve as a powerful catalyst to amend prevailing sedentary lifestyle.
Contributor Information
Mr. Shih-Chun Kao, University of Illinois at Urbana-Champaign, Kinesiology and Community Health, 316 Louise Freer Hall 906 S. Goodwin Avenue, Urbana, 61801 United States, skao5@illinois.edu
Mr. Eric Drollette, University of Illinois at Urbana-Champaign, Department of Kinesiology and Community Health, Urbana, United States, drollet1@illinois.edu
Mr. Mark Scudder, University of Illinois at Urbana-Champaign, Department of Kinesiology and Community Health, Urbana, United States, mscudde2@illinois.edu
Miss Lauren Raine, University of Illinois at Urbana-Champaign, Department of Kinesiology and Community Health, Urbana, United States, lraine2@illinois.edu.
Mr. Daniel Westfall, University of Illinois at Urbana-Champaign, Department of Kinesiology and Community Health, Urbana, United States, dwestfa2@illinois.edu
Mr. Matthew Pontifex, Michigan State University, Department of Kinesiology, East Lansing, United States, pontifex@msu.edu
Dr. Charles Hillman, University of Illinois at Urbana-Champaign, Department of Kinesiology and Community Health, Urbana, United States, chhillma@illinois.edu
References
- American College of Sports Medicine. (2014). ACSM’s guidelines for exercise testing and prescription (9th ed.). Lippincott Williams & Wilkins. [DOI] [PubMed] [Google Scholar]
- Bar-Or O (1983). Pediatric sports medicine for the practitioner: From physiologic principles to clinical applications. New York: Springer-Verlag. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, & Cohen JD (2001). Conflict monitoring and cognitive control. Psychological Review, 108, 624–652. [DOI] [PubMed] [Google Scholar]
- Braver TS (2012). The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Sciences, 16, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum AS, Lytle LA, Murray DM, Story M, Perry CL, Boutelle KN (2002). Survey development for assessing correlates of young adolescents’ eating. American Journal of Health Behavior, 26, 284–295. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Hillman CH, Pontifex MB, Raine LB, Johnson CR, & Kramer AF (2012). Childhood aerobic fitness predicts cognitive performance one year later. Journal of Sports Sciences, 30, 421–430. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Pontifex MB, Hillman CH, & Kramer AF (2011). A review of the relation of aerobic fitness and physical activity to brain structure and function in children. Journal of the International Neuropsychological Society, 17, 1–11. [DOI] [PubMed] [Google Scholar]
- Chaddock-Heyman L, Erickson KI, Voss MW, Knecht AM, Pontifex MB, Castelli DM, Hillman CH, & Kramer AF (2013). The effects of physical activity on functional MRI activation associated with cognitive control in children: a randomized controlled intervention. Frontiers in Human Neuroscience, 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase-Lansdale PL, Gordon RA, Brooks-Gunn J, & Klebanov PK (1997). Neighborhood and family influences on the intellectual and behavioral competence of preschool and early school-age children. Neighborhood Poverty, 1, 79–118. [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, & Smith EE (1997). Temporal dynamics of brain activation during a working memory task. Nature, 386, 604–608. [DOI] [PubMed] [Google Scholar]
- Coles MG, Gratton G, Bashore TR, Eriksen CW, & Donchin E (1985). A psychophysiological investigation of the continuous flow model of human information processing. Journal of Experimental Psychology: Human Perception and Performance, 11, 529–553. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, & Diamond A (2006). Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia, 44, 2037–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2013). Executive functions. Annual review of psychology, 64, 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, & Reid R (1998). ADHD Rating Scale-IV: Checklists, norms, and clinical interpretation. New York: Guilford Press. [Google Scholar]
- Eriksen CW, & Eriksen BA (1974). Effects of noise letters upon the identification of a target letter in a non-search task. Perception & Psychophysics, 16, 143–149. [Google Scholar]
- Eriksen CW, & James JDS (1986). Visual attention within and around the field of focal attention: A zoom lens model. Perception & psychophysics, 40, 225–240. [DOI] [PubMed] [Google Scholar]
- Etnier JL, & Chang YK (2009). The effect of physical activity on executive function: A brief commentary on definitions, measurement issues, and the current state of the literature. Journal of Sport & Exercise Psychology, 31, 469–483. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, & Yeh YY (1985). Allocation of attention in the visual field. Journal of Experimental Psychology: Human Perception and Performance, 11, 583. [DOI] [PubMed] [Google Scholar]
- Freedson PS, & Goodman TL (1993). Measurement of oxygen consumption In Rowland TW (Ed.), Pediatric laboratory exercise testing: Clinical guidelines (pp. 91–113). Champaign, IL: Human Kinetics [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1992). Optimizing the use of information: strategic control of activation of responses. Journal of Experimental Psychology: General, 121, 480–506. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Sirevaag EJ, Eriksen CW, & Donchin E (1988). Pre-and poststimulus activation of response channels: a psychophysiological analysis. Journal of Experimental Psychology: Human perception and performance, 14, 331–344. [DOI] [PubMed] [Google Scholar]
- Heitz RP, & Engle RW (2007). Focusing the spotlight: individual differences in visual attention control. Journal of Experimental Psychology: General, 136, 217–240. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Römmler J, Ehlis AC, Heidrich A, & Fallgatter AJ (2004). Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe). Cognitive Brain Research, 20, 294–299. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Pontifex MB, Castelli DM, Khan NA, Raine LB, Scudder MR, ... & Kamijo K (2014). Effects of the FITKids randomized controlled trial on executive control and brain function. Pediatrics, 134, e1063–e1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Buck SM, Themanson JR, Pontifex MB, & Castelli D (2009). Aerobic fitness and cognitive development: Event-related brain potential and task performance indices of executive control in preadolescent children. Developmental Psychology, 45, 114–129. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, & Kramer AF (2008). Be smart, exercise your heart: Exercise effects on brain and cognition. Nature Reviews Neuroscience, 9, 58–65. [DOI] [PubMed] [Google Scholar]
