Abstract
The tumor microenvironment (TME) plays a major role in the pathogenesis of multiple cancer types, including upper-gastrointestinal (GI) cancers that currently lack effective therapeutic options. Cancer-associated fibroblasts (CAF) are an essential component of the TME, contributing to tumorigenesis by secreting growth factors, modifying the extracellular matrix, supporting angiogenesis, and suppressing anti-tumor immune responses. Through an unbiased approach, we have established that IL-6 mediates crosstalk between tumor cells and CAF not only by supporting tumor cell growth, but also by promoting fibroblast activation. As a result, IL-6 receptor (IL-6Rα) and downstream effectors offer opportunities for targeted therapy in upper-GI cancers. IL-6 loss suppressed tumorigenesis in physiologically relevant 3D organotypic and 3D tumoroid models and murine models of esophageal cancer. Tocilizumab, an anti-IL-6Rα antibody, suppressed tumor growth in vivo in part via inhibition of STAT3 and MEK/ERK signaling. Analysis of a pan-cancer TCGA dataset revealed an inverse correlation between IL-6 and IL-6Rα overexpression and patient survival. Therefore, we expanded evaluation of tocilizumab to head-and-neck squamous cell carcinoma patient-derived xenografts and gastric adenocarcinoma xenografts, demonstrating suppression of tumor growth and altered STAT3 and ERK1/2 gene signatures. We used small molecule inhibitors of STAT3 and MEK1/2 signaling to suppress tumorigenesis in the 3D organotypic model of esophageal cancer. We demonstrate that IL-6 is a major contributor to the dynamic crosstalk between tumor cells and CAF in the TME. Our findings provide a translational rationale for inhibition of IL-6Rα and downstream signaling pathways as a novel targeted therapy in oral-upper-GI cancers.
Introduction
The dynamic interplay between multiple cell types (e.g. cancer associated fibroblasts, immune cells, endothelial cells, pericytes, neurons, adipocytes) in the tumor microenvironment (TME) has gained attention as a promising target for cancer therapy (1). Chronic inflammation and its consequences, such as immunosuppression and induction of the reactive stroma (a result of cancer-related activation of stromal fibroblasts, followed by reorganization of the extracellular matrix), contribute to tumorigenesis on multiple levels and are amongst the most prominent features of the TME (1). Furthermore, these are often the key factors that foster resistance to therapy, whether it is conventional chemoradiotherapy (2) or targeted approaches such as receptor tyrosine kinase inhibitors (3).
Cancer-associated fibroblasts (CAFs) occupy a central position in the TME architecture, and are believed to be indispensable for both formation and function of the TME (2). CAFs are among the most prominent cell types in the tumor microenvironment and have been reported to predict poor outcome and promote tumorigenesis (2). It is believed that therapeutic disruption of the cross-talk between CAFs and tumor cells would be beneficial in multiple types of cancer (1,2), but this has not been achieved yet in a rigorous fashion, thereby representing a critical gap in the preclinical-clinical continuum.
In this study, we have used esophageal cancer as an example of two lethal types of carcinoma: squamous cell carcinoma and adenocarcinoma. Esophageal cancer is a major cause of cancer-related death worldwide: 455,800 new cases were reported and 400,200 deaths occurred from this disease in 2012 (4). There are two major subtypes of esophageal cancer: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), and while the latter is more prevalent in North America and Europe, ESCC is the subtype that accounts for 90% of all esophageal cancers worldwide (5). According to the SEER database, 16,910 new esophageal cancer cases (1% of all cancers) and 15,690 deaths (2.6% of all cancer-related deaths) are predicted to occur in the US in 2017 (6). Therapeutic options are severely limited and there is a compelling need to overcome these barriers that are attributable to several reasons, such as the persistence of cancer stem cells (7), presence of desmoplasia (8), and resistance to chemotherapy (9) and radiation therapy (10). Furthermore, the majority of these esophageal cancers, regardless of subtype, present at advanced stages, when tumor cells have metastasized to other organs, such as lung, liver and bone marrow (5).
It is necessary to understand the role of certain factors in the tumor microenvironment. Interleukin 6 (IL-6) is a pleiotropic cytokine, widely appreciated as a major regulator of the acute phase response, yet harboring numerous functions outside of the immune system, including, but not limited to lipid metabolism, insulin resistance, mitochondrial function and neuroendocrine regulation (11). IL-6 signaling is mediated by a heterodimeric receptor complex comprised of the ligand-binding subunit (IL-6Rα, or CD126 (12)) and the signal-transducing subunit (gp130, or CD130 (13,14)). Herein, we demonstrate through an unbiased approach a novel role for IL-6 in mediating the interaction between tumor cells and CAFs. We also identify STAT3 and ERK1/2 as key signaling pathways enabling the effects of IL-6 in esophageal cancer. Furthermore, we reveal through 3D culture models and in vivo murine models that IL-6Rα is a novel and promising target for esophageal cancer therapy. IL-6 signaling can be targeted by tocilizumab, a neutralizing antibody to human IL-6Rα. We also provide evidence of involvement of IL-6 signaling in other related cancers, including gastric adenocarcinoma and head and neck squamous cell carcinoma (HNSCC), and demonstrate the potential of using tocilizumab for treatment of these cancers. There is a strong need for novel therapeutic options for these interrelated HNSCC-upper-digestive cancers (15), and we propose targeting IL-6 signaling, in either the neoadjuvant and/or the adjuvant setting, as a meritorious pursuit in phase I/II clinical trials for these specific cancers.
Materials and Methods
Cell lines
The ESCC, EAC, gastric cancer and FEF cell lines were described previously (16–19). The TE cell line series and HCE7 cells were generous gifts from Dr. Tetsuro Nishihira (Tohuko University, Sendai, Japan) and Dr. Curtis Harris (Laboratory of Human Carcinogenesis, National Cancer Institute, National Institutes of Health, Bethesda, MD), respectively, and were extensively characterized by us. The OE-19, OE-33, FLO-1, EsoAd1 and SKGT4 cell lines were a generous gift from Dr. Kishore Guda (Case Western Reserve University School of Medicine, Cleveland, OH). The NCI-N87 cells were a generous gift from Dr. Sandra Ryeom (University of Pennsylvania, Philadelphia, PA). The earliest frozen stocks of all cell lines have been stored at the Cell Culture Core of the University of Pennsylvania. We have propagated cells from frozen stocks of original vials that were authenticated by short tandem repeat profiling (ATCC) for highly polymorphic microsatellites to validate the identity of cells by comparing cells at the earliest stocks and those grown >8–12 passages. All cell lines undergo routine mycoplasma testing.
The ESCC-Fb-1 and ESCC-CAF-J1 cell lines were generated from esophageal biopsies by collagenase/dispase digestion, followed by mechanical disruption. The culture media (DMEM supplemented with 15% FBS (HyClone) and 1x penicillin/streptomycin) was replaced every 48 hours or as needed.
3D culture
3D tumoroids were generated by seeding trypsinized cells in matrigel and culturing in CnT Prime 3D Barrier Media for Epidermal Models (ZenBio). Tumoroid size was quantified by measuring the cross-section area in brightfield images using ImageJ (20).
3D organotypic culture
TE11 cells were grown in 3D organotypic culture as described previously (21).
