Abstract
Holoprosencephaly (HPE) is partial or complete failure of the forebrain to divide into hemispheres and can be an isolated finding or associated with a syndrome. Most cases of HPE are associated with a syndrome and roughly 40–60% of fetuses with HPE have trisomy 13 which is the most common etiology of HPE. Other syndromes associated with HPE include additional aneuploidies like trisomy 18 and single gene disorders such as Smith-Lemli-Opitz syndrome. There are a number of syndromes such as pseudotrisomy 13 which do not have a known molecular etiology; therefore, this review has two parts: syndromes with a molecular diagnosis and syndromes where the etiology is yet to be found. As most HPE is syndromic, this review provides a comprehensive list and description of syndromes associated with HPE that may be used as a differential diagnosis and starting point for evaluating individuals with HPE.
INTRODUCTION
Holoprosencephaly is a relatively common forebrain malformation, occurring in 1 in 1298 fetuses (1st and 2nd trimesters) (Kagan, Staboulidou, Syngelaki, Cruz, & Nicolaides, 2010). Holoprosencephaly (HPE) is characterized by complete or partial failure of the prosencephalon (forebrain) to separate in early embryogenesis into two cerebral hemispheres. HPE is often accompanied by midline facial anomalies such as hypotelorism, cleft lip/palate, and in severe cases, cyclopia and a proboscis (Figure 1A). HPE is a relatively common finding in fetal surveys with one large prenatal ultrasound screening study finding 44 cases in 55,117 cases screened or 1:1298 (Kagan et al., 2010). HPE may occur in isolation (nonsyndromic) and is often associated with pathogenic variants in the genes SHH, SIX3, and ZIC2; however, HPE most commonly occurs as part of a syndrome. The most common syndromes include aneuploidies such as trisomies 13, 18 and 22, Smith-Lemli-Opitz syndrome, and Hartsfield syndrome (Dubourg et al., 2007; Solomon, Gropman, & Muenke, 1993).
Figure 1.
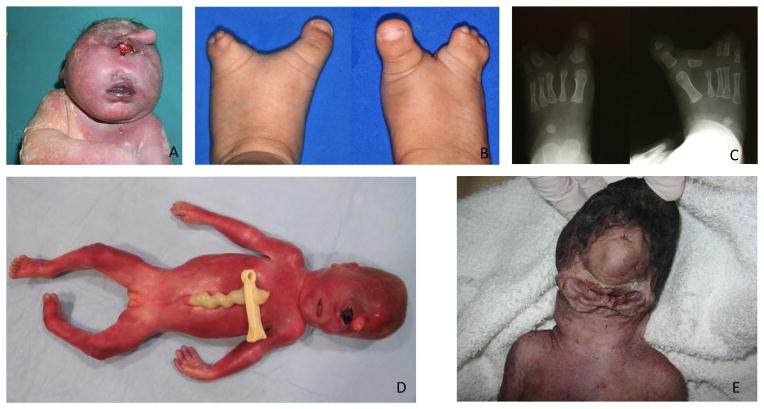
A) Trisomy 13 fetus with cyclopia and proboscis. Reprinted by permission from Springer Nature (Capobianco et al., 2007). B) Bilateral split foot. Reprinted by permission from Oxford University Press (Hong et al., 2016). C) Bilateral split foot and the absence of second digit phalanges bilaterally on X-ray. Reprinted by permission from Oxford University Press (Hong et al., 2016). D) Frontal view of a 24-week-old fetus. Note cyclopia (synophthalmia), ambiguous genitalia, and partial syndactyly of 2nd and 3rd toes on the right Reprinted by permission from John Wiley and Sons (Weaver et al., 2010). E) Agnathia–otocephaly complex with cyclopia, agnathia, microstomia and ventromedial ear position. Reprinted by permission from BMJ Publishing Group Ltd. (Wai & Chandran, 2017).
Advances in genomic technology during the last three decades has allowed for a molecular diagnosis to accompany many of the syndromes comprising HPE. As with any major malformation such as HPE, the clinician is obligated to look for findings in other organ systems that may lead to a syndromic diagnosis. The purpose of this review is to summarize syndromes associated with holoprosencephaly.
METHODS
PubMed was queried for the terms holoprosencephaly, syndromes, and the list of specific syndromes known by our group to be associated with HPE. Additionally, reference lists of journal articles where queried for relevant studies. Table I summarizes reported syndromes in the medical literature that have had at least one case of holoprosencephaly.
Table I.
Syndromic cases of holoprosencephaly (HPE) with a molecular diagnosis. Case reports are not listed. In the “cases screened column”, the phenotype is clarified in parentheses to specify how a cohort was ascertained.
