Abstract
Previous studies of ethanol drinking in rodents have shown greater intake in females than in males, but the reasons behind this difference are unknown. To address one possible interpretation of the drinking difference, these studies tested the hypothesis that female and male mice differ in sensitivity to the rewarding effects of ethanol using the conditioned place preference (CPP) procedure. To increase the generalizability of the results, sex differences were examined in two inbred mouse strains known to differ in their sensitivity to ethanol reward: C57BL/6J (B6) and DBA/2J (D2). Mice were conditioned in an unbiased CPP procedure using either 1 or 2 g/kg ethanol. To detect possible differences in learning rate, they were tested once at the midpoint of conditioning and again after conditioning ended. As expected, CPP was stronger with 2 g/kg than with 1 g/kg, and D2 mice generally showed stronger CPP than B6 mice. However, there were no sex differences in the rate of CPP acquisition or in CPP magnitude, suggesting no sex difference in ethanol reward sensitivity as indexed by CPP. Nevertheless, there were sex differences in locomotor activity. B6 females were generally more active than B6 males during CPP acquisition whereas D2 females were slightly less active than D2 males during both CPP acquisition and preference testing. Unexpectedly, female mice showed more variability than males in the behavioral measures recorded in these studies, encouraging greater attention to variability in the design, analysis and interpretation of future studies of sex differences in mice.
Keywords: sex; alcohol; reward; activity; learning; variability; inbred strains (DBA/2J, C57BL/6J)
1. Introduction
Female rats and mice from a wide range of genotypes generally drink more ethanol than males (e.g., Li & Lumeng, 1984; Yoneyama et al., 2008), a finding that suggests females find ethanol more rewarding than males. However, relatively few studies have systematically assessed sex differences in ethanol reward using the conditioned place preference (CPP) procedure. Moreover, the results are sometimes contradictory. In an extensive dose-response study using Wistar rats, Torres et al. (2014) found a biphasic dose-effect in both adult and adolescent females, observing CPP at 1 g/kg, but conditioned place aversion (CPA) at 2.5 g/kg. Males at both ages, however, showed only CPA (at the highest dose), suggesting that males are less sensitive than females to low-dose rewarding effects of ethanol. In contrast, other studies have reported no sex differences in the CPP produced by a 1 g/kg dose in adult Sprague-Dawley rats (Nentwig et al., 2017) or in adolescent Wistar (Acevedo et al., 2013) or Sprague-Dawley (Nizhnikov et al., 2010) rats. Whether these discrepancies across rat studies are due to differences in genotype, apparatus or procedure is currently unknown.
Studies in mice have generally been more consistent in showing no sex differences in ethanol-induced CPP across many different genotypes. For example, male and female mice have shown similar levels of CPP in studies involving two different genetically heterogeneous strains (Barkley-Levenson et al., 2015; Song et al., 2007), two standard inbred strains and their reciprocal cross (Gabriel & Cunningham, 2008; Nocjar et al., 1999), six different selectively bred mouse lines (Barkley-Levenson et al., 2015; Phillips et al., 2005), and six different knockout or wildtype lines (Bechtholt et al., 2004; Cunningham et al., 2000; Giardino et al., 2011; Hill et al., 2003; Itzhak et al., 2009). One exception to this pattern is a study that tested early and late adolescent outbred (OF1) mice (Roger-Sánchez et al., 2012). Females at both ages showed CPP only at the highest dose (2.5 g/kg), while early adolescent males showed CPP at that high dose as well as at a lower (1.25 g/kg) dose. Unexpectedly, late adolescent males failed to show CPP at any dose. These data raise the possibility of opposing sex differences in sensitivity to ethanol reward that depend on dose and age in at least one outbred mouse strain.
