Abstract
Background
Biomarkers of tobacco exposure have a central role in studies of tobacco use and nicotine intake. The most significant exposure markers are nicotine itself and its metabolites in urine. Therefore, it is important to evaluate the performance of laboratories conducting these biomarker measurements.
Methods
This report presents the results from a method performance study involving 11 laboratories from 6 countries which are currently active in this area. Each laboratory assayed blind replicates of 7 human urine pools at various concentrations on 3 separate days. The samples included 5 pools blended from smoker and nonsmoker urine sources, and 2 additional blank urine samples fortified with pure nicotine, cotinine and hydroxycotinine standards. All laboratories used their own methods, and all were based on some form of liquid chromatography / tandem mass spectrometry.
Results
Overall, good agreement was found among the laboratories in this study. Intralaboratory precision was good, and in the fortified pools the mean bias observed was < + 3.5% for nicotine, approximately 1.2% for hydroxycotinine, and less than 1% for cotinine (1 outlier excluded in each case). Both indirect and direct methods for analyzing the glucuronides gave comparable results.
Conclusions
This evaluation indicates that the experienced laboratories participating in this study can produce reliable and comparable human urinary nicotine metabolic profiles in samples from people with significant recent exposure to nicotine.
Impact
This work supports the reliability and agreement of an international group of established laboratories measuring nicotine and its metabolites in urine in support of nicotine exposure studies.
Keywords: urine, biomarker, interlaboratory, nicotine, cotinine
INTRODUCTION
The health risks of smoking and of other forms of tobacco use are well-established. Although the proportion of the population that smokes cigarettes continues to decline both in the United States and in other developed countries (1,2), smoking remains a significant public health issue, particularly regarding its initiation among young people. Tobacco use also continues to present a problem in many less developed areas of the world (3,4). In recognition of the extreme harmfulness of cigarette smoking, there have been efforts in recent years to develop tobacco products that are less hazardous (5–7). In 2009 the United States Food and Drug Administration (FDA) was granted statutory authority over the regulation of tobacco products in the United States. This led to the formation of the FDA’s Center for Tobacco Products (CTP) which has the responsibility for monitoring tobacco and related nicotine delivery systems, and regulating the development and marketing of newer, potentially less-hazardous products. One significant aspect of this oversight function depends upon biomarker data. Both Public Health studies and manufacturer’s product development research will typically rely in part on the measurement of various biomarkers.
Biomarkers have a dual role as exposure and/or dosage indicators, and as sentinels for potential future harm, customarily referred to as “biomarkers of exposure” and “biomarkers of effect”, respectively. Regardless of the form and method, the purpose of cigarettes and other dosage devices is the delivery of nicotine to the user. Therefore, nicotine itself is the most significant, specific and reliable exposure marker (and dosimeter) for both smoking and for nicotine intake in other forms, and measurement of nicotine and its metabolites in people is perhaps the most fundamental biomarker assay of tobacco exposure (8). Since nicotine is rapidly metabolized in the body, cotinine -- the primary proximate metabolite of nicotine with both a significantly longer half-life and the same high specificity as nicotine -- has been the most commonly measured biomarker of exposure. However, the metabolism of nicotine is complex and subject to genetic variability (9–12), so cotinine measurements alone may have limitations. For example, genetically slow metabolizers of nicotine have higher blood levels of cotinine for a given systemic dose of nicotine than normal metabolizers (13).
The best index of recent nicotine intake over the previous few days is currently believed to be the molar sum of nicotine and its major metabolites measured in a urine sample, referred to as the Total Nicotine Equivalents (TNE) (14–15). Although the definition may occasionally vary to include other minor metabolites, most commonly TNE has referred to a sum based on the measurement of the three primary metabolites in the urine: total nicotine, cotinine, and trans-3′-hydroxycotinine, where the term “total” refers to the inclusion of both the “free” and glucuronide forms of each of these analytes. Together, nicotine and these 5 additional metabolites constitute about 80% to 90% of the metabolites of nicotine in the body (16) and the TNE based on their measurement is believed to provide a reliable index of recent (past several days) nicotine exposures.
Our purpose in this work was to conduct a method performance study of the current inter-laboratory comparability of measurements including as many active laboratories as possible. Laboratories known to be active in urinary nicotine biomarker assays (based primarily on publication activity) from the tobacco industry, universities, government laboratories, and contract labs were invited to participate in a study coordinated by the US Centers for Disease Control and Prevention (CDC) and investigators from the University of California, San Francisco (UCSF). The basic design and objectives were the same as in our previous study of serum cotinine (17), although that prior study was focused primarily on nonsmokers exposed to secondhand smoke whereas the current study includes multiple markers among active users of tobacco and related nicotine delivery products. The number of separate analytes reported by individual laboratories in this study ranged from 1 (free cotinine only) to a maximum of 18, including 9 metabolites and their corresponding glucuronides.
MATERIALS AND METHODS
Preparation of Standards
Three high-purity standards of nicotine, cotinine and hydroxycotinine as their perchlorate or salicylate salts were used to prepare the fortified pools used in this study. Additional information on the source, purity and evaluation of those standards is provided in the Supplementary Materials.