| Condition | HPE cases | Cases screened (denominator) | Holoprosencephaly type | Extra-CNS anomalies | Molecular findings | Reference |
|---|---|---|---|---|---|---|
| Trisomy 13 | 1 | 13 (trisomy 13) | holoprosencephaly not specified | multiple congenital anomalies | karyotype | (Hsu & Hou, 2007) |
| Trisomy 13 | 13 | 33 (trisomy 13) | holoprosencephaly not specified | multiple congenital anomalies | karyotype | (Lehman et al., 1995) |
| Trisomy 13 | 4 | 23 (trisomy 13) | holoprosencephaly not specified | multiple congenital anomalies | karyotype | (H. Y. Lin et al., 2007) |
| Trisomy 13 | 5 | 28 (trisomy 13) | fetal ultrasound: holoprosencephaly | multiple congenital anomalies | karyotype | (Papp et al., 2006) |
| Trisomy 13 | 12 | 28 (HPE) | fetal ultrasound: holoprosencephaly | multiple congenital anomalies | karyotype | (Petracchi et al., 2011) |
| Trisomy 18 | 2 | 28 (HPE) | fetal ultrasound: holoprosencephaly | multiple congenital anomalies | karyotype | (Petracchi et al., 2011) |
| Trisomy 18 | 1 | 14 (trisomy 18) | fetal ultrasound: holoprosencephaly | multiple congenital anomalies | karyotype | (Rosa et al., 2017) |
| Trisomy 16 | 1 | 28 (HPE) | fetal ultrasound holoprosencephaly | multiple congenital anomalies | karyotype | (Petracchi et al., 2011) |
| Triploidy | 3 | 54 (triploidy) | holoprosencephaly not specified | multiple congenital anomalies | karyotype | (Toufaily et al., 2016) |
| 13q deletion syndrome | 4 | 12 (13q deletion) | holoprosencephaly not specified | multiple congenital anomalies | 13q31.1–13qter; 13q31.1–13qter; 13q31.3–13q33.1; 13q32.3 | (Quelin et al., 2009) |
| Smith-Lemli-Opitz syndrome | 1 | 18 (SLOS) | holoprosencephaly not specified | multiple congenital anomalies | (elevation of 7-DHC and 8-DHC/decreased cholesterol | (Caruso et al., 2004) |
| Smith-Lemli-Opitz syndrome | 1 | 50 (sporadic cases of HPE) | semi-lobar | polydactyly of the right hand and mild cutaneoussyndactyly of toes 2 and 3 | increased 7-DHC/cholesterol ratio | (Kelley et al., 1996) |
| Smith-Lemli-Opitz syndrome | 2 | 28 (HPE) | fetal ultrasound holoprosencephaly | multiple congenital anomalies | DHCR7: c.964-1G > C; c.440 G>A | (Petracchi et al., 2011) |
| Hartsfield syndrome | 7 | 200 (HPE) | semi-lobar; microform; not specified; microform; lobar; semi-lobar/lobar (sisters) | limb anomalies | FGFR1: p.Asp641Asn; p.Arg627Thr; p.Asp623Glu; p.Met535Lys; p.Gly487Asp; Glu294Lys | (Hong et al., 2016) |
| Hartsfield syndrome | 6 | 7 (Hartsfield syndrome) | alobar; lobar; semi-lobar; lobar; semi-lobar; lobar | limb anomalies | p.L165S*; p.L191S*; p.G490R; p.D623Y; p.N628K; p.C725Y | (Simonis et al., 2013) |
SYNDROMES ASSOCIATED WITH MOLECULAR DIAGNOSES
Trisomy 13
Trisomy 13 is the most common cause of HPE. Multiple organ systems can be affected in trisomy 13 and common physical exam findings include microphthalmia/anophthalmia, cleft lip and palate, postaxial polydactyly, and rocker bottom feet. Holoprosencephaly has been reported in 8%–39% of individuals with trisomy 13 (Hsu & Hou, 2007; Lehman et al., 1995; H. Y. Lin et al., 2007; Papp et al., 2006). Much of the data associating trisomy 13 HPE has come from prenatal testing. In a screen of over 50,000 pregnancies, Kagan et al. found that 65.9% of fetuses with holoprosencephaly had abnormal karyotypes and the majority (86%) of these abnormal karyotypes were trisomy 13 (Kagan et al., 2010). In another large study in Argentina of 13,883 ultrasounds with prenatal genetic testing, 28 fetuses were found to have holoprosencephaly (0.2%) and 12 cases (43%) had trisomy 13. (Petracchi, Crespo, Michia, Igarzabal, & Gadow, 2011). Figure 1A shows the most severe form of holoprosencephaly in a patient with trisomy 13, born with cyclopia and a proboscis (Capobianco et al., 2007). Due to the high incidence of trisomy 13 in HPE cases, trisomy 13 is at the top of the differential diagnosis for HPE. The mechanism of trisomy 13’s relationship to HPE is not understood. ZIC2, a gene commonly associated with HPE is located at 13q32.3, but ZIC2 variants are loss of function (Roessler et al., 2009). There currently is no available mouse model for trisomy 13, but human chromosome 13 has synteny with six mouse chromosome segments, making a model possible (Sheppard, Wiseman, Ruparelia, Tybulewicz, & Fisher, 2012). Promising models for understanding the pathogenicity of trisomy 13 are stem cell and gene expression research (Biancotti et al., 2010; Zhang et al., 2013).
Other aneuploidies
After trisomy 13, the most common aneuploidies associated with HPE are trisomy 18, trisomy 21 and trisomy 22. Central nervous system anomalies found in trisomy 18 include corpus callosum agenesis, spina bifida, and cerebellar hypoplasia (Cereda & Carey, 2012). Holoprosencephaly is a less common CNS manifestation in trisomy 18. In a study of 14 fetuses with trisomy 18, only one (7.1%) had holoprosencephaly (Rosa et al., 2017). Petracchi et al. in their study of 13,883 prenatal diagnoses, found 2 cases of trisomy 18 in 28 fetuses with holoprosencephaly (Petracchi et al., 2011). More research is needed to know why trisomy 18 infrequently presents with holoprosencephaly. TGIF, which is one of the known gene associations with HPE (Gripp et al., 2000), is located at 18p11.31; additionally 10–15% of individuals with monosomy 18p present with HPE (Turleau, 2008). At least 5 cases of Down syndrome with holoprosencephaly have been individually reported (Basu, Kumar, & Das, 2009; Epstein, Seto, & Golabi, 1988; Hamada et al., 1991; Martinez-Frias, 1989; Urioste et al., 1988). Down syndrome is common and holoprosencephaly associated with Down syndrome is very rare; thus, the question of coincidence versus causation remains to be answered (Epstein et al., 1988; Martinez-Frias, 1989). Trisomy 22 is a rare condition. Three cases of trisomy 22 and HPE has been reported (Fahmi, Schmerler, & Hutcheon, 1994; Isada, Bolan, Larsen, & Kent, 1990; Kehinde et al., 2014). One case of trisomy 16 has been associated with HPE, Petracchi et al. in their study of 13,883 prenatal diagnoses, found 1 case of trisomy 16 presenting with holoprosencephaly.
Triploidy
Common findings in triploidy include lethality, microcephaly, limb anomalies and rarely holoprosencephaly. In a study of 54 triploidy fetuses at Brigham and Women’s Hospital in Boston, 3 were found to have holoprosencephaly (Toufaily, Roberts, Westgate, & Holmes, 2016). In the Kagan et al. study, 3 of the 29 cases of holoprosencephaly with abnormal karyotypes were found to be triploidy (Kagan et al., 2010).
13q deletion syndrome
Not surprisingly, there have been multiple reports of chromosome 13q (location of ZIC2) deletions associated with holoprosencephaly, known as 13q deletion syndrome (Araujo Junior, Filho, Pires, & Filho, 2006; Garcia-Rodriguez, Garcia-Garcia, Perez-Sanchez, & Pavon-Delgado, 2015; Marcorelles et al., 2002; Mimaki et al., 2015; Quelin et al., 2009). In a series of 12 patients with varying 13q deletions, the 4 cases with holoprosencephaly all had ZIC2 in the deletion interval (Quelin et al., 2009). Other manifestations of 13q deletion syndrome include cognitive impairment, growth delay, facial anomalies, and limb, kidney, eye, and heart malformations (Quelin et al., 2009). The phenotype and genotype of 13q deletion syndrome is variable; the Online Medelian in Man ((Online Mendelian Inheritance in Man)) website defines 13q deletion syndrome as a 13q14 deletion syndrome comprising a 16Mb interval, which does not contain ZIC2 [OMIM, accessed 3/15/2018]; however, the medical literature in general labels most deletions on 13q as part of this syndrome (Araujo Junior et al., 2006; Mimaki et al., 2015; Quelin et al., 2009).
