Abstract
Background:
Immediate placement of an intrauterine device (IUD) after vaginal delivery is safe and convenient, but longitudinal data describing clinical outcomes have been limited.
Objectives:
To determine the proportion of TCu380A (copper) IUDs devices expelled, partially expelled, malpositioned, and retained, as well as contraceptive use by 6 months postpartum. To determine risk factors for expulsion and partial expulsion.
Study Design:
In this prospective, observational study, women who received a postplacental TCu380A IUD at vaginal delivery were enrolled postpartum. Participants returned for clinical follow-up at 6 weeks, and for a research visit with a pelvic exam and ultrasound at 6 months. We recorded IUD outcomes and 6-month contraceptive use. Partial expulsion was defined as an IUD protruding from the external cervical os, or a transvaginal ultrasound showing the distal end of the IUD below the internal os of the cervix. Multinomial logistic regression models identified risk factors associated with expulsion and partial expulsion by 6 months. The area under the receiver-operating characteristics (ROC) curve was used to assess the ability of a string check to predict the correct placement of a postplacental IUD. The primary outcome was the proportion of IUDs expelled at 6 months.
Results:
We enrolled 200 women. Of 162 participants with follow-up data at 6 months, 13 (8.0%, 95% CI 4.7%–13.4%), experienced complete expulsion and 26 (16.0%, 95% CI 11.1%–22.6%) partial expulsion (see Figure 1). Of 25 malpositioned IUDs (15.4%, 95% CI 10.2%–21.9%), 14 were not at the fundus (8.6%, 95% CI 5.2%–14.1%) and 11 were rotated within the uterus (6.8%, 95% CI 3.8%–11.9%). Multinomial logistic regression modeling indicated that higher parity (OR 2.05; 95% CI 1.21–3.50, p=0.008) was associated with expulsion. Provider specialty (Obstetrics vs. Family Medicine; OR 5.31, 95% CI 1.20–23.59; p=0.03) and gestational weight gain (normal vs. excess; OR 9.12, 95% CI 1.90–43.82; p=0.004) were associated with partial expulsion. Long-acting reversible contraceptive method use at 6 months was 80.9% (95% CI 74.0%–86.6%).
At 6 weeks postpartum, 35 of 149 (23.5%, 95% CI 16.9%–31.1%) participants had no IUD strings visible. Sensitivity of a string check to detect an incorrectly positioned intrauterine device was 36.2%, and specificity of the string check to predict a correctly positioned intrauterine device was 84.5%. This corresponds to an area under the ROC curve of 0.5.
Conclusion:
This prospective assessment of postplacental TCu380A intrauterine device placement, with ultrasound to confirm device position, finds a complete intrauterine device expulsion proportion of 8.0% at 6 months. The association of increasing parity with expulsion is consistent with prior research. The clinical significance of covariates associated with partial expulsion (provider specialty and gestational weight gain) is unclear. Due to the observational study design, any associations cannot imply causality.
The proportion of partially expelled and malpositioned IUDs was high, and the area under the ROC curve of 0.5 indicates that a string check is a poor test for assessing device position. Women considering a postplacental IUD should be counseled about the risk of position abnormalities, as well as the possibility of non-visible strings, which may complicate clinical follow-up. The clinical significance of IUD position abnormalities is unknown; future research should evaluate the influence of malposition and partial expulsion on contraceptive effectiveness and side effects.
Keywords: TCu380A IUD, copper IUD, intrauterine device, IUD complication, IUD expulsion, IUD retention, LARC, long-acting reversible contraception, postpartum contraception, postplacental IUD, postpartum IUD, vaginal delivery
Introduction
Facilitation of reproductive life planning and commensurate contraception counseling and provision are key elements of postpartum care.1 The use of a postplacental intrauterine device (IUD) for postpartum contraception offers several advantages: the IUD is a highly effective method,2,3 women are often highly motivated to begin contraception after giving birth, and most have ready access to health care during delivery.4 Offering long-acting reversible contraception (LARC) at delivery has become increasingly popular in the United States, and 35 states have proposed or accepted guidelines to enable Medicaid coverage of LARC placement during the hospitalization for delivery.5
Although postplacental IUD placement has a long safety record,6 literature describing TCu380A (copper) IUD expulsion after immediate insertion at vaginal delivery has been limited to self-reported outcomes,7 small sample sizes,8,9 and international data that may not be generalizable to the U.S.10–12 Reports of 3- to 6-month expulsion rates range from 7.0–19.5% after vaginal delivery.9–12
This prospective, observational study of IUD position outcomes after postplacental placement of copper IUDs after vaginal delivery was designed to determine the proportion of IUDs expelled, partially expelled, malpositioned, and continued by 6 months postpartum, to evaluate contraceptive method use at 6 months, and to determine risk factors for IUD expulsion and partial expulsion.
Materials and Methods
All study activities were approved by the Hospital of the University of Pennsylvania Institutional Review Board. We recruited women between April 2015 and August 2016. We included English-speaking women who were 18 years of age or older, delivered vaginally at 34 weeks 0 days’ gestation or more, received a postplacental TCu380A IUD, and who were willing to participate in study follow-up after hospital discharge. We excluded women who were unwilling or unable to comply with the study protocol.
