Abstract
Purpose
Allogeneic hematopoietic stem-cell transplantation (HSCT) is a curative treatment for many hematologic cancers. Use of haploidentical (mismatched) donors increases HSCT availability but is limited by severe graft-versus-host disease (GvHD) and delayed immune reconstitution. Alloanergization of donor T-cells is a simple approach to rebuild immunity whilst limiting GvHD after haploidentical HSCT but the optimal T-cell dose and impact on immune reconstitution remain unknown.
Experimental Design
We performed a multicentre Phase 1 trial of alloanergized donor lymphocyte infusion (aDLI) after CD34-selected myeloablative haploidentical HSCT. The primary aim was feasibility and safety with secondary aims of assessing the less frequently addressed issue of impact on immune reconstitution.
Results
Nineteen high-risk acute leukemia or myelodysplasia patients were enrolled. Engraftment occurred in 18/19 patients (95%). Pre-aDLI, twelve patients (63%) had bacteremia, 9/17 at-risk patients (53%) reactivated CMV and one developed acute GvHD. Sixteen patients received aDLI at dose-levels 1 (103 T-cells/kg, n=4), 2 (104, n=8), and 3 (105, n=4). Post-aDLI, 5 patients developed clinically significant acute GvHD and 4/14 at-risk patients (29%) reactivated CMV. T-cell recovery was significantly greater and functional virus- and tumor-associated antigen-specific T-cells were detectable earlier in patients receiving dose-level 2 or 3 vs dose-level 1/no aDLI. Alloanergization of donor cells expanded the CD4+ T-regulatory cell frequency within aDLI which increased further in vivo without impeding expansion of virus- and tumor-associated antigen-specific T-cells.
Conclusion
These data demonstrate safety and a potential role for aDLI in contributing to immune reconstitution and expanding tolerogenic regulatory T-cells in vivo after CD34-selected myeloablative haploidentical HSCT.
Keywords: HEMATOLOGICAL CANCERS/Leukemias, IMMUNOTHERAPY/Stem Cell Transplantation, CLINICAL TRIAL, IMMUNOLOGY/Graft versus host disease, IMMUNOLOGY/Tumor ANTIGENS
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) remains the only curative therapy for many patients with high-risk hematologic malignancies, but not all will have fully human leucocyte antigen (HLA)-matched donors. In contrast, most patients will have haploidentical family donors, the use of which would significantly increase availability of HSCT as a treatment modality.1 Historically, haploidentical HSCT has resulted in increased frequency and severity of acute graft-versus-host disease (aGvHD),2,3 mediated by alloreactive donor T-cells.4 Various methodologies to deplete donor T-cells have significantly reduced incidences of severe aGvHD after haploidentical HSCT.5,6 However, these non-selective T-cell depletion (TCD) strategies also remove pathogen- and tumor-antigen-reactive T-cells, which contributes to delayed immune reconstitution, and increased infection and disease relapse rates.7–9
Several approaches have therefore been developed to selectively deplete T-cells most likely to mediate GvHD from haploidentical donors, including depletion of α/β T-cells,10 or selective removal of alloreactive T-cells.11,12 Although some of these strategies have been shown to be feasible in early clinical studies,13,14 they are technically complex and may also deplete desirable effector and CD4+ regulatory T-cells (Treg) from the donor pool. More recently, post-transplant cyclophosphamide (PTC) after haploidentical HSCT has been successfully adopted to inhibit proliferation of alloreactive donor T-cells in vivo.15 Although early reports suggest this approach results in less viral reactivation and infection than non-selective TCD strategies,16 infections common to immunocompromised populations continue to be reported and the degree and temporal sequence of broad immune reconstitution after PTC-haploidentical HSCT have yet to be determined.
An unmet need therefore remains for strategies that facilitate haploidentical transplantation without negatively impacting beneficial immune reconstitution. One such approach is alloanergization, the induction of alloantigen-specific hyporesponsiveness in donor T-cells by recipient alloantigen presentation with concurrent co-stimulatory blockade.17 This strategy was successfully employed in 2 prior clinical studies in which very large doses of haploidentical alloanergized donor T-cells were infused en mass with donor bone marrow (BM), resulting in less severe aGvHD than historical control recipients of non-TCD haploidentical BM transplantation (BMT).18,19 Furthermore, the approach does not require the generation of specialized recipient antigen-presenting cells or sorting technologies, retains the majority of pathogen-and tumor-antigen specific T-cells in vitro,19 and results in expansion of allospecific donor Tregs in vivo which may in turn contribute to the control of harmful alloresponses.20
However, the previous studies of alloanergized BMT were not designed to determine the impact of alloanergized donor T-cells on immune reconstitution or to define the minimal or optimal dose of alloanergized donor T-cells able to confer clinically beneficial virus-specific immunity whist maintaining an acceptable toxicity profile. To address these issues, we therefore conducted a novel multicenter Phase 1 clinical study examining toxicity and immune reconstitution after administration of escalating doses of alloanergized donor lymphocyte infusion (aDLI) in patients with high-risk acute leukemia or myelodysplasia after CD34+-selected (i.e. non-selectively T-cell depleted) myeloablative HSCT from haploidentical related donors.
Materials and methods
Study design and eligibility
This was an open-label phase I trial to establish the feasibility and safety of delayed administration of aDLI after CD34+-selected myeloablative haploidentical HSCT in patients with hematopoietic malignancies. The primary aim of the study was to assess feasibility and safety of this strategy during two consecutive study stages defined for each patient. The first comprised the period prior to aDLI infusion during which the toxicity of the conditioning regimen, the likelihood of engraftment and the subsequent percentage of individuals who would be eligible to receive aDLI were determined. The second comprised the period from aDLI infusion through D+100 (as the at-risk period for aGVHD). Preliminary efficacy was addressed in the secondary aim with determination of immune reconstitution and viral infection rates in patients receiving aDLI. Adult and pediatric patients with high-risk hematological malignancies (acute leukemia in first remission with high-risk cytogenetics or second or subsequent remission, high-risk myelodysplasia or acute myelogenous leukemia with induction failure after at most 2 regimens) without either available genotypically HLA-matched (6/6) unrelated donors (after a search of longer than 2 months or with high-risk of progression during a search) or fully-matched/single HLA-antigen-mismatched related donors, but with available haploidentical related donors were eligible for inclusion at 5 US centers (Supplementary Methods). The study was conducted in accordance with the Belmont Report and all patients and/or legal guardians gave consent and/or assent as age-appropriate. The study was approved by local institutional review boards and by the US Food and Drug Administration and registered at ClinicalTrials.gov (Identifier: NCT00475384).
Patient characteristics and treatment regimens
Nineteen patients received either total body irradiation (TBI)-based (n=8) or chemotherapy-based (n=11) myeloablative conditioning at the site investigator’s discretion. After conditioning, patients received CliniMACS-selected CD34+ G-CSF-mobilized peripheral blood cells from haploidentical related donors (median age 39 years, range 19–50) at D0. Patient and donor characteristics and conditioning regimens are shown in Table S1. Of the 10 male and 9 female patients, five were children (median age 7 years, range 2–17) of whom 3 were white/non-Latino and 2 white/Latino; fourteen were adults (median age 39 years, range 22–50) of whom 6 were white/non-Latino, 3 black non-Latino and 5 white/Latino. No post-transplant immune suppression was used. Neutrophil engraftment was defined as the first of three consecutive days on which the patient’s absolute neutrophil count was > 500/μL. Chimerism was assessed by D+28 on unselected and CD3+ fractions of peripheral blood using DNA polymerase chain reaction (PCR) of short tandem repeats. Supportive care included antibacterial, antifungal and PCP prophylaxis per institutional practice. For recipients at risk of CMV reactivation (donor and/or recipient CMV IgG-seropositive) systemic anti-CMV prophylaxis was recommended from the initiation of conditioning through D+75 unless contraindicated and thereafter at the discretion of the treating physician. Physicians were encouraged to use a strategy of CMV-DNA monitoring and pre-emptive therapy upon reactivation for all at-risk patients.
