Abstract
Docetaxel resistance remains a major obstacle in the treatment of prostate cancer (PCa) bone metastasis. In this study, we demonstrate that the dopamine D2 receptor (DRD2) agonist bromocriptine effectively enhances docetaxel efficacy and suppresses skeletal growth of PCa in preclinical models. DRD2 is ubiquitously expressed in PCa cell lines, and DRD2 is significantly reduced in PCa tissues with high Gleason score. Bromocriptine has weak to moderate cytotoxicity in PCa cells, but effectively induces cell cycle arrest. At the molecular level, bromocriptine inhibits the expression of c-Myc, E2F-1 and survivin, and increases the expression of p53, p21 and p27. Intriguingly, bromocriptine markedly reduces androgen receptor (AR) levels, partially through heat-shock protein 90 (Hsp90)-mediated protein degradation. The combination of bromocriptine and docetaxel demonstrates enhanced in vitro cytotoxicity in PCa cells and significantly retards the skeletal growth of C4-2-Luc tumors in mice. Collectively, these results provide the first experimental evidence for repurposing bromocriptine as an effective adjunct therapy to enhance docetaxel efficacy in PCa.
Keywords: Prostate cancer, bone metastasis, docetaxel, dopamine D2 receptor, bromocriptine, androgen receptor
Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer in the US and the third leading cause of cancer-related deaths. The American Cancer Society estimated that 164,690 new cases will be diagnosed and 29,430 patients die in 2018 (1). More than 85% of PCa patients have bone metastasis at autopsy, and their quality of life can be significantly compromised by skeletal complications (2). Docetaxel treatment remains a major type of chemotherapy with survival benefits in PCa patients with bone metastasis. Unfortunately, docetaxel therapy eventually leads to chemoresistance, posing a major obstacle in the treatment of PCa bone metastasis (3). It is imperative to develop novel strategies to enhance docetaxel chemotherapy and improve the survivorship and quality of life of patients.
Dopamine is a major neurotransmitter involved in a wide variety of physiological processes, such as reward and motor functions (4). Aberrant dopamine secretion and signaling may lead to various neurological disorders, including Parkinson’s disease, schizophrenia and depression (5). Dopamine exerts its functions in target cells via selective ligation and activation of distinct dopamine receptors (DRs), referred to as the D1-like (D1, D5) and D2-like (D2, D3, D4) classes. Both classes are G protein-coupled receptors (GPCRs), whose signaling is primarily mediated by coupling with specific heterotrimeric G proteins: the D1-like DRs bind the adenylate cyclase stimulatory G protein Gαs, increase cyclic AMP (cAMP) and activate protein kinase A (PKA), while the D2-like DRs couple to the Gαi/o protein, inhibit adenylyl cyclase, suppress cAMP and inactivate PKA (6).
DRD2 is the major subtype of D2-like DRs, playing a prominent role in psychiatric and neurological disorders and being the primary target for most antipsychotic drugs (7). Bromocriptine, a semi-synthetic ergot alkaloid, is a potent DRD2 agonist and has been widely used in the treatment of Parkinson’s disease, hyperprolactinemia, pituitary tumors and neuroleptic malignant syndrome (8–10). A quick release formulation of bromocriptine (Bromocriptine-QR) was approved for the treatment of type 2 diabetes mellitus (11). Although the underlying mechanisms associated with these clinical benefits remain poorly understood, it is generally thought that they are primarily attributed to the specific DRD2 agonist activity of bromocriptine. For example, bromocriptine directly stimulates specific pituitary cell membrane DRD2 and inhibits the synthesis and secretion of prolactin, the major driver of prolactinoma (9).
Recent studies have implicated DRD2 in the progression of malignant tumors, although the association between DRD2 signaling and these diseases appears to be tissue-specific and sometimes remains inconclusive. On the one hand, elevated levels of DRD2 are associated with acute myeloid leukemia (AML), glioblastoma, esophageal squamous cell carcinoma, pancreatic ductal adenocarcinoma and neuroendocrine tumors (12–17). In line with these observations, several DRD2 antagonists were found to be capable of inhibiting the growth of hematopoietic and solid tumors and enhancing chemotherapy in metastatic breast cancer cells (14–16,18). On the other hand, however, it has been reported that DRD2 agonism demonstrated inhibitory effects in preclinical models of lung cancer, gastric cancer and leukemia, suggesting that re-activation of DRD2 signaling could have therapeutic benefits in these malignancies (19–23). Intriguingly, bromocriptine exhibits promising anticancer activity in AML, presumably via the inhibition of leukemia stem cells (24,25).
The expression profile of DRD2 and its targeting potential in PCa have not been investigated. In this study, we present the first evidence demonstrating the correlation between DRD2 expression and PCa progression. We have determined the mechanism of action and in vivo efficacy of bromocriptine in enhancing docetaxel chemotherapy and treating PCa bone metastasis using preclinical models.
Materials and Methods
Cell Culture and Reagents
Human PCa cell lines LNCaP, PC-3 and DU145 were obtained from American Type Culture Collection (ATCC), C4-2 and luciferase-tagged C4-2-Luc cells were provided by Dr. Leland WK Chung (Cedars-Sinai Medical Center) in 2004, C4-2B cells and its docetaxel-resistant derivative C4-2B-TaxR subline were provided by Dr. Allen C. Gao (University of California Davis) in 2016. The above PCa cells were routinely maintained in T-medium (Life Technologies, Carlsbad, CA) with 5% fetal bovine serum (FBS; Atlanta Biologicals, Atlanta, GA). CWR22Rv1 cells were provided by Dr. Jin-Tang Dong (Emory University) in 2016, and maintained in RPMI1640 2% L-glutamine (Thermo Fisher Scientific., Waltham, MA) supplemented with 10% FBS, 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 10 mM HEPES and 10 mM sodium pyruvate. All cell lines were authenticated by the providers and were tested negative for mycoplasma using a detection kit from Lonza (Morristown, NJ). All cells were passaged for less than 3 months before renewal from frozen, early-passage stocks. Cycloheximide (CHX), dimethyl sulfoxide (DMSO), propidium iodide (PI) and sulpiride were purchased from Sigma-Aldrich (St. Louis, MO). Bromocriptine mesylate was obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and docetaxel was obtained from LC Laboratories (Woburn, MA).
In Vivo Efficacy of Bromocriptine and Docetaxel in Intratibial Xenografts
All animal procedures were approved by Augusta University Institutional Animal Care and Use Committee (IACUC). A total of 2.0 × 106 C4-2-Luc cells per mouse were inoculated into the bilateral tibia of male athymic nude mice (Hsd: athymic nude-nu; 5 weeks; Harlan Laboratories, Indianapolis, IN). Following the confirmation of tumor formation by rising PSA levels in mouse sera (≥ 1.0 ng/ml), tumor-bearing mice were randomized, divided into 4 groups and treated with vehicle (DMSO; n = 5), docetaxel (5 mg/kg, once per week; n = 5), bromocriptine (5 mg/kg, 3 times per week; n = 6), or the combination of bromocriptine and docetaxel (n = 7), via intraperitoneal (i.p) injection. Mice were weighed and tumor growth in bilateral tibia was followed by serum PSA once a week using an enzyme-linked immunosorbent assay (ELISA) kit from United Biotech, Inc (Mountain View, CA). At the end point, the bilateral tibia bones were removed, fixed and decalcified, then paraffin embedded for hematoxylin and eosin (H&E) stain and immunohistochemistry (IHC) analyses.
Immunohistochemistry
Human prostate cancer tissue microarray (TMA) was purchased from US Biomax (Rockville, MD). IHC staining on TMA and PCa xenograft specimens was performed following standard procedures. The antibodies are listed in Table S1.