Provision of postplacental IUDs was initiated as a part of clinical care at our university hospital starting in January 2014. We made efforts to increase awareness by providers and patients of the option for postplacental IUD placement from January 2014 onward, unrelated to the research study setting. Levonorgestrel IUDs were not available on our obstetrics ward. Obstetric providers were trained in postplacental IUD placement with both ring forceps and manual insertion, and booster trainings were provided to the Labor and Delivery service monthly. Transabdominal ultrasound to guide or confirm IUD placement was used at the discretion of the provider. IUDs were provided through philanthropic funding13 or, after April 2015, through a combination of philanthropic and research funding. Medical assistance did not cover immediate postpartum LARC during the study period.
Potentially eligible participants were approached prior to postpartum discharge by a study coordinator. Eligible women provided written informed consent in the postpartum unit, or were given the option to enroll by telephone after discharge. Women wishing to enroll after discharge were contacted within 6 weeks by a study coordinator and provided verbal consent. A baseline questionnaire including demographic information, obstetric and contraceptive history, and satisfaction with the postplacental IUD was administered at the time of enrollment. Labor characteristics, delivery information, and neonatal information were abstracted from the medical record after delivery using a standardized form.
The primary outcome for this study was the proportion of IUDs expelled at 6 months. Secondary outcomes were IUD position (partial expulsion, malposition, or correct position), elective removal, and contraceptive method use at 6 months postpartum. We defined a partial expulsion as an IUD protruding from the external cervical os, or a transvaginal ultrasound showing the distal end of the IUD below the internal os of the cervix. Malposition was defined as an IUD that was lower than 1cm from the fundus, or in an abnormal orientation, but not partially expelled.
IUD location and participant satisfaction with the IUD were assessed at 6 weeks and 6 months postpartum. At 6 weeks postpartum, the research staff extracted data from the medical record to obtain information about IUD position. Participants with incomplete documentation of IUD status in the medical record (that is, no documentation of strings on exam, documentation of absent or long strings but no ultrasound ordered, or ultrasound ordered but not performed), and those who did not follow up with their provider, were recalled for a visit in the research office at 6 weeks. Research visits included a pelvic examination and transvaginal ultrasound to evaluate IUD position. Participants who were diagnosed with an IUD problem during this visit were offered a same-day clinical appointment for contraceptive counseling, and if necessary, IUD removal and initiation of a new method, including all LARC methods. Additionally, a questionnaire was administered either in person or over the phone to assess satisfaction (using a 5-item Likert scale, “How happy are you with your choice to get the IUD immediately,” with the bounds “extremely unhappy” to “extremely happy”), participant-reported IUD status (reported as “in place” or “expelled”), and performance of self-string check (reported as “yes” or “no”).
Participants returned at 6 months for an in-person study visit with a research clinician. Procedures at this visit included a pelvic exam with string check and a transvaginal ultrasound. Participants diagnosed with an IUD problem at 6 months were also offered a clinical appointment for same-day contraceptive management. We administered the same study questionnaire to assess satisfaction, participant-reported IUD status, and performance of self-string check. Participants were compensated for time and participation.
Our sample size was computed assuming that 15% of IUDs would be expelled by 6 months postpartum.11,14,15 A sample size of 150 participants provided a narrow 95% confidence interval of +/− 5.3% around this expected expulsion percentage. We planned to enroll 200 participants, anticipating 25% loss to follow up by 6 months.
We analyzed baseline demographic and reproductive health variables for all participants using standard descriptive statistics. We distinguished between full and partial expulsion and computed the proportion of IUDs in each category. For the primary outcome of IUD expulsion at 6 months postpartum, we used one-way Analysis of Variance or Kruskal-Wallis tests for continuous variables and Fisher’s exact test for categorical variables, to initially assess associations. We performed a multinomial (polytomous) logistic regression analysis, including demographic variables (age, BMI category, race/ethnicity, income category, and relationship status) as well as variables where p≤0.20 from bivariate tests (parity, gestational age at delivery, IUD placement method, provider specialty, maternal weight gain, time to IUD placement) were considered. From this set, we used a backward elimination strategy to confirm all factors that were significantly associated with complete IUD expulsion or partial IUD expulsion or confounded associations of interest. The area under the receiver-operating characteristics (ROC) curve was used to assess the ability of a string check to predict the correct placement of a postplacental IUD.
Study data were collected and managed using electronic standardized data abstraction forms in REDCap (Research Electronic Data Capture, Vanderbilt University). We used Stata 14.2 (StataCorp, College Station, TX) for all statistical analyses. This prospective cohort study was conducted in accordance with the protocol registered on ClinicalTrials.gov, NCT02706340.
Results
We approached 234 women, and enrolled 200. The most common reason for declining participation was unwillingness to participate in research (n=31). Three potential subjects were ineligible, 5 women were found to be ineligible after enrollment (screen fail), and 4 participants withdrew prior to 6 months (Figure 1). Baseline characteristics including demographic characteristics for the entire cohort are shown in Table 1. The median time between delivery of the placenta and placement of the IUD was 4 minutes (range 0–26 min). A total of 11 IUDs (5.5%) were inserted greater than 10 minutes after placental delivery. Ultrasound guidance assisted in the placement of 4 IUDs for study participants.
Figure 1:
Study flow: recruitment, enrollment, and intrauterine device status.
Table 1.