- Jonides J (1983). Further toward a model of the mind’s eye’s movement. Bulletin of the Psychonomic Society, 21, 247–250. [Google Scholar]
- Kane MJ, & Engle RW (2002). The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review, 9, 637–671. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, & Kaufman NL (1990). Kaufman Brief Intelligence Test manual. Circle Pines, MN: American Guidance Service [Google Scholar]
- Khan NA, & Hillman CH (2014). The relation of childhood physical activity and aerobic fitness to brain function and cognition: a review. Pediatric Exercise Science, 26, 138–146. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, ... & Johnson CL (2000). CDC growth charts: United States. Advance data, 314, 1–27. [PubMed] [Google Scholar]
- Lappin JS, & Disch K (1972). The latency operating characteristic: I. Effects of stimulus probability on choice reaction time. Journal of Experimental Psychology, 92, 419–427. [DOI] [PubMed] [Google Scholar]
- Lappin JS, & Disch K (1972). The latency operating characteristic: II. Effects of visual stimulus intensity on choice reaction time. Journal of Experimental Psychology, 93, 367–372 [DOI] [PubMed] [Google Scholar]
- Nelson MC, Neumark-Stzainer D, Hannan PJ, Sirard JR, & Story M (2006). Longitudinal and secular trends in physical activity and sedentary behavior during adolescence. Pediatrics, 118, e1627–e1634. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, & Kok A (2001). Error‐related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology, 38, 752–760. [PubMed] [Google Scholar]
- Paridon SM, Alpert BS, Boas SR, Cabrera ME, Caldarera LL, Daniels SR, ... Yetman AT (2006). Clinical stress testing in the pediatric age group a statement from the american heart association council on cardiovascular disease in the young, committee on atherosclerosis, hypertension, and obesity in youth. Circulation, 113, 1905–1920. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Raine LB, Johnson CR, Chaddock L, Voss MW, Cohen NJ, Kramer AF, & Hillman CH (2011). Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. Journal of Cognitive Neuroscience, 23, 1332–1345 [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Scudder MR, Drollette ES, & Hillman CH (2012). Fit and vigilant: The relationship between poorer aerobic fitness and failures in sustained attention during preadolescence. Neuropsychology, 26, 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof RK (2002). Micro-and macro-adjustments of task set: Activation and suppression in conflict tasks. Psychological Research, 66, 312–323. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, & Molen MW (1995). A psychophysiological analysis of developmental differences in the ability to resist interference. Child Development, 66, 1040–1056. [Google Scholar]
- Scudder MR, Lambourne K, Drollette ES, Herrmann S, Washburn R, Donnelly JE, & Hillman CH (2014). Aerobic capacity and cognitive control in elementary school-age children. Medicine & Science in Sports & Exercise, 46, 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvartz E, & Reibold RC (1990). Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviation, Space, and Environmental Medicine, 61, 3–11. [PubMed] [Google Scholar]
- Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, & Cook DG (2001). Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatric & Perinatal Epidemiology, 15, 88–94. [DOI] [PubMed] [Google Scholar]
- Thomas S, Reading J, & Shephard RJ (1992). Revision of the physical activity readiness questionnaire (PAR-Q). Canadian Journal of Sport Sciences, 17, 338–345. [PubMed] [Google Scholar]
- Tomkinson GR, Léger LA, Olds TS, & Cazorla G (2003). Secular trends in the performance of children and adolescents (1980–2000). Sports Medicine, 33, 285–300. [DOI] [PubMed] [Google Scholar]
- Utter AC, Roberson RJ, Nieman DC, & Kang J (2002). Childrenʼs OMNI scale of perceived exertion: Walking/running evaluation. Medicine & Science in Sports & Exercise, 34, 139–144. [DOI] [PubMed] [Google Scholar]
- van der Niet AG, Smith J, Scherder EJ, Oosterlaan J, Hartman E, & Visscher C (2015). Associations between daily physical activity and executive functioning in primary school-aged children. Journal of Science & Medicine in Sport, 18, 673–677. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, & van der Molen MW (2004). Developmental trends in simple and selective inhibition of compatible and incompatible responses. Journal of Experimental Child Psychology, 87, 201–220. [DOI] [PubMed] [Google Scholar]
- Vaynman S, & Gomez‐Pinilla F (2006). Revenge of the “sit”: how lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. Journal of Neuroscience Research, 84, 699–715. [DOI] [PubMed] [Google Scholar]
- Voss MS, Chaddock L, Kim JS, VanPatter M, Pontifex MB, Raine LB, … Kramer AF (2011). Aerobic fitness is associated with greater efficiency of the network underlying cognitive control in preadolescent children. Neuroscience, 199, 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, Ridderinkhof KR, Bashore TR, & van den Wildenberg WP (2010). The effect of Parkinson’s disease on the dynamics of on-line and proactive cognitive control during action selection. Journal of Cognitive Neuroscience, 22, 2058–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, Van Den Wildenberg WPM, Ridderinkhof KR, Bashore TR, Powell VD, Manning CA, & Wooten GF (2009). The effect of speed-accuracy strategy on response interference control in Parkinson’s disease. Neuropsychologia, 47, 1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]