Data Analysis
All data represent three or more experiments. Errors are S.E values of averaged results, and values of p < 0.05 were taken as a significant difference between means. To assess the longitudinal effect of treatments on tumor growth in mouse bones, two-way ANOVA analysis was performed to test the overall difference across the control and treatment groups during the whole study period. GraphPad Prism 7.0 program (GraphPad Software Inc., La Jolla, CA) was used to perform the statistical analyses. The significance levels were set at 0.05 for all tests.
Supplementary materials and methods for cell proliferation assay, cell cycle and apoptosis analyses, gene transfer, quantitative PCR, Western blot analysis, protein half-life determination and immunoprecipitation are described in Supplementary Data. The antibodies and primers are described in Tables S1 and S2.
Results
Expression of DRD2 in human PCa cell lines and tissues
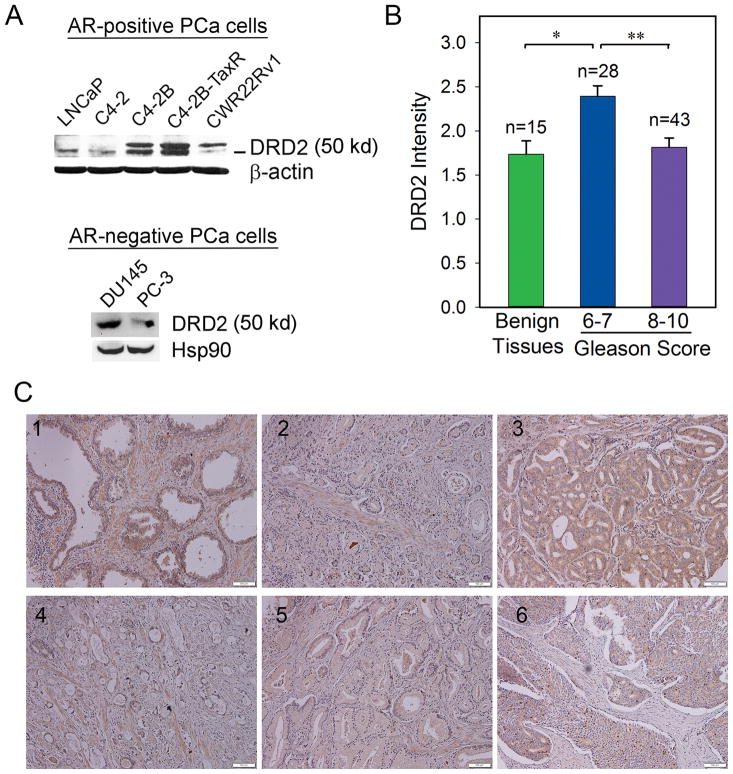
Previous studies have reported that DRD2 is expressed in several types of human cancers (12–17). However, it remains unclear regarding the expression pattern of DRD2 in PCa (26). We first examined the protein levels of DRD2 in established PCa cell lines, including androgen receptor (AR)-positive (LNCaP, C4-2, C4-2B, C4-2B-TaxR, CWR22Rv1) and -negative (DU145, PC-3) cells. Western blot analyses showed that all the examined PCa cells express DRD2, although the levels of expression vary among these cells (Fig. 1A).
Figure 1. Expression of DRD2 in human PCa cell lines and prostate tissue specimens.
(A) Western blot analysis of DRD2 protein expression in PCa cell lines. β-actin or Hsp90 was used as loading control. (B) Relative DRD2 intensity in a human TMA containing normal/benign prostate tissues and PCa. *, ** p < 0.001 (t-test). (C) Representative IHC staining of DRD2 expression in normal/benign human prostate tissues (#1) and PCa tissues with different Gleason scores of 6 (#2), 7 (#3), 8 (#4), 9 (#5) and 10 (#6).
We further examined tissue expression of DRD2 in a human prostate TMA. IHC study found that DRD2 is expressed in both normal/benign and cancerous prostate tissues (Fig. 1B, 1C). The average intensity of DRD2 expression is 1.63 ± 0.15 in normal/benign tissues (n = 19), 2.39 ± 0.12 in PCa with Gleason score 6–7 (n = 28), and 1.81 ± 0.11 in PCa with Gleason score 8–10 (n = 43), respectively. Compared with the low-grade tumors, high-grade PCa express significantly increased DRD2 (p < 0.001), indicating that the tissue expression of DRD2 is inversely associated with clinical PCa progression.
In vitro effects of bromocriptine on PCa cell viability and proliferation
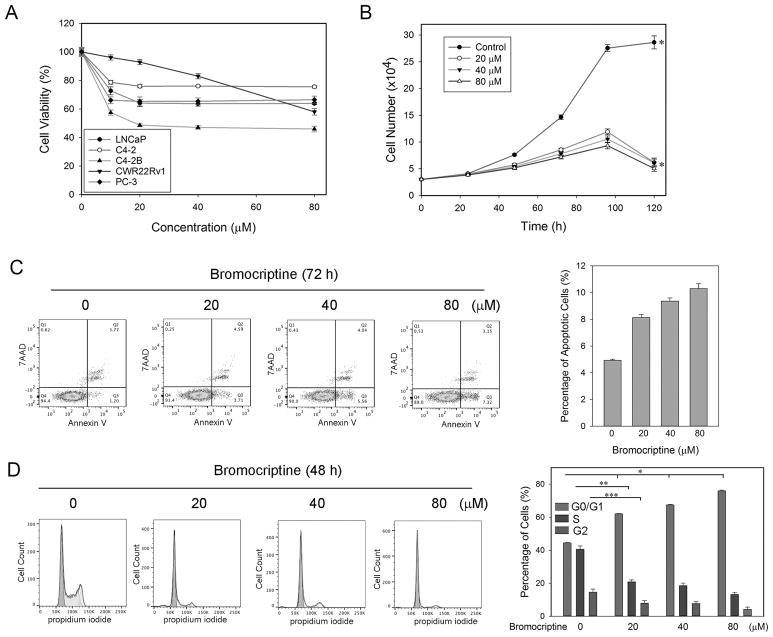
Given the observation that DRD2 is reduced in high-grade PCa tissues, we postulated that re-activation of DRD2 signaling may have inhibitory effects on the proliferation and/or viability of PCa cells. To test this hypothesis, we first treated PCa cells with escalating doses of bromocriptine for 72 h and examined its cytotoxicity in vitro. Interestingly, the viabilities of both AR-positive and -negative PCa cells were significantly affected only by treatments of low-concentration (≤ 20 μM) bromocriptine, then reached a plateau with the percentages of viable cells over 50% at higher concentrations of bromocriptine (up to 80 μM) (Fig. 2A). These results suggested that bromocriptine is not a potent cytotoxic agent in PCa cells. We then examined the effects of bromocriptine on the in vitro proliferation of C4-2 cells, an AR-positive PCa cell line that closely mimics the clinical pathology of human PCa (27), at extended time points of up to 5 days. Cell counting results showed that compared with the vehicle control, bromocriptine effectively suppressed C4-2 proliferation at the concentrations of as low as 20 μM (Fig. 2B). These results indicated that bromocriptine is an inhibitor of PCa cell proliferation.
Figure 2. In vitro effects of bromocriptine on PCa cells.
(A) MTS assay of the viability of C4-2 cells treated with varying concentrations of bromocriptine for 72 h. (B) Proliferation of C4-2 cells in response to varying concentrations of bromocriptine. Cell numbers were counted with an automated cell counter at different time points. * p < 0.05. (C) Flow cytometry assay of Annexin V expression in C4-2 cells treated with varying concentrations of bromocriptine (72 h). p < 0.05 in all pairwise comparisons (t-test). (D) Flow cytometry assay of the cell cycle of C4-2 cells treated with varying concentrations of bromocriptine (48 h). *, **, ***: p < 0.05 (t-test).