Baseline characteristics and IUD expulsion or partial expulsion at 6 months postpartum.
| Characteristic | Total (n=162) | Complete Expulsion (n=13) | Partial Expulsion (n=26) | Other IUD Outcomea (n=123) | P-value |
|---|---|---|---|---|---|
| Age (years) | 27.7 ± 5.1 | 29.9 ± 4.8 | 27.7 ± 4.8 | 27.5 ± 5.1 | 0.26 |
| Parity | 2 (2, 2) | 4 (2, 4) | 2 (2, 3) | 2 (2, 3) | 0.02 |
| Gestational age at delivery | 39 1/7 (38 0/7, 40 1/7) | 38 1/7 (37 0/7, 40 1/7) | 39 2/7 (39 0/7, 40 3/7) | 39 1/7 (38 0/7, 40 0/7) | 0.04 |
| BMI (kg/m2) | |||||
| Unknown | 21 (13.0) | 2 (15.4) | 3 (11.5) | 16 (13.0) | 0.68 |
| Normal (<24.9) | 50 (30.9) | 3 | 6 (23.1) | 41 (33.) | |
| Overweight (25–29.9) | 35 (21.6) | 5 (38.5) | 6 (23.1) | 24 (19.5) | |
| Obese (>30) | 56 (34.6) | 3 (23.1) | 11 (42.3) | 42 (34.2) | |
| Race / Ethnicity American | |||||
| Black / African | 122 (75.3) | 9 (69.2) | 20 (76.9) | 93 (75.6) | 0.67 |
| White | 20 (12.4) | 2 (15.4) | 2 (7.7) | 16 (13.0) | |
| Asian/Indian | 6 (3.7) | 1 (7.7) | 0 | 5 (4.1) | |
| Hispanic | 11 (6.8) | 1 (7.7) | 3 (11.5) | 7 (5.7) | |
| Other | 3 (1.9) | 0 | 1 (3.9) | 2 (1.6) | |
| Annual income | |||||
| <$10,000 | 46 (28.4) | 3 (23.1) | 4 (15.4) | 39 (31.7) | 0.50 |
| 10,001–30,000 | 39 (24.1) | 4 (30.8) | 7 (26.9) | 28 (22.8) | |
| >30,001 | 77 (47.5) | 6 (46.2) | 15 (57.7) | 56 (45.5) | |
| Relationship status | |||||
| Single | 51 (31.5) | 5 (38.5) | 8 (30.8) | 38 (30.9) | 0.82 |
| With a partner | 69 (42.6) | 4 (30.8) | 10 (38.5) | 55 (44.7) | |
| Married or divorced | 42 (25.9) | 4 (30.8) | 8 (30.8) | 30 (24.4) | |
| IUD placement method | |||||
| Manual | 131 (80.9) | 7 (53.9) | 18 (69.2) | 106 (86.2) | <0.01 |
| Ring forceps | 16 (9.3) | 4 (30.8) | 2 (7.7) | 9 (7.3) | |
| Unknown | 16 (9.9) | 2 (15.4) | 6 (23.1) | 8 (6.5) | |
| Provider specialty | |||||
| OB | 143 (88.3) | 13 (100) | 19(73.1) | 111 (90.2) | 0.03 |
| Family Medicine | 19 (11.7) | 0 | 7 (26.9) | 12 (9.8) | |
| Maternal weight gainb | |||||
| Normal | 102 (68.5) | 8 (66.7) | 20 (87.0) | 74 (64.9) | 0.10 |
| Above recommendation | 47 (31.5) | 4 (33.3) | 3 (13.0) | 40 (35.1) | |
| Time to IUD placement | |||||
| ≤ 10 minutes | 136 (84.0) | 11 (84.6) | 22 (84.6) | 103 (83.7) | 0.23 |
| > 10 minutes | 9 (5.5) | 2 (15.4) | 0 | 7 (5.7) | |
| Unknown | 17 (10.5) | 0 | 4 (15.4) | 13 (10.6) | |
Data are mean ± standard deviation, median (IQR), or n (%).
Other outcomes at 6 months were: correctly positioned IUD, malpositioned IUD, perforation, and IUD removed without ultrasound assessment.
According to Institute of Medicine recommendations14 based on maternal BMI.
Thirty-three subjects did not have the status of their IUD confirmed at 6 months, either due to loss to follow-up or withdrawn consent. A sensitivity analysis evaluating these participants’ baseline characteristics found no difference in comparison to participants who did complete the study, in all categories except BMI. Participants without outcome data were more likely to have an unknown BMI (36.4% vs 13.0%) and more likely to be overweight (30.3% vs 21.6%, p<0.01).