Generation of alloanergized donor lymphocyte infusions
ADLI were generated ex vivo under Good Manufacturing Practice conditions by co-culture of fresh non-mobilized peripheral blood mononuclear cells (PBMC) from the stem cell donor with gamma-irradiated allostimulator PBMC. In order to both ensure adequate numbers of allostimulator cells were available, and reduce theoretical concerns regarding possible anergization to tumor-associated antigens present in the patient, where the patient had a suitable second haploidentical family member sharing the haplotype possessed by the patient but not by the stem cell/aDLI donor, fresh, non-mobilized PBMC obtained from the second haploidentical family member were used as allostimulators. Where the patient lacked such a suitable second haploidentical family member, non-mobilized patient PBMC were obtained prior to transplant and cryopreserved for later thaw and use as allostimulators. Non-mobilized PBMC from stem cell/aDLI donors were obtained on D+32/39 and alloanergized by co-culture with equal numbers of irradiated (33 Gy) allostimulators in the presence of humanized anti-B7.1/2 antibodies as described (clones h1F1 and h3D1, Wyeth, Madison, NJ).19 Control flasks with donor PBMC and irradiated allostimulators without anti-B7 antibodies were set up in parallel. After 72 hours, alloanergized donor lymphocytes were harvested, washed twice and sampled for viability, contamination (Gram stain, Mycoplasma testing, bacterial and fungal cultures), endotoxin levels, and flow cytometric phenotype (see Supplementary Methods). Residual alloreactivity was determined by measuring residual proliferative stimulator-specific alloresponses in secondary mixed lymphocyte reactions, as previously described and calculated as
ADLI were infused fresh, prior to phenotypic assessment and determination of residual alloreactivity, if they met release criteria (sufficient T-cell numbers with >/=70% viability, no evidence of bacterial contamination and endotoxin levels </= 5 EU/kg/). A schema for the study is shown in Figure S1.
Dosing and administration of aDLI
Patients who were stably engrafted with no active aGVHD or uncontrolled infection received aDLI on D+35. Patients who did not fulfil these criteria could be delayed for one week and reassessed. Twelve patients received aDLI on D+35 as scheduled, while 4 received their aDLI after a one week delay (3 due to viral reactivation and one due to active aGVHD). A Bayesian, adaptive study design based on efficacy and toxicity seen in prior patients was used to assign the aDLI dose for each patient.21 For this purpose, efficacy was defined as stable engraftment (absolute neutrophil count > 500/μL by D+100) and toxicity was defined as severe refractory aGVHD (see below) or a grade 4 adverse event (graded per The Common Terminology Criteria for Adverse Events (CTCAE v.3) between the time of aDLI and D+100 that was possibly, probably or definitely related to the aDLI. The aDLI consisted of PBMC with the total cell number adjusted to contain the indicated dose of CD3+ T-cells/kg. The initial starting aDLI dose for the first patient was 103 CD3+ T-cells/kg (dose-level 1) and subsequent available dose-levels were 104 CD3+ T-cells/kg (dose-level 2) and 105 CD3+ T-cells/kg (dose-level 3). In order to implement this adaptive trial design, prior patients had to reach D+100 before the subsequent aDLI dose assignment. The study design mandated stopping if no acceptable aDLI dose could be identified for a patient based on prior patient outcomes.
Acute and chronic GvHD
AGVHD was graded according to the Consensus Grading Scale.22 Hyperacute GvHD was defined as occurring within 14 days of aDLI. Refractory aGVHD was defined as aGVHD that did not resolve within 3 weeks of treatment with corticosteroids. AGVHD treatment was at the discretion of site investigators. Patients who were placed onto other randomized studies or given unconventional therapy for refractory aGVHD were removed from study at the time of these interventions and followed only for survival. Chronic GvHD was diagnosed and graded as per contemporaneous NIH criteria.23
Measurement of immune reconstitution
Immune reconstitution assays were performed on patient PBMC at 1, 2, 3, 4, 6, 9 and 12 months post-transplant. Patients were eligible for immune reconstitution analysis if they had engrafted and had samples available for a minimum of 2 time points. Patients were excluded from subsequent immune reconstitution studies from time of relapse or administration of experimental or unconventional therapy for refractory aGvHD. T-cell subsets, naive and memory, regulatory and effector cell phenotypes and antigen-specific T-cell responses were determined by surface and intracellular cytokine flow cytometry as previously described.19,20 The frequencies of CD4+ Treg and T effectors (Teff) were measured by flow cytometry and defined as the percentage of CD4+ T-cells with a CD25hiCD127lo and CD25hiCD127+ phenotype respectively. Further details are contained in Supplementary Methods.
Statistical analysis
For correlations of phenotypic characteristics of aDLI and residual alloresponses the non-parametric test of significance was used. For assessing the significance of differences between groups, two-tailed t tests were used throughout. For unpaired t tests, equal variance was not assumed and Welch’s correction was used where appropriate. Area-under-the-curve (AUC) was calculated using the trapezoid rule. Statistical analysis was performed on Graphpad Prism V4. Overall survival was calculated using the method of Kaplan and Meier.
Results
Engraftment and chimerism
Stem cell infusions contained a median CD34+ cell dose of 9.9 × 106/kg (range 5.2–17.5) with a median residual CD3+ T-cell dose of 2.0 × 104/kg (range 0–3). Eighteen of 19 patients (95%) engrafted (median time to neutrophil engraftment D+12, range 8–19). Only one of 18 engrafted patients received G-CSF. All patients who engrafted achieved full donor chimerism at first testing (median D+28, range D+17-D+40). The patient who failed to engraft had autologous reconstitution. (Table S2).
Characteristics of aDLI
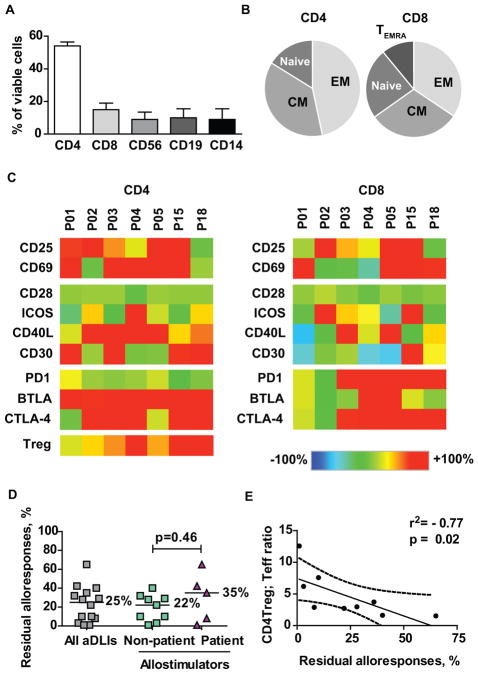
We analysed the phenotypic and functional characteristics of aDLI prior to infusion. Half of the viable infused cells were CD4+ T-cells, with smaller proportions of CD8+ T-cells, CD56+ NK cells, CD19+ B cells and CD14+ monocytes. Infused T-cells were predominantly central and effector memory cells (Figure 1 A–B). A more detailed phenotypic characterisation was performed on 7 aDLI products and on corresponding unmanipulated donor PBMC. Consistent phenotypic changes seen in alloanergized CD4+ T-cells included increased expression of activation markers CD25 and CD69, positive costimulatory receptors CD40L and CD30 and negative costimulatory receptors BTLA and CTLA4. Additionally, there was a consistent increase in the frequency of CD25+CD127lo CD4+ Treg in accordance with our previous observations.20 In contrast, less consistent changes occurred in CD8+ T-cells, although 5 of 7 products had increased expression of PD1 on CD8+ T-cells (Figure 1C). We determined residual alloreactivity in aDLI to quantify the efficiency of alloanergization. The median residual alloreactivity was 25% (range 1–65%) for all assessable products. There was no significant difference in residual alloreactivity in products generated at different study sites or for different dose levels (Figure S2) or using patient or non-patient allostimulators (Figure 1D). Finally, as aDLI were infused fresh before residual alloreactivity results were available, we correlated phenotypic characteristics of aDLI with residual alloreactivity to identify real time correlates of residual alloreactivity. Levels or changes in expression of individual molecules did not correlate closely with residual alloresponses. However, there was a significant negative correlation with the ratio of CD4+ Treg to CD4+ Teff in aDLI products and residual alloresponses (Figure 1E).