Xenograft tumors
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania and the Wistar Institute. A total of 6×106 cells (all epithelial or 5×106 epithelial cells with 106 fibroblasts), resuspended in 50 μl Matrigel, were implanted. Tocilizumab (purchased at the pharmacy of the Hospital of the University of Pennsylvania) or human IgG control (Sigma) were delivered intraperitoneally at 10mg/kg three times a week. Patient-derived xenograft (PDX) tumors were described previously (22,23). Persistence of human CAFs in xenograft tumors upon implantation has been confirmed by immunofluorescence staining (Fig. S1)
Generation of IL-6 knockout cell lines
TE11 and OE-33 cell line derivatives were generating using the IL-6 CRISPR/Cas9 KO (h) kit (Santa-Cruz; TE11) or using the pLentiCRISPRv2 vector (with the same gRNA sequences as in the IL-6 CRISPR/Cas9 KO (h) kit). The levels of IL-6 in cell culture conditioned media were measured by ELISA (Fig. S2). The stability of knockout was confirmed by ELISA for up to passage 12 post-transfection.
Real-time qPCR
Real-time qPCR was performed using validated SYBR Green primers and ABI7000 and ABI StepOne instruments (Applied Biosystems). The primer sequences can be found in Table S1. The STAT3 target gene panel was selected based on the results of STAT3 ChIP-Seq (Dr. David Frank lab, unpublished data, GEO submission pending). The ERK1/2 target genes were selected based upon published research (24). Heatmaps were generated using the Heatmapper tool (25).
Flow cytometry (FACS)
Cells were labeled with CFDA-SE (Invitrogen), and the samples were analyzed on a FACScalibur (BD) or Accuri (BD). Data were analyzed using FlowJov10.1 (Treestar).
Cytokine array
The cells were grown in mono- or co-cultures for 48 hours, and relative concentration of 42 cytokines was measured using the Human cytokine array G3 (Raybiotech). The list of targets included in the array can be found in Table S2.
ELISA
IL-6 levels in culture supernatants were quantified using the Human IL-6 ELISA MAX Standard kit (BioLegend), according to the manufacturer’s instructions. Absorbance was measured on the Sunrise Microplate Reader (TECAN).
Histology
Clinical materials were obtained from patients who provided written informed consent, according to the Institutional Review Board standards and guidelines. ESCC tissue samples were obtained as surgical biopsies from Kagoshima University Hospital, as described previously (26). EAC and gastric cancer tissue samples were obtained as surgical biopsies from Dr. Kenneth K. Wang, M.D. at the Mayo Clinic (Rochester, MN; IRB protocol 15-009292) and Dr. Matthew D. Stachler M.D., Ph.D. at the Brigham and Women’s Hospital (Boston, MA; IRB protocol 2012P002411). HNSCC tissue samples were obtained as biopsies from Dr. Devraj Basu, M.D., Ph.D. at the University of Pennsylvania (IRB protocol 417200). Fluorescent in situ hybridization was performed as described previously (27). The studies were conducted in accordance with recognized ethical guidelines (Declaration of Helsinki, CIOMS, Belmont Report, U.S. Common Rule). The list of antibodies used for staining can be found in Table S3.
Quantification and Statistical analysis
Student’s t-test was used to determine significance (p<0.05). Unless noted otherwise, the p-values are listed in the graphs (statistically-significant values in bold black, insignificant – in grey). ANOVA were performed in R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
The significance in differences in tumor growth rates in vivo was determined by mixed models regression in STATA v14.2 using tumor size as the outcome variable, and drug treatment (tocilizumab versus control) and day as predictors. Statistical significance changes in STAT3 and ERK1/2 gene expression was determined using linear mixed models in STATA v14.2. Gene expression (RNAseqV2) and survival data from The Cancer Genome Atlas (TCGA) were sorted and downloaded via the UCSC Cancer Browser (28) and analyzed in GraphPad Prism 7.0.
Additional details can be found in Supplemental Materials and Methods.
Results
Interaction between tumor cells and CAFs induces changes in tumor cells and the TME
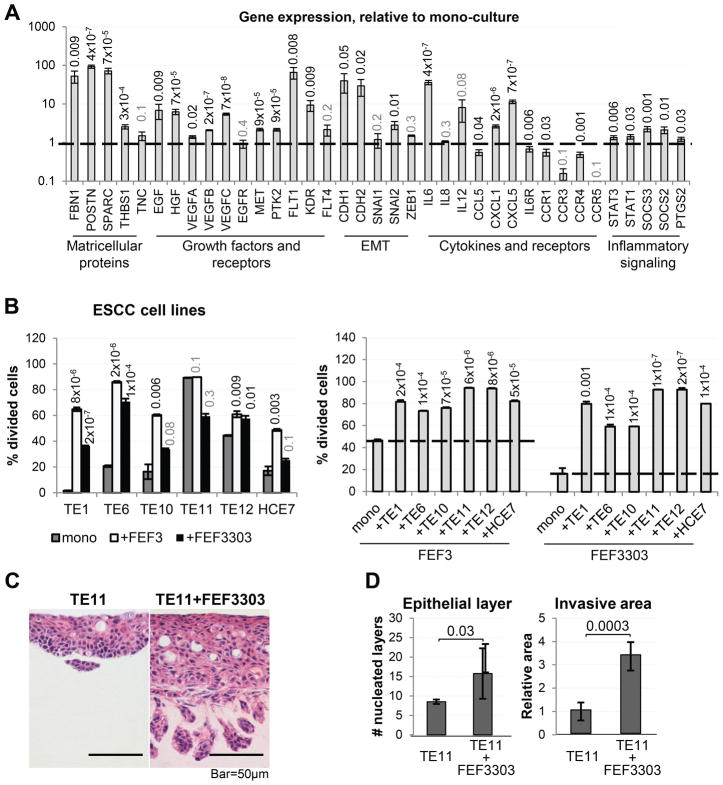
Desmoplasia is one of the key characteristics of ESCC (8), which prompted us to investigate the consequences of interaction between tumor cells and activated fibroblasts. First, we evaluated changes in gene expression in TE11 human ESCC cells grown in mono-culture, compared to co-culture with the FEF3303 human esophageal CAFs. Upon magnetic bead-based purification from co-culture, RNA was extracted from the tumor cells, followed by qPCR analysis. We observed significant changes in expression of multiple genes encoding matricellular proteins (FBN1, POSTN, SPARC, THBS1), growth factors and their receptors (EGF, HGF, VEGFA, VEGFB, VEGFC, MET, PTK2, FLT1, KDR), EMT-related genes (CDH1, CDH2, SNAI2), cytokines, chemokines and their receptors (IL6, CCL5, CXCL1, CXCL5, IL6R, CCR1, CCR4), as well as components of inflammatory signaling pathways (STAT1, STAT3, SOCS3, SOCS2, PTGS2) (Fig. 1A).
Figure 1. Direct interaction between tumor cells and CAFs induces changes in both the tumor cells and CAFs.