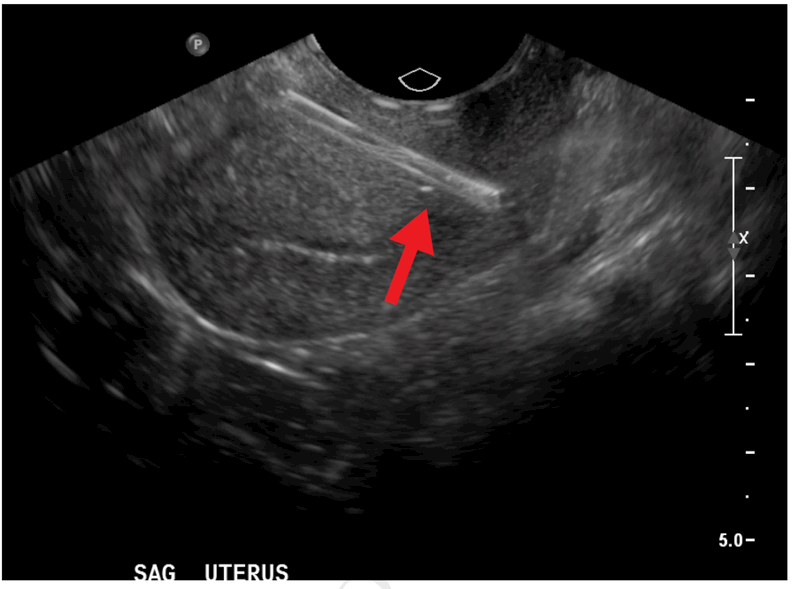
Among the 162 participants with available data on IUD status at 6 months, 13 (8.0%, 95% CI 4.7%–13.4%) had a complete IUD expulsion, and 26 (16.0%; 11.1%–22.6%) had a partial IUD expulsion. Of 25 malpositioned IUDs (15.4%, 95% CI 10.2%–21.9%), 14 were not at the fundus (8.6%, 95% CI 5.2%–14.1%) and 11 were rotated within the uterus (6.8%, 95% CI 3.8%–11.9%). Five IUDs (3.1%, 95% CI 1.3%–7.3%) were removed electively, and 2 (1.2%, 95% CI 0.3%–4.9%) were removed because of infection. Ultrasound examination was not consistently performed to establish IUD position prior to elective removal, as these removals were performed in the clinical, not research, setting. One participant (0.6%, 95% CI 0%–4.3%), whose provider visualized and trimmed IUD strings at a 6-week postpartum visit, was diagnosed by ultrasound at 6 months with an IUD perforation. Her IUD was noted to be upside-down, with the stem partially perforated through the anterior lower uterine segment of the uterus and the arms in the endocervix (Figure 2). This device was subsequently removed via hysteroscopy and she opted against a new contraceptive method.
Figure 2:
Midsaggital transvaginal ultrasound demonstrating intrauterine device perforated through anterior uterine wall (arrow).
Ninety participants (55.6%, 95% CI 44.7%–63.1%) had their original IUD in the correct position and continued to use it for contraception after the 6-month study visit. Of women with a compete expulsion, 9 of 13 (69.2%; 95% CI 38.6%–90.9%) were recognized by the participant prior to clinical examination. Most women diagnosed with an abnormally positioned IUD had both the desire and the access to have a new LARC placed at the time of this diagnosis. Thus, despite the overall low proportion of women at 6 months continuing with their original IUD, a total of 132 (80.9%) women in our cohort were using a highly effective method at 6 months (Table 2). Two subjects (1.2%, 95% CI 0%–4.4%) experienced a new pregnancy within 6 months of delivery.
Table 2.
Contraceptive outcomes at 6 months postpartum among 162 women receiving a postplacental IUD after vaginal delivery.
| Contraceptive outcome | n | % | (95% CI) |
|---|---|---|---|
| TCu380A IUD | 121 | 74.7 | (67.5–80.8) |
| Original IUD, correctly positioned | 90 | 55.6 | (44.7–63.1) |
| Replacement TCu380A IUD | 21 | 13.0 | (8.6–19.0) |
| Abnormal position, continuing abnormally placed IUDa | |||
| Partial expulsion | 3 | 1.9 | (0.6–5.3) |
| Malpositioned | |||
| Not at fundus | 4 | 2.5 | (1.0–6.2) |
| Rotated | 3 | 1.9 | (0.6–5.34) |
| Levonorgestrel IUD | 9 | 5.6 | (3.0–10.2) |
| Etonogestrel implant | 1 | 0.6 | (0.1–3.4) |
| Hormonal method | 7 | 4.3 | (2.1–8.7) |
| Condoms | 9 | 5.6 | (3.0–10.2) |
| Emergency contraception | 1 | 0.6 | (0.1–3.4) |
| None, abstinence, withdrawal | 13 | 8.0 | (4.8–13.2) |
| Tubal sterilization | 1 | 0.6 | (0.1–3.4) |
Unable to replace IUD due to logistical barriers.