Figure 1. Phenotypic and functional characteristics of alloanergized donor lymphocyte infusions.
(A) Cellular composition of alloanergized donor lymphocyte infusions (aDLI) prior to infusion. Values shown are mean (+/− sd) percentages of viable cells for aDLI generated for 9 different patients.
(B) Proportions of CD4+ and CD8+ T-cells in aDLI with CD45RA+CD62L+ naive, CD45RA−CD62L+ central memory (CM), CD45RA−CD62L− effector memory (EM) and CD45RA+CD62L− T effector memory-RA (TEMRA) phenotypes. Mean percentages of viable CD4+ or CD8+ T-cells are shown for aDLI generated for 9 different patients.
(C) Phenotypic heat map of viable CD4+ and CD8+ cells in aDLI compared to expression levels in unmanipulated donor PBMC. Expression patterns are shown for aDLI for 7 different patients. Treg frequencies were determined in 10 aDLI products; data is shown for the 7 products where full phenotypic analysis was available. ICOS, inducible T-cell costimulator; PD1, programmed death 1; BTLA, B and T lymphocyte attenuator; CTLA4, cytotoxic T lymphocyte antigen 4; Treg, CD25+CD127lo regulatory T-cell.
(D) Residual alloproliferative responses of aDLI after ex vivo alloanergization. Lines and adjacent numbers are medians. Results are shown for aDLI products generated for 14 patients.
(E) Correlation between CD4+ Treg:Teffector ratio and residual alloresponses in aDLI. R2 value is for Spearman correlation coefficient and p value is for two-tailed t test. Dashed line represents 95% confidence limits. Results are shown for aDLI products generated for 9 different patients.
GvHD before and after aDLI
Incidences of aGvHD pre-aDLI (from the start of conditioning to immediately prior to aDLI infusion) and post-aDLI (from aDLI infusion to D+100) are shown in Table 1. One of 18 evaluable patients developed aGVHD pre-aDLI (Grade 2, D+22). Three patients did not receive aDLI; one died of bacterial sepsis at D+32, one donor was unable to donate and one patient failed to engraft. Sixteen patients received aDLI at dose-levels 1 (n=4), 2 (n=8), and 3 (n=4), Table S3. No infusional reactions or hyperacute GvHD occurred after aDLI. Six patients developed aGVHD of any grade post-aDLI at a median D+63 (range D+52-D+98). One patient (P005) had developed and resolved grade 2 aGvHD pre-aDLI, and then presented again after aDLI with grade 2 on D+98. Five additional patients developed aGVHD post-aDLI: 2 patients at dose-level 2, (one grade 1 and one grade 4); and 3 patients at dose-level 3 (all grade 3). There was a trend to greater residual alloreactivity of aDLI in patients who went on to develop aGvHD than those who did not but this did not reach statistical significance (median 35% versus 20%, p=0.15, Figure S3). None of the 4 patients receiving aDLI at dose-level 1 and only 2 of the 8 patients who received aDLI at dose-level 2 developed Grade 2 or higher aGVHD, compared to 3 of 4 patients who received aDLI at dose-level 3. Two patients had refractory aGvHD while the others responded to steroids/other systemic immunosuppressive therapies. Four patients went on to develop chronic GVHD.
Table 1.
acute GvHD pre-and post-aDLI
| Patient | aDLI dose level and day | Pre-aDLI | Post-aDLI |
|---|---|---|---|
| Acute GvHD Time, Grade (stage) | |||
| 001 | Level 1, D+35 | None | None |
| 002 | Level 1, D+35 | None | None |
| 003 | Level 1, D+35 | None | None |
| 004 | Level 1, D+42 | None | None |
| 005 | Level 2, D+42 | D+22 Grade 2 (S2,GI 1, L0) | D+98 Grade 2 (S3,GI 0, L1) |
| 010 | Level 2, D+34 | None | None |
| 012 | Level 2, D+35 | None | None |
| 013 | None given† | None | N/E |
| 025 | None given§ | None | N/E |
| 026 | Level 2, D+35 | None | D+52 Grade 4 (S0,GI 4, L4) |
| 027 | Level 2, D+42 | None | None |
| 028 || | None Given | N/E | N/E |
| 030 | Level 2, D+42 | None | None |
| 033 | Level 2, D+35 | None | D+54 Grade 1 (S2,GI 0, L0) |
| 035 | Level 2, D+35 | None | None |
| 015 | Level 3, D+36 | None | D+63 Grade 3 (S3,GI 3, L0) |
| 016 | Level 3, D+35 | None | D+62 Grade 3 (S2,GI 3, L0) |
| 018 | Level 3, D+35 | None | None |
| 023 | Level 3, D+35 | None | D+89 Grade 3 (S0,GI 1, L3) |
Level 1, 103 CD3 T-cells/Kg, Level 2 104 CD3 T-cells/kg, Level 3, 105 CD3 T-cells/kg; S, skin; GI, gastrointestinal; L, liver
died D+32 of bacterial sepsis prior to infusion of aDLI.
Donor not able to donate.
non-engraftment followed by autologous reconstitution
Infections before and after aDLI
A high proportion of patients (twelve of 19, 63%) had documented bacteremia and CMV reactivation occurred in 9 of 17 (53%) at-risk patients during the pre-aDLI period. None of these 9 had received CMV prophylaxis, and all developed peripheral blood CMV reactivation prior to aDLI at median D+24 (range 16–33 days). Eight at-risk patients did not develop CMV reactivation pre-aDLI. Five of these received CMV prophylaxis as recommended, 2 received prophylaxis after stem cell infusion but not during conditioning and one received HSV prophylaxis with famcyclovir and valacyclovir. Five patients had BK viruria and five patients had symptomatic viral infections in the pre-aDLI period; 3 had oral/nasal HSV infection, one had nasopharyngeal RSV infection and one had systemic adenoviral infection.
Only 4/14 at-risk patients (29%) reactivated CMV post-aDLI with an overall cumulative incidence of CMV reactivation below 60% at D+180. Of these, 3 were receiving immunosuppression for GvHD treatment and 3 successfully cleared CMV with antiviral therapy. CMV disease (retinitis) was confirmed in one patient. Four patients had EBV reactivation in peripheral blood with no confirmed cases of post-transplant lymphoproliferative disorder. Two patients were electively treated with rituximab. One had become EBV DNA-negative prior to treatment while the other did so on treatment. Two patients became EBV DNA-negative without any treatment. Other viral reactivations/infections occurred in 4 of 4 assessable patients who received aDLI at dose-level 1/no aDLI, compared to 4 of 8 patients receiving dose-level 2 and 3 of 4 patients who received aDLI at dose-level 3. Two patients developed aspergillus pneumonia and one patient was positive for aspergillus by PCR in broncho-alveolar lavage.