(A) qPCR analysis of gene expression in ESCC cells (TE11) co-cultured with CAFs (FEF3303), compared to mono-culture (n=2 for each culture condition). (B) Relative proliferation rates of ESCC cells (TE1, TE6, TE10, TE11, TE12, HCE7) and CAFs (FEF3, FEF3303) in mono- or co-culture, measured by CFDA-SE fluorescence dilution (n=3 for each culture condition). (C) Representative images of 3D organotypic cultures where epithelial cells have been seeded alone (TE11) or mixed directly with CAFs (TE11+FEF3303). Number of nucleated epithelial layers and relative invasive area are quantified (n=2/condition). See also Figure S3, S4.
We next decided to investigate the effect of co-culture on cell proliferation. Evaluated by flow cytometry (dilution of the CFDA-SE dye), five out of six ESCC cells lines (TE1, TE6, TE10, TE12, HCE7) had increased proliferation rates when co-cultured with fibroblasts (Fig. 1B). Furthermore, proliferation of either FEF3 or FEF3303 esophageal CAFs was significantly enhanced by any of the ESCC cells tested (Fig. 1B).
In a 3D organotypic culture model of ESCC, addition of FEF3303 CAFs to the epithelial layer (TE11) resulted in a dramatic increase in epithelial layer thickness (hyperplasia), as well as enhanced invasion into the ECM (Fig. 1C, 1D). Importantly, co-injection of TE11 cells with FEF3303, ESCC-CAF-J1 or ESCC-Fb-1 CAFs subcutaneously into flanks of nude mice significantly enhanced tumor growth rate, when compared to TE11 cells alone (Fig. S3). These observations underscore the importance of CAFs in ESCC progression.
IL-6 is a potential mediator of cross-talk between tumor cells and CAFs in esophageal cancer
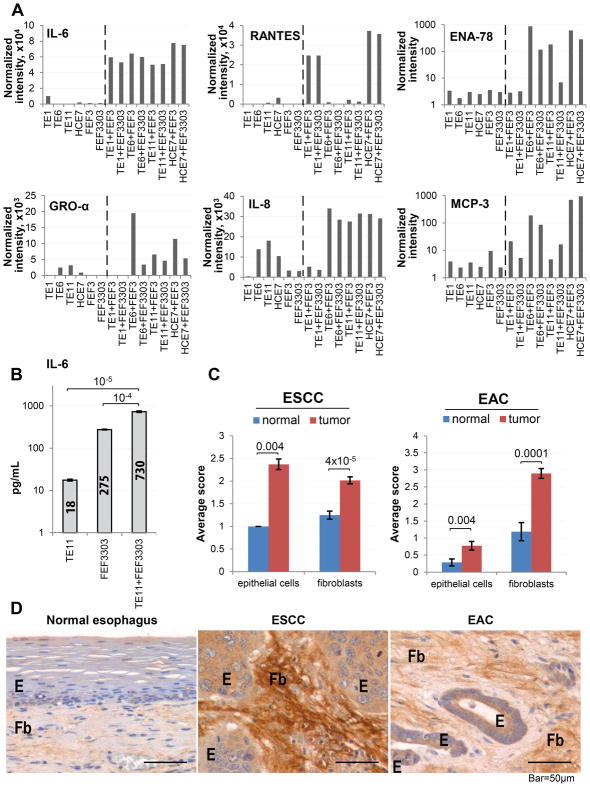
To identify potential mediators of cross-talk between tumor cells and CAFs in esophageal cancer, we conducted an unbiased cytokine array analysis of conditioned media from co-cultures of ESCC cells with esophageal CAFs. We identified six cytokines (IL-6, RANTES, ENA-78, GRO-α, IL-8 and MCP-3) with enhanced secretion in co-culture, compared to either cell line in mono-culture (Fig. 2A, Table S2). Among these, IL-6 was observed to have the most dramatic and specific increase in secretion in co-culture, which was confirmed by ELISA (Fig. 2B). We also found that direct co-culture was necessary for an increase in IL-6 mRNA levels (Fig. S4A). Interestingly, treatment of 3D esophageal tumoroid cultures with recombinant IL-6 resulted in increased tumoroid size and elevated expression of IL-6 gene, as well as of some of the targets identified in Fig. 1A (Fig. S4B, C). This reveals the mechanistic and functional involvement of IL-6 in mediating changes induced by co-culture of tumor cells and CAFs.
Figure 2. IL-6 is a potential mediator of cross-talk between tumor cells and CAFs in ESCC.
(A) Representative results of the cytokine array conducted on conditioned media from mono- and co-cultures of ESCC cells (TE1, TE6, TE11, HCE7) and CAFs (FEF3, FEF3303) (n=2 per culture condition). (B) Concentration of IL-6 in conditioned media from mono- and co-culture of ESCC cells and CAFs, measured by ELISA (n=3 per culture condition, 1/4 independent experiments shown). (C) Representative images of normal esophagus (n=14), ESCC (n=40) and EAC (n=30) stained immunohistochemically for IL-6. (D) Histopathological scoring of samples from (C). See also Figures S2, S4, S5 and S6.
To evaluate the clinical relevance of the in vitro findings and their applicability to esophageal adenocarcinoma (EAC), in addition to ESCC, we immunohistochemically (IHC) stained tissue sections of paraffin-embedded samples of human ESCC, EAC or normal esophagus for IL-6. Interestingly, we observed intense staining in both fibroblasts and epithelial cells in ESCC and EAC, while only smooth muscle cells expressed IL-6 in normal esophagus (Fig. 2C, D). We also confirmed that both epithelial cells and fibroblasts are the source of IL-6 in patient tissue by conducting fluorescent in situ hybridization for IL-6 mRNA (Fig. S5). Finally, we detected expression of both IL-6 (Fig. S6A) and its ligand-specific receptor subunit (IL-6Rα; Fig S6B, C) in 12 human ESCC and EAC cell lines. These data suggest that IL-6 may be relevant to ESCC and EAC pathogenesis.
Tumor cell-derived IL-6 promotes tumorigenic properties of esophageal cancer cells
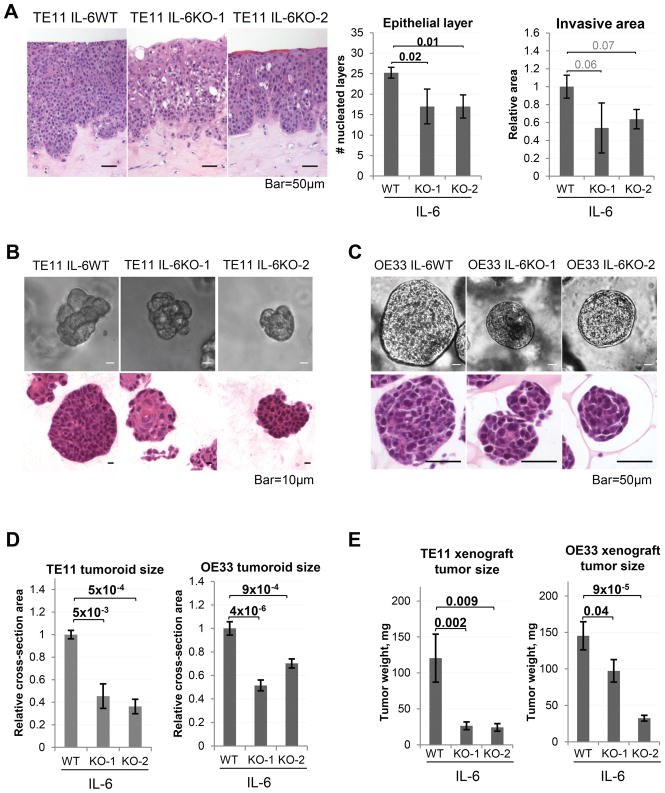
Prompted by the preceding novel findings, we used CRISPR/Cas9 to knock out IL-6 in TE11 and OE33 cells, and refer to the resulting subclones as IL-6WT, IL-6KO-1, and IL-6KO-2 (Fig. S2). While these cell lines did not seem to differ morphologically from the IL-6 wild-type parental lines in 2D culture, we observed significant differences in two types of independent 3D culture systems. The 3D organotypic cultures formed by TE11 IL-6KO cells were characterized by decreased epithelial hyperplasia and a trend of reduced invasion (Fig. 3A). Furthermore, we found that IL-6KO cells formed smaller 3D tumoroids with normalized morphology, compared to IL-6WT TE11 and OE33 cells (Fig. 3B–D). This phenotype was rescued by addition of recombinant human IL-6 to tumoroid culture media (Fig. S7). Importantly, subcutaneous xenograft tumors formed by the TE11 and OE33 IL-6KO cells were characterized by stalled growth rates (Fig. 3E).