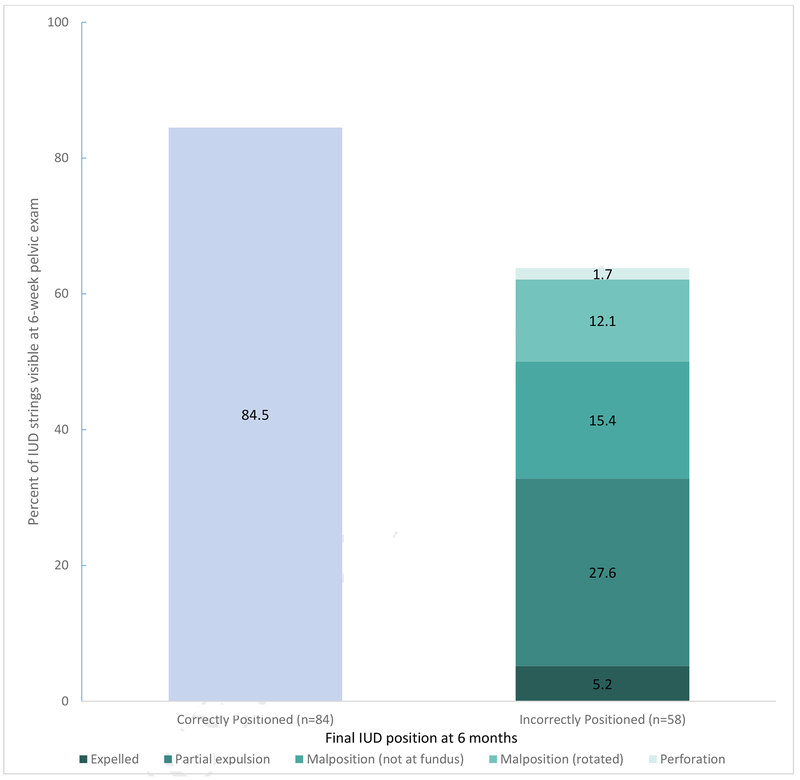
Thirty-four of 142 participants (23.9%, 95% CI 17.2%–31.8%) who were seen in the office at 6 weeks, and had not had an IUD removal, had no strings visible. Sixty-three percent (95% CI 55.0%–76.0%) of women who were later confirmed to have an abnormally positioned IUD had strings visible, and 84.5% (95% CI 75.0%–91.5%) of women who were later confirmed to have a correctly positioned IUD had strings visible. Thus, the sensitivity and specificity of an IUD string check for predicting correct 6-month positioning of the device was 36.2% and 84.5%, respectively (Figure 3). This corresponds to an area under the receiver operating characteristics curve of 0.5. Among the 51 participants who had a partial IUD expulsion or malposition, additional follow up to assess or correct the IUD was required.
Figure 3:
Visibility of intrauterine device strings at 6 week exam and final intrauterine device position outcome at 6 months.
Unadjusted associations between risk factors and our outcome (IUD expulsion, IUD partial expulsion, and all other IUD outcomes) are presented in Table 1. Parity, gestational age, IUD placement method (ring forceps compared with manual insertion), provider specialty (Obstetrics and Gynecology compared with Family Medicine), time to IUD placement (greater than 10 minutes from placental delivery as compared with 10 minutes or fewer), and gestational weight gain categories as defined by the Institute of Medicine16 were all associated with IUD expulsion and partial expulsion with a p-value of 0.2 or less. Thus, we included these variables in a multinomial logistic regression model where only significant risk factors and identified confounding variables were retained, to assess factors associated with complete and partial expulsion. In this adjusted analysis, each additional prior delivery (higher parity) increased the odds of complete expulsion twofold (OR 2.05; 95% CI 21–3.49, p=0.008). Covariates associated with partial expulsion included insertion by an Obstetrics and Gynecology provider (as compared to a Family Medicine provider; OR 5.31, 95% CI 1.20–23.60; p=0.03) and gestational weight gain category (normal compared to excess; OR 9.12, 95% CI 1.90–43.82; p=0.006). These associations were adjusted for gestational age and time to IUD placement, which were noted to be confounders.
Of the 143 women who completed a satisfaction survey at 6 months, 110 (76.9%, 95% CI 69.1%–83.6%) were happy or extremely happy with the IUD. The majority of survey respondents (36 of 62, 61.3%, 95% CI 44.8%–70.5%) who experienced an IUD problem (including expulsion, partial expulsion, malposition, and removal for other reasons) and completed the study were happy or extremely happy with the IUD, and 12 (19.4%, 95% CI 10.4%–31.4%) were neither happy nor unhappy with the IUD.
Comment
In this prospective cohort study of women who received a copper IUD immediately after vaginal delivery, 8.0% had a complete IUD expulsion, and 24.0% had complete or partial expulsion by 6 months. The 6-month complete expulsion rate was 8.0% (95% CI 4.7%–13.4%), 69.2% of which were recognized by the participants prior to clinical examination. When also considering women who had partially expelled or malpositioned IUDs, and those who had their IUDs removed, only 55.6% of participants had the original IUD in place at 6 months. We allowed same-day clinical appointments for contraceptive management, including LARC, for subjects diagnosed with an IUD problem during a study visit; so despite relatively low continuation of the original IUD, 80.9% of women with follow-up were using an IUD or an implant at 6 months postpartum.