18p deletion syndrome
TGIF1, associated a very small fraction of HPE, is located on at this locus (Gripp et al., 2000; Mercier et al., 2011). Adding to evidence of pathogenicity are multiple reports of 18p deletions involving TGIF1 found with HPE (Chen et al., 2013; Portnoi et al., 2007; Yi et al., 2014).
Smith-Lemli-Opitz syndrome
Smith-Lemli-Opitz syndrome (SLOS) is a multiple congenital anomaly syndrome that presents with intellectual disability, facial dysmorphisms, congenital heart anomalies, and external genitalia defects in males. Multiple case reports have associated SLOS with HPE (Caruso et al., 2004; Kelley et al., 1996; Nowaczyk et al., 2001; Travessa, Dias, Rocha, & Sousa, 2017; Weaver, Solomon, Akin-Samson, Kelley, & Muenke, 2010). Even though only 5% of individuals with Smith-Lemli-Opitz syndrome (SLOS) present with HPE (Caruso et al., 2004), it remains the classic example of a single gene variant associated with syndromic HPE, and its cholesterol metabolism perturbation makes this syndrome more interesting. Low cholesterol has been proposed to affect the SHH signaling pathway (Haas & Muenke, 2010) and SLOS is caused by a deficiency of the enzyme 7-dehyrocholestol reductase resulting in a block in the last step of cholesterol synthesis where 7-DHC is not converted to cholesterol. Figure 1D demonstrates clinical findings in a 24 week gestation fetus with SLOS and HPE including cyclopia, ambiguous genitalia, and syndactyly of toes 2 and 3 on the right (Weaver et al., 2010).
Hartsfield syndrome
Hartsfield syndrome is characterized by holoprosencephaly, cleft lip and palate, and unilateral or bilateral split hands/feet (Figure 1B–C), also known as ectrodactyly (Hong et al., 2016; Simonis et al., 2013). Simonis et al. found heterozygous variants in FGFR1 in six of seven patients with Hartsfield syndrome, four with heterozygous variants in the intracellular kinase domain and two individuals with homozygous variants located in the extracellular domain (Simonis et al., 2013). Our group recently screened 200 probands with holoprosencephaly and found 7 cases of pathogenic variants in FGFR1 (Hong et al., 2016). In Hong et al., zebrafish studies demonstrated that the variants in FGFR1 acted in a dominant negative fashion.
Steinfeld syndrome
Steinfeld first reported a female child with HPE, bilateral reduction defects in upper limbs, midline cleft lip and palate, congenital heart disease, and renal anomalies (Steinfeld, 1982). Four further cases have been reported since this report (Jones et al., 2016; Nothen, Knopfle, Fodisch, & Zerres, 1993; Siebert, Schoenecker, Resta, & Kapur, 2005; Stevens, 2010). Steinfeld syndrome is characterized by holoprosencephaly and limb anomalies. One report has connected Steinfeld to a variant in CDON (Jones et al., 2016); however, this was not classic HPE, but microform. Another report has associated isolated (nonsyndromic) HPE with CDON variants but did not classify the patients as having Steinfeld syndrome, as the patients did not have limb anomalies (Bae et al., 2011).
Culler-Jones syndrome
Special mention is made of Culler-Jones syndrome, a syndrome that is associated with GLI2, a gene which has been associated with “holoprosencephaly-like” features in the past (Roessler et al., 2003). Culler-Jones syndrome (CJS) is characterized by hypopituitarism and postaxial polydactyly. Bear et al. screened approximately 400 individuals with HPE spectrum phenotype for GLI2 variants and found variants in 112 individuals, with 43 of these variants being truncating variants. Of the 43 individuals with truncating variants, only one had a brain malformation consistent with HPE (Bear et al., 2014). Thus, individuals with GLI2 variants and CJS have a well-defined phenotype that does not usually include HPE.
SYNDROMES WITHOUT MOLECULAR DIAGNOSES
Pseudotrisomy 13
Pseudotrisomy 13 syndrome, also known as holoprosencephaly-polydactyly syndrome, refers to HPE associated with postaxial polydactyly and a normal karyotype. Because HPE and polydactyly are features of trisomy 13, Hewitt et al. suggested the term pseudotrisomy 13 for this presentation given the normal karyotype (Hewitt, Seller, Bennett, & Maxwell, 1989), and Cohen and Gorlin later “coined” the term pseudotrisomy 13 syndrome (Cohen & Gorlin, 1991). Bous et al. reviewed 40 cases of pseudotrisomy 13 syndrome and reported 80% of cases with classic HPE (MRI evidence of HPE), 80% with polydactyly, and 58% with a cardiac anomaly (Bous et al., 2012). There is no known molecular diagnostic association and research with next generation sequencing is needed to more thoroughly interrogate pseudotrisomy 13 for a molecular diagnosis.
Hydrolethalus syndrome
Hydrolethalus syndrome is characterized by a lethal form of brain malformations, usually hydrocephalus and absent midline structures, micrognathia, polydactyly, and defective lobation of the lungs (Salonen, Herva, & Norio, 1981). We mention hydrolethalus syndrome as Bachman et al. found a case of holoprosencephaly, hydrocephalus, and polydactyly in a consanguineous Mexican-American family and proposed that pseudotrisomy 13 cases were part of hydrolethalus syndrome (Bachman, Clark, & Salahi, 1990). Subsequent to Bachman et al.’s 1990 study, hydrolethalus syndrome was found to be an autosomal recessive condition associated with two genes, HYLS1 and KIF7 (Mee et al., 2005; Putoux et al., 2011). However, no cases of hydrolethalus syndrome with a molecular diagnosis have been associated with HPE. A review of 21 cases with molecularly confirmed variants in the gene HYLS1 found that all cases had a complete interhemispheric fissure, and in a few cases, a hypothalamic hamartoma was found (Paetau et al., 2008). Holoprosencephaly is not a common finding in hydrolethalus syndrome.