Immune reconstitution of T-cell subsets
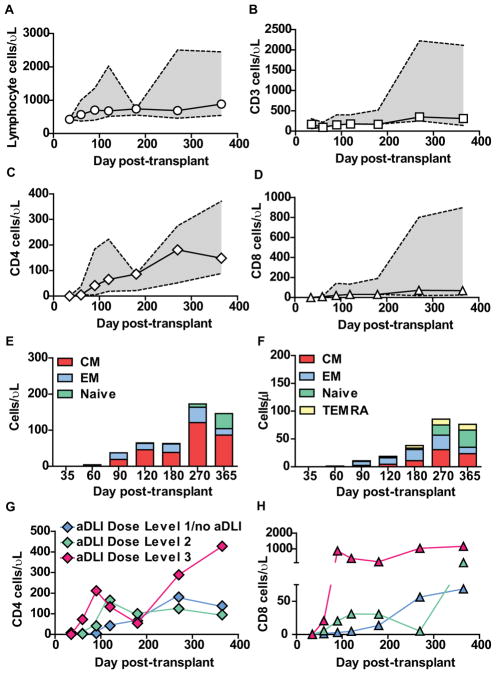
Sixteen patients were eligible for immune reconstitution studies. Median values and ranges for absolute lymphocyte count, CD3+, CD4+ and CD8+ T-cell recovery are shown in Figure 2 (A–D). CD4+ T-cells recovered more rapidly than CD8+ T-cells. Reconstituting T-cells early post-transplant were predominantly central and effector memory cells reflecting the phenotype of infused aDLI, with no emergence of naive T-cells until 9–12 months (Figure 2 E–F).
Figure 2. Reconstitution of immune cell subsets after T-cell depleted haploidentical HSCT and infusion of aDLI.
(A)–(D) Absolute numbers of lymphocytes (A), CD3+ (B), CD4+ (C) and CD8+ T-cells (D) in patient peripheral blood after T-cell depleted haploidentical HSCT and infusion of aDLI. Medians and interquartile ranges are shown for 16 patients eligible for immune reconstitution studies.
(E)–(F) Absolute numbers of CD4+ (E) and CD8+ (F) T-cells in patient peripheral blood after T-cell depleted haploidentical HSCT and infusion of aDLI with naive, central memory (CM), effector memory (EM) and T effector memory-RA (TEMRA) phenotypes. Medians are shown for 16 patients eligible for immune reconstitution studies.
(G)–(H) Absolute numbers of CD4+ (G) and CD8+ T-cells (H) in patient peripheral blood after T-cell depleted haploidentical HSCT and infusion of aDLI. Median values are shown for 16 patients eligible for immune reconstitution studies grouped according to aDLI dose.
We next analysed immune reconstitution in relation to the dose of aDLI received. Reconstitution of CD4+ and CD8+ T-cell numbers was accelerated in patients receiving aDLI at dose-levels 2 and 3 compared to patients receiving dose-level 1/no aDLI (Figure 2G–H), although differences in T-cell counts at individual time points did not reach statistical significance. In order to better evaluate the impact of aDLI on reconstitution of T-cells over the first 4 months post-transplant, we also performed an AUC analysis in patients surviving until this point. The D+120 AUC for both CD4+ T-cell and CD8+ T-cell recovery was significantly greater in patients who received aDLI at either dose-level 2 or dose-level 3 compared to those who received dose-level 1/no aDLI (p=0.04 and 0.02 (CD4+) and p=0.04 and p=0.04 (CD8+) respectively). Additionally, D+120 AUC for both CD4+ and CD8+ T-cell recovery in patients receiving aDLI at dose-level 3 was significantly greater than patients receiving aDLI at dose-level 2 (p=0.03 and 0.01 respectively). Importantly, there was no significant difference in D+120 AUC for either CD4+ or CD8+ T-cell reconstitution in patients grouped by age, donor age, TBI or chemotherapy-only conditioning, or higher or lower contaminating T-cell doses within the stem cell transplant. Beyond D+120, CD4+ T-cell counts in recipients of aDLI at dose-level 3 fell transiently, reaching levels similar to those of recipients of aDLI at dose-levels 1 and 2, although a similar dip in CD8+ T-cell reconstitution was not observed. This dip in CD4+ T-cell levels may have reflected the lympholytic effect of systemic steroid therapy for aGvHD initiated in 3 of 4 recipients of aDLI at dose-level 3.
Recovery of virus-specific T-cells
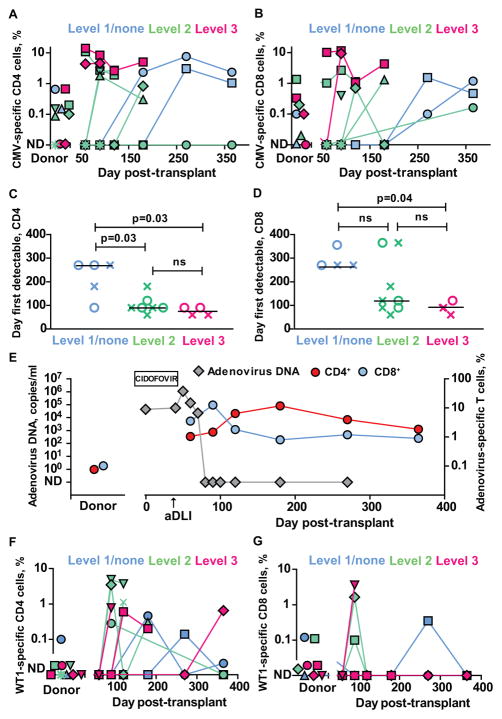
As virus-specific T-cells identified by multimer technology may lack functionality after HSCT,24 we assessed the frequency of functional CMV- and VZV-specific CD4+ and CD8+ T-cells by measuring intracellular IFN-γ accumulation after stimulation with virus-infected lysates. Thirteen patients who received transplants from CMV and/or VZV-seropositive donors were studied. In several patients, greatly expanded (>50-fold) frequencies of IFN-γ+ virus-specific T-cells were detectable post-transplant compared to levels determined in donor PBMC. None of 3 assessable patients receiving Dose 1/no aDLI had detectable functional CMV-specific T-cells in the first 4 months after transplant despite 3 of 4 having detectable peripheral blood CMV DNA. In contrast, 3 of 6 patients receiving aDLI at dose-level 2, and all assessable patients receiving aDLI at dose-level 3 had functional CMV-specific T-cells detectable by this time point (Figure 3A–B).
Figure 3. Reconstitution of virus- and tumor antigen-specific T-cells after T-cell depleted haploidentical HSCT and aDLI.
(A)–(B) Frequencies of CMV-specific IFN-γ+ CD4+ (A) and CD8+ (B) T-cells in donor and patient peripheral blood after T-cell depleted haploidentical HSCT and infusion of aDLI. Individual results are shown for patients who received transplants from CMV-seropositive donors and were eligible for immune reconstitution studies and one patient who received a transplant from a CMV-seronegative donor (P02030). Patients are colour coded according to the dose of aDLI. ND= not detectable.