Prior studies have shown that IUD expulsion occurs more frequently when placed immediately after placental delivery as compared with standard placement, but many of these studies have been limited by small sample sizes that prevented accurate determination of expulsion rates.17 In studies of IUD expulsion, the method of detection is central to the interpretation of the partial and complete expulsion rate. Previous similar studies of IUD expulsion after placement immediately postpartum have assessed expulsion via a variety of methods: provider pelvic exam and string check,18 participant self-report,7,18,19 ultrasound to confirm expulsion,10,11 or ultrasound for all participants.9,20,21 Study protocols also have used a combination of these strategies.10,18,19 The expulsion rate in our study is concordant with other studies that used ultrasound universally for assessment of IUD position, although the complete expulsion rate in our study is lower than that noted for the LNG IUD.9,20,22
Without using ultrasound as a detection modality, partial expulsion is not well predicted clinically; even ultrasound 24 hours postpartum does not predict complete expulsion.9 Although the degree to which a partially expelled copper IUD compromises contraceptive efficacy is not fully understood, prior studies comparing the copper T380A to the T380S have shown that moving the copper closer to the cornua is associated with higher efficacy, and that IUDs with no copper near the cornua have lower efficacy.23–25 Thus, partially expelled and even malpositioned copper IUDs may result in compromised efficacy. In a case-control study of women found to be pregnant with a copper IUD in situ, the odds of pregnancy for a woman with an IUD in the endocervix versus an IUD in the endometrial canal was 13.93 (95% CI 4.13–48.96).26
Our study found that the sensitivity and specificity of the IUD string check as a test for correct IUD positioning are overall low; only 36.2% of women who eventually were diagnosed with an abnormally positioned IUD had no strings visible, and 84.5% of women with a correctly positioned IUD had strings visible. Although string visibility confirms the presence of the IUD, identifying strings on pelvic examination does not ensure correct intrauterine placement, and cannot be considered clinically reassuring. Similarly, the absence of strings on examination does not diagnose an expulsion. Our finding that the area under the receiver operating characteristics curve was 0.5 demonstrates that a string check is a poor test for assessing whether a postplacental IUD is correctly positioned. Even for women who have an interval IUD insertion, many women are unable and/or unwilling to perform monthly IUD string palpation,27 and in a population of women who have had a postplacental IUD, recommending self-palpation of strings would be especially unhelpful.
Our findings suggest that women who have had a postplacental IUD should have a health care provider assess their IUD position at a postpartum visit, preferably with sonographic imaging. However, there are many potential limitations to this recommendation. Ultrasound is unlikely to be readily available in low-resource settings, and many women do not return for postpartum visits28–30. Underinsured women enrolled in Emergency Medicaid for their pregnancy may face restrictions on additional procedures after delivery, such as pelvic ultrasound or removal and replacement of an abnormally positioned IUD, especially because Emergency Medicaid covers only a finite number of weeks postpartum. In our study population, 10 women continued an IUD with a positional problem due to insurance barriers to replacement, illustrating this disappointing void in healthcare access. Patients insured by Emergency Medicaid should be counseled in the antenatal period regarding these logistics, and on issues such as the cost of IUD removal services, if needed outside of the insured period. The American College of Obstetricians and Gynecologists and others have recommended revising the timing of the initial postpartum visit to two or three weeks postpartum,1,31 which could provide sufficient time for confirmation of IUD position, and correction if needed, prior to loss of insurance.
Our multivariable analysis of risk factors contributing to postplacental IUD expulsion by 6 months postpartum found that higher parity and ring forceps insertion of the IUD were associated with complete IUD expulsion. Each prior delivery was associated with a twofold increase in odds of complete IUD expulsion, which is consistent with prior research.21 The clinical significance of the covariates associated with partial IUD expulsion in our study, provider specialty and gestational weight gain, is unclear.
Strengths of this study include its prospective nature, the diverse participant population, and longitudinal follow-up with a high retention rate. Importantly, in contrast to many prior studies of postplacental IUDs, detection of the IUD location outcome was obtained in person and with ultrasound, which allowed for a detailed description and understanding of the varied positions that may result after an IUD is placed immediately following placental delivery. Our study also had limitations. We constrained the study to the copper IUD, as levonorgestrel IUDs were not available for postplacental placement during this time period at our institution. Thus, our outcomes are not generalizable to women receiving postplacental levonorgestrel IUDs, for which partial expulsion or malposition might not result in significant decreases in efficacy32. Satisfaction in our study was measured via a 5-item Likert scale; there are likely to be additional nuances in patient satisfaction that were not captured via our survey. Finally, our study is subject to the biases of observational studies, and any associations cannot imply causality.
Unlike interval IUDs, retained postplacental IUDs have high frequencies of abnormal positioning at 6 months, including complete expulsion, partial expulsion, and malposition. Patients who choose an IUD are counseled that that they are receiving the highest tier of contraceptive effectiveness in a low-maintenance method. Women considering a postplacental IUD, especially those with limited time periods of insurance coverage and in low-resource settings, should be made aware of the risk of IUD position abnormalities after postplacental placement, as well as the possibility of non-visible IUD strings complicating follow-up and IUD removal. Thus, we recommend that a detailed consent process for postplacental IUD placement, including the risks, benefits, alternatives (including interval IUD placement) and logistics occur in an outpatient antenatal setting, in order to allow sufficient time for informed decision-making. Furthermore, the clinical significance of IUD position abnormalities and partial expulsion is currently unknown, and future research should evaluate the effectiveness of postplacental IUDs and resultant variations in IUD position in a large, multicenter prospective trial.
Table 3.
Risk factorsa associated with complete and partial IUD expulsion among women receiving immediate postplacental copper IUDs.
| Odds Ratio | 95% CI | p-value | |
|---|---|---|---|
| Complete Expulsion compared to other IUD outcomeb | |||
| Parity | 2.05 | 1.21–3.49 | <0.01 |
| Partial Expulsion compared to other IUD outcomeb | |||
| Provider Specialty | |||
| Family Medicine | 1 | ||
| Obstetrics & Gynecology | 5.31 | 1.20–23.60 | 0.03 |
| Maternal Weight Gainc | |||
| Excess | 1 | ||
| Normal | 9.12 | 1.90–43.82 | <0.01 |
Covariates in multinomial logistic regression model were: IUD status at 6 months postpartum, parity, gestational age at delivery, provider specialty, IUD placement method, time to IUD placement, and maternal weight gain.