One of the difficulties in interpreting the mouse studies of sex differences in CPP is that most have only used doses in the plateau of the monotonic increasing ethanol dose-effect curve for male mice. Thus, greater sensitivity to ethanol reward in females might be obscured due to ceiling effects (Groblewski et al., 2008). Additionally, most studies have only tested CPP after a relatively large number of conditioning trials when performance is already asymptotic, eliminating the ability to detect sex differences in the rate of CPP acquisition. Finally, many previous studies were not designed with sufficient statistical power to detect sex differences. The present studies were designed specifically to address these shortcomings by testing two commonly used inbred mouse strains that are known to differ in their sensitivity to ethanol CPP—C57BL/6J (B6) and DBA/2J (D2) (Cunningham, 2014; Cunningham et al., 1992). Several strategies were used to enhance our ability to detect possible sex differences in sensitivity to ethanol reward. First, we used two ethanol doses, one known to be near the threshold for producing CPP (1 g/kg) and another at the beginning of the dose-effect plateau (2 g/kg) for D2 male mice (Groblewski et al., 2008). Second, we tested all mice twice, once at the midpoint of training (after only two ethanol conditioning trials) and again after training ended. Finally, we selected group sizes for each sex (n = 11–12/conditioning subgroup) that were expected to have sufficient power (β = 0.8; α = 0.05) to detect conditioning subgroup differences in mean test scores of 10–12 sec/min based on variability measured in previous studies with D2 male mice in our lab. If female mice are more sensitive to ethanol reward, we expected that they would develop CPP at a lower dose and/or after fewer conditioning trials than male mice. Based on past studies, we also expected that D2 mice would generally be more sensitive to ethanol reward than B6 mice, regardless of sex (Cunningham, 2014; Cunningham et al., 1992; Gabriel & Cunningham, 2008).
2. Methods
2.1. Subjects
Six-week old female and male C57BL/6J and DBA/2J mice (n = 46–48 of each sex from each strain, i.e., a total of 94–96 per strain) were shipped from the Jackson Laboratory (Sacramento, CA) and housed in same Strain x Sex groups of four in polycarbonate cages (27.9 x 9.5 x 12.7 cm) with cob bedding in a colony room maintained on a normal 12 h light-dark cycle. Water and mouse chow were continuously available in the home cage. Behavioral testing began when mice were about 8 weeks old and all testing occurred during the light phase of the circadian cycle. Estrus cycle phases were not monitored and it is likely that several estrus phases are represented in the dataset. The Oregon Health & Science University IACUC approved the protocol, which followed the National Institutes of Health (NIH) “Principles of Laboratory Animal Care.”
2.2. Apparatus
The apparatus consisted of 12 identical acrylic and aluminum boxes (30 x 15 x 15 cm) contained in individual light- and sound-attenuating enclosures (Coulbourn Instruments Model E10-20). These enclosures had ventilation fans but no internal lighting. General activity and position were detected by six sets of infrared sensors and light sources mounted opposite each other at 5-cm intervals 2.2 cm above the floor on the long walls of each box. LabVIEW 2014 software stored data to computer during all sessions.
The floor of each box consisted of reversible halves made of two distinct textures: a "grid" floor composed of 2.3 mm stainless-steel rods mounted 6.4 mm apart in acrylic rails, and a "hole" floor made from stainless steel (16 GA) perforated with 6.4-mm round holes on 9.5-mm staggered centers (Cunningham et al., 2006). These floor textures were selected on the basis of previous studies showing that drug-naive (saline only) groups of male B6 and D2 mice spend about half their time on each floor type during preference tests (Cunningham, 1995; Cunningham et al., 1992). The apparatus and floors were wiped with a damp sponge and the litter paper was changed after each animal.
2.3. Procedure
Two identical experiments were completed, one with B6 mice and the other with D2 mice. To facilitate detection of possible sex or strain differences in sensitivity on the rising limb of the dose-effect curve (Groblewski et al., 2008), half of the mice in each Strain x Sex group were randomly assigned to receive a relatively low ethanol dose (1 g/kg) while the other half received our standard conditioning dose (2 g/kg). Each experiment included three phases: pretest (one session), conditioning (four ethanol trials and four saline trials) and preference testing (two sessions). Experimental sessions were conducted during the light cycle, 5 days per week.
2.3.1. Pretest
The primary purpose of the pretest session was to determine whether B6 or D2 female mice showed any bias for the floor textures before conditioning. Although previous studies have shown no apparatus bias in male mice from these strains (Cunningham, 1995; Cunningham et al., 1992, 2003), female mice have not been systematically tested. Mice were weighed and injected (IP) with saline (12.5 ml/kg) immediately before placement in the conditioning apparatus with a different floor texture on each side of the apparatus. Floor positions were counterbalanced within each conditioning subgroup. The amount of time spent on each floor texture was recorded during a 30-min session before returning mice to their home cages.