Urine Pool Sample Collection
Anonymized urine samples were obtained from smokers participating in on-going research studies at UCSF, and anonymous non-smoker samples were collected from employees, contractors and staff located in several buildings on a CDC campus in Atlanta Georgia. This study and the sample collection protocols were approved by both CDC and UCSF IRB panels. Because these were anonymous collections, limited demographic data are available. All participants were adults and either regular cigarette smokers (UCSF) or self-identified nonsmokers (CDC).
Selection of Urine Samples and Pool Preparation
We first performed a preliminary screening of all samples collected at CDC to exclude and discard any urine sample that had a total cotinine concentration judged to be inconsistent with nonsmoker status, and to rank the background levels of total cotinine for the remaining samples. A sub-group of samples from these nonsmokers with the lowest background levels (NS1) was preferentially selected and pooled for use as the matrix for the two fortified pools with known analyte concentrations which allowed for both accuracy and precision assessments; the remaining nonsmoker samples were combined as NS2. The concentrations of total cotinine and total hydroxycotinine in the NS1 matrix pool were estimated to be 0.010 ng/mL and 0.014 ng/mL, respectively. Those results were below the Lower Limit of Detection (LLOD) of 0.030 ng/mL for both analytes and more than 104 lower than the lowest fortified concentration used in this study, so any endogenous matrix contribution was disregarded when preparing the fortified pools using NS1.
Smoker samples were similarly prescreened and divided into two groups (low=SML and high=SMH) based on their total cotinine concentration, with SMH including those samples with the highest concentration levels available among the smokers. This was done because SMH defined the high-end concentration of all analytes for the blended pool series and was used to prepare Pool E. Pools D through A were then prepared by blending SML and NS2 in varying proportions to form a range of intermediate concentrations.
For the two fortified pools at known concentration which were labeled as Pools F and G, we prepared stock solutions of the 3 standards in water and used them to prepare the final mixed standard pool. The matrix used for these two pools was NS1 as described above. The final target concentrations of nicotine, cotinine and hydroxycotinine (as the free base) in Pool F was 286, 605, and 1,066 ng/mL, respectively. For Pool G, the corresponding values were 714, 1,729 and 3,999 ng/mL.
Sample Analysis
We requested that each laboratory measure all of the analytes that they normally included in their urinary nicotine metabolite assays. Samples were run in duplicate (using separate vials) in a total of 3 runs on different days. We did not require that the days of analysis be contiguous, only that each of the three runs be conducted on separate days. Each laboratory used its own established methods for analysis, but all 11 used some form of LC tandem mass spectrometry. All participants, including analytical personnel in the two coordinating labs, were blinded to the specific identities and concentrations of the samples used in this study.
The results were sent to the coordinating laboratory in Atlanta and the data were processed and formatted as needed, and then combined into a single database for evaluation. Some participants reported results for free and total forms by measuring samples twice, once with and once without a prior hydrolysis of the glucuronides. Other laboratories measured both the free and glucuronide forms directly in a single analytical run. To establish free, glucuronide, and total values for each laboratory we used one of two approaches. For those labs that reported data for free and total concentrations, we calculated the glucuronide contribution (as the free base) by the simple difference: total – free. Results from laboratories using direct assays were processed by converting the reported glucuronide values to the equivalent free base concentration, and then calculating the total as the sum of free + glucuronide forms. TNE were calculated as the molar sum of total nicotine, cotinine and hydroxycotinine in each case when data for all three analytes were available. The molecular weights and calculations used to prepare these results are described in Supplementary Table S1.
Homogeneity
To test for homogeneity of the pools prior to sending them to participating laboratories, we analyzed 3 aliquots selected from the beginning, middle and end of the dispensing runs for each pool. In addition, a more rigorous evaluation of homogeneity was subsequently conducted using the results of free cotinine measurements from each participating laboratory in an analysis of covariance model for each pool. These analyses were designed to separate out among-laboratory variation from among-sample variation due to time of dispensing and then test for any significant increase or decrease in free cotinine levels during the dispensing process. The results of this extended homogeneity confirmation analysis are provided in Supplementary Table S2 and confirmed homogeneity of the pools.
Statistical Analysis
Summary statistics and statistical evaluations were calculated using Statistical Analysis System (SAS) software. Standard deviations and standard errors were obtained from analysis of variance models with multiple sources of variance. As in our previous study of serum cotinine (17), we followed the definitions and procedures outlined in International Standardization Organization (ISO) documents (ISO 5725-1, ISO 5725-2, and ISO 5725-4). The relevant concepts and specific approaches used in these analyses are the same as were described in detail in our prior report (17). A series of Mandel’s h and k charts were prepared for each pool as in our prior study, arranged by laboratory and analyte. Mandel’s h and k statistics were used to examine the consistency of the interlaboratory results, by measuring the accuracy (h statistic) and repeatability (k statistic) of each laboratory relative to the mean and average repeatability, respectively, of all participating laboratories. In the h and k plots, critical values at the 5% probability level were obtained to quantify significant departures from average behavior.