(D–E) First time points when virus-specific IFN-γ+ T-cells were detectable in peripheral blood of individual patients after transplant. Crosses denote CMV-specific T-cells and circles denote VZV-specific T-cells. Results are shown for 13 patients with CMV- and/or VZV-seropositive donors who were eligible for immune reconstitution studies. Values are grouped and colour coded according to the dose of aDLI. Horizontal lines are medians.
(F–G) Frequencies of WT1-specific IFN-γ+ CD4+ (A) and CD8+ (B) T-cells in donor and patient peripheral blood after T-cell depleted haploidentical HSCT and infusion of aDLI. Individual results are shown for 14 patients eligible for immune reconstitution studies, colour coded according to the infused dose of viable donor CD3+ T-cells within aDLI/kg body weight.
To assess a broader spectrum of virus-specific T-cell reconstitution, we grouped the earliest time points post-transplant, according to dose of aDLI, that functional CMV or VZV-specific T-cells were detectable in patient peripheral blood. (Figure 3C–D). The median time when virus-specific CD4+ IFN-γ+ T-cells were first detectable was 3 months and 2.5 months for patients who received aDLI at dose-levels 2 and 3 respectively, significantly earlier than patients receiving Dose 1/no aDLI (median 9 months, p=0.03 for comparison with either dose-level 2 or dose-level 3). Similarly, functional virus-specific CD8+ T-cells were first detectable at a median of 4 and 3 months in recipients of aDLI at dose-levels 2 and 3, considerably earlier than in patients receiving dose 1/no DaLI (median 9 months) although only dose-level 3 versus dose-level 1/no aDLI reached statistical significance.
We also determined the frequency of adenovirus-specific T-cells in P018 who prior to aDLI had developed symptomatic adenoviral infection with a high and rising copy number of adenovirus by PCR despite systemic antiviral treatment with cidofovir. aDLI was followed by marked expansion of donor-derived adenovirus-specific T-cells co-incident with a fall and subsequent eradication of adenovirus DNA. (Figure 3E).
Tumor-associated antigen-specific T-cells
Donor-derived T-cells specific for the tumor-associated antigen WT1 can provide graft-versus-leukemia effects after HSCT.25,26 We therefore measured WT1-specific CD4+ and CD8+ IFN-γ+ T-cells in patient peripheral blood. WT1-specific T-cells were largely undetectable in peripheral blood from donors. However, large expansions of WT1-specific CD4+ and CD8+ T-cells were detectable in patient peripheral blood after HSCT. Furthermore, WT1-specific T-cells were detectable earlier and at higher frequencies in patients receiving aDLI at dose-levels 2 and 3 (Figure 3F–G).
Regulatory T-cell expansion
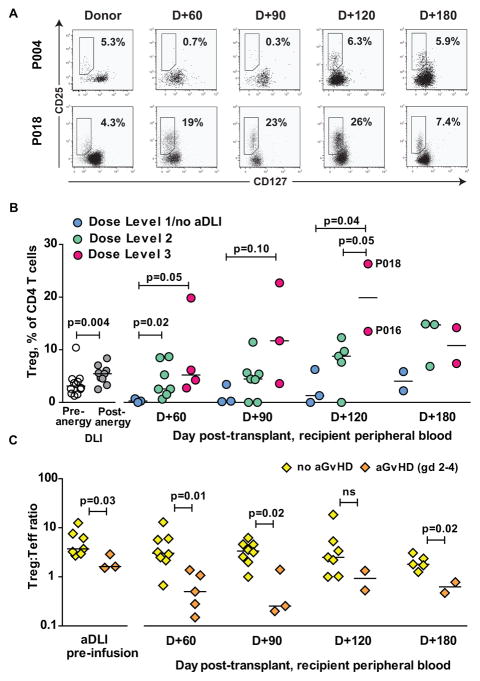
We have previously shown that infusion of alloanergized T-cells en bloc with haploidentical BM results in an expansion of CD4+ Treg in patient peripheral blood post-transplant, and that expanded populations of Treg may contribute to the control of alloresponses in this setting.20 We therefore measured the frequencies of CD4+ Treg in aDLI pre- and post-alloanergization and in peripheral blood of patients after aDLI. CD4+ Treg frequencies were significantly increased in aDLI compared to unmanipulated donor PBMC (median 6% versus 3.5%, p=0.004). A further increase in the frequencies of CD4+ Treg occurred in patient peripheral blood after infusion of aDLI, with a peak frequency occurring at D+120 post-HSCT. Infusion of higher dose-levels of aDLI was associated with significantly higher frequencies of CD4+ Treg in patient peripheral blood at D+60 and D+120 when compared to frequencies in patients receiving dose-level 1/no aDLI (Figure 4 A–B). Expanded populations of CD4+CD25hiCD127lo Treg in patient peripheral blood post-transplant expressed high levels of FOXP3 consistent with suppressive function (Figure S5). Several patients who had demonstrated large expansions of virus-specific T-cells in peripheral blood post-transplant (including P016, CMV-specific T-cell expansion and P018, adenovirus-specific T-cell expansion) also had large expansions of peripheral blood CD4+ Treg.
Figure 4. Reconstitution of CD4+ regulatory T-cells (Treg) after T-cell depleted haploidentical HSCT and aDLI.
(A) CD4+ Treg in donor and patient peripheral blood after T-cell depleted haploidentical HSCT and infusion of aDLI. Representative dot plots are shown for patients who received aDLI at a dose of 103 CD3+ T-cells/kg (P004) and aDLI at a dose of 105 CD3+ T-cells/kg (P018). Boxed regions contain CD25+CD127lo Tregs and numbers denote the frequencies of Treg expressed as a percentage of CD4+ T-cells.
(B) Frequencies of CD4+ Treg in DLI before and after alloanergization, and patient peripheral blood after T-cell depleted haploidentical HSCT and infusion of aDLI. Values are shown for 14 patients eligible for immune reconstitution studies grouped according to dose of aDLI. Horizontal lines are medians. Treg frequencies at D+120 in two patients who also had large expansions of peripheral blood virus-specific T-cells post-transplant (P016, CMV-specific T-cell expansion) and P018, adenovirus-specific T-cell expansion) are highlighted.
(C) Ratio of frequencies of CD4+ Treg;Teffector cells in aDLI, and in patient peripheral blood after T-cell depleted haploidentical HSCT and infusion of aDLI. Values are shown for 13 patients eligible for immune reconstitution studies grouped according to whether they did or did not develop acute GvHD (grades 2–4). Horizontal lines are medians.
However, there was no clear association with relative or absolute numbers of CD4+ Treg in patient peripheral blood post-transplant and occurrence of aGvHD. As recent studies have shown that aGvHD is more clearly associated with the ratio of CD4+ Treg: Teff than with CD4+ Treg numbers alone after T-cell depleted HSCT,27 we calculated the CD4+ Treg: Teff ratio in aDLI pre-infusion, and in patient peripheral blood after haploidentical HSCT and aDLI. Patients who developed Grade 2 or higher aGvHD had significantly lower CD4+ Treg: Teff ratios in the peripheral blood post-transplant. In addition, the CD4+ Treg: Teff ratio was also significantly lower in aDLI products administered to patients who went on to develop aGvHD (Figure 4C).
Patient Outcomes
All 16 patients who received HSC and aDLI were evaluable for survival (Table S4). Two patients relapsed post-transplant (P010 at D+180, P012 at D+150); both subsequently died. Only one patient (P023) died as a result of viral infection. One patient died from aGvHD (P026) after receiving aDLI at dose-level 2. There were 7 patient deaths from regimen-related toxicity (pulmonary veno-occlusive disease (1 patient, D+78), respiratory distress syndrome (5 patients, D+59-D313) and sepsis/multiorgan failure (1 patient at D+312). An additional patient (P002) died from bleeding after a liver biopsy 2 years after transplant. Four of 13 assessable patients developed chronic GVHD- limited in one (P027) and extensive in three patients (P015, P016, P033). The actuarial overall survival of the 16 patients receiving aDLI was 38% at 1 year. Three patients (P005, P015 and P018) are known to be currently alive and disease-free with a median follow up of 9 years (range 8.9–9.3). Patient 004 was alive and disease-free through 5.8 years post-transplant after which she was lost to follow up.