Pallister-Hall syndrome
Pallister-Hall syndrome is diagnosed in the presence of a hypothalamic hamartoma and mesoaxial polydactyly, with confirmation in a heterozygous pathogenic variant in GLI3. Verloes et al. presented a case with alobar HPE, hypothalamic hamartoma, and polydactyly, and reviewed an additional 27 cases of hypothalamic hamartoma with other congenital anaomalies (Verloes, Gillerot, Langhendries, Fryns, & Koulischer, 1992). This group proposed a classification that would combine cases with hypothalamic hamartomas and HPE (i.e. Pallister-Hall syndrome) and cases of HPE and polydactyly without hypothalamic hamartomas under a classification of Cebro-Acro-Visceral Early Lethality (CAVE) Multiplex Syndrome (Verloes et al., 1992). Additionally, SLOS type II, hydrolethalus, pseudotrisomy 13 (holoprosencpehaly-polydactyly syndrome), orofacialdigital type IV were included in this umbrella. Since the availability of a molecular test for Pallister-Hall syndrome, there has been no confirmed PHS case with HPE. We conclude that HPE is not a frequent finding in HPS.
Agnathia-Otocephaly Complex
Agnathia-Otocephaly complex (AGOTC) is a rare malformation with failure of the first arch development and is characterized by agnathia, ventromedial ear position, microstomia, and holoprosencephaly (Figure 1E) (Faye-Petersen et al., 2006). The gene PRRX1 (Celik et al., 2012; Dasouki, Andrews, Parimi, & Kamnasaran, 2013) has been associated with AGOC; however, there are no cases of AGOTC with HPE associated with PPRX1 (Dasouki et al., 2013).
CHARGE syndrome
Lin et al. evaluated 144 patients with CHARGE syndrome, and three were found to have holoprosencephaly (Lin, Siebert, & Graham, 1990); however, these patients did not have a molecular diagnosis as CHD7 would not be associated with CHARGE syndrome for another 14 years.
Genoa syndrome
Camera et al. reported 2 siblings with semilobar HPE and craniosynostosis involving the coronal and lambdoid sutures and called this Genoa syndrome (Camera, Lituania, & Cohen, 1993). There have been other reports of individuals with both HPE and craniosynostosis; however, and etiology remains elusive (Hacihamdioglu et al., 2010; Lapunzina, Musante, Pedraza, Prudent, & Gadow, 2001; C. H. Lin, Tsai, Ho, & Lin, 2009; Raam, Solomon, Shalev, & Muenke, 2010).
Lambotte syndrome
Lambotte et al. in 1978 first reported a new multiple congenital anomaly condition in two siblings with microcephaly, intrauterine growth retardation (IUGR), cerebral malformation, and early lethality in two siblings. Verloes et al. later reported 4 siblings from one family with Lambotte syndrome where one of the siblings had semilobar HPE (Verloes, Dodinval, Beco, Bonnivert, & Lambotte, 1990). Subsequently, an unaffected sister in the family described by Verloes et al. gave birth to an affected child, and a t(2;4)(q37.1;p16.2) translocation was found in the mother, suggesting a combination of 2q/4p trisomy/monosomy in all of the affected children of this family (Herens et al., 1997).
Agnathia-microstomia-synotia syndrome
Agnathia-microstomia-synotia syndrome is a rare lethal congenital malformation of the first branchial arch that presents with agnathia, mandibular hypoplasia, anteromedial malposition of ears, microstomia, and aglossia or microglossia. A number of case reports of agnathia-microstomia-synotia have shown HPE (Chaoui, Heling, Thiel, & Karl, 2011; Faye-Petersen et al., 2006; Wai & Chandran, 2017).
Amelia, cleft lip, and holoprosencephaly
Five cases of amelia, cleft lip, and holoprosencephaly have been reported (Kariminejad, Goodarzi, Asghari-Roodsari, & Kariminejad, 2009; Thomas & Donnai, 1994; Zimpfer et al., 2007). The association of HPE with limb defects is of special interest as SHH signaling directs digit number and identity in the vertebrate limb (Vokes, Ji, Wong, & McMahon, 2008); however, the mechanism responsible for the association between holoprosencephaly and amelia remains unknown.
DISCUSSION
Most cases of holoprosencephaly are associated with a syndrome and trisomy 13 is the most common etiology. Syndromes associated with single gene variants are much less common and make up a limited amount of cases. Over the last two decades, the advancement of molecular diagnoses attached to syndromes has better defined genetic syndromes. As next generation sequencing becomes more available (Roessler, Hu, & Muenke, 2018), larger sets of genes will be able to be tested per sample, allowing for expansion of syndrome associated with HPE. As noted above, there are still a number of syndromes associated with HPE that do not have a molecular etiology such as pseudotrisomy 13, Genoa syndrome and Agnathia-microstomia-synotia syndrome. Additionally, there are syndromes such as CHARGE syndrome with a known genetic etiology that was associated with HPE before available genetic testing and a case with HPE and a pathogenic variant is missing from the medical literature. In the coming years, many of these questions will be answered from the research community.
In addition to the diagnostic value to families and patients of associating syndromes with HPE, the connection of HPE with multiple congenital anomaly syndromes inform us about the pathophysiology of embryonic brain development. HPE is a midline malformation disorder; however, HPE syndromes are associated with other midline brain anomalies. As an example, Smith-Lemli-Opitz syndrome (SLOS) is often associated with brain malformations other than HPE. In the largest brain imaging study of 55 individuals with SLOS, Lee et al. found 53 of 55 (96%) individuals to have aberrant brain MRI scans (Lee, Conley, Gropman, Porter, & Baker, 2013). Abnormalities of the septum pellucidum were found in 42/55 (76%) and of the corpus callosum in 38/55 (69%). Knowing that sonic hedgehog processing and signaling is dependent on cholesterol (Cooper et al., 2003; Porter, Young, & Beachy, 1996), Lee et al. correlated the severity of the brain malformations to sterol levels in these patients with SLOS. We next direct our attention to Hartsfield syndrome and FGFR1 variants as a second example of midline brain malformations (Lee et al., 2013). Although Hartsfield syndrome is defined by the co-occurrence of HPE, ectrodactyly, and FGFR1 variants (Simonis et al., 2013), there a number of other conditions caused by FGFR1 variants including Pfeiffer syndrome, Kallmann syndrome, Hartsfield syndrome, and normosmic hypogonadotropic hypogonadism (nIHH) (Hong et al., 2016). Kallmann syndrome may be associated with agenesis of the corpus callosum and dysgenesis of the olfactory bulbs, neural structures in vertebrate forebrains. Klein et al. reported a case of choanal atresia in a family with Kallmann syndrome, suggesting that Kallmann syndrome may be the least severe form of the holoprosencephaly-hypopituitarism complex (Klein, Friedman, Brookshire, Brown, & Edman, 1987). The anterior neural ridge located at the rostral edge of the embryonic neural plate is one of three organizing centers in the developing prosencephalon (embryonic forebrain) and secretes fgf ligands and reduction of fgf signaling in the mouse model in the telencephalon results in HPE-like malformations (Paek, Gutin, & Hebert, 2009; Storm et al., 2006). Hong et al. determined that in families affected by Hartsfield syndrome, FGFR1 acted in a dominant negative fashion which is consistent with known loss of function mechanism in Kallmann syndrome (Hong et al., 2016). SLOS and Hartsfield are excellent examples of how syndromic disease has advance our knowledge of brain development and there is room for much more to learn.