Figure 3. Tumor cell-derived IL-6 promotes tumorigenic properties in ESCC.
(A) Representative images of 3D organotypic cultures (Hematoxylin-Eosin stained paraffin-embedded sections) formed by wild-type or IL-6 knockout TE11 cells (n=3, results of 1/3 independent experiments shown). (B) Representative images of 3D ESCC tumoroids (brightfield and Hematoxylin-Eosin staining of paraffin-embedded sections) formed by wild-type of IL-6 knockout TE11 cells (n=3, results of 1/4 independent experiments shown). (C) Representative images of 3D EAC tumoroids (brightfield and Hematoxylin-Eosin staining of paraffin-embedded sections) formed by wild-type of IL-6 knockout OE33 cells (n=3, results of 1/2 independent experiments shown). (D) Tumoroid size is quantified as relative cross-section area. (E) Weights of subcutaneous xenograft tumors formed by wild-type or IL-6 knockout TE11 (n= 6 (KO) or 4 (WT) per cohort) or OE33 cells (n=6/cohort). See also Figure S7.
Targeting IL-6Rα as a therapeutic approach in esophageal cancer
Since we found that IL-6 drives esophageal cancer progression, we conducted a pilot therapeutic study with tocilizumab, a neutralizing antibody against the human IL-6Rα, in our in vitro 3D tumoroid model of ESCC and EAC. Strikingly, tocilizumab treatment suppressed growth of 100% (7/7) ESCC tumoroids and 80% (4/5) EAC tumoroids tested (Fig. S8A and B, respectively).
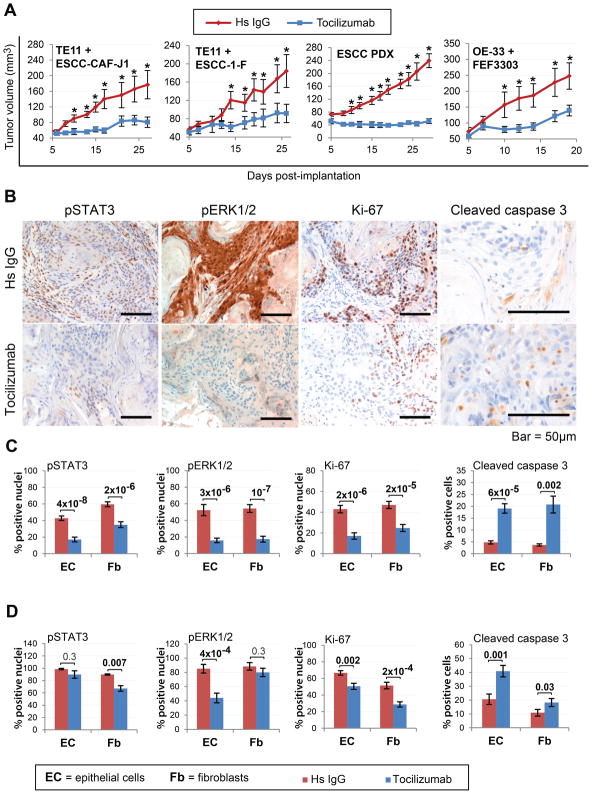
Next, we proceeded to in vivo therapeutic studies in athymic nude mice bearing subcutaneous xenograft ESCC or EAC tumors (formed by co-implantation of appropriate CAFs with TE11 or OE33 cells, respectively), as well as two ESCC patient-derived xenograft (PDX) tumor lines. Interestingly, tocilizumab treatment resulted in stalled tumor growth with a significant difference in the temporal trends between the two experimental groups (Fig. 4A; Table S4A–F), which was accompanied by reduced STAT3 and ERK1/2 signaling (Fig. 4B–D, S9). We observed a different impact of inhibition of IL-6 signaling on STAT3 and ERK1/2 activation in the epithelial and stromal compartments in ESCC versus EAC (Fig. 4C, D). Specifically, in ESCC, tocilizumab treatment significantly suppressed STAT3 phosphorylation in both the epithelia and stroma, while in EAC only the fibroblasts were affected. Interestingly, in ESCC, pERK1/2 levels were reduced significantly in the epithelium and stroma, while in EAC, pERK1/2 was only reduced in epithelial cells. These compartment-specific effects of tocilizumab could point to potential differential aspects of the ESCC vs. the EAC microenvironment, however, different model systems are required to address this issue.
Figure 4. Targeting IL-6 is a promising novel approach for esophageal cancer therapy.
(A) Growth kinetics of subcutaneous ESCC (TE11 or PDX) and EAC (OE-33) xenograft tumors treated with Tocilizumab or isotype control (i.c.) antibody (n=10/cohort, *p≤0.01). (B) Representative images of TE11+ESCC-CAF-J1 tumor sections stained for pSTAT3, pERK1/2, Ki-67 and cleaved caspase 3. (C) Quantification of staining from (B). (D) Quantification of IHC staining of OE-33+FEF3303 tumors. See also Figures S8–10.
We also evaluated cell proliferation and apoptosis rates in the tumor samples via IHC staining for Ki-67 and cleaved caspase 3, respectively. We observed decreased Ki-67 staining and increased activation of caspase 3 in both the epithelial and fibroblast compartments in response to tocilizumab treatment (Fig. 4B–D, S9B–E). We have observed a 1.5–2-fold decrease in angiogenesis in response to tocilizumab (Fig. S10), however, additional studies are required to investigate this.
We have investigated the possible interactions between STAT3/ERK1/2 signaling and downstream target expression with interesting findings (Fig. S9F). We found a positive correlation between pSTAT3 levels and pERK1/2, and a negative correlation between pSTAT3 levels and caspase 3 cleavage in fibroblasts, all of which were uniform for all tumor types evaluated. Interestingly, several observations were specific to ESCC xenografts: a positive correlation between Ki-67 levels and pSTAT3 or ERK1/2, and a negative correlation between pERK1/2 levels and caspase 3 cleavage in fibroblasts. These findings provide new insights into the role of IL-6 signaling in regulating cell proliferation and apoptosis in the TME in certain cancers.