Discussion
We have shown that generation and administration of aDLI after CD34-selected myeloablative haploidentical HSCT in patients with acute leukemia and myelodysplasia are feasible across multiple clinical sites. While this study highlights the potential influence of regimen-related toxicities in high-risk patients on the interpretation of early phase studies, we demonstrated that a modest dose of aDLI is well tolerated and positively impacts on a range of immune reconstitution parameters.
We observed a relatively low incidence of clinically significant aGvHD at dose-levels 1 and 2 (2/12 patients) whereas aGvHD occurred in 3 of 4 recipients of aDLI at dose-level 3 (105 CD3+ T-cells/kg). This should be set within the context of reported frequencies of aGVHD after CD34-selected haploidentical HSCT without DLI of between 0 and 10% in adults and children.9,28 The maximum tolerated dose of aDLI given in the context of this study was therefore 104 T-cell/kg (dose-level 2), lower than T-cell doses administered in published reports of CD25-allodepleted DLI and our previous studies using alloanergization to reduce alloreactivity of T-cells within BM grafts.18,19,29 Several potential explanations are possible. Firstly, in our prior studies, 2–3-log greater doses of alloanergized cells were infused at the time of BMT which may have optimized homeostatic expansion and the presence and activity of relevant immunomodulatory cell populations such as Treg. Secondly, pro-inflammatory environments have been shown to both reverse anergy in alloreactive T cells in vitro and adversely influence the functional stability of potentially tolerogenic CD4+ Treg.30,31 In our current study, a notably large proportion of these high-risk patients (63% overall and 56% who went on to get aDLI) had proven bacteremia in the first period of the study (pre-aDLI) and all at-risk patients who did not receive CMV prophylaxis demonstrated reactivation. This high rate of infectious comorbidity could have created a pro-inflammatory, cytokine-rich micro-environment impacting adversely on both passive and active tolerance mechanisms in the period post-aDLI resulting in an increased frequency of aGvHD. Finally, alloanergization of DLI resulted in less efficient and more variable reduction in residual alloreactivity compared to our pre-clinical scale up studies19 or alloanergization of T cell-replete donor BM in our previous clinical studies.18,19 This may in turn have contributed to breakthrough aGvHD at lower doses of infused T-cells. It is possible that the increased variability in the efficiency of alloanergization resulted from either the use of non-patient allostimulators or from center effects, although the small numbers in this study are insufficient to firmly support or reject these hypotheses. The most generalizable means to address the variability would be to implement a real-time assay of residual alloreactivity prior to infusion of aDLI. As the ratio of CD4+ Treg:Teff in aDLI significantly correlated with residual alloreactivity, use of this phenotypic correlate might improve quality control of aDLI whilst facilitating the use of fresh cells to preserve optimal viability.
Infusion of aDLI at a dose above 103 CD3+ T-cells/kg resulted in faster T-cell reconstitution than reported after CD34-selected haploidentical HSCT without T-cell addback,9,32 and comparable reconstitution to that reported after TCR α/β depletion prior to haploidentical HSCT.33 While CD34-selected grafts infused prior to aDLI contained up to 3 × 104/kg contaminating CD3+ T-cells, these unmanipulated donor T-cells should not have contributed to immune reconstitution as they would likely have been destroyed by ATG administered in the conditioning regimen.
We observed a high incidence of CMV reactivation (53% of at-risk patients) pre-aDLI, similar to that reported (60% by D+42) in the largest published study of CMV reactivation rates in the early post-transplant period after CD34-selected TCD haploidentical HSCT.34 Only 29% of at-risk patients on our study had further episodes of CMV reactivation after aDLI, with one first reactivation occurring in this period so the cumulative incidence of CMV reactivation remained below 60% at D+180, in contrast to the study reported by Lilleri et al who found that CMV reactivation continued beyond D+40 to reach a cumulative incidence above 80% by D+100 post-transplant.34
Encouragingly, functional virus-specific T-cells became detectable early after aDLI. In contrast, virus-specific T-cell responses remained undetectable until 12 months after TCD haploidentical HSCT in the absence of additional T-cell infusions in previously published studies.35 The median time to reconstitution of detectable CD4+ and CD8+ CMV and VZV-specific T-cells in recipients of aDLI at the lowest dose-level (103 CD3+ T-cells/kg) was 9 months and was significantly earlier in recipients of 104 CD3+ T-cells/kg (median 3 and 4 months, CD4+ and CD8+ T-cells). This is consistent with the provision of a threshold number of viral-specific T-cells contained within aDLI that are able to undergo antigen-specific expansion in vivo. Although variance in the strategies used for CMV prophylaxis at different clinical centres make it impossible to draw firm conclusions regarding the impact of aDLI on CMV reactivation and CMV-specific T-cell reconstitution, the rates of CMV reactivation after aDLI are comparable to that recently reported with the PTC approach,16 suggesting that the emergence of virus-specific T-cells after aDLI might have potential to provide functional virus-specific immunity.
Importantly we also observed expansion of donor-derived tumor–antigen-specific T-cells which could contribute to Graft-versus-Leukemia (GvL) effects. While our study was not designed to assess the impact of aDLI on disease relapse and there were confounding effects of early mortality, only 2 of 16 patients who received aDLI subsequently relapsed, despite uniformly high-risk disease characteristics. This contrasts with some reported outcomes after haploidentical HSCT with PTC, where relapse rates of 35–50% have been reported suggesting that this approach may result in impairment of donor-derived GvL effects.15,36 However, it is important to stress that our study was not designed to determine the clinical efficacy of aDLI in terms of provision of functional immunity post-transplant. Thus, while no conclusions can be drawn on the impact of aDLI on infections or relapse, the observed results support the hypothesis that aDLI may exert beneficial effects on these endpoints and provides a rationale for addressing these endpoints in larger subsequent studies.
Our study also demonstrated a dose-dependent expansion of donor-derived CD4+ Treg in the peripheral blood of patients after aDLI, confirming our earlier findings after alloanergized BMT. We and others have previously shown that donor CD4+ Treg generated by alloanergization play an important role in the control of alloresponses mediated by human and murine T-cells.20,37 Observational studies have shown higher numbers of CD4+ Treg post-transplant are associated with freedom from aGvHD.38 Adoptive therapy with donor-derived CD4+ Tregs has recently been shown to reduce the incidence of aGvHD after haploidentical HSCT.39 Higher ratios of CD4+ Treg:Teff in patients who did not develop aGvHD after aDLI suggest that CD4+ Treg may indeed have a central role in controlling clinically significant alloresponses in this setting. While limiting numbers of cells prevented us from characterizing the suppressive function of expanded populations of CD4+ Tregs, large expansions of CD4+ Treg were seen in several patients who also had vigorous expansion of pathogen-specific T-cells demonstrated that expanded CD4+ Treg did not result in global immunosuppression. Strategies to potentiate the expansion of CD4+ Treg after aDLI may further reduce toxicity and improve the therapeutic window of this approach. Additional studies of the suppressive capacity and in vivo stability of these Treg would be of interest.