2.3.2. Conditioning
All mice were exposed to a one-compartment place conditioning procedure (Cunningham et al. 2003, 2006, 2011). Within each Strain x Sex x Dose group, mice were assigned to one of two conditioning subgroups (n = 11–12/subgroup) that were matched on the basis of their pretest scores, i.e., approximately equal numbers of mice showing a preference or aversion for each cue were assigned to each subgroup. Thus, the subject assignment procedure was unbiased in that there was no systematic relationship between initial bias and the floor cue that was later paired with ethanol. Mice in one subgroup (GRID+) received IP injections of ethanol (1 or 2 g/kg, 20% v/v in saline) immediately before placement on the grid floor whereas mice in the other subgroup (GRID-) received ethanol before placement on the hole floor. Injection of saline preceded placement on the opposite floor type within each of these subgroups. The difference between these counterbalanced conditioning subgroups during preference testing is used to index CPP strength, with mice in the GRID+ subgroups expected to spend more time on the grid floor during testing than mice in the GRID- subgroups (Cunningham et al. 2003, 2006, 2011). The floor texture was identical on both sides of the apparatus during conditioning trials and mice had free access to the entire apparatus (one-compartment procedure). A 5-min conditioning trial duration was selected on the basis of previous studies showing that it produces stronger CPP than longer trial durations in male D2 mice (Cunningham and Prather, 1992). Mice received only one trial per day, with ethanol (CS+) and saline (CS-) trials alternating across days (counterbalanced order within conditioning subgroups). Mice were trained and tested in a darkened enclosure because previous research has shown that illumination reduces CPP to tactile cues in D2 mice in the one-compartment CPP procedure (Cunningham & Zerizef, 2014).
2.3.3. Preference Tests
All mice received two 30-min preference tests that were procedurally identical to the pretest. The first test occurred 24 h after mice had received two conditioning trials of each type and the second test occurred 24 h after the fourth (and final) pair of trials. The position of each floor type matched that used during the pretest for each mouse.
3. Results
3.1. Pretest
Mean sec/min (±SEM) spent on the grid floor by B6 females, B6 males, D2 females and D2 males during the pretest session were 30.4 (± 0.8), 30.1 (± 0.8), 30.4 (± 0.8), and 28.1 (± 1.1), respectively. Expressed as the mean percentage of time spent on the floor that would later serve as the CS+, both B6 (49.5 ± 1.0%) and D2 (50 ± 1.2%) mice spent about half of the session on each floor, consistent with previous studies indicating a lack of bias in this apparatus (Cunningham et al., 2003). Three-way ANOVA (Strain x Sex x Conditioning Subgroup) applied to grid time scores showed no significant group differences in initial biases for the floors within either strain.
3.2. Conditioning
3.2.1. B6 Mice
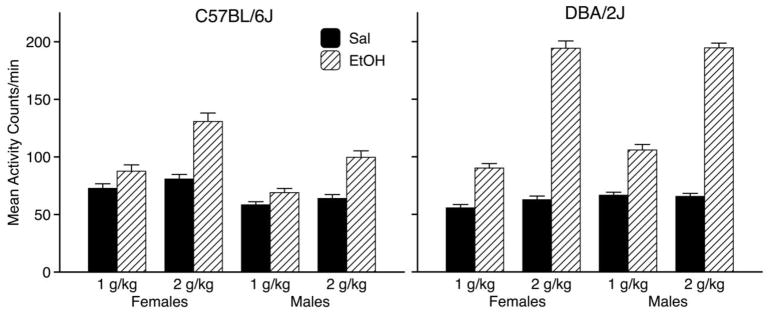
Mean activity rates (±SEM) collapsed over the four conditioning trials of each type are shown for each Sex x Dose group in the left panel of Fig. 1. As can be seen, activity was consistently higher on ethanol trials than on saline trials and activity on ethanol trials was positively related to dose. In addition, females were generally more active than males. Three-way ANOVA (Sex x Dose x Trial Type) supported these observations, yielding significant main effects of Sex [F(1,90) = 22.8, p < .001], Dose [F(1, 90) = 26.8, p < .001] and Trial Type [F(1, 90] = 187.4, p < .001), as well as significant Sex x Trial Type [F(1, 90) = 5.2, p = .03] and Dose x Trial Type [F(1, 90] = 59.9 p < .001) interactions.