We performed flow cytometry analyses of apoptosis and the cell cycle in C4-2 cells treated with bromocriptine. Compared with the vehicle control, increased concentrations of bromocriptine (20, 40 and 80 μM) did not affect surface expression of Annexin V, a marker of apoptosis (Fig. 2C). On the other hand, starting from a concentration of 20 μM, bromocriptine treatment significantly induced cell cycle arrest at the G1/S checkpoint and to a lesser degree, the G2/M checkpoint (Fig. 2D). Taken together, these results are in line with the notion that bromocriptine mainly inhibits PCa cell proliferation through the induction of cell cycle arrest.
Effects of bromocriptine on the expression of DRD2 and cell cycle regulators in PCa cells
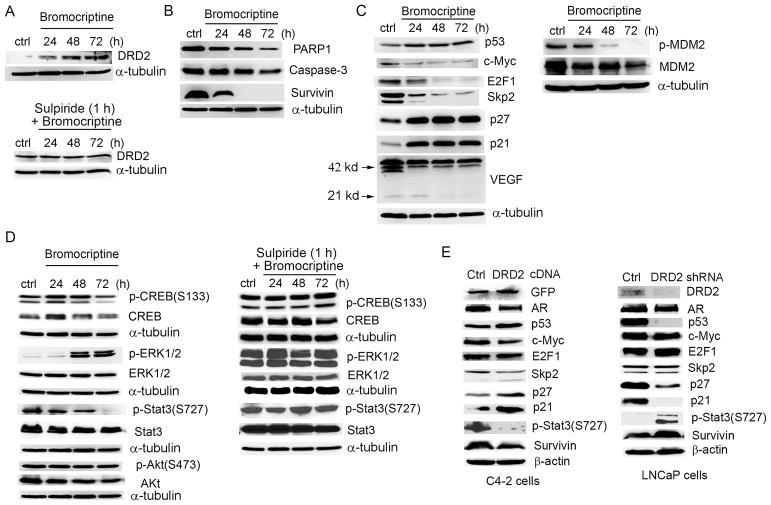
At the molecular level (Fig. 3A), a significant change following bromocriptine treatment (for example, at 20 μM) was an increase in endogenous expression of DRD2 in a time-dependent manner, as early as at 24 h. Pre-incubation of C4-2 cells with the DRD2 antagonist sulpiride attenuated this inductive effect of bromocriptine on DRD2 expression, suggesting a possible autoregulation mechanism. Western blot analyses of apoptotic markers found that bromocriptine could reduce protein expression of poly (ADP-ribose) polymerase 1 (PARP1) and caspase-3 at 72 h. Markedly, bromocriptine treatment resulted in a rapid reduction of survivin (Fig. 3B), an important anti-apoptotic protein implicated in PCa progression and therapeutic response (28). Quantitative PCR (qPCR) analysis showed that survivin mRNA levels were also significantly suppressed after 24 h-treatment with bromocriptine in a dose-dependent manner (Fig. S1).
Figure 3. Effects of bromocriptine on the expression of DRD2 and cell signaling molecules.
(A) Upper panel: Western blot analysis of protein expression of DRD2 in C4-2 cells treated with 20 μM bromocriptine; Lower panel: Cells were pre-incubated with sulpiride (1 μM) for 1 h prior to bromocriptine treatment. (B) Western blot analysis of protein expression of survivin, PARP1 and caspase-3 in C4-2 cells treated with 20 μM bromocriptine for varying times. (C) Left: Western blot analysis of protein expression of cell cycle regulators in C4-2 cells treated with 20 μM bromocriptine for varying times; Right: Western blot analysis of p-MDM2(S166) and MDM2 in C4-2 cells treated with 20 μM bromocriptine for varying times. (D) Left: Western blot analysis of protein expression of key DRD2 downstream effectors in C4-2 cells treated with 20 μM bromocriptine for varying times; Right: Cells were pre-incubated with sulpiride (1 μM) for 1 h prior to bromocriptine treatment, then cell lysates were analyzed using Western blotting. (E) Left: Western blot analysis of protein expression of potential bromocriptine targets in C4-2 cells transfected with control or expression vector for GFP-tagged human DRD2 (48 h); Right: Western blot analysis of protein expression of potential bromocriptine targets in LNCaP cells transfected with a DRD2 shRNA or scramble control construct.
Since bromocriptine induces cell cycle arrest in PCa cells, we determined protein expression of several key regulators of the cell cycle, particularly those involved in the G1/S checkpoint. Bromocriptine significantly inhibited the expression of c-Myc, S-phase kinase associated protein 2 (Skp2) and E2F transcription factor 1 (E2F1), and increased the expression of p53, p27 Kip1 and p21, with most events starting at 24 h (Fig. 3C, left panel). A close examination of the protein expression of mouse double minute 2 homolog (MDM2), a p53-specific E3 ubiquitin ligase, and its phosphorylation at serine 166 found that bromocriptine reduced the levels of total MDM2 and to a greater degree, p-MDM2, which may be responsible for the increase in p53 protein expression (29) (Fig. 3C, right panel).
Effects of bromocriptine on DRD2 downstream signaling pathways in PCa cells
As a classical GPCR, DRD2 transmits dopamine signals to several downstream effectors, including cAMP response element binding protein (CREB), extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) and Akt (6). We examined the effects of bromocriptine on these intracellular signaling pathways and found that bromocriptine inhibited the expression of total CREB and p-CREB (Ser133) in a time-dependent manner, suggesting an inhibition of cAMP synthesis in the presence of bromocriptine. Bromocriptine treatment also significantly increased the phosphorylation of ERK1/2 kinases without affecting the expression of total ERK1/2. Pre-incubation of C4-2 cells with sulpiride antagonized the effects of bromocriptine on p-CREB and p-ERK1/2. In comparison, neither total Akt nor phosphorylated Akt was markedly changed following bromocriptine treatment. These results indicated that bromocriptine may act as a DRD2 agonist by inhibiting cAMP signaling and activating the ERK1/2 pathway in PCa cells. Interestingly, bromocriptine also significantly inhibited the phosphorylation of Stat3, a transcription factor involved in the regulation of multiple genes (such as survivin). The presence of sulpiride reversed the suppression of p-Stat3 by bromocriptine, in a similar manner to that on the expression of p-CREB and p-ERK1/2 (Fig. 3D).
Effects of DRD2 expression on PCa cell signaling
To determine whether the effects of bromocriptine on PCa cell signaling is DRD2-specific, we performed “gain-of-function” in DRD2-low C4-2 cells and “loss-of-function” in DRD2-high LNCaP cells using gene manipulation approaches. As shown in Fig. 3E, ectopic expression of DRD2 in C4-2 cells led to decreased expression of AR, c-Myc, Skp2, p-Stat3(S727) and survivin, and increased expression of p53, p27 and p21. Conversely, short hairpin RNA (shRNA) depletion of DRD2 in LNCaP cells significantly reduced the expression of p53, p27 and p21 and increased the expression of p-Stat3(S727) and survivin. Taken together, these results in general support the notion that DRD2 affects the expression of key regulators of proliferation and survival in PCa cells.