We are encouraged that only one patient in the study died from aGvHD and only one patient from viral infection. However, despite using a chemotherapy-only conditioning regimen in the majority of patients, the incidence of non-GvHD treatment-related mortality remained disappointingly high. The high rate of infection observed in patients during conditioning and immediately thereafter suggests that the baseline risk of the study population was high and may have affected study endpoints. With the greater acceptance of haploidentical HSCT since the study was conducted, due in large part to the successes of the approaches reviewed above, it should become increasingly easier to study novel approaches at times when patients are earlier in their disease course.
In summary, we have shown that low doses of aDLI can provide beneficial immune reconstitution after CD34-selected myeloablative haploidentical HSCT with acceptable rates of aGvHD. As beneficial effects can be observed after aDLI at low cell numbers, this may be a valuable strategy to bolster anti-viral and potentially anti-tumor effects after HSCT undertaken using one of a variety of available approaches to the problem of aGvHD. The approach has the advantage of rapid in vivo expansion of donor CD4+ Treg which may provide an additional mechanism to control alloreactivity in this setting.
Supplementary Material
Table 2.
Infections pre- and post-aDLI
| Patient | aDLI dose/day |
CMV IgG (D/R) |
Pre-aDLI | Post-aDLI | |||||
|---|---|---|---|---|---|---|---|---|---|
| Bacterial Infection | Viral Reactivation | Viral Infn | Fungal Infn | Viral Reactivation | Viral Infn | Fungal Infn | |||
| 001 | Level 1, D+35 | −/− | None | None | None | None | VZV skin D+76 | None | None |
| 002 | Level 1, D+35 | +/− | Stomatococcua blood D+1 | BK urine D-8 | None | None | EBV blood D+147,BK urine D+49,254,331, VZV skin D+260 | EBV D+147 VZV D+260 |
None |
| 003 | Level 1, D+35 | −/+ | E.Coli blood D-3 VRE blood D0 |
HSV oral D+28 CMV blood D+33 |
HSV D+28 | None | None | None | None |
| 004 | Level 1, D+42 | +/+ | Enterobact. blood D-6 Proteus Urine D+32 |
BK urine D+5, CMV blood D+26, RSV NP D+26 | RSV D+26 | None | EBV blood D+234 | None | None |
| 005 | Level 2, D+42 | +/+ | None | None | None | None | RSV NP D+209 | RSV D+209 | None |
| 010 | Level 2, D+34 | +/+ | Staph Epi blood D-8 | CMV blood D+20,30 | None | None | CMV blood D+74,84,96, HHV6 BM D+78 | None | None |
| 012 | Level 2, D+35 | −/+ | C.Diff colitis D-2 | CMV blood D+20 | None | None | None | None | None |
| 013 | None given† | −/+ | Stomatococcus + VRE blood D+11 | N/E | N/E | N/E | N/E | N/E | N/E |
| 025 | None given§ | +/+ | Corynebacterium blood D+27 | HSV nasal D+24 CMV blood D+27 |
HSV nasal D+24 | N/E | N/E | N/E | |
| 026 | Level 2, D+35 | −/+ | None | None | None | None | None | None | None |
| 027 | Level 2, D+42 | +/+ | None | CMV blood D+16 | None | Aspergillus BAL D+13 | CMV blood ends D+73 then reactivates D+209 | CMV retinitis D+114 to D+176 | None |
| 028 || | None Given | +/+ | Fusobact + α-haem strep blood D+11 | N/E | N/E | N/E | N/E | N/E | N/E |
| 030 | Level 2, D+42 | −/+ | Staph Epi blood D-1 | HSV oral D+35 | HSV oral | None | None | None | None |
| 033 | Level 2, D+35 | +/+ | None | BK urine D+13 | None | None | BK blood D+53, EBV blood D+137. CMV blood D+179 | None | None |
| 035 | Level 2, D+35 | +/+ | VRE blood D+3 | CMV blood D+23 | None | None | None | None | Presumed fungal pneumonia D+62 |
| 015 | Level 3, D+36 | +/− | α-haem strep blood D+1, C.Diff colitis D+8 | None | None | None | EBV blood D+124 | EBV D+124 | None |
| 016 | Level 3, D+35 | +/+ | α-haem strep blood D+1 | BK urine D+13, CMV blood D+27 | None | None | CMV blood D+106, cleared after D+141 | None | Aspergillus pneumonia D+172 |
| 018 | Level 3, D+35 | −/− | None | Adeno blood, urine, stool D+18 BK urine D+25 | Adenov. | None | Adenovirus (cleared by D+73) | None | None |
| 023 | Level 3, D+35 | +/+ | VRE Blood D+5 | CMV blood D+24 | None | None | BK blood D+76, Adenovirus urine, stool, sputum D+106 | None | Aspergillus in BAL D+96 |
Level 1, 103 CD3 T-cells/Kg, Level 2 104 CD3 T-cells/kg, Level 3, 105 CD3 T-cells/kg; D, donor; R, recipient; BKV, BK virus, VRE, vancomycin-resistant enterococcus; HSV, herpes simplex virus; RSV, respiratory syncytial virus; CMV, cytomegalovirus; C Diff, clostridium difficile; BAL; Bronch-alveolar lavage,
died D+32 of bacterial sepsis prior to infusion of aDLI.
Donor not able to donate.
non-engraftment followed by autologous reconstitutiion
Statement of Translational Relevance.
Allogeneic hematopoetic stem-cell transplantation (HSCT) remains the most effective form of cellular immunotherapy for patients with acute myeloid leukemia and myelodysplasia but the lack of fully-matched donors currently limits applicability of this treatment. The use of more widely available haploidentical (half-matched) donors has been associated with delayed immune reconstitution and increased graft-versus-host disease (GvHD). We previously used the approach of alloanergization of T-cells within donor bone marrow to facilitate haploidentical HSCT. We now show in a novel Phase 1 study that infusion of low doses of alloanergized donor lymphocyte infusions (aDLI) after CD34-selected myeloablative haploidentical HSCT is feasible at multiple clinical sites and improves immune reconstitution without excess GvHD. Importantly we also show administration of aDLI expands frequencies of potentially tolerogenic regulatory T cells in vivo, a mechanism which could further improve post-transplant immune recovery in this setting.
Acknowledgments
Financial Support: This study was funded by NIH R21CA137645 and U19CA100265. JKD was funded by Leukemia and Lymphoma Society (Special fellow in Clinical Research), MRC (Clinician Scientist Fellowship G0902269) and CRUK centre grant (C16420/A18066). CRC was awarded Scholar in Clinical Research by the Leukemia & Lymphoma Society (2400-13).
DFCI Cell Manipulation Core facility (CMCF), John Daley, John McMannis, cell processing centers at all sites
Footnotes
Declaration: The authors declare no potential conflicts of interest
Author Contributions
JKD designed, supervised, processed and analyzed the immune reconstitution studies, assisted in clinical study design and wrote the manuscript, LLB collected the clinical data, PFT contributed to the statistical design of the clinical study, JRW, CRC, NK, AS, BD, TRS, MdeL, LC, REC and ECG recruited patients, LMN developed and provided critical reagents and assisted in study design and manuscript preparation, ECG designed and oversaw the clinical study and wrote the manuscript.