Figure 1.
Mean activity rates (activity counts/min ± SEM) on CS- (saline) and CS+ (ethanol) conditioning trials in female and male C57BL/6J mice (left panel) and DBA/2J mice (right panel). Each bar depicts data from 23–24 mice.
3.2.2. D2 Mice
Fig. 1 (right panel) depicts mean activity rates (±SEM) collapsed over the four conditioning trials of each type for each Sex x Dose group. Consistent with previous studies, ethanol-induced activation was greater in D2 mice than in B6 mice (Cunningham et al., 1992; Cunningham, 1995). As in B6 mice, D2 mice were more active on ethanol trials, especially at the higher dose. However, in contrast to B6 mice, D2 females were slightly less active than males. Three-way ANOVA indicated significant main effects of Sex [F(1,92) = 5.3, p = .02], Dose [F(1, 92) = 232.4, p < .001] and Trial Type [F(1, 92) = 1347.1, p < .001], as well as a significant Dose x Trial Type interaction [F(1, 92) = 419.3 p < .001].
3.3. Preference Tests
3.3.1. B6 Mice
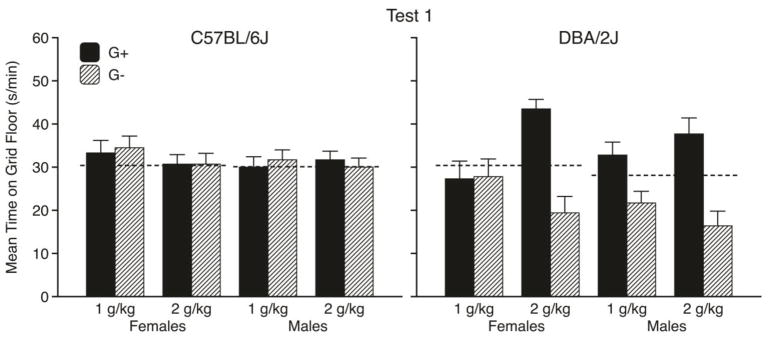
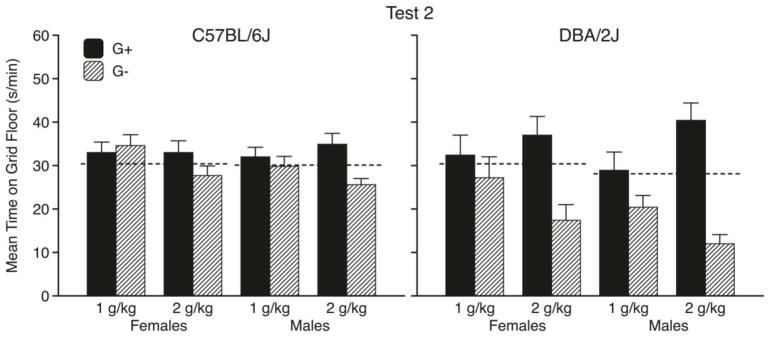
Mean times (s/min ± SEM) spent on the grid floor during the first and second preference tests are shown in the left-hand panels of Figs. 2 and 3, respectively. As can be seen, there was no evidence of place conditioning in B6 mice after the first two pairs of conditioning trials (Fig. 2, left panel). However, after all four pairs of trials, there was a modest CPP in the 2 g/kg groups (but not in the 1 g/kg groups), which did not differ across sexes (Fig. 3, left panel). These conclusions were supported by three-way ANOVAs that were applied separately to the data from each test. More specifically, the Test 1 ANOVA showed no significant main effects or interactions, whereas the Test 2 ANOVA yielded a significant main effect of Conditioning Subgroup [F(1, 86) = 5.4, p = .02] and a significant Dose x Conditioning Subgroup interaction [F(1, 86) = 4.7, p = .03]. Separate follow-up analyses of each dose group on Test 2 indicated that the interaction was explained by a significant effect of Conditioning Subgroup in the 2 g/kg groups [F(1, 43) = 10.6, p = .002], but no significant effect in the 1 g/kg groups. Two-way (Sex x Dose) ANOVAs applied to activity rates during each test showed no significant main effects or interaction. Overall activity rates (counts/min ± SEM) were 46.6 ± 1.8 and 45.0 ± 1.7 in females and males, respectively.
Figure 2.