Other outcomes at 6 months were: correctly positioned IUD, malpositioned IUD, perforation, and IUD removed without ultrasound assessment.
As defined by the Institute of Medicine.
Condensation:
Of women who choose and receive a postplacental copper IUD after vaginal delivery, 8.0% (95% CI 4.7%–13.4%) experience complete expulsion and 16.0% (95% CI 11.1%–22.6%) have partial expulsion of the device at 6 months postpartum.
Implications and Contributions:
To describe positional outcomes of postplacental copper IUDs placed after vaginal delivery.
Eight percent of immediate postplacental IUDs were completely expelled, and 16% partially expelled, by 6 months postpartum. Only 55.6% of participants continued using their original IUD at 6 months, but 80.9% were using a LARC method. The sensitivity of a string check to detect an incorrectly positioned IUD was 36.2%, and the specificity of a string check to predict a correctly positioned IUD was 84.5%. Three-quarters of immediate postplacental IUD users were happy or extremely happy with the IUD.
This study provides a detailed description of postplacental IUD position at 6 months postpartum, and finds that a string check is a poor test to confirm correct IUD position.
Acknowledgements:
The authors would like to acknowledge the contributions of Lauren Kus, University of Pennsylvania, Perelman School of Medicine (financial compensation provided by the Society of Family Planning), Brittany Lang, MA, University of Pennsylvania, Perelman School of Medicine (financial compensation provided by the Society of Family Planning), Camille McCallister, University of Pennsylvania, Perelman School of Medicine (financial compensation provided by the University of Pennsylvania), Jessica McClusky, University of Pennsylvania, Perelman School of Medicine (financial compensation provided by the Society of Family Planning), Clarissa O’Conor, University of Pennsylvania, Perelman School of Medicine (financial compensation provided by the Society of Family Planning), Chierika Ukogu, University of Pennsylvania, Perelman School of Medicine (financial compensation provided by the Society of Family Planning), and Veronica Chavez, University of Pennsylvania, Perelman School of Medicine (financial compensation provided by the Society of Family Planning), in conducting this research.
Financial support for the research:
This research was sponsored by a grant from the Society of Family Planning Research Fund (SFPRF15-15). This study was funded in part by Women’s Reproductive Health Research award (Sonalkar): K12-HD001265-18, and a Society of Family Planning Research Fund Midcareer Mentor Award (Schreiber). Intrauterine devices were donated, in part, by Teva Pharmaceuticals, the Ryan LARC Grant, and the philanthropic efforts of Dr. Ann Steiner’s LARC Project.
Role of the funding source:
The funding sources had no role in study design; data collection, analysis or interpretation; manuscript writing, or the decision to submit for publication.
Clinialtrials.gov: This work was registered and conducted as described under NCT02706340.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
GURNEY. The author is a Nexplanon® trainer for Merck.
SONALKAR. The author reports no conflict of interest.
MCALLISTER. The author reports no conflict of interest.
SAMMEL. The author reports no conflict of interest.
SCHRIEBER. The author is a consultant for Bayer Pharmaceuticals and receives research funding from Bayer Pharmaceuticals, ContraMed, Medicines360 and NICHD.
Paper presentation information: Results from this research were presented as a poster presentation at the American College of Obstetrics and Gynecology Annual Clinical Meeting on May 6–9, 2017, San Diego, CA.
Authors employed by the Federal Government or Armed Forces:
None.
References
- 1.ACOG Committee Opinion No. 736: Optimizing Postpartum Care. Obstetrics and gynecology 2018;131:e140–e50. [DOI] [PubMed] [Google Scholar]
- 2.Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012;366:1998–2007. [DOI] [PubMed] [Google Scholar]
- 3.Washington CI, Jamshidi R, Thung SF, Nayeri UA, Caughey AB, Werner EF. Timing of postpartum intrauterine device placement: a cost-effectiveness analysis. Fertil Steril 2015;103:131–7. [DOI] [PubMed] [Google Scholar]
- 4.Speroff L, Mishell DR Jr. The postpartum visit: it’s time for a change in order to optimally initiate contraception. Contraception 2008;78:90–8. [DOI] [PubMed] [Google Scholar]
- 5.Medicaid reimbursement for postpartum LARC by state: American Congress of Obstetricians and Gynecologists; 2017. October 18, 2017. [Google Scholar]
- 6.Sonalkar S, Kapp N. Intrauterine device insertion in the postpartum period: a systematic review. Eur J Contracept Reprod Health Care 2015;20:4–18. [DOI] [PubMed] [Google Scholar]
- 7.Eggebroten JL, Sanders JN, Turok DK. Immediate postpartum intrauterine device and implant program outcomes: a prospective analysis. American journal of obstetrics and gynecology 2017;217:51 e1–e7. [DOI] [PubMed] [Google Scholar]
- 8.Jatlaoui TC, Marcus M, Jamieson DJ, Goedken P, Cwiak C. Postplacental intrauterine device insertion at a teaching hospital. Contraception 2014;89:528–33. [DOI] [PubMed] [Google Scholar]