IL-6 signaling as a therapeutic target in head and neck squamous cell carcinoma (HNSCC) and gastric adenocarcinoma
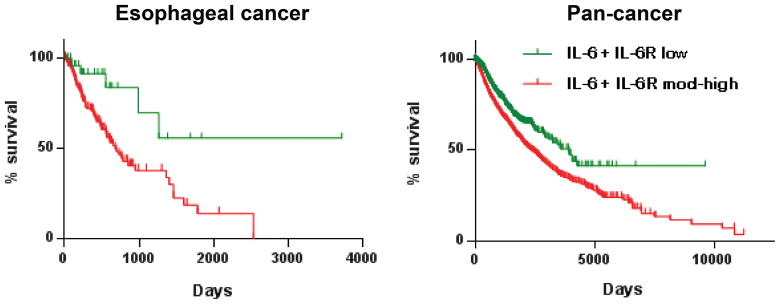
To determine the potential of targeting IL-6 signaling in other related cancers, we performed in silico survival analysis using IL-6+IL-6Rα expression (RNAseq) data acquired from the TCGA database (http://cancergenome.nih.gov/). Remarkably, we found that low IL-6+IL-6Rα expression correlated with improved survival not only in esophageal cancer (ESCA), but in the TCGA pan-cancer (PANCAN) dataset as well (Fig. 5).
Figure 5. Co-expression of IL-6 and IL-6R correlates with decreased survival in cancer.
Kaplan-Meier survival analysis based on differential expression of IL-6 and IL-6R in esophageal cancer and pan-cancer (RNAseq data, TCGA; p=0.0065 (esophageal, n=184), 3×10−11 (pan-cancer, n=8,901)).
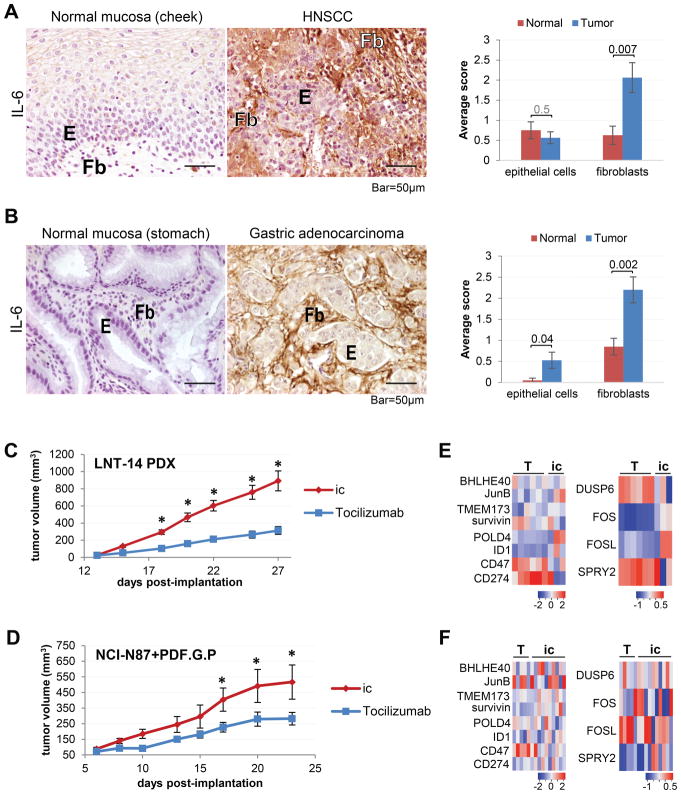
Given the overlapping genomic, genetic and epigenetic features of oral-upper digestive cancers, and shared environmental risk factors (15), we decided to investigate the role of IL-6 signaling in head and neck squamous carcinoma (HNSCC) and gastric adenocarcinoma. IHC staining for IL-6 in HNSCC and gastric adenocarcinoma patient samples demonstrated overexpression in tumor sections (both epithelial and stromal compartments), compared to matched normal oral and gastric mucosa, respectively (Fig. 6A, B). We next tested tocilizumab treatment in a patient-derived xenograft (PDX) model of HNSCC (LNT-14 (22,23)) and a subcutaneous xenograft model of gastric cancer (NCI-C87 cell line, co-injected with the PDF.G.P gastric CAFs) and observed significant reduction of tumor growth rates with a significant difference in the temporal trends between the two experimental groups (Fig. 6C, D; Table S5A–B). Histologically, tocilizumab treatment resulted in a reduced fraction of viable tumor cells and increased necrosis in the HNSCC PDX (Fig. S11A), while the gastric adenocarcinoma xenografts contained a decreased percentage of viable tumor cells, but no changes in necrosis were observed (Fig. S11B). Such variability in response may be attributable to the differentiation status of the tumor.
Figure 6. Potential of targeting IL-6Rα in several types of cancer.
(A) Representative images of normal oral mucosa and head and neck cancer (n=8 each) stained for IL-6 by immunohistochemistry, with quantification. (B) Representative images of normal gastric mucosa and gastric adenocarcinoma (n=10 each) stained for IL-6 by immunohistochemistry, with quantification. (C) Growth kinetics of patient-derived xenograft (PDX) HNSCC tumors (LNT-14) treated with tocilizumab or isotype control (i.c.) antibody (n=8/cohort; *p ≤10−4). (D) Growth kinetics of subcutaneous gastric carcinoma xenografts (NCI-N87 co-implanted with PDF.G.P CAF cell line) treated with tocilizumab (n=7) or isotype control (i.c., n=6) antibody (*p≤0.03). (E) Heat maps illustrating changes in expression of select STAT3 and ERK1/2 targets in the HNSCC PDX tumors treated with tocilizumab (T, n=6) or a control antibody (ic, n=6). (D) (F) Heat maps illustrating changes in expression of select STAT3 and ERK1/2 targets in xenograftgastric tumors treated with tocilizumab (T, n=7) or a control antibody (ic, n=7). See also Figures S11–12.
IL-6-induced STAT3 and ERK1/2 signaling contributes to tumorigenesis
Since the anti-tumorigenic effect of tocilizumab treatment is likely to be mediated in part by the STAT3 and ERK1/2 signaling pathways (Fig. 4), we sought to determine whether genes regulated by these pathways were modulated by tocilizumab treatment. To that end, we analyzed the expression levels of select STAT3 and ERK1/2 target genes (please refer to Materials and Methods for target gene selection) in tumor samples from therapeutic studies in HNSCC and gastric cancer (Fig. 6). We observed a change in expression for several key genes regulated by either pathway in response to tocilizumab treatment (Fig. 6E, F, S12). While for many targets significant changes in gene expression were not observed, an overall trend in decreased expression of STAT3 targets was detected. Interestingly, ID1 gene was significantly downregulated in response to tocilizumab in both HNSCC and gastric adenocarcinoma. For ERK1/2 targets, tocilizumab-induced changes in expression were more complex: in case of the HNSCC PDX, FOS and FOSL1 were down-regulated, and the ERK1/2 targets, DUSP6 and SPRY2, were up-regulated in the tocilizumab-treated cohort. At the same time, in the gastric adenocarcinoma xenografts, the ERK1/2 targets, namely DUSP6 and SPRY2, were significantly downregulated, and a trend in decreased expression was observed for FOS and FOSL1. Another intriguing observation is that for HNSCC, a significant change in expression in response to tocilizumab was observed for only 12.5% (1/8) of STAT3 targets, and 100% (4/4) ERK1/2 target genes. Yet, in the case of gastric adenocarcinoma, 50% (4/8) STAT3 targets and 50% (2/4) ERK1/2 targets were differentially expressed.