Mean time spent on the grid floor (s/min ± SEM) by C57BL/6J mice (left panel) and DBA/2J mice (right panel) during the first preference test (after the first two ethanol conditioning trials) in each Sex x Dose conditioning subgroup. Each bar depicts data from 11–12 mice. (G+ = GRID+; G- = GRID-). Dashed lines depict overall mean times spent on the grid floor during the pretest by each Strain x Sex group. Analyses based on individual changes from the pretest baseline scores can be found in the supplementary material (Figure S1).
Figure 3.
Mean time spent on the grid floor (s/min ± SEM) by C57BL/6J mice (left panel) and DBA/2J mice (right panel) during the second preference test (after all four ethanol conditioning trials) in each Sex x Dose conditioning subgroup. Each bar depicts data from 11–12 mice. (G+ = GRID+; G− = GRID−). Dashed lines depict overall mean times spent on the grid floor during the pretest by each Strain x Sex group. Analyses based on individual changes from the pretest baseline scores can be found in the supplementary material (Figure S1).
3.3.2. D2 Mice
The right-hand panels of Figs. 2 and 3 depict the results of the preference tests for D2 mice. In contrast to B6 mice, D2 mice showed CPP at 2 g/kg after only two conditioning trials of each type (Fig. 2, right panel), reflecting their greater sensitivity to ethanol’s rewarding effect in this procedure (Cunningham et al., 1992; Cunningham, 2014). There were no apparent sex differences in CPP at 2 g/kg and only a weak trend toward CPP at the lower dose. Three-way ANOVAs showed significant main effects of Conditioning Subgroup [both F(1,88) > 30.9, p < .001] and significant Dose x Conditioning Subgroup interactions [both F(1, 88) > 9.6, p < .005] on both tests, but no significant effects involving Sex. Separate follow-up analyses of each dose group on each test were conducted to interpret the interactions. Those analyses showed significant Conditioning Subgroup effects in the 2 g/kg groups on both tests [both F(1,44) > 43.7, p < .001], but no significant effects in the 1 g/kg groups on either test. Males were more active than females on both tests [both F(1, 92) > 6.3, p < .02]. Overall activity rates were 45.1 ± 1.1 and 40.2 ± 1.4 in males and females, respectively.
3.4. Variability
To address whether there were sex differences in behavioral variability, we adopted the approach described in two recent meta-analyses (Becker et al., 2016; Prendergast et al., 2014). More specifically, we calculated the Coefficient of Variation (CV) (defined as the group standard deviation divided by the group mean) for each of the eight independent groups in each experiment for all six of the following dependent variables: activity rates on CS+ (ethanol) and CS- (saline) conditioning trials (collapsed across conditioning trials 1–4), activity rates on the first and second CPP tests, and grid floor times for the first and second CPP tests. Thus, we generated a total of 96 CVs, which included 24 from the female groups of each strain (4 groups x 6 variables/group) and 24 from the male groups of each strain (4 groups x 6 variables/group). Consistent with the approach of Becker et al., we compared sexes by pairing the female and male scores obtained for a given measure from each Strain x Dose x Conditioning Subgroup and treating Sex as a within-group variable. Two-way ANOVA (Strain x Sex) applied to the CVs yielded a significant main effect of Sex [F(1, 46) = 9.2, p < .01], reflecting greater variability in females (mean CV = 0.299 ± 0.018) than in males (mean CV = 0.266 ± 0.017). Computation of effect size yielded a Cohen’s d value of 0.44, which is just short of the 0.50 value specified for “medium” effects (Cohen, 1988, pp. 24–27). Neither the main effect of Strain nor the interaction was significant. Box plots of the CVs for each Strain x Sex condition are provided in the supplementary material (Fig. S1).
As in the published meta-analyses, we also calculated the ratios of female to male CVs within each treatment group, i.e., CV female/(CV female + CV male). A test (Shapiro-Wilk) of the distribution of ratios indicated no significant deviation from normality. The CV ratios were then compared to the theoretical mean of 0.5 (i.e., no sex difference) using a one-sample t-test. This test indicated that the overall mean ratio (0.53 ± 0.01) differed significantly from 0.50 [t(47) = 3.0, p < .005)], confirming that female mice showed greater variability in CPP-related behaviors. An independent-samples t-test showed no significant strain difference in the mean ratios [p > .20], although D2 mice showed a slightly higher mean ratio (0.54 ± 0.01) than B6 mice (0.52 ± 0.02).