In summary, we present a comprehensive list and description of syndromic HPE in this review. As most HPE is syndromic, this paper will serve as a differential diagnosis in individuals diagnosed with HPE.
Acknowledgments
P.K. and M.M are supported by the Division of Intramural Research at the National Human Genome Research Institute, NIH. The authors have no conflicts of interest to declare.
Biographies
Paul S. Kruszka, MD, MPH, is a board-certified family physician and clinical geneticist at the Medical Genetics Branch, National Human Genome Research Institute, National Institutes of Health (Bethesda, MD). His research interest includes early embryonic errors in brain, craniofacial, and heart development.
Maximilian Muenke, MD, is a board-certified pediatric geneticist and the Branch Chief of the Medical Genetics Branch, National Human Genome Research Institute, National Institutes of Health, Bethesda, MD. He has worked on the genetics of HPE for the past 25 years. His group has discovered numerous genes contributing to HPE, including the first and most well-known gene, Sonic Hedgehog. His other research interests include cardiac disease, craniofacial malformation syndromes, attention-deficit-hyperactivity disorder (ADHD), and the genetics of diverse populations.
References
- Araujo E, Junior, Filho HA, Pires CR, Filho SM. Prenatal diagnosis of the 13q-syndrome through three-dimensional ultrasonography: a case report. Arch Gynecol Obstet. 2006;274(4):243–245. doi: 10.1007/s00404-006-0128-0. [DOI] [PubMed] [Google Scholar]
- Bachman H, Clark RD, Salahi W. Holoprosencephaly and polydactyly: a possible expression of the hydrolethalus syndrome. J Med Genet. 1990;27(1):50–52. doi: 10.1136/jmg.27.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae GU, Domene S, Roessler E, Schachter K, Kang JS, Muenke M, Krauss RS. Mutations in CDON, encoding a hedgehog receptor, result in holoprosencephaly and defective interactions with other hedgehog receptors. Am J Hum Genet. 2011;89(2):231–240. doi: 10.1016/j.ajhg.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Kumar A, Das BK. Down syndrome (trisomy 21) with premaxillary agenesis and semilobar holoprosencephaly. Am J Med Genet A. 2009;149A(11):2578–2580. doi: 10.1002/ajmg.a.33072. [DOI] [PubMed] [Google Scholar]
- Bear KA, Solomon BD, Antonini S, Arnhold IJ, Franca MM, Gerkes EH, … Muenke M. Pathogenic mutations in GLI2 cause a specific phenotype that is distinct from holoprosencephaly. J Med Genet. 2014;51(6):413–418. doi: 10.1136/jmedgenet-2013-102249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancotti JC, Narwani K, Buehler N, Mandefro B, Golan-Lev T, Yanuka O, … Lavon N. Human embryonic stem cells as models for aneuploid chromosomal syndromes. Stem Cells. 2010;28(9):1530–1540. doi: 10.1002/stem.483. [DOI] [PubMed] [Google Scholar]
- Bous SM, Solomon BD, Graul-Neumann L, Neitzel H, Hardisty EE, Muenke M. Holoprosencephaly-polydactyly/pseudotrisomy 13: a presentation of two new cases and a review of the literature. Clin Dysmorphol. 2012;21(4):183–190. doi: 10.1097/MCD.0b013e3283551fd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camera G, Lituania M, Cohen MM., Jr Holoprosencephaly and primary craniosynostosis: the Genoa syndrome. Am J Med Genet. 1993;47(8):1161–1165. doi: 10.1002/ajmg.1320470806. [DOI] [PubMed] [Google Scholar]
- Capobianco G, Cherchi PL, Ambrosini G, Cosmi E, Andrisani A, Dessole S. Alobar holoprosencephaly, mobile proboscis and trisomy 13 in a fetus with maternal gestational diabetes mellitus: a 2D ultrasound diagnosis and review of the literature. Arch Gynecol Obstet. 2007;275(5):385–387. doi: 10.1007/s00404-006-0264-6. [DOI] [PubMed] [Google Scholar]
- Caruso PA, Poussaint TY, Tzika AA, Zurakowski D, Astrakas LG, Elias ER, … Irons MB. MRI and 1H MRS findings in Smith-Lemli-Opitz syndrome. Neuroradiology. 2004;46(1):3–14. doi: 10.1007/s00234-003-1110-1. [DOI] [PubMed] [Google Scholar]
- Celik T, Simsek PO, Sozen T, Ozyuncu O, Utine GE, Talim B, … Kamnasaran D. PRRX1 is mutated in an otocephalic newborn infant conceived by consanguineous parents. Clin Genet. 2012;81(3):294–297. doi: 10.1111/j.1399-0004.2011.01730.x. [DOI] [PubMed] [Google Scholar]
- Cereda A, Carey JC. The trisomy 18 syndrome. Orphanet J Rare Dis. 2012;7:81. doi: 10.1186/1750-1172-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaoui R, Heling KS, Thiel G, Karl K. Agnathia-otocephaly with holoprosencephaly on prenatal three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2011;37(6):745–748. doi: 10.1002/uog.9009. [DOI] [PubMed] [Google Scholar]
- Chen CP, Huang JP, Chen YY, Chern SR, Wu PS, Su JW, … Wang W. Chromosome 18p deletion syndrome presenting holoprosencephaly and premaxillary agenesis: prenatal diagnosis and aCGH characterization using uncultured amniocytes. Gene. 2013;527(2):636–641. doi: 10.1016/j.gene.2013.06.081. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Jr, Gorlin RJ. Pseudo-trisomy 13 syndrome. Am J Med Genet. 1991;39(3):332–335. doi: 10.1002/ajmg.1320390316. discussion 336–337. [DOI] [PubMed] [Google Scholar]
- Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, … Beachy PA. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33(4):508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- Dasouki M, Andrews B, Parimi P, Kamnasaran D. Recurrent agnathia-otocephaly caused by DNA replication slippage in PRRX1. Am J Med Genet A. 2013;161A(4):803–808. doi: 10.1002/ajmg.a.35879. [DOI] [PubMed] [Google Scholar]