Effects of bromocriptine on AR expression in PCa cells
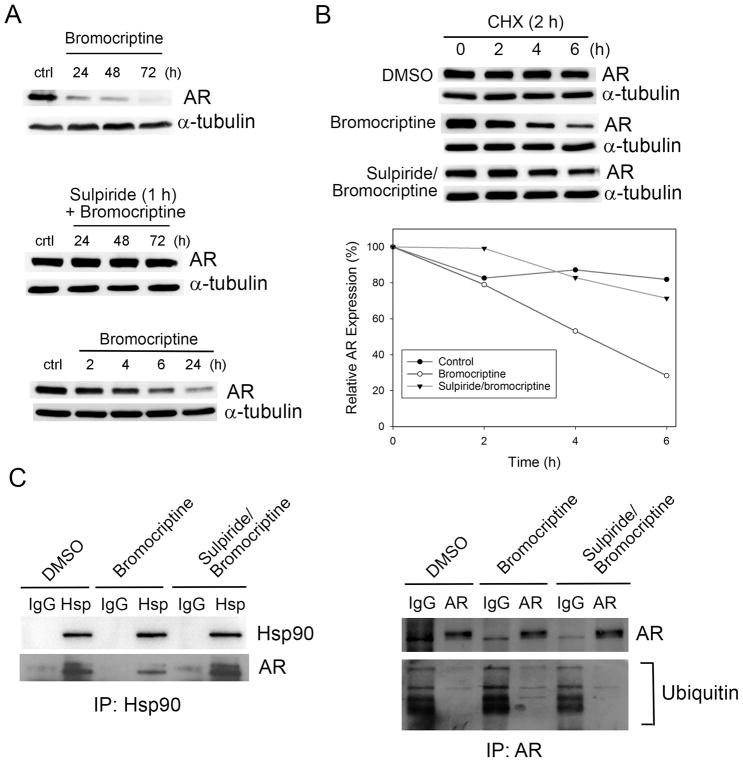
AR plays a central role in PCa progression and remains one of the most significant targets for PCa treatment. Intriguingly, bromocriptine treatment resulted in a significant reduction of AR proteins, an effect that was antagonized by pre-treatment with sulpiride (Fig. 4A). It appears that bromocriptine can also inhibit AR expression at the RNA levels (Fig. S1). Since the decline in AR proteins occurs shortly after bromocriptine treatment (less than 4 h) (Fig. 4A), it is plausible that bromocriptine may affect the stability of AR proteins. Indeed, bromocriptine promoted AR degradation in the presence of CHX, an inhibitor of de novo protein synthesis. The half-life (T1/2) of AR protein was reduced from 20.7 h to 4.1 h upon bromocriptine treatment, whereas the pre-incubation with sulpiride prior to bromocriptine treatment attenuated the effect of bromocriptine on AR stability (T1/2 = 12.9 h) (Fig. 4B).
Figure 4. Effects of bromocriptine on AR stability and ubiquitination.
(A) Upper panel: Western blot analysis of AR protein expression in C4-2 cells treated with 20 μM bromocriptine for varying times; Middle panel: Cells were pre-incubated with sulpiride (1 μM) for 1 h prior to bromocriptine treatment; Lower panel: Western blot analysis of AR protein expression in C4-2 cells treated with 20 μM bromocriptine for short term. (B) Upper paenel: Western blot analysis of AR expression in C4-2 cells pre-treated with CHX (50 μg/ml; 2 h) followed by treatment with DMSO or bromocriptine (20 μM) for the indicated times, or pre-treated with CHX for 1 h, followed by sulpiride for 1h and bromocriptine for the indicated times. Lower panel: T1/2 of AR proteins was calculated using SigmaPlot 13.0. (C) Left: Western blot analysis of protein expression of AR and Hsp90 in Hsp90 immunoprecipitates from C4-2 cells treated with DMSO (6 h), bromocriptine (20 μM, 6 h) or sulpiride (1 μM, 1 h) followed by bromocriptine (20 μM, 6 h). Right: Western blot analysis of expression of AR and ubiquitin in AR immunoprecipitates from C4-2 cells treated with DMSO (6 h), bromocriptine (20 μM, 6 h) or sulpiride (1 μM, 1 h) followed by bromocriptine (20 μM, 6 h).
As a chaperon protein, heat-shock protein 90 (Hsp90) binds and protects AR from proteasome-dependent degradation (30). To determine whether Hsp90 is involved in the bromocriptine’s regulation of AR stability, co-immunoprecipitation (co-IP) was performed using an anti-Hsp90 antibody. Compared with that in control cells, AR expression in the immunoprecipitates from bromocriptine-treated C4-2 cells (for 6 h) was significantly decreased, whereas the pre-incubation with sulpiride (for 1 h) prior to bromocriptine treatment antagonized the reduction in AR expression. As the loading control, Hsp90 levels in the three Hsp90 immunoprecipitates remained unchanged. Consistently, compared with that in control cells, ubiquitinated AR was significantly increased in C4-2 cells treated with bromocriptine, an effect attenuated by the sulpiride pretreatment (Fig. 4C). These results indicated that bromocriptine activation of DRD2 may interrupt the physical association between Hsp90 and AR, thereby promoting AR degradation in PCa cells.
In vitro cytotoxicity of the combination of bromocriptine and docetaxel in PCa cells
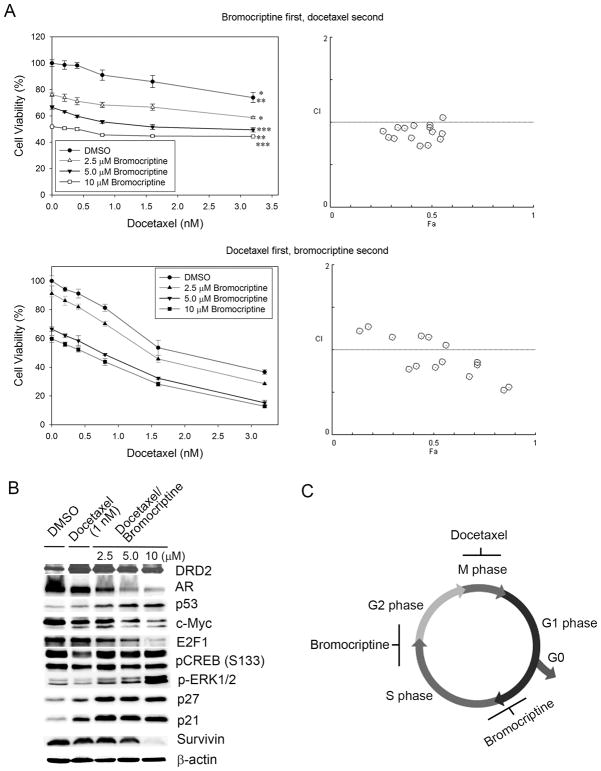
As a single agent, bromocriptine lacks potent cytotoxicity in PCa cells but significantly suppresses their proliferation, indicating a novel function of bromocriptine as a cell cycle inhibitor. Given the fact that the chemotherapeutic docetaxel mainly targets the mitotic phase, it is plausible that the combination of bromocriptine (an inhibitor of the G1/S and G2/M checkpoints) and docetaxel (an apoptosis inducer at the M phase) may synergize in interrupting multiple checkpoints and demonstrate superior activity to either drug in PCa cells. To test this hypothesis, C4-2 cells were treated with two different combination orders of bromocriptine and docetaxel: (1) treatment with bromocriptine at the indicated concentrations for 48 h prior to the addition of docetaxel for 24 h; and (2) treatment with docetaxel for 24 h prior to bromocriptine treatment for a further 48 h. Compared with the bromocriptine-first, docetaxel-second order, the addition of bromocriptine at the concentrations of as low as 2.5 μM to docetaxel-pretreated C4-2 cells significantly enhanced the cytotoxicity of docetaxel (p < 0.001) (Fig. 5A). The interaction between bromocriptine and docetaxel was further evaluated by the combination index (CI) isobologram method using the CompuSyn program, which demonstrated that the docetaxel-first, bromocriptine-second order achieved significant synergy between the two agents than the reverse order, particularly when both agents were used at relatively high concentrations (Fig. 5A, Table S3). Western blot analyses showed that compared with the docetaxel-only treatment, the docetaxel-first, bromocriptine-second treatment synergistically inhibited the expression of AR, c-Myc, E2F-1 and survivin, and increased the expression of DRD2, p53, p21 and p27 (Fig. 5B). These results indicate that bromocriptine can be an adjuvant agent to enhance the cytotoxicity of docetaxel, presumably via the induction of cell cycle arrest in the PCa cells escaping docetaxel treatment and entering next the cell cycle (Fig. 5C).
Figure 5. In vitro cytotoxicity of the combination of bromocriptine and docetaxel in C4-2 cells.