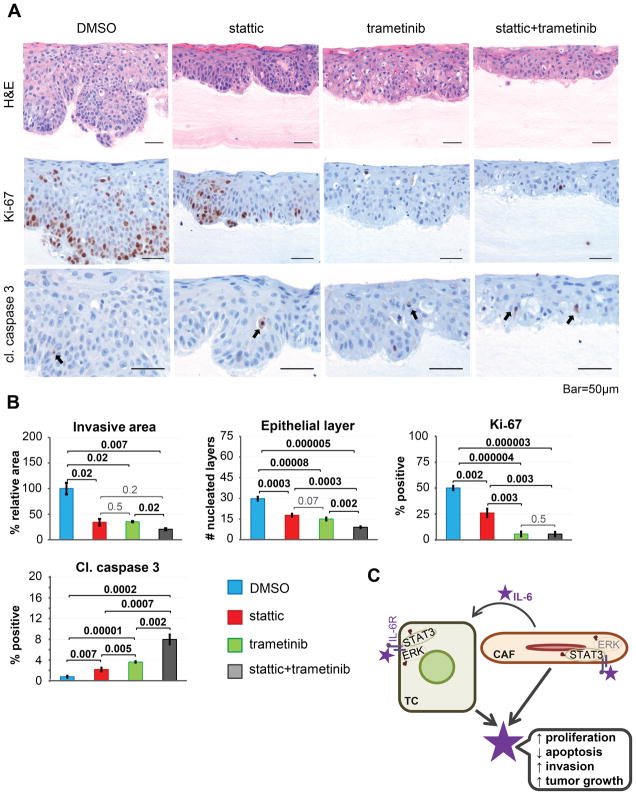
We next treated 3D organotypic cultures of ESCC with small molecule inhibitors of STAT3 and MEK1/2 (stattic and trametinib, respectively). Surprisingly, while treatment with each individual drug suppressed tumor cell invasion and led to a reduced number of nucleated cells in the epithelial layer, the combination of stattic and trametinib affected these phenotypes even more potently (Fig. 7A, B). MEK1/2 inhibition was most potent for suppression of cell proliferation, as measured by Ki-67 IHC staining (Fig. 7A, B). Importantly, the combination of stattic and trametinib promoted apoptosis when compared to monotherapy, as revealed by IHC staining for cleaved caspase 3 (Fig. 7A, B). These data suggest that IL-6-induced STAT3 and MEK1/2 signaling contribute to tumor cell invasion, proliferation and evasion of apoptosis in ESCC.
Figure 7. STAT3 and MEK/ERK signaling mediate ESCC tumorigenesis in vitro.
(A) Representative images of 3D organotypic ESCC cultures treated with stattic, trametinib or a combination of the two (Hematoxylin-Eosin, cleaved caspase 3 and Ki-67 staining; representative results from 1/3 independent experiments are shown). (B) Quantification of images from (A). (C) The dynamic IL-6-mediated crosstalk between tumor cells and CAFs in esophageal cancer.
Discussion
The importance of the tumor microenvironment in tumor initiation and progression is well appreciated (8). Yet, there is a compelling need for new perspectives on ways to target the TME, including factors that mediate the cross-talk between different TME cell types with tumor cells. We focused our attention on CAFs and identification of novel mediators of interactions between CAFs and esophageal tumor cells. A previous study ablated stromal cells by targeting the FAP-expressing CAFs (29). However, there are potential risks associated with targeting the TME as demonstrated by suicide gene-mediated depletion of αSMA-expressing cells, which led to invasive tumors and enhanced Treg recruitment in a murine model of pancreatic adenocarcinoma (30). Herein, we undertook an alternative approach by targeting the pro-tumorigenic signaling events induced by the interaction between CAFs and tumor cells, while preserving the barrier function of the stroma.
Based upon the unbiased cytokine array results, IL-6 was selected as a candidate, since it was upregulated in co-cultures of ESCC and esophageal CAFs, compared to monocultures. This was strengthened by the IHC staining of human esophageal cancers, where IL-6 expression was significantly stronger in both tumor cells and fibroblasts in ESCC or EAC, compared to adjacent normal tissues (Fig. 2C–D). Importantly, we demonstrated that IL-6 expression in tumor cells is likely induced through direct interaction with CAFs (Fig. 1A, 2B, S4A). Our data suggest that besides paracrine signaling induced by fibroblast-derived IL-6 in co-cultures, autocrine IL-6 signaling serves as an additional mechanism for the induction of IL-6 (Fig. S7). In support of this, CRISPR/Cas9-mediated knockout of IL-6 in the tumor cells resulted in stalled growth and normalized morphology of the 3D tumoroids, which lack a stromal compartment (Fig. 3B). To our knowledge, this is the first report of such tumor cell-specific function of IL-6 in esophageal cancer. According to our findings, chronic inflammation leads to activation of normal fibroblasts and their conversion into cancer-associated fibroblasts (CAF). CAFs acquire a new secretory profile, producing pro-tumorigenic cytokines, including IL-6. CAFs also interact with the tumor cells (TC) directly and alter their gene expression profile, enabling tumor cells to secrete high levels of IL-6. This cytokine activates the receptor (IL-6R) on both tumor cells and CAFs in an autocrine-paracrine manner, which results in activation (to a different extent) of the STAT3 and MEK/ERK signaling pathways. These events result in increased tumor cell proliferation, decreased apoptosis, increased invasion, and promote overall tumor growth (Fig. 7C).
Furthermore, our data strengthen the case for targeting IL-6 signaling in cancer. We showed that tocilizumab, an anti-IL-6Rα antibody approved by the FDA for treatment of autoimmune disease, inhibits tumor growth in in vivo models of ESCC and EAC, as well as related cancers, such as HNSCC and gastric adenocarcinoma (Fig. 4,6), and perhaps could be extended to other cancer types, given the observed inverse correlation between IL-6+IL-6Rα expression and survival (Fig. 5). Anti-IL-6Rα therapy could also provide benefits when used in combination with chemotherapy or immune checkpoint inhibitors (as suggested by downregulation of CD274, which encodes the programmed death-ligand 1 (PD-L1) protein (Fig. 6F, S12B) in gastric adenocarcinoma xenografts). For instance, tocilizumab was found to enhance gemcitabine antitumor activity in mice bearing skin squamous cell tumors through the reduction of myeloid-derived suppressor cells (MDSCs) (31). In colon cancer, anti-IL-6Rα treatment resulted in decreased tumor growth and 5-fluorouracil resistance (32). Despite these pre-clinical data, however, there remains a dearth of clinical trials investigating targeted approaches, such as IL-6 signaling inhibition, in cancers spanning the oral cavity and upper digestive tract.