- Dubourg C, Bendavid C, Pasquier L, Henry C, Odent S, David V. Holoprosencephaly. Orphanet J Rare Dis. 2007;2:8. doi: 10.1186/1750-1172-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein CJ, Seto S, Golabi M. Chance vs. causality in the association of Down syndrome and holoprosencephaly. Am J Med Genet. 1988;30(4):939–942. doi: 10.1002/ajmg.1320300410. [DOI] [PubMed] [Google Scholar]
- Fahmi F, Schmerler S, Hutcheon RG. Hydrocephalus in an infant with trisomy 22. J Med Genet. 1994;31(2):141–144. doi: 10.1136/jmg.31.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye-Petersen O, David E, Rangwala N, Seaman JP, Hua Z, Heller DS. Otocephaly: report of five new cases and a literature review. Fetal Pediatr Pathol. 2006;25(5):277–296. doi: 10.1080/15513810601123417. [DOI] [PubMed] [Google Scholar]
- Garcia-Rodriguez E, Garcia-Garcia E, Perez-Sanchez A, Pavon-Delgado A. A NEW OBSERVATION OF 13q DELETION SYNDROME: SEVERE UNDESCRIBED FEATURES. Genet Couns. 2015;26(2):213–217. [PubMed] [Google Scholar]
- Gripp KW, Wotton D, Edwards MC, Roessler E, Ades L, Meinecke P, … Elledge SJ. Mutations in TGIF cause holoprosencephaly and link NODAL signalling to human neural axis determination. Nat Genet. 2000;25(2):205–208. doi: 10.1038/76074. [DOI] [PubMed] [Google Scholar]
- Haas D, Muenke M. Abnormal sterol metabolism in holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C(1):102–108. doi: 10.1002/ajmg.c.30243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacihamdioglu B, Siklar Z, Savas Erdeve S, Berberoglu M, Deda G, Tiras ST, … Ocal G. Genoa syndrome and central diabetes insipidus: a case report. J Clin Res Pediatr Endocrinol. 2010;2(2):89–91. doi: 10.4274/jcrpe.v2i2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Arinami T, Koresawa M, Kubo T, Hamaguchi H, Iwasaki H. A case of trisomy 21 with holoprosencephaly: the fifth case. Jinrui Idengaku Zasshi. 1991;36(2):159–163. doi: 10.1007/BF01876579. [DOI] [PubMed] [Google Scholar]
- Herens C, Jamar M, Alvarez-Gonzalez ML, Lesenfants S, Lombet J, Bonnivert J, … Verloes A. Private multiple congenital anomaly syndromes may result from unbalanced subtle translocations: t(2q;4p) explains the Lambotte syndrome. Am J Med Genet. 1997;73(2):127–131. doi: 10.1002/(sici)1096-8628(19971212)73:2<127::aid-ajmg5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hewitt BG, Seller MJ, Bennett CP, Maxwell DM. Holoprosencephaly, polydactyly and normal chromosomes: pseudo-trisomy 13? Clin Genet. 1989;36(2):141–143. doi: 10.1111/j.1399-0004.1989.tb03177.x. [DOI] [PubMed] [Google Scholar]
- Hong S, Hu P, Marino J, Hufnagel SB, Hopkin RJ, Toromanovic A, … Muenke M. Dominant-negative kinase domain mutations in FGFR1 can explain the clinical severity of Hartsfield syndrome. Hum Mol Genet. 2016;25(10):1912–1922. doi: 10.1093/hmg/ddw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HF, Hou JW. Variable expressivity in Patau syndrome is not all related to trisomy 13 mosaicism. Am J Med Genet A. 2007;143A(15):1739–1748. doi: 10.1002/ajmg.a.31835. [DOI] [PubMed] [Google Scholar]
- Isada NB, Bolan JC, Larsen JW, Jr, Kent SG. Trisomy 22 with holoprosencephaly: a clinicopathologic study. Teratology. 1990;42(4):333–336. doi: 10.1002/tera.1420420402. [DOI] [PubMed] [Google Scholar]
- Jones GE, Robertson L, Maniyar A, Shammas C, Phelan MM, Vasudevan PC, Tanteles GA. Microform holoprosencephaly with bilateral congenital elbow dislocation; increasing the phenotypic spectrum of Steinfeld syndrome. Am J Med Genet A. 2016;170(3):754–759. doi: 10.1002/ajmg.a.37511. [DOI] [PubMed] [Google Scholar]
- Kagan KO, Staboulidou I, Syngelaki A, Cruz J, Nicolaides KH. The 11–13-week scan: diagnosis and outcome of holoprosencephaly, exomphalos and megacystis. Ultrasound Obstet Gynecol. 2010;36(1):10–14. doi: 10.1002/uog.7646. [DOI] [PubMed] [Google Scholar]
- Kariminejad A, Goodarzi P, Asghari-Roodsari A, Kariminejad MH. Amelia, cleft lip, and holoprosencephaly: a distinct entity. Am J Med Genet A. 2009;149A(12):2828–2831. doi: 10.1002/ajmg.a.32933. [DOI] [PubMed] [Google Scholar]
- Kehinde FI, Anderson CE, McGowan JE, Jethva RN, Wahab MA, Glick AR, … Liu J. Co-occurrence of non-mosaic trisomy 22 and inherited balanced t(4;6)(q33;q23.3) in a liveborn female: case report and review of the literature. Am J Med Genet A. 2014;164A(12):3187–3193. doi: 10.1002/ajmg.a.36778. [DOI] [PubMed] [Google Scholar]
- Kelley RL, Roessler E, Hennekam RC, Feldman GL, Kosaki K, Jones MC, … Muenke M. Holoprosencephaly in RSH/Smith-Lemli-Opitz syndrome: does abnormal cholesterol metabolism affect the function of Sonic Hedgehog? Am J Med Genet. 1996;66(4):478–484. doi: 10.1002/(SICI)1096-8628(19961230)66:4<478::AID-AJMG22>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Klein VR, Friedman JM, Brookshire GS, Brown OE, Edman CD. Kallmann syndrome associated with choanal atresia. Clin Genet. 1987;31(4):224–227. doi: 10.1111/j.1399-0004.1987.tb02800.x. [DOI] [PubMed] [Google Scholar]
- Lapunzina P, Musante G, Pedraza A, Prudent L, Gadow E. Semilobar holoprosencephaly, coronal craniosynostosis, and multiple congenital anomalies: a severe expression of the Genoa syndrome or a newly recognized syndrome? Am J Med Genet. 2001;102(3):258–260. doi: 10.1002/ajmg.1467. [DOI] [PubMed] [Google Scholar]