(A) In vitro effects of different combination orders of bromocriptine and docetaxel. Upper panel: C4-2 cells were first treated with bromocriptine at the indicated concentrations for 48 h prior to the addition of docetaxel for 24 h. (Left) MTS assay of cell viability. *, **, ***: p < 0.05 (two-way ANOVA); (Right) combination index (CI) between docetaxel and bromocriptine. Fa: the fraction affected. Lower panel: C4-2 cells were first treated with docetaxel for 24 h prior to bromocriptine treatment at the indicated concentrations for 48 h. (Left) MTS assay of cell viability. p < 0.05 for all pairwise comparisons (two-way ANOVA); (Right) CI between docetaxel and bromocriptine. (B) Western blot analysis of protein expression of key cell cycle and survival regulators in C4-2 cells first treated with docetaxel (1 nM) for 24 h prior to bromocriptine treatment for 48 h. (C) A possible mechanism of synergy between bromocriptine and docetaxel in PCa cells. Bromocriptine may primarily induce cell cycle arrest at the G1/S and G2/M checkpoints; therefore the combined treatment in the order of docetaxel-first (mainly causing apoptosis by targeting the M phase), bromocriptine-second may have better efficacy in PCa cells than the combination with the order of bromocriptine-first, docetaxel-second.
In vivo efficacy of bromocriptine and its combination with docetaxel against bone metastatic growth of PCa in athymic nude mice
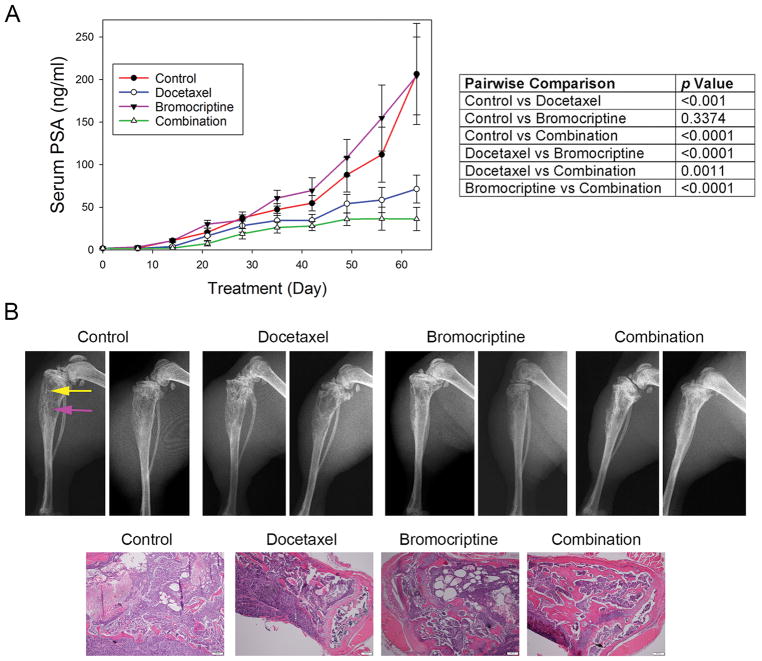
We evaluated the in vivo efficacy of bromocriptine and its combination with docetaxel in treating bone metastatic PCa. Athymic nude mice bearing intratibial C4-2-Luc xenografts were randomized into 4 groups and treated with vehicle control, bromocriptine (5 mg/kg, three times per week), docetaxel (5 mg/kg, once per week) or the combination, respectively. Serum PSA level was used as an indicator of C4-2-Luc tumor growth in mouse skeletons. Following a 9-week treatment, the average PSA level for each treatment group was 206.52 ± 59.32 ng/ml (control), 204.37 ± 45.65 ng/ml (bromocriptine), 71.49 ± 16.34ng/ml (docetaxel) and 36.23 ± 13.67 ng/ml (combination), respectively (Fig. 6A, left). The pairwise comparisons in PSA values between any two groups are summarized in Fig. 6A, right panel. Statistical analyses did not find significant differences between the control and bromocriptine alone (p = 0.3374) groups. However, the combination regimen resulted in a significant reduction in PSA values, when compared with the vehicle control (p < 0.0001). Importantly, there was a significant difference in the PSA levels between the combination regimen and the single treatment with either bromocriptine (p < 0.0001) or docetaxel (p = 0.0011) respectively, indicating a synergistic effect between the two agents in suppressing the bone metastatic growth of C4-2-Luc cells. Consistently, X-ray radiography showed that, compared with the vehicle control, tumor-bearing bones treated with either docetaxel or the combination displayed improved skeletal architecture with reduced osteolytic destruction and osteoblastic lesions (Fig. 6B), indicating an inhibitory effect of docetaxel or the combination treatment on tumor-induced bone turnover. Although the combination regimen slightly reduced the average body weight of mice (Fig. S2), neither the combination nor bromocriptine alone exhibited significant toxicities, as demonstrated by normal animal behaviors during the treatments and ex vivo examination of major organs. Taken together, these results indicated that bromocriptine can enhance the in vivo efficacy of docetaxel and retard the skeletal growth of PCa.
Figure 6. In vivo anticancer activity of bromocriptine and its combination with docetaxel in C4-2-Luc intratibial xenografts.
(A) Left: serum PSA levels of tumor-bearing mice under different treatments; Right: Two-way ANOVA of pairwise comparison of the PSA values between any two treatment groups. p< 0.05 was considered as statistically significant. (B) Upper panel: X-ray radiography of tumor-bearing tibias under different treatments. Yellow arrow: osteoblastic lesion; pink arrow: osteolytic lesion. Lower panel: H&E staining of bone tumor tissues. Bar scale: 200 μm.
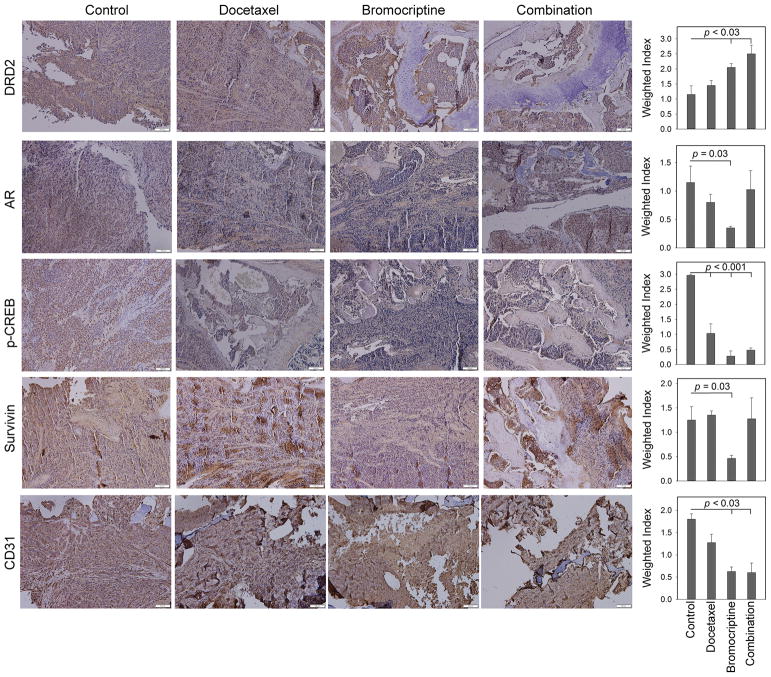
IHC analyses were performed to determine the in vivo effects of long-term bromocriptine treatment on several putative targets in C4-2-Luc bone tumor specimens (Fig. 7). Compared with the control group, bromocriptine significantly increased the tissue level of DRD2 (p = 0.03) and reduced the expression of AR (p = 0.02), p-CREB (p < 0.001) and survivin (p < 0.001). Docetaxel treatment also suppressed p-CREB expression (p < 0.001), with no significant effects on the levels of DRD2 or AR. Interestingly, survivin was slightly elevated in the tissues treated with docetaxel, an effect we have observed previously (31). The combination treatment resulted in increased expression of DRD2 (p = 0.02) and decreased level of p-CREB (p = 0.001) in bone tumors. In addition, the treatments with bromocriptine (p < 0.001) or the combination (p = 0.003) significantly reduced the expression of CD31, an indicator of angiogenesis, as well as tumor-associated microvessels. These results indicated that bromocriptine is effective in affecting its molecular targets in the C4-2 xenografts.