We find that the pro-tumorigenic role of IL-6 in esophageal cancer is mediated in part via STAT3 and ERK1/2 signaling pathways (Fig. 4B–C, 6E–F, 7, S9, S11). In addition to suppression of apoptosis, we also found that these pathways promote tumor cell proliferation and invasion (Fig. 7). This finding can provide a rationale for yet another targeted therapy approach. Trametinib is a MEK1/2 inhibitor currently approved by the FDA for the treatment of BRAF-mutant melanoma (33), and another small molecule with a known safety profile in humans – pyrimethamine – has been identified recently as a potent STAT3 inhibitor (34,35). A combination of these two drugs could be used to more efficiently target some of the downstream mediators of IL-6 signaling, as well as prevent any compensatory activation of these pathways by other cytokines, such as RANTES or IL-8, which are also secreted in co-culture of tumor cells and CAFs (Fig. 2A)
Our analysis of changes in expression of STAT3 and ERK1/2 target genes in response to tocilizumab treatment (Fig. 6E–F, S12) reveals some interesting observations. For example, while a trend of downregulation of STAT3 target genes was observed for HNSCC PDX and gastric cancer xenograft tumors, the specific genes that were suppressed significantly in the two types of cancer were quite distinct: ID1 in HNSCC versus Survivin (BIRC5), TMEM173, ID1 and CD274 in gastric adenocarcinoma. The only STAT3 target gene that was significantly downregulated in both cancers was ID1. Interestingly, Id1, the protein encoded by the ID1 gene, is known to have multiple functions in cancer progression (36).
CD47 is a STAT3 target gene which is upregulated (albeit not significantly) in tocilizumab-treated tumors (Fig. 6E–F, S12; Table S6A–B). The function of CD47 largely depends on co-expression of its partners, such as SIRPα, thrombospondin-1, and integrins (37). The CD47-SIRPα axis has gained a lot of attention in cancer research field due to its role in evading phagocytosis (37). The trend of increased CD47 expression in response to tocilizumab could be part of the tumor cell’s strategy for survival and may be partially responsible for the lack of complete tumor clearance.
In the case of the ERK1/2 targets, the differences between the two cancer types are even more dramatic: in HNSCC PDX DUSP6 and SPRY2 are upregulated in tocilizumab-treated tumors, while in gastric adenocarcinoma xenografts these genes are downregulated. Interestingly, for another pair of ERK1/2 target genes, FOS and FOSL1, a significant downregulation in HNSCC, as well as a trend of decreased expression in gastric cancer were observed in response to tocilizumab. Lastly, the overall ratios of genes with significant changes in gene expression varied as well: in HNSCC, 12.5% of STAT3 and 100% of ERK1/2 targets were significantly changed upon tocilizumab treatment, while in gastric adenocarcinoma the ratio was 50% – 50%.
The finding that expression of immune-inhibitory genes, such as CD274, is altered by tocilizumab in gastric adenocarcinoma (Fig. 6F, S12B) raises the possibility of synergy of this agent in combination with immune modulatory therapy such as immune checkpoint inhibitors; in fact, CD274 has been found to be increased in EAC. (38). However, since CD247 expression was affected differentially in response to tocilizumab, based on the tumor type (upregulated in HNSCC PDX and downregulated in gastric adenocarcinoma xenografts), detailed approaches to patient eligibility criteria will be necessary prior to implication of such treatment approaches. Finally, it is important to note that the tumor samples used in gene expression analysis were obtained at the latest time points in the therapeutic studies, at which time there may have been enrichment for resistant cells with persistent or altered STAT3 or ERK1/2 activity.
Targeting the dynamic interaction between tumor cells and various cell types in the TME is a much-needed approach to cancer therapy. Herein we describe how one of the mediators of such an interaction, namely IL-6, can be antagonized by a neutralizing antibody to its receptor, which significantly reduces tumor growth in murine models of oral and upper digestive cancers. Importantly, although our findings were focused on the interaction between tumor cells and CAFs, IL-6 is known to influence multiple cell types in the TME, from immune cells to adipocytes to osteoclasts (11,39–42). This strongly indicates that neutralization of IL-6 signaling could have even more potent anti-tumor effects. Our work provides sufficient rationale for testing tocilizumab or potentially second-generation anti-IL-6Rα antibodies (such as NI-1201 (11)), in oral-upper digestive cancers, perhaps in combination with inhibitors of STAT3 and/or MEK/ERK. This novel approach fills a major gap in current therapeutic options for advanced, metastatic oral-upper digestive cancers.
Supplementary Material
SIGNIFICANCE.
Findings demonstrate the interaction of esophageal cancer cells and cancer-associated fibroblasts through IL-6 signaling providing rationale for a novel therapeutic approach to target these cancers.
Acknowledgments
Financial support: This work was supported by the National Cancer Institute P01-CA14305603 (A.K. Rustgi, T.A. Karakasheva, E.W. Lin, Q. Tang, E. Qiao, M. Soni, T.J. Waldron, A. Klein-Szanto, A.J. Bass, J.A. Diehl), the National Institutes of Health Center for Molecular Studies in Digestive and Liver Diseases P30-DK050306 (A.K. Rustgi, T.A. Karakasheva). Parts of this work were supported by the National Institutes of Health K08-DE02284 (D. Basu, V. Sahu), the National Institutes of Health R21-DE024396 (D. Basu, V. Sahu) and the National Institutes of Health R01-CA196932 02 (A.J. Bass). This work was supported in part by P30-ES013508 (A.K. Rustgi) funded by the National Institute of Environmental Health Sciences (NIEHS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NIH.
We thank the Molecular Pathology and Imaging Core (J. Katz, A. Bedenbaugh, D. Budo, and C. O’Donnel), the Human and Microbial Analytic and Repository Core (G. Wu and L. Chau), the Genetically Modified Mouse Core, the Cell Culture and iPS Core, the Penn Microarray and Flow Cytometry and Cell Sorting Facilities, and Penn Biostatistics Core. We thank members of the Rustgi, Nakagawa and Basu laboratories for discussions.
Footnotes
Conflict of interest: the authors have no conflicts of interest to disclose.