- 9.Goldthwaite LM, Sheeder J, Hyer J, Tocce K, Teal SB. Postplacental intrauterine device expulsion by 12 weeks: a prospective cohort study. American journal of obstetrics and gynecology 2017;217:674 e1–e8. [DOI] [PubMed] [Google Scholar]
- 10.Xu JX, Rivera R, Dunson TR, et al. A comparative study of two techniques used in immediate postplacental insertion (IPPI) of the Copper T-380A IUD in Shanghai, People’s Republic of China. Contraception 1996;54:33–8. [DOI] [PubMed] [Google Scholar]
- 11.Celen S, Moroy P, Sucak A, Aktulay A, Danisman N. Clinical outcomes of early postplacental insertion of intrauterine contraceptive devices. Contraception 2004;69:279–82. [DOI] [PubMed] [Google Scholar]
- 12.Shukla M, Qureshi S, Chandrawati. Post-placental intrauterine device insertion--a five year experience at a tertiary care centre in north India. Indian J Med Res 2012;136:432–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Simonson K, Gerard N, Pomerantz T, Mullersman K, Landy U Abstract: Improving access and training for LARC: evaluation of the Ryan LARC program. Contraception 2014;90:323–4. [Google Scholar]
- 14.Xu J, Yang X, Gu X, et al. Comparison between two techniques used in immediate postplacental insertion of TCu 380A intrauterine device: 36-month follow-up. Reproduction and contraception 1999;10:156–62. [PubMed] [Google Scholar]
- 15.Eroglu K, Akkuzu G, Vural G, et al. Comparison of efficacy and complications of IUD insertion in immediate postplacental/early postpartum period with interval period: 1 year follow-up. Contraception 2006;74:376–81. [DOI] [PubMed] [Google Scholar]
- 16.Weight gain during pregnancy: reexamining the guidelines. . Washington, DC: Institute of Medicine; 2009. [PubMed] [Google Scholar]
- 17.Lopez LM, Bernholc A, Hubacher D, Stuart G, Van Vliet HA. Immediate postpartum insertion of intrauterine device for contraception. Cochrane Database Syst Rev 2015:CD003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levi EE, Stuart GS, Zerden ML, Garrett JM, Bryant AG. Intrauterine Device Placement During Cesarean Delivery and Continued Use 6 Months Postpartum: A Randomized Controlled Trial. Obstetrics and gynecology 2015;126:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colwill AC, Schreiber CA, Sammel MD, Sonalkar S. Six-week retention after postplacental copper intrauterine device placement. Contraception 2017. [DOI] [PubMed] [Google Scholar]
- 20.Chen BA, Reeves MF, Hayes JL, Hohmann HL, Perriera LK, Creinin MD. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstetrics and gynecology 2010;116:1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sucak A, Ozcan S, Celen S, Caglar T, Goksu G, Danisman N. Immediate postplacental insertion of a copper intrauterine device: a pilot study to evaluate expulsion rate by mode of delivery. BMC Pregnancy Childbirth 2015;15:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitaker AK, Chen BA. Society of Family Planning Guidelines: Postplacental insertion of intrauterine devices. Contraception 2018;97:2–13. [DOI] [PubMed] [Google Scholar]
- 23.Sivin I, Shaaban M, Odlind V, et al. A randomized trial of the Gyne T 380 and Gyne T 380 Slimline Intrauterine Copper devices. Contraception 1990;42:379–89. [DOI] [PubMed] [Google Scholar]
- 24.Bahamondes L, Diaz J, Petta C, Monteiro I, Monteiro CD, Regina CH. Comparison of the performances of TCu380A and TCu380S IUDs up to five years. Adv Contracept 1999;15:275–81. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien PA, Kulier R, Helmerhorst FM, Usher-Patel M, d’Arcangues C. Copper-containing, framed intrauterine devices for contraception: a systematic review of randomized controlled trials. Contraception 2008;77:318–27. [DOI] [PubMed] [Google Scholar]
- 26.Anteby E, Revel A, Ben-Chetrit A, Rosen B, Tadmor O, Yagel S. Intrauterine device failure: relation to its location within the uterine cavity. Obstetrics and gynecology 1993;81:112–4. [PubMed] [Google Scholar]
- 27.Melo J, Tschann M, Soon R, Kuwahara M, Kaneshiro B. Women’s willingness and ability to feel the strings of their intrauterine device. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2017;137:309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilcox A, Levi EE, Garrett JM. Predictors of Non-Attendance to the Postpartum Follow-up Visit. Matern Child Health J 2016;20:22–7. [DOI] [PubMed] [Google Scholar]
- 29.Thiel de Bocanegra H, Braughton M, Bradsberry M, Howell M, Logan J, Schwarz EB. Racial and ethnic disparities in postpartum care and contraception in California’s Medicaid program. American journal of obstetrics and gynecology 2017;217:47 e1–e7. [DOI] [PubMed] [Google Scholar]
- 30.Bennett WL, Chang HY, Levine DM, et al. Utilization of primary and obstetric care after medically complicated pregnancies: an analysis of medical claims data. J Gen Intern Med 2014;29:636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen MJ, Hou MY, Hsia JK, Cansino CD, Melo J, Creinin MD. Long-Acting Reversible Contraception Initiation With a 2- to 3-Week Compared With a 6-Week Postpartum Visit. Obstetrics and gynecology 2017;130:788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratsula K Clinical performance of a levonorgestrel-releasing intracervical contraceptive device during the first two years of use. Contraception 1989;39:187–93. [DOI] [PubMed] [Google Scholar]