4. Discussion
Despite using procedures intended to enhance our ability to detect sex differences, male and female mice within each strain developed similar levels of conditioned preference for ethanol-paired tactile cues, suggesting there are no sex differences in sensitivity to the rewarding effects of ethanol at 1 or 2 g/kg in B6 and D2 mice. Moreover, there were no sex differences in the rate of CPP learning. Although these studies were not designed to directly compare B6 and D2 mice, our results are also consistent with several previous studies showing that D2 mice are more sensitive to ethanol reward than B6 mice (e.g., Cunningham et al., 1992; Cunningham, 2014) and they extend the generalizability of those findings by confirming that the genetic difference in sensitivity is similar in both sexes.
The failure to find a significant sex difference in ethanol-induced CPP argues against the idea that higher ethanol intakes in female mice reflect a sex difference in sensitivity to ethanol reward. One possible alternative interpretation of the ethanol drinking difference is that female mice are less sensitive to post-absorptive aversive effects of ethanol. Several studies support this idea, showing weaker ethanol-induced conditioned taste aversion in female rodents than in males (Cailhol & Mormède, 2002; Phillips et al., 2005; Schramm-Sapyta et al., 2014; Sherrill et al., 2011). Another possible interpretation of the drinking difference is a sex difference in taste sensitivity or preference (Martin & Sollars, 2017). However, additional research is needed to more fully address the role that sex differences in ethanol’s aversive effects or taste sensitivity/preference play in mediating the well-established sex difference in ethanol drinking.
Although these studies showed no sex differences in CPP, they revealed sex differences in locomotor activity, albeit in opposite directions in the two strains. More specifically, B6 females were generally more active than B6 males during CPP acquisition whereas D2 females were slightly less active than D2 males during both CPP acquisition and preference testing. The findings of greater activity in B6 females (Podhorna & Brown, 2002; Van Swearingen et al., 2013) and less activity in D2 females (Bolivar et al., 2000) are consistent with some studies, but not with other studies showing no sex differences in locomotor activity in either strain (Cook et al., 1998; Logue et al., 1997; Morse et al., 1995). The reasons behind these discrepancies are unknown, but may be related to differences in the apparatus, lighting conditions or procedure.
The finding of greater variability in behavioral responding in females than in males was unexpected because recent meta-analyses had not shown such differences in either rats (Becker et al., 2016) or mice (Prendergast et al., 2014). In fact, male mice were found to exhibit greater variability in several physiological trait categories (Prendergast et al., 2014). One possibility is that the sex difference in variability seen here was somehow related to estrus cycling in female mice. However, in the absence of data on sex differences in variability across a wider range of behavioral phenotypes and mouse genotypes, it is difficult to know whether the differences observed here are unique to these behaviors and strains or are more broadly representative. Moreover, the mechanisms underlying the sex difference in variability are unknown and must be determined by future research.
One simple implication of the sex difference in behavioral variability is that more female than male mice may be needed to detect group mean differences of the same magnitude. For example, using the means of the standard deviations averaged separately for the male and female groups (from both strains) on the second CPP test, power analyses (G*Power 3.1) indicate one would need to test 16 female mice per group to detect a 12-sec mean group difference in grid time, but only 11 male mice per group (two-tailed tests, α = 0.05, β = 0.80). Another way to characterize the sex differences is in terms of effect size. More specifically, these data suggest that even when group mean differences are similar in males and females, effect size will be smaller in females. For example, if both sexes showed a mean difference of 12 sec between the GRID+ and GRID- groups, the effect size (Cohen’s d) would be 1.29 for males but only 1.02 for females. It is important to note that the absence of sex differences in ethanol-induced CPP in these studies does not provide a rationale for studying only one sex in future studies with this procedure.
It is possible, for example, that changes in the apparatus, conditioning parameters or a variety of other variables (e.g., housing, light cycle, diet) would reveal sex differences not seen under the “standard” conditions tested here (i.e., a sex x environment interaction). Furthermore, the observed sex differences in variability suggest that closer attention must be given to variability in the design, analysis and interpretation of future studies that compare female and male mice.
Supplementary Material
Research Highlights.