- Lee RW, Conley SK, Gropman A, Porter FD, Baker EH. Brain magnetic resonance imaging findings in Smith-Lemli-Opitz syndrome. Am J Med Genet A. 2013;161A(10):2407–2419. doi: 10.1002/ajmg.a.36096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman CD, Nyberg DA, Winter TC, 3rd, Kapur RP, Resta RG, Luthy DA. Trisomy 13 syndrome: prenatal US findings in a review of 33 cases. Radiology. 1995;194(1):217–222. doi: 10.1148/radiology.194.1.7997556. [DOI] [PubMed] [Google Scholar]
- Lin AE, Siebert JR, Graham JM., Jr Central nervous system malformations in the CHARGE association. Am J Med Genet. 1990;37(3):304–310. doi: 10.1002/ajmg.1320370303. [DOI] [PubMed] [Google Scholar]
- Lin CH, Tsai JD, Ho YJ, Lin WC. Alobar holoprosencephaly associated with cebocephaly and craniosynostosis. Acta Neurol Taiwan. 2009;18(2):123–126. [PubMed] [Google Scholar]
- Lin HY, Lin SP, Chen YJ, Hsu CH, Kao HA, Chen MR, … Chan WT. Clinical characteristics and survival of trisomy 13 in a medical center in Taiwan, 1985–2004. Pediatr Int. 2007;49(3):380–386. doi: 10.1111/j.1442-200X.2007.02377.x. [DOI] [PubMed] [Google Scholar]
- Marcorelles P, Loget P, Fallet-Bianco C, Roume J, Encha-Razavi F, Delezoide AL. Unusual variant of holoprosencephaly in monosomy 13q. Pediatr Dev Pathol. 2002;5(2):170–178. doi: 10.1007/s10024-001-0200-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Frias ML. Association of holoprosencephaly and Down syndrome. Am J Med Genet. 1989;32(3):435. doi: 10.1002/ajmg.1320320334. [DOI] [PubMed] [Google Scholar]
- Mee L, Honkala H, Kopra O, Vesa J, Finnila S, Visapaa I, … Peltonen L. Hydrolethalus syndrome is caused by a missense mutation in a novel gene HYLS1. Hum Mol Genet. 2005;14(11):1475–1488. doi: 10.1093/hmg/ddi157. [DOI] [PubMed] [Google Scholar]
- Mercier S, Dubourg C, Garcelon N, Campillo-Gimenez B, Gicquel I, Belleguic M, … Odent S. New findings for phenotype-genotype correlations in a large European series of holoprosencephaly cases. J Med Genet. 2011;48(11):752–760. doi: 10.1136/jmedgenet-2011-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimaki M, Shiihara T, Watanabe M, Hirakata K, Sakazume S, Ishiguro A, … Mizuguchi M. Holoprosencephaly with cerebellar vermis hypoplasia in 13q deletion syndrome: Critical region for cerebellar dysgenesis within 13q32.2q34. Brain Dev. 2015;37(7):714–718. doi: 10.1016/j.braindev.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Nothen MM, Knopfle G, Fodisch HJ, Zerres K. Steinfeld syndrome: report of a second family and further delineation of a rare autosomal dominant disorder. Am J Med Genet. 1993;46(4):467–470. doi: 10.1002/ajmg.1320460426. [DOI] [PubMed] [Google Scholar]
- Nowaczyk MJ, Farrell SA, Sirkin WL, Velsher L, Krakowiak PA, Waye JS, Porter FD. Smith-Lemli-Opitz (RHS) syndrome: holoprosencephaly and homozygous IVS8-1G-->C genotype. Am J Med Genet. 2001;103(1):75–80. doi: 10.1002/1096-8628(20010915)103:1<75::aid-ajmg1502>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University. Baltimore, MD: [accessed: 3/15/2018]. Online Mendelian Inheritance in Man. Retrieved Date. https://omim.org/ [Google Scholar]
- Paek H, Gutin G, Hebert JM. FGF signaling is strictly required to maintain early telencephalic precursor cell survival. Development. 2009;136(14):2457–2465. doi: 10.1242/dev.032656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetau A, Honkala H, Salonen R, Ignatius J, Kestila M, Herva R. Hydrolethalus syndrome: neuropathology of 21 cases confirmed by HYLS1 gene mutation analysis. J Neuropathol Exp Neurol. 2008;67(8):750–762. doi: 10.1097/NEN.0b013e318180ec2e. [DOI] [PubMed] [Google Scholar]
- Papp C, Beke A, Ban Z, Szigeti Z, Toth-Pal E, Papp Z. Prenatal diagnosis of trisomy 13: analysis of 28 cases. J Ultrasound Med. 2006;25(4):429–435. doi: 10.7863/jum.2006.25.4.429. [DOI] [PubMed] [Google Scholar]
- Petracchi F, Crespo L, Michia C, Igarzabal L, Gadow E. Holoprosencephaly at prenatal diagnosis: analysis of 28 cases regarding etiopathogenic diagnoses. Prenat Diagn. 2011;31(9):887–891. doi: 10.1002/pd.2796. [DOI] [PubMed] [Google Scholar]
- Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274(5285):255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- Portnoi MF, Gruchy N, Marlin S, Finkel L, Denoyelle F, Dubourg C, … Houang M. Midline defects in deletion 18p syndrome: clinical and molecular characterization of three patients. Clin Dysmorphol. 2007;16(4):247–252. doi: 10.1097/MCD.0b013e328235a572. [DOI] [PubMed] [Google Scholar]
- Putoux A, Thomas S, Coene KL, Davis EE, Alanay Y, Ogur G, … Attie-Bitach T. KIF7 mutations cause fetal hydrolethalus and acrocallosal syndromes. Nat Genet. 2011;43(6):601–606. doi: 10.1038/ng.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelin C, Bendavid C, Dubourg C, de la Rochebrochard C, Lucas J, Henry C, … Pasquier L. Twelve new patients with 13q deletion syndrome: genotype-phenotype analyses in progress. Eur J Med Genet. 2009;52(1):41–46. doi: 10.1016/j.ejmg.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Raam MS, Solomon BD, Shalev SA, Muenke M. Holoprosencephaly and craniosynostosis: A report of two siblings and review of the literature. Am J Med Genet C Semin Med Genet. 2010;154C(1):176–182. doi: 10.1002/ajmg.c.30234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Du YZ, Mullor JL, Casas E, Allen WP, Gillessen-Kaesbach G, … Muenke M. Loss-of-function mutations in the human GLI2 gene are associated with pituitary anomalies and holoprosencephaly-like features. Proc Natl Acad Sci U S A. 2003;100(23):13424–13429. doi: 10.1073/pnas.2235734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Hu P, Muenke M. Holoprosencephaly in the genomics era. American Journal of Medical Genetics Part C (Seminars in Medical Genetics) 2018 doi: 10.1002/ajmg.c.31615. [DOI] [PubMed] [Google Scholar]