Figure 7. IHC analyses of tissue expression of potential bromocriptine targets in C4-2-Luc bone tumors.
Bar scale: 100 μm. Weighted index was calculated as the average (intensity x percentage of positive cells) from 4 random tissue areas. p values were calculated using Student’s t-test.
Discussion
Aberrant DRD2 signaling plays a prominent role in neurological disorders. However, the implication of DRD2 in human cancers remains inconclusive, and sometimes controversial. In this study, we demonstrated that DRD2 is ubiquitously expressed in PCa cell lines and tissues. Significantly, DRD2 expression is reduced in clinical PCa specimens with high Gleason scores. We further found that bromocriptine can increase DRD2 expression and activate DRD2 signaling in PCa cells, and significantly affect the expression of critical regulators of PCa cell proliferation and survival, including AR. Importantly, bromocriptine exhibits strong anticancer activity when combined with docetaxel, resulting in the suppression of tumor growth in mouse skeletons. These findings support the promise of bromocriptine as a novel agent to enhance docetaxel chemotherapy and treat PCa bone metastasis. To our knowledge, this is the first study investigating the existence of DRD2 in PCa and its therapeutic significance in treating PCa bone metastasis.
Previous studies showed that bromocriptine exerted anticancer efficacy in the xenograft models of AML and small cell lung cancer (SCLC). Although the mechanisms of action were not thoroughly elucidated, it appeared that a primitive subpopulation of leukemia cells with the CD34+CD38− profile is more sensitive to bromocriptine treatment than the bulk population, suggesting that bromocriptine may selectively target leukemia stem cells (24). In SCLC models, bromocriptine dose-dependently suppressed the growth of patient-derived xenografts and NCI-H69 tumors in mice, and the effects seemed to be mediated via DRD2 expressed by these tumors (32). Given the expression profile of DRD2 in PCa cells, we postulated that bromocriptine may activate classical DRD2 signaling. Indeed, bromocriptine significantly inhibited the expression of p-CREB and increased ERK1/2 phosphorylation, two common consequences of DRD2 activation (6). Bromocriptine also suppressed the phosphorylation of Stat3 at Serine 727, in a similar manner to that of the DRD2 agonist quinpirole in mouse hypothalamus (33). Pre-incubation with the DRD2 antagonist sulpiride effectively abrogated these effects, suggesting that DRD2 is required for the inhibition of p-CREB and p-Stat3 and the activation of p-ERK1/2. In comparison, bromocriptine did not affect the phosphorylation of Akt, another known downstream effector of the DRD2-phosphoinositide 3-kinase (PI-3K) pathway (6). These results suggested that bromocriptine may act as a “biased agonist” of DRD2 in PCa cells, i.e., inhibiting the CREB and Stat3 pathways while activating ERK1/2 signaling.
The inhibitory effect of bromocriptine on p-CREB and p-Stat3 may be of significant relevance to its biological functions in PCa cells, since the activation of both signaling pathways has been implicated in PCa progression towards an invasive and aggressive status (34,35). Our previous studies showed that bone metastatic PCa expresses elevated levels of p-CREB when compared to primary PCa. We further observed a close correlation between serum VEGF and clinical bone metastasis, and elucidated a mechanism by which p-CREB activates VEGF expression via a hypoxia-inducible factor (HIF-1)-dependent mechanism in normoxic PCa cells (36). Consistent with the role of p-CREB as a transcription activator of VEGF, the suppression of p-CREB by bromocriptine is associated with reduced protein expression of VEGF in PCa cells, which may lead to the inhibition of angiogenesis in bone metastatic tumors (Fig. 7). Interestingly, it was unexpected for us to observe a significant inhibition of survivin expression in the presence of bromocriptine, since bromocriptine does not induce massive apoptosis and significantly inhibit the viability of PCa cells. Mechanistically, previous studies from us and others have demonstrated Stat3 as a direct regulator of survivin transcription (31,37), which may provide an explanation on the inhibitory effect of bromocriptine on survivin transcription (Fig. S1). At the cellular levels, survivin functions not only as an anti-apoptotic protein but also a critical regulator of proliferation, specifically by initiating cell cycle entry and accelerating the S phase shift (38). Therefore, the inhibition of survivin by bromocriptine could have a two-fold effect in PCa cells: sensitizing them to programmed death and blocking their entry into the cell cycle, eventually enhancing the cytotoxicity of chemotherapeutics (Fig. 5C).
Our study identified AR as a novel molecular target of bromocriptine in PCa cells. As a major driver of PCa progression, AR is tightly regulated at multiple levels (39). Bromocriptine appears to inhibit AR expression at both the RNA (Fig. S1) and protein (Fig. 4A) levels. Since AR protein is rapidly decreased following bromocriptine treatment, we hypothesized that bromocriptine may promote the degradation of AR in PCa cells. Post-translationally, the molecular chaperone Hsp90 binds AR protein and protects it from proteasome-dependent degradation (40). Although bromocriptine did not affect Hsp90 expression, it may disrupt the physical interaction between Hsp90 and AR proteins and subject AR to ubiquitination and degradation. In search of the priming signal(s) leading to AR polyubiquitination and degradation, we further found that bromocriptine treatment led to a rapid increase in the phosphorylation of AR at residue serine 650 (Fig. S3). Previously, it has been shown that protein phosphatase 1α (PP1α) promotes the dephosphorylation of AR at serine 650, which plays a crucial role in stabilizing AR proteins (41,42). It would be interesting to examine whether bromocriptine promotes AR degradation via a PP1α-dependent mechanism, thereby contributing to its inhibition on PCa cell proliferation.
Although it exhibited relatively weak cytotoxicity in PCa cells, bromocriptine effectively induces cell cycle arrest at both the G1/S and G2/M checkpoint. This novel characteristic of bromocriptine as a cell cycle inhibitor may be an underlying mechanism by which bromocriptine synergizes with docetaxel, a potent apoptosis inducer mainly affecting the M phase (Fig. 5C), and demonstrates enhanced cytotoxicity in vitro and suppresses PCa growth in vivo. At the molecular level, bromocriptine treatment consistently resulted in a significant change in the expression profile of a panel of key cell cycle regulators, including c-Myc, E2F-1, p21 and p27. Skp2, an F-box protein responsible for the ubiquitination and degradation of p27 (43), was also suppressed by bromocriptine. Interestingly, as an oncoprotein frequently upregulated in PCa, Skp2 is associated with paclitaxel resistance in PCa cells (44). Therefore, bromocriptine-induced reduction of Skp2 may restore chemosensitivity in PCa cells. It is worth noting that bromocriptine may rescue p53 from degradation in an MDM2-dependent manner, as the treatment decreased the phosphorylated form of MDM2 that is required for the ubiquitination and degradation of p53 (29). Subsequently, elevated p53 expression may not only disrupt cell cycle progression but also inhibit the expression of certain oncogenes, such as survivin (45), resulting in the suppression of cellular proliferation and sensitization to docetaxel treatment.
At a low dose of 5 mg/kg, bromocriptine significantly enhances the in vivo efficacy of docetaxel against C4-2 growth in mouse bones. These results provided the first “proof-of-concept” evidence demonstrating the potential of bromocriptine in the treatment of PCa bone metastasis. Given the fact that bromocriptine and docetaxel may have different mechanisms of action in PCa cells, it would be interesting to test whether the use of bromocriptine, docetaxel or both drugs at lower doses (such as 2.5 mg/kg) could achieve similar levels of tumor suppression as the 5 mg/kg dosage while exhibiting improved safety profile in animals. It would also be interesting to test whether bromocriptine treatment via oral gavage, a more clinically-relevant route than intraperitoneal injection, delivers sufficient bromocriptine within bone tumors and effectively inhibits PCa growth. These studies will provide important pharmacological knowledge for clinical testing of bromocriptine in PCa patients.