Ethanol induced conditioned place preference (CPP) in both C57BL/6J and DBA/2J mice
DBA/2J mice developed CPP more strongly and more quickly than C57BL/6J mice
Females and males did not differ in rate of CPP learning or magnitude in either strain
Females and males differed in activity rates in opposite directions across strains
Females showed greater variability than males
Acknowledgments
Research reported in this paper was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number R01AA007702. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Lee Bakner for helpful comments on an earlier draft of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo MB, Nizhnikov ME, Spear NE, Molina JC, Pautassi RM. Ethanol-induced locomotor activity in adolescent rats and the relationship with ethanol-induced conditioned place preference and conditioned taste aversion. Developmental Psychobiology. 2013;55(4):429–442. doi: 10.1002/dev.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley-Levenson AM, Cunningham CL, Smitasin PJ, Crabbe JC. Rewarding and aversive effects of ethanol in High Drinking in the Dark selectively bred mice. Addiction Biology. 2015;20(1):80–90. doi: 10.1111/adb.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtholt AJ, Smith R, Raber J, Cunningham CL. Enhanced ethanol-, but not cocaine-induced, conditioned place preference in Apoe(-/-) mice. Pharmacology, Biochemistry & Behavior. 2004;77(4):783–792. doi: 10.1016/j.pbb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ. 2016;7(1):34. doi: 10.1186/s13293-016-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Caldarone BJ, Reilly AA, Flaherty L. Habituation of activity in an open field: A survey of inbred strains and F1 hybrids. Behavior Genetics. 2000;30(4):285–293. doi: 10.1023/A:1026545316455. [DOI] [PubMed] [Google Scholar]
- Cailhol S, Mormède P. Conditioned taste aversion and alcohol drinking: Strain and gender differences. Journal of Studies on Alcohol. 2002;63(1):91–99. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cook MN, Ware DD, Boone EM, Hou X, Morse AC, Reed CL, et al. Ethanol modulates cocaine-induced behavioral change in inbred mice. Pharmacology, Biochemistry & Behavior. 1998;59(3):567–575. doi: 10.1016/s0091-3057(97)00449-8. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Localization of genes influencing ethanol-induced conditioned place preference and locomotor activity in BXD recombinant inbred mice. Psychopharmacology. 1995;120(1):28–41. doi: 10.1007/BF02246142. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Genetic relationship between ethanol-induced conditioned place preference and other ethanol phenotypes in 15 inbred mouse strains. Behavioral Neuroscience. 2014;128(4):430–445. doi: 10.1037/a0036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Prather LK. Conditioning trial duration affects ethanol-induced conditioned place preference in mice. Animal Learning & Behavior. 1992;20(2):187–194. [Google Scholar]
- Cunningham CL, Zerizef CL. Effects of combining tactile with visual and spatial cues in conditioned place preference. Pharmacology, Biochemistry & Behavior. 2014;124:443–450. doi: 10.1016/j.pbb.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology. 2003;170(4):409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nature Protocols. 2006;1(4):1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Groblewski PA, Voorhees CM. Place conditioning. In: Olmstead MC, editor. Animal Models of Drug Addiction, Neuromethods. Vol. 53. Springer Science+Business Media, LLC; 2011. pp. 167–189. [DOI] [Google Scholar]
- Cunningham CL, Howard MA, Gill SJ, Rubinstein M, Low MJ, Grandy DK. Ethanol-conditioned place preference is reduced in dopamine D2 receptor-deficient mice. Pharmacology, Biochemistry & Behavior. 2000;67(4):693–699. doi: 10.1016/s0091-3057(00)00414-7. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology. 1992;107(2–3):385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Cunningham CL. Effects of maternal strain on ethanol responses in reciprocal F1 C57BL/6J and DBA/2J hybrid mice. Genes, Brain and Behavior. 2008;7(3):276–287. doi: 10.1111/j.1601-183X.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- Giardino WJ, Cocking DL, Kaur S, Cunningham CL, Ryabinin AE. Urocortin-1 within the Centrally-Projecting Edinger-Westphal Nucleus Is Critical for Ethanol Preference. PLoS ONE. 2011;6(10):e26997. doi: 10.1371/journal.pone.0026997.g003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groblewski PA, Bax LS, Cunningham CL. Reference-dose place conditioning with ethanol in mice: empirical and theoretical analysis. Psychopharmacology. 