- Roessler E, Lacbawan F, Dubourg C, Paulussen A, Herbergs J, Hehr U, … Muenke M. The full spectrum of holoprosencephaly-associated mutations within the ZIC2 gene in humans predicts loss-of-function as the predominant disease mechanism. Hum Mutat. 2009;30(4):E541–554. doi: 10.1002/humu.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa RFM, Correia EPE, Bastos CS, da Silva GS, Correia JD, da Rosa EB, … Zen PRG. Trisomy 18 and holoprosencephaly. Am J Med Genet A. 2017 doi: 10.1002/ajmg.a.38129. [DOI] [PubMed] [Google Scholar]
- Salonen R, Herva R, Norio R. The hydrolethalus syndrome: delineation of a “new”, lethal malformation syndrome based on 28 patients. Clin Genet. 1981;19(5):321–330. doi: 10.1111/j.1399-0004.1981.tb00718.x. [DOI] [PubMed] [Google Scholar]
- Sheppard O, Wiseman FK, Ruparelia A, Tybulewicz VL, Fisher EM. Mouse models of aneuploidy. ScientificWorldJournal. 2012;2012:214078. doi: 10.1100/2012/214078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert JR, Schoenecker KA, Resta RG, Kapur RP. Holoprosencephaly and limb reduction defects: a consideration of Steinfeld syndrome and related conditions. Am J Med Genet A. 2005;134(4):381–392. doi: 10.1002/ajmg.a.30648. [DOI] [PubMed] [Google Scholar]
- Simonis N, Migeotte I, Lambert N, Perazzolo C, de Silva DC, Dimitrov B, … Vilain C. FGFR1 mutations cause Hartsfield syndrome, the unique association of holoprosencephaly and ectrodactyly. J Med Genet. 2013;50(9):585–592. doi: 10.1136/jmedgenet-2013-101603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon BD, Gropman A, Muenke M. Holoprosencephaly Overview. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, editors. GeneReviews((R)) Seattle (WA): 1993. [Google Scholar]
- Steinfeld H. Holoprosencephaly and visceral defects with familial limb anomalies. Syndr Ident. 1982;8:1–2. [Google Scholar]
- Stevens CA. Steinfeld syndrome: Further delineation. Am J Med Genet A. 2010;152A(7):1789–1792. doi: 10.1002/ajmg.a.33440. [DOI] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, … Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133(9):1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Thomas M, Donnai D. Bilateral brachial amelia with facial clefts and holoprosencephaly. Clin Dysmorphol. 1994;3(3):266–269. [PubMed] [Google Scholar]
- Toufaily MH, Roberts DJ, Westgate MN, Holmes LB. Triploidy: Variation of Phenotype. Am J Clin Pathol. 2016;145(1):86–95. doi: 10.1093/ajcp/aqv012. [DOI] [PubMed] [Google Scholar]
- Travessa A, Dias P, Rocha P, Sousa AB. Prenatal diagnosis of holoprosencephaly associated with Smith-Lemli-Opitz syndrome (SLOS) in a 46,XX fetus. Taiwan J Obstet Gynecol. 2017;56(4):541–544. doi: 10.1016/j.tjog.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Turleau C. Monosomy 18p. Orphanet J Rare Dis. 2008;3:4. doi: 10.1186/1750-1172-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urioste M, Valcarcel E, Gomez MA, Pinel I, Garcia de Leon R, Diaz de Bustamante A, … Martinez-Frias ML. Holoprosencephaly and trisomy 21 in a child born to a nondiabetic mother. Am J Med Genet. 1988;30(4):925–928. doi: 10.1002/ajmg.1320300408. [DOI] [PubMed] [Google Scholar]
- Verloes A, Dodinval P, Beco L, Bonnivert J, Lambotte C. Lambotte syndrome: microcephaly, holoprosencephaly, intrauterine growth retardation, facial anomalies, and early lethality--a new sublethal multiple congenital anomaly/mental retardation syndrome in four sibs. Am J Med Genet. 1990;37(1):119–123. doi: 10.1002/ajmg.1320370128. [DOI] [PubMed] [Google Scholar]
- Verloes A, Gillerot Y, Langhendries JP, Fryns JP, Koulischer L. Variability versus heterogeneity in syndromal hypothalamic hamartoblastoma and related disorders: review and delineation of the cerebro-acro-visceral early lethality (CAVE) multiplex syndrome. Am J Med Genet. 1992;43(4):669–677. doi: 10.1002/ajmg.1320430404. [DOI] [PubMed] [Google Scholar]
- Vokes SA, Ji H, Wong WH, McMahon AP. A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog-mediated patterning of the mammalian limb. Genes Dev. 2008;22(19):2651–2663. doi: 10.1101/gad.1693008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai LT, Chandran S. Cyclopia: isolated and with agnathia-otocephaly complex. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-220159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DD, Solomon BD, Akin-Samson K, Kelley RI, Muenke M. Cyclopia (synophthalmia) in Smith-Lemli-Opitz syndrome: First reported case and consideration of mechanism. Am J Med Genet C Semin Med Genet. 2010;154C(1):142–145. doi: 10.1002/ajmg.c.30241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z, Yingjun X, Yongzhen C, Liangying Z, Meijiao S, Baojiang C. Prenatal diagnosis of pure partial monosomy 18p associated with holoprosencephaly and congenital heart defects. Gene. 2014;533(2):565–569. doi: 10.1016/j.gene.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Zhang R, Hao L, Wang L, Chen M, Li W, Li R, … Wu J. Gene expression analysis of induced pluripotent stem cells from aneuploid chromosomal syndromes. BMC Genomics. 2013;14(Suppl 5):S8. doi: 10.1186/1471-2164-14-S5-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimpfer A, Miny P, Dombrowski U, Tolnay M, Meyer P, Bruder E. Upper limb amelia, facial clefts, holoprosencephaly, and interrupted aortic arch. Fetal Pediatr Pathol. 2007;26(4):169–176. doi: 10.1080/15513810701696874. [DOI] [PubMed] [Google Scholar]