Bromocriptine is a standard treatment for prolactinoma, the most frequent pituitary adenoma characterized by excessive and autonomic production of prolactin by lactotroph cells (46). Interestingly, activation of prolactin receptor signaling has been associated with prostate tumorigenesis and cancer progression, leading to the hypothesis that the blockade of pituitary prolactin production by bromocriptine could benefit PCa patients (47). Indeed, several pilot trials have been performed in castration-resistant PCa (48–50), which showed that bromocriptine administration (for example, 2.5 mg, three times per day) was well tolerated and significantly reduced the plasma levels of prolactin and testosterone. However, the bromocriptine-associated responses varied among these studies from an overall objective regression of 22.2% (and 50% of the patients had a prompt relief of bone pain) to no complete or partial responses in evaluable patients. While well-designed, large-scale trials are clearly needed to determine the clinical efficacy of bromocriptine, the lack of significant therapeutic benefits in these pilot trials suggest the necessity of a better understanding of the mechanism of action of bromocriptine in PCa cells.
Our study demonstrated, for the first time, the existence of functional DRD2 signaling in PCa cells, which can be exploited as a new prognostic marker indicative of PCa progression and a therapeutic target to design effective treatment for PCa. The targeting strategy of DRD2 agonism is particularly attractive when considering the availability of large numbers of DRD2 agonists, many of them marketed as antipsychotic drugs (13). Novel molecular targets of bromocriptine, including AR, Skp2, p53, c-Myc and survivin, could serve as reliable surrogate biomarkers to evaluate the clinical effects of bromocriptine in humans. Importantly, the results from our xenograft models showed that bromocriptine significantly enhances the in vivo efficacy of docetaxel treatment. Therefore, it is plausible to postulate that the adjunctive administration of bromocriptine in PCa patients along with docetaxel chemotherapy could demonstrate improved therapeutic responses in this particular population. Given its favorable safety profile and extensive use in neurological and endocrinological disorders, the efficacy of bromocriptine against PCa can be readily evaluated in clinical settings. Taken together, our study provides a strong rationale to repurpose bromocriptine, an FDA-approved non-cancerous drug, as a novel adjunct therapy to enhance docetaxel chemotherapy and treat PCa bone metastasis. Clinical trials investigating the combination of docetaxel and bromocriptine are warranted.
Supplementary Material
Acknowledgments
This work is partially supported by the National Cancer Institute grants 1R41CA186498-01A1, 1R41CA206725-01A1 and 1R41CA217491-01A1, University of Georgia-Augusta University Cancer Research Initiative Award, and Georgia Cancer Center Startup Fund (D. Wu), National Natural Science Foundation of China grant 81401759 (Y. Chen). We thank the Electron Microscopy and Histology Core at Medical College of Georgia, Augusta University for technical assistance in IHC studies, and the Histomorphometry and Molecular Analysis Core at Department of Pathology, University of Alabama at Birmingham for technical assistance in bone specimen preparation and analyses. Dr. Rhea-Beth Markowitz at Georgia Cancer Center kindly provided editorial assistance.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs SC. Spread of prostatic cancer to bone. Urology. 1983;21(4):337–44. doi: 10.1016/0090-4295(83)90147-4. [DOI] [PubMed] [Google Scholar]
- 3.Attard G, Parker C, Eeles RA, Schroder F, Tomlins SA, Tannock I, et al. Prostate cancer. Lancet. 2016;387(10013):70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 4.Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30(5):194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17(8):524–32. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408(6809):199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 7.Noble EP. The DRD2 gene in psychiatric and neurological disorders and its phenotypes. Pharmacogenomics. 2000;1(3):309–33. doi: 10.1517/14622416.1.3.309. [DOI] [PubMed] [Google Scholar]
- 8.Calne DB, Teychenne PF, Leigh PN, Bamji AN, Greenacre JK. Treatment of parkinsonism with bromocriptine. Lancet. 1974;2(7893):1355–6. doi: 10.1016/s0140-6736(74)92219-3. [DOI] [PubMed] [Google Scholar]
- 9.Walker S, Groom G, Davis RH, Hibbard BM, Griffiths K. Controlled trial of bromocriptine, quinoestrol, and placebo in suppression of puerperal lactation. Lancet. 1975;2(7940):842–4. doi: 10.1016/s0140-6736(75)90235-4. [DOI] [PubMed] [Google Scholar]
- 10.Sachdev Y, Gomez-Pan A, Tunbridge WM, Duns A, Weightman DR, Hall R, et al. Bromocriptine therapy in acromegaly. Lancet. 1975;2(7946):1164–8. doi: 10.1016/s0140-6736(75)92655-0. [DOI] [PubMed] [Google Scholar]
- 11.Chamarthi B, Gaziano JM, Blonde L, Vinik A, Scranton RE, Ezrokhi M, et al. Timed Bromocriptine-QR Therapy Reduces Progression of Cardiovascular Disease and Dysglycemia in Subjects with Well-Controlled Type 2 Diabetes Mellitus. J Diabetes Res. 2015;2015:157698. doi: 10.1155/2015/157698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grossrubatscher E, Veronese S, Ciaramella PD, Pugliese R, Boniardi M, De Carlis L, et al. High expression of dopamine receptor subtype 2 in a large series of neuroendocrine tumors. Cancer Biol Ther. 2008;7(12):1970–8. doi: 10.4161/cbt.7.12.6957. [DOI] [PubMed] [Google Scholar]
- 13.Pawlikowski M, Pisarek H, Winczyk K. Immunohistochemical detection of dopamine D2 receptors in neuroendocrine tumours. Endokrynol Pol. 2011;62(5):388–91. [PubMed] [Google Scholar]
- 14.Sachlos E, Risueno RM, Laronde S, Shapovalova Z, Lee JH, Russell J, et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149(6):1284–97. doi: 10.1016/j.cell.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 15.Shin JH, Park SJ, Kim ES, Jo YK, Hong J, Cho DH. Sertindole, a potent antagonist at dopamine D(2) receptors, induces autophagy by increasing reactive oxygen species in SH-SY5Y neuroblastoma cells. Biol Pharm Bull. 2012;35(7):1069–75. doi: 10.1248/bpb.b12-00009. [DOI] [PubMed] [Google Scholar]
- 16.Jandaghi P, Najafabadi HS, Bauer AS, Papadakis AI, Fassan M, Hall A, et al. Expression of DRD2 Is Increased in Human Pancreatic Ductal Adenocarcinoma and Inhibitors Slow Tumor Growth in Mice. Gastroenterology. 2016;151(6):1218–31. doi: 10.1053/j.gastro.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Miyamoto M, Ebihara Y, Mega S, Takahashi R, Hase R, et al. DRD2/DARPP-32 expression correlates with lymph node metastasis and tumor progression in patients with esophageal squamous cell carcinoma. World J Surg. 2006;30(9):1672–9. doi: 10.1007/s00268-006-0035-3. discussion 80-1. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Yao QY, Xue JS, Wang LJ, Yuan Y, Tian XY, et al. Dopamine D2 receptor antagonist sulpiride enhances dexamethasone responses in the treatment of drug-resistant and metastatic breast cancer. Acta Pharmacol Sin. 2017;38(9):1282–96. doi: 10.1038/aps.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Q, Yuan LB. Dopamine inhibits proliferation, induces differentiation and apoptosis of K562 leukaemia cells. Chin Med J (Engl) 2007;120(11):970–4. [PubMed] [Google Scholar]