2008;201(1):97–106. doi: 10.1007/s00213-008-1251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KG, Alva H, Blednov YA, Cunningham CL. Reduced ethanol-induced conditioned taste aversion and conditioned place preference in GIRK2 null mutant mice. Psychopharmacology. 2003;169(1):108–114. doi: 10.1007/s00213-003-1472-4. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Roger-Sánchez C, Anderson KL. Role of the nNOS gene in ethanol-induced conditioned place preference in mice. Alcohol (Fayetteville, NY) 2009;43(4):285–291. doi: 10.1016/j.alcohol.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Lumeng L. Alcohol preference and voluntary alcohol intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcoholism: Clinical and Experimental Research. 1984;8(5):485–486. doi: 10.1111/j.1530-0277.1984.tb05708.x. [DOI] [PubMed] [Google Scholar]
- Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997;80(4):1075–1086. doi: 10.1016/S0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Sollars SI. Contributory role of sex differences in the variations of gustatory function. Journal of Neuroscience Research. 2017;95(1–2):594–603. doi: 10.1002/jnr.23819. [DOI] [PubMed] [Google Scholar]
- Morse AC, Erwin VG, Jones BC. Behavioral responses to low doses of cocaine are affected by genetics and experimental history. Physiology & Behavior. 1995;58(5):891–897. doi: 10.1016/0031-9384(95)00144-8. [DOI] [PubMed] [Google Scholar]
- Nentwig TB, Myers KP, Grisel JE. Initial subjective reward to alcohol in Sprague-Dawley rats. Alcohol (Fayetteville, NY) 2017;58:19–22. doi: 10.1016/j.alcohol.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizhnikov ME, Pautassi RM, Truxell E, Spear NE. Opioid antagonists block the acquisition of ethanol-mediated conditioned tactile preference in infant rats. Alcohol (Fayetteville, NY) 2010;43(5):347–358. doi: 10.1016/j.alcohol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, Middaugh LD, Tavernetti M. Ethanol consumption and place-preference conditioning in the alcohol-preferring C57BL/6 mouse: relationship with motor activity patterns. Alcoholism: Clinical and Experimental Research. 1999;23(4):683–692. [PubMed] [Google Scholar]
- Phillips TJ, Broadbent J, Burkhart-Kasch S, Henderson C, Wenger CD, McMullin C, et al. Genetic correlational analyses of ethanol reward and aversion phenotypes in short-term selected mouse lines bred for ethanol drinking or ethanol-induced conditioned taste aversion. Behavioral Neuroscience. 2005;119(4):892–910. doi: 10.1037/0735-7044.119.4.892. [DOI] [PubMed] [Google Scholar]
- Podhorna J, Brown RE. Strain differences in activity and emotionality do not account for differences in learning and memory performance between C57BL/6 and DBA/2 mice. Genes, Brain and Behavior. 2002;1(2):96–110. doi: 10.1034/j.1601-183X.2002.10205.x. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neuroscience and Biobehavioral Reviews. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Roger-Sánchez C, Aguilar MA, Rodríguez-Arias M, Aragon CM, Miñarro J. Age- and sex-related differences in the acquisition and reinstatement of ethanol CPP in mice. Neurotoxicology and Teratology. 2012;34(1):108–115. doi: 10.1016/j.ntt.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O’Neill L, Kuhn CM. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology. 2014;231(8):1831–1839. doi: 10.1007/s00213-013-3319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill LK, Berthold C, Koss WA, Juraska JM, Gulley JM. Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behavioural Brain Research. 2011;225(1):104–109. doi: 10.1016/j.bbr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Wang XY, Zhao M, Wang XY, Zhai HF, Lu L. Role of Stress in Acquisition of Alcohol-Conditioned Place Preference in Adolescent and Adult Mice. Alcoholism: Clinical and Experimental Research. 2007;31(12):2001–2005. doi: 10.1111/j.1530-0277.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, O'Dell LE. Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcoholism: Clinical and Experimental Research. 2014;38(1):108–115. doi: 10.1111/acer.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Swearingen AED, Walker QD, Kuhn CM. Sex differences in novelty- and psychostimulant-induced behaviors of C57BL/6 mice. Psychopharmacology. 2013;225(3):707–718. doi: 10.1007/s00213-012-2860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol (Fayetteville, NY) 2008;42(3):149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.