References
- 1.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012 Mar 24;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016:16. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 3.Alderton GK. Therapeutic resistance: Fibroblasts restrain drug sensitivity. Nat Rev Cancer. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. 2015;15:318–9. doi: 10.1038/nrc3965. [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Rustgi AK, El-Serag HB. Esophageal Carcinoma. N Engl J Med. 2014;371:2499–509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJCK, editors. SEER Cancer Stat Rev 1975–2013. Natl. Cancer Institute; Bethesda, MD: 2016. Cancer Statistics Review, 1975–2013 - SEER Statistics [Internet] Available from: http://seer.cancer.gov/csr/1975_2013/ [Google Scholar]
- 7.Sato F, Kubota Y, Natsuizaka M, Maehara O, Hatanaka Y, Marukawa K, et al. EGFR inhibitors prevent induction of cancer stem-like cells in esophageal squamous cell carcinoma by suppressing epithelial-mesenchymal transition. Cancer Biol Ther. 2015;16:933–40. doi: 10.1080/15384047.2015.1040959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin EW, Karakasheva TA, Hicks PD, Bass AJ, Rustgi AK. The tumor microenvironment in esophageal cancer. Oncogene. 2016;35:5337–49. doi: 10.1038/onc.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilson D. Esophageal cancer chemotherapy: recent advances. Gastrointest cancer Res GCR. 2008:85–92. [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Zhang C, He J, Guo Q, Hu D, Yang X, et al. STAT3 inhibitor stattic enhances radiosensitivity in esophageal squamous cell carcinoma. Tumour Biol. 2014 doi: 10.1007/s13277-014-2823-y. [DOI] [PubMed] [Google Scholar]
- 11.Hunter Ca, Jones Sa. Nat Immunol. Vol. 16. Nature Publishing Group; 2015. IL-6 as a keystone cytokine in health and disease; pp. 448–57. [DOI] [PubMed] [Google Scholar]
- 12.Yamasaki K, Taga T, Hirata Y, Yawata H, Kawanishi Y, Seed B, et al. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988;241:825–8. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- 13.Karakasheva TA, Waldron TJ, Eruslanov E, Lee J-S, O’Brien S, Hicks PD, et al. CD38-Expressing Myeloid-Derived Suppressor Cells Promote Tumor Growth in a Murine Model of Esophageal Cancer. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-14-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M-F, Kuan F-C, Yen T-C, Lu M-S, Lin P-Y, Chung Y-H, et al. IL-6-stimulated CD11b+ CD14+ HLA-DR- myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget. 2014;5:8716–28. doi: 10.18632/oncotarget.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dotto GP, Rustgi AK. Squamous Cell Cancers: A Unified Perspective on Biology and Genetics. Cancer Cell. 2016;29:622–37. doi: 10.1016/j.ccell.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okano J, Gaslightwala I, Birnbaum MJ, Rustgi AK, Nakagawa H. Akt/protein kinase B isoforms are differentially regulated by epidermal growth factor stimulation. J Biol Chem. 2000;275:30934–42. doi: 10.1074/jbc.M004112200. [DOI] [PubMed] [Google Scholar]
- 17.Boonstra JJ, van Marion R, Beer DG, Lin L, Chaves P, Ribeiro C, et al. Verification and unmasking of widely used human esophageal adenocarcinoma cell lines. J Natl Cancer Inst. 2010;102:271–4. doi: 10.1093/jnci/djp499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JG, Frucht H, LaRocca RV, Bliss DP, Jr, Kurita Y, Chen TR, et al. Characteristics of cell lines established from human gastric carcinoma. Cancer Res. 1990;50:2773–80. [PubMed] [Google Scholar]
- 19.Okawa T, Michaylira CZ, Kalabis J, Stairs DB, Nakagawa H, Andl CD, et al. The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev. 2007;21:2788–803. doi: 10.1101/gad.1544507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalabis J, Wong GS, Vega ME, Natsuizaka M, Robertson ES, Herlyn M, et al. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat Protoc. 2012 Jan 14;7:235–46. doi: 10.1038/nprot.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Facompre ND, Harmeyer KM, Sole X, Kabraji S, Belden Z, Sahu V, et al. JARID1B Enables Transit between Distinct States of the Stem-like Cell Population in Oral Cancers. Cancer Res. 2016;76:5538–49. doi: 10.1158/0008-5472.CAN-15-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basu D, Bewley AF, Sperry SM, Montone KT, Gimotty PA, Rasanen K, et al. EGFR Inhibition Promotes an Aggressive Invasion Pattern Mediated by Mesenchymal-like Tumor Cells within Squamous Cell Carcinomas. Mol Cancer Ther. 2013:12. doi: 10.1158/1535-7163.MCT-12-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deschênes-Simard X, Gaumont-Leclerc M-F, Bourdeau V, Lessard F, Moiseeva O, Forest V, et al. Tumor suppressor activity of the ERK/MAPK pathway by promoting selective protein degradation. Genes Dev Cold Spring Harbor Laboratory Press. 2013;27:900–15. doi: 10.1101/gad.203984.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 2016;44:W147–53. doi: 10.1093/nar/gkw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natsuizaka M, Kinugasa H, Kagawa S, Whelan KA, Naganuma S, Subramanian H, et al. IGFBP3 promotes esophageal cancer growth by suppressing oxidative stress in hypoxic tumor microenvironment. Am J Cancer Res. 2014;4:29–41. [PMC free article] [PubMed] [Google Scholar]
- 27.Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods. 2008;5:877–9. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res Cold Spring Harbor Laboratory Press. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo A, Wang LCS, Scholler J, Monslow J, Avery D, Newick K, et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 2015;75:2800–10. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–34. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sumida K, Wakita D, Narita Y, Masuko K, Terada S, Watanabe K, et al. Anti-IL-6 receptor mAb eliminates myeloid-derived suppressor cells and inhibits tumor growth by enhancing T-cell responses. Eur J Immunol. 2012;42:2060–72. doi: 10.1002/eji.201142335. [DOI] [PubMed] [Google Scholar]
- 32.Ying J, Tsujii M, Kondo J, Hayashi Y, Kato M, Akasaka T, et al. The effectiveness of an anti-human IL-6 receptor monoclonal antibody combined with chemotherapy to target colon cancer stem-like cells. Int J Oncol. 2015;46:1551–9. doi: 10.3892/ijo.2015.2851. [DOI] [PubMed] [Google Scholar]
- 33.Chopra N, Nathan PD. Trametinib in metastatic melanoma. Expert Rev Anticancer Ther. 2015;15:749–60. doi: 10.1586/14737140.2015.1060127. [DOI] [PubMed] [Google Scholar]
- 34.Takakura A, Nelson EA, Haque N, Humphreys BD, Zandi-Nejad K, Frank DA, et al. Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet. 2011;20:4143–54. doi: 10.1093/hmg/ddr338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan MW, Saadalla A, Ewida AH, Al-Katranji K, Al-Saoudi G, Giaccone ZT, et al. The STAT3 inhibitor pyrimethamine displays anti-cancer and immune stimulatory effects in murine models of breast cancer. Cancer Immunol Immunother. 2017 Jan;:1–11. doi: 10.1007/s00262-017-2057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ling M-T, Wang X, Zhang X, Wong Y-C. The multiple roles of Id-1 in cancer progression. Differentiation. 2006;74:481–7. doi: 10.1111/j.1432-0436.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- 37.Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPα axis. Eur J Cancer. 2017;76:100–9. doi: 10.1016/j.ejca.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Derks S, Nason KS, Liao X, Stachler MD, Liu KX, Bin Liu J, et al. Epithelial PD-L2 Expression Marks Barrett’s Esophagus and Esophageal Adenocarcinoma. Cancer Immunol Res. 2015:3. doi: 10.1158/2326-6066.CIR-15-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Luca A, Lamura L, Gallo M, Maffia V, Normanno N. Mesenchymal stem cell-derived interleukin-6 and vascular endothelial growth factor promote breast cancer cell migration. J Cell Biochem. 2012;113:3363–70. doi: 10.1002/jcb.24212. [DOI] [PubMed] [Google Scholar]
- 40.Ara T, Declerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46:1223–31. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neiva KG, Warner KA, Campos MS, Zhang Z, Moren J, Danciu TE, et al. Endothelial cell-derived interleukin-6 regulates tumor growth. BMC Cancer. 2014;14:99. doi: 10.1186/1471-2407-14-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bochet L, Meulle A, Imbert S, Salles B, Valet P, Muller C. Cancer-associated adipocytes promotes breast tumor radioresistance. Biochem Biophys Res Commun. 2011;411:102–6. doi: 10.1016/j.bbrc.2011.06.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.