- 20.Ganguly S, Basu B, Shome S, Jadhav T, Roy S, Majumdar J, et al. Dopamine, by acting through its D2 receptor, inhibits insulin-like growth factor-I (IGF-I)-induced gastric cancer cell proliferation via up-regulation of Kruppel-like factor 4 through down-regulation of IGF-IR and AKT phosphorylation. Am J Pathol. 2010;177(6):2701–7. doi: 10.2353/ajpath.2010.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang H, Wu K, Ma J, Du Y, Cao C, Nie Y. Dopamine D2 receptor suppresses gastric cancer cell invasion and migration via inhibition of EGFR/AKT/MMP-13 pathway. Int Immunopharmacol. 2016;39:113–20. doi: 10.1016/j.intimp.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Mu J, Huang W, Tan Z, Li M, Zhang L, Ding Q, et al. Dopamine receptor D2 is correlated with gastric cancer prognosis. Oncol Lett. 2017;13(3):1223–7. doi: 10.3892/ol.2017.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoeppner LH, Wang Y, Sharma A, Javeed N, Van Keulen VP, Wang E, et al. Dopamine D2 receptor agonists inhibit lung cancer progression by reducing angiogenesis and tumor infiltrating myeloid derived suppressor cells. Mol Oncol. 2015;9(1):270–81. doi: 10.1016/j.molonc.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lara-Castillo MC, Cornet-Masana JM, Etxabe A, Banus-Mulet A, Torrente MA, Nomdedeu M, et al. Repositioning of bromocriptine for treatment of acute myeloid leukemia. J Transl Med. 2016;14:261. doi: 10.1186/s12967-016-1007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberante FG, Pouryahya T, McMullin MF, Zhang SD, Mills KI. Identification and validation of the dopamine agonist bromocriptine as a novel therapy for high-risk myelodysplastic syndromes and secondary acute myeloid leukemia. Oncotarget. 2016;7(6):6609–19. doi: 10.18632/oncotarget.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arvigo M, Gatto F, Ruscica M, Ameri P, Dozio E, Albertelli M, et al. Somatostatin and dopamine receptor interaction in prostate and lung cancer cell lines. J Endocrinol. 2010;207(3):309–17. doi: 10.1677/JOE-10-0342. [DOI] [PubMed] [Google Scholar]
- 27.Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, et al. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998;77(6):887–94. doi: 10.1002/(SICI)1097-0215(19980911)77:6<887::AID-IJC15>3.0.CO;2-Z. [pii] [DOI] [PubMed] [Google Scholar]
- 28.Seo SI, Gera L, Zhau HE, Qian WP, Iqbal S, Johnson NA, et al. BKM1740, an acyl-tyrosine bisphosphonate amide derivative, inhibits the bone metastatic growth of human prostate cancer cells by inducing apoptosis. Clin Cancer Res. 2008;14(19):6198–206. doi: 10.1158/1078-0432.CCR-08-1023. [DOI] [PubMed] [Google Scholar]
- 29.Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol Cell. 2006;21(3):307–15. doi: 10.1016/j.molcel.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang Y, Fliss AE, Robins DM, Caplan AJ. Hsp90 regulates androgen receptor hormone binding affinity in vivo. J Biol Chem. 1996;271(45):28697–702. doi: 10.1074/jbc.271.45.28697. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Gera L, Mamouni K, Li X, Chen Z, Kucuk O, et al. Inhibition of skeletal growth of human prostate cancer by the combination of docetaxel and BKM1644: an aminobisphosphonate derivative. Oncotarget. 2016;7(19):27489–98. doi: 10.18632/oncotarget.8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishibashi M, Fujisawa M, Furue H, Maeda Y, Fukayama M, Yamaji T. Inhibition of growth of human small cell lung cancer by bromocriptine. Cancer Res. 1994;54(13):3442–6. [PubMed] [Google Scholar]
- 33.Kim KS, Yoon YR, Lee HJ, Yoon S, Kim SY, Shin SW, et al. Enhanced hypothalamic leptin signaling in mice lacking dopamine D2 receptors. J Biol Chem. 2010;285(12):8905–17. doi: 10.1074/jbc.M109.079590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steven A, Seliger B. Control of CREB expression in tumors: from molecular mechanisms and signal transduction pathways to therapeutic target. Oncotarget. 2016;7(23):35454–65. doi: 10.18632/oncotarget.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu P, Wu D, Zhao L, Huang L, Shen G, Huang J, et al. Prognostic role of STAT3 in solid tumors: a systematic review and meta-analysis. Oncotarget. 2016;7(15):19863–83. doi: 10.18632/oncotarget.7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu D, Zhau HE, Huang WC, Iqbal S, Habib FK, Sartor O, et al. cAMP-responsive element-binding protein regulates vascular endothelial growth factor expression: implication in human prostate cancer bone metastasis. Oncogene. 2007;26(35):5070–7. doi: 10.1038/sj.onc.1210316. 1210316 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Kanda N, Seno H, Konda Y, Marusawa H, Kanai M, Nakajima T, et al. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23(28):4921–9. doi: 10.1038/sj.onc.1207606. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki A, Hayashida M, Ito T, Kawano H, Nakano T, Miura M, et al. Survivin initiates cell cycle entry by the competitive interaction with Cdk4/p16(INK4a) and Cdk2/cyclin E complex activation. Oncogene. 2000;19(29):3225–34. doi: 10.1038/sj.onc.1203665. [DOI] [PubMed] [Google Scholar]
- 39.Culig Z, Santer FR. Androgen receptor signaling in prostate cancer. Cancer Metastasis Rev. 2014;33(2–3):413–27. doi: 10.1007/s10555-013-9474-0. [DOI] [PubMed] [Google Scholar]
- 40.Georget V, Terouanne B, Nicolas JC, Sultan C. Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry. 2002;41(39):11824–31. doi: 10.1021/bi0259150. [DOI] [PubMed] [Google Scholar]
- 41.Chen S, Kesler CT, Paschal BM, Balk SP. Androgen receptor phosphorylation and activity are regulated by an association with protein phosphatase 1. J Biol Chem. 2009;284(38):25576–84. doi: 10.1074/jbc.M109.043133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Han W, Gulla S, Simon NI, Gao Y, Liu J, et al. Androgen ablation elicits PP1-dependence for AR stabilization and transactivation in prostate cancer. Prostate. 2016;76(7):649–61. doi: 10.1002/pros.23157. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Gao D, Fukushima H, Inuzuka H, Liu P, Wan L, et al. Skp2: a novel potential therapeutic target for prostate cancer. Biochim Biophys Acta. 2012;1825(1):11–7. doi: 10.1016/j.bbcan.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y, Lu Y, Wang L, Mizokami A, Keller ET, Zhang J, et al. Skp2 is associated with paclitaxel resistance in prostate cancer cells. Oncol Rep. 2016;36(1):559–66. doi: 10.3892/or.2016.4809. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277(5):3247–57. doi: 10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]
- 46.Ciccarelli E, Camanni F. Diagnosis and drug therapy of prolactinoma. Drugs. 1996;51(6):954–65. doi: 10.2165/00003495-199651060-00004. [DOI] [PubMed] [Google Scholar]
- 47.Goffin V. Prolactin receptor targeting in breast and prostate cancers: New insights into an old challenge. Pharmacol Ther. 2017 doi: 10.1016/j.pharmthera.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Jacobi GH, Sinterhauf K, Kurth KH, Altwein JE. Testosterone metabolism in patients with advanced carcinoma of the prostate: a comparative in vivo study of the effects of oestrogen and antiprolactin. Urol Res. 1978;6(3):159–65. doi: 10.1007/BF00261317. [DOI] [PubMed] [Google Scholar]
- 49.Jacobi GH, Altwein JE. Bromocriptine for palliation of advanced prostatic carcinoma. Experimental and clinical profile of a drug (author’s’ transl) Urol Int. 1979;34(4):266–90. doi: 10.1159/000280272. [DOI] [PubMed] [Google Scholar]
- 50.Horti J, Figg WD, Weinberger B, Kohler D, Sartor O. A phase II study of bromocriptine in patients with androgen-independent prostate cancer. Oncol Rep. 1998;5(4):893–6. doi: 10.3892/or.5.4.893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.