References
- 1.Spitzer TR. Haploidentical stem cell transplantation: the always present but overlooked donor. Hematology Am Soc Hematol Educ Program. 2005:390–5. doi: 10.1182/asheducation-2005.1.390. [DOI] [PubMed] [Google Scholar]
- 2.Powles RL, Morgenstern GR, Kay HE, et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet. 1983;1(8325):612–5. doi: 10.1016/s0140-6736(83)91793-2. [DOI] [PubMed] [Google Scholar]
- 3.Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313(13):765–71. doi: 10.1056/NEJM198509263131301. [DOI] [PubMed] [Google Scholar]
- 4.Kernan NA, Collins NH, Juliano L, Cartagena T, Dupont B, O’Reilly RJ. Clonable T lymphocytes in T cell-depleted bone marrow transplants correlate with development of graft-v-host disease. Blood. 1986;68(3):770–3. [PubMed] [Google Scholar]
- 5.Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339(17):1186–93. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 6.Mehta J, Singhal S, Gee AP, et al. Bone marrow transplantation from partially HLA-mismatched family donors for acute leukemia: single-center experience of 201 patients. Bone Marrow Transplant. 2004;33(4):389–96. doi: 10.1038/sj.bmt.1704391. [DOI] [PubMed] [Google Scholar]
- 7.Ash RC, Horowitz MM, Gale RP, et al. Bone marrow transplantation from related donors other than HLA-identical siblings: effect of T cell depletion. Bone Marrow Transplant. 1991;7(6):443–52. [PubMed] [Google Scholar]
- 8.Henslee PJ, Thompson JS, Romond EH, et al. T cell depletion of HLA and haploidentical marrow reduces graft-versus-host disease but it may impair a graft-versus-leukemia effect. Transplant Proc. 1987;19(1 Pt 3):2701–6. [PubMed] [Google Scholar]
- 9.Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23(15):3447–54. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 10.Chaleff S, Otto M, Barfield RC, et al. A large-scale method for the selective depletion of alphabeta T lymphocytes from PBSC for allogeneic transplantation. Cytotherapy. 2007;9(8):746–54. doi: 10.1080/14653240701644000. [DOI] [PubMed] [Google Scholar]
- 11.Guimond M, Balassy A, Barrette M, Brochu S, Perreault C, Roy DC. P-glycoprotein targeting: a unique strategy to selectively eliminate immunoreactive T cells. Blood. 2002;100(2):375–82. doi: 10.1182/blood-2001-12-0353. [DOI] [PubMed] [Google Scholar]
- 12.Amrolia PJ, Muccioli-Casadei G, Yvon E, et al. Selective depletion of donor alloreactive T cells without loss of antiviral or antileukemic responses. Blood. 2003;102(6):2292–9. doi: 10.1182/blood-2002-11-3516. [DOI] [PubMed] [Google Scholar]
- 13.Handgretinger R, Lang R, Feuchtinger T, et al. Transplantation of TcR{alpha}{beta}/CD19 depleted stem cells from haploidentical donors: robust engraftment and rapid immune reconstitution in children with high risk leukemia. Blood. 2011;118:1005. [Google Scholar]
- 14.Amrolia PJ, Muccioli-Casadei G, Huls H, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108(6):1797–808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10):1310–6. doi: 10.1200/JCO.2012.44.3523. [DOI] [PubMed] [Google Scholar]
- 16.Tischer J, Engel N, Fritsch S, et al. Virus infection in HLA-haploidentical hematopoietic stem cell transplantation: incidence in the context of immune recovery in two different transplantation settings. Annals of hematology. 2015;94(10):1677–88. doi: 10.1007/s00277-015-2423-y. [DOI] [PubMed] [Google Scholar]
- 17.Gribben JG, Guinan EC, Boussiotis VA, et al. Complete blockade of B7 family-mediated costimulation is necessary to induce human alloantigen-specific anergy: a method to ameliorate graft-versus-host disease and extend the donor pool. Blood. 1996;87(11):4887–93. [PubMed] [Google Scholar]
- 18.Guinan EC, Boussiotis VA, Neuberg D, et al. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340(22):1704–14. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]
- 19.Davies JK, Gribben JG, Brennan LL, Yuk D, Nadler LM, Guinan EC. Outcome of alloanergized haploidentical bone marrow transplantation after ex vivo costimulatory blockade: results of 2 phase 1 studies. Blood. 2008;112(6):2232–41. doi: 10.1182/blood-2008-03-143636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies JK, Nadler LM, Guinan EC. Expansion of allospecific regulatory T cells after anergized, mismatched bone marrow transplantation. Science translational medicine. 2009;1(1):1ra3. doi: 10.1126/scitranslmed.3000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thall PF, Cook JD. Dose-finding based on efficacy-toxicity trade-offs. Biometrics. 2004;60(3):684–93. doi: 10.1111/j.0006-341X.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 23.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Ozdemir E, St John LS, Gillespie G, et al. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood. 2002;100(10):3690–7. doi: 10.1182/blood-2002-05-1387. [DOI] [PubMed] [Google Scholar]
- 25.Rezvani K, Grube M, Brenchley JM, et al. Functional leukemia-associated antigen-specific memory CD8+ T cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation. Blood. 2003;102(8):2892–900. doi: 10.1182/blood-2003-01-0150. [DOI] [PubMed] [Google Scholar]
- 26.Rezvani K, Yong AS, Savani BN, et al. Graft-versus-leukemia effects associated with detectable Wilms tumor-1 specific T lymphocytes following allogeneic stem cell transplantation for acute lymphoblastic leukemia (ALL) Blood. 2007 doi: 10.1182/blood-2007-03-076844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews K, Lim Z, Afzali B, et al. Imbalance of effector and regulatory CD4 T cells is associated with graft-versus-host disease after hematopoietic stem cell transplantation using a reduced intensity conditioning regimen and alemtuzumab. Haematologica. 2009;94(7):956–66. doi: 10.3324/haematol.2008.003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handgretinger R, Klingebiel T, Lang P, et al. Megadose transplantation of purified peripheral blood CD34(+) progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant. 2001;27(8):777–83. doi: 10.1038/sj.bmt.1702996. [DOI] [PubMed] [Google Scholar]
- 29.Andre-Schmutz I, Le Deist F, Hacein-Bey-Abina S, et al. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a phase 1/2 study. Lancet. 2002;360(9327):130–7. doi: 10.1016/S0140-6736(02)09413-8. [DOI] [PubMed] [Google Scholar]
- 30.Boussiotis VA, Barber DL, Nakarai T, et al. Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science. 1994;266(5187):1039–42. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- 31.Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29(1):44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eyrich M, Lang P, Lal S, et al. A prospective analysis of the pattern of immune reconstitution in a paediatric cohort following transplantation of positively selected human leucocyte antigen-disparate haematopoietic stem cells from parental donors. British journal of haematology. 2001;114(2):422–32. doi: 10.1046/j.1365-2141.2001.02934.x. [DOI] [PubMed] [Google Scholar]
- 33.Bertaina A, Merli P, Rutella S, et al. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B-cells in children with non-malignant disorders. Blood. 2014 doi: 10.1182/blood-2014-03-563817. [DOI] [PubMed] [Google Scholar]
- 34.Lilleri D, Gerna G, Fornara C, et al. Human cytomegalovirus-specific T cell reconstitution in young patients receiving T cell-depleted, allogeneic hematopoietic stem cell transplantation. The Journal of infectious diseases. 2009;199(6):829–36. doi: 10.1086/597123. [DOI] [PubMed] [Google Scholar]
- 35.Kanda Y, Oshima K, Kako S, et al. In vivo T-cell depletion with alemtuzumab in allogeneic hematopoietic stem cell transplantation: Combined results of two studies on aplastic anemia and HLA-mismatched haploidentical transplantation. American journal of hematology. 2013;88(4):294–300. doi: 10.1002/ajh.23392. [DOI] [PubMed] [Google Scholar]
- 36.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2008;14(6):641–50. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. The Journal of experimental medicine. 2001;193(11):1311–8. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108(4):1291–7. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–8. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.