As noted above, all laboratories in this study used some form of LC/MS/MS for their measurements, but with two different approaches used for measuring the individual metabolites. To test for a difference between these two methods, we examined results from six labs divided between these two approaches, and used a nested analysis of variance procedure with laboratories nested in method.
RESULTS
The consensus mean values (and 95% confidence intervals) for the blended pools A-E are provided in Table 1. These data represent the aggregate results from all participating laboratories except for one lab (#11) which was omitted because it’s average CV estimates across pools ranged from 2 to 18 times larger than the average CVs across pools for the other labs. The total n for each result represents the number of contributing laboratories times 6 (for duplicate samples measured in 3 runs each). There were two exceptions including hydroxycotinine glucuronide in pool A in which one laboratory measured values below that laboratory’s detection limit, and a different laboratory’s results for free cotinine in one aliquot of pool E that exceeded its upper quantification limit. In both cases, those values were omitted from the summary in Table 1.
Table 1. Mean Results for Blended Pools A – E from All Laboratories.
All values are ng/mL except for TNE which is nmol/mL
| Pool | Labs | N | NICFREE | 95% CI | Labs | N | NICGLUC | 95% CI | Labs | N | NICTOT | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 8 | 48 | 48.8 | 29.0 – 68.7 | 7 | 42 | 10.2 | 7.6 – 12.9 | 8 | 48 | 61.2 | 40.0 – 82.4 |
| B | 8 | 48 | 165.4 | 149.6 – 181.3 | 7 | 42 | 32.7 | 29.0 – 36.4 | 8 | 48 | 206 | 183.9 – 228.1 |
| C | 8 | 48 | 404.1 | 374.5 – 433.7 | 7 | 42 | 86.0 | 81.5 – 90.4 | 8 | 48 | 504.7 | 467.6 – 541.8 |
| D | 8 | 48 | 779.3 | 713.4 – 845.3 | 7 | 42 | 158.9 | 140.0 – 177.7 | 8 | 48 | 950.1 | 893.1 – 1007 |
| E | 8 | 48 | 1039.6 | 940 – 1139 | 7 | 42 | 439.5 | 420.3 – 458.7 | 8 | 48 | 1460.3 | 1345 – 1575 |
| Pool | Labs | N | COTFREE | 95% CI | Labs | N | COTGLUC | 95% CI | Labs | N | COTTOT | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 9 | 54 | 49.4 | 45.4 – 53.4 | 7 | 42 | 45.2 | 34.7 – 55.8 | 8 | 48 | 93.5 | 79.8 – 107.2 |
| B | 9 | 54 | 191.4 | 185.3 – 197.6 | 7 | 42 | 177.6 | 153.3 – 201.8 | 8 | 48 | 362.4 | 333.2 – 391.7 |
| C | 9 | 54 | 475.1 | 464.5 – 484.8 | 7 | 42 | 443.3 | 396.5 – 490.2 | 8 | 48 | 895.8 | 832.5 – 959.1 |
| D | 9 | 54 | 955.3 | 910 – 1001 | 7 | 42 | 878.7 | 755.9 – 1002 | 8 | 48 | 1762.7 | 1584 – 1942 |
| E | 8 * | 48 | 1781.9 | 1714 – 1850 | 7 | 42 | 2220.7 | 1866 – 2576 | 8 | 48 | 3854.2 | 3396 – 4312 |
| Pool | Labs | N | HCOTFREE | 95% CI | Labs | N | HCOTGLUC | 95% CI | Labs | N | HCOTTOT | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 8 | 42 | 149.2 | 128.9 – 169.5 | 7 | 41 * | 50.4 | 32.8 – 68.0 | 8 | 48 | 196.2 | 163.8 – 228.5 |
| B | 8 | 42 | 559 | 535.5 – 582.6 | 7 | 42 | 184.4 | 144.1 – 224.8 | 8 | 48 | 736.1 | 681.8 – 790.5 |
| C | 8 | 42 | 1433.1 | 1362 – 1504.5 | 7 | 42 | 463.7 | 358.1 – 569.2 | 8 | 48 | 1871.1 | 1729 – 2013 |
| D | 8 | 42 | 2819 | 2719 – 2918 | 7 | 42 | 938.9 | 757.3 – 1120.7 | 8 | 48 | 3689.9 | 3427 – 3953 |
| E | 8 | 42 | 5479 | 5108 – 5850 | 7 | 42 | 1396.4 | 1015 – 1778 | 8 | 48 | 6711.2 | 6072 – 7350 |
| Pool | Labs | N | TNE | 95% CI |
|---|---|---|---|---|
| A | 8 | 48 | 1.93 | 1.56 – 2.29 |
| B | 8 | 48 | 7.16 | 6.67 – 7.64 |
| C | 8 | 48 | 17.9 | 17.0 – 18.8 |
| D | 8 | 48 | 35.0 | 32.9 – 37.2 |
| E | 8 | 48 | 65.8 | 59.9 – 71.7 |
One outlier laboratory (#11) was omitted
Excluded a result that was outside a labs calibration limits
95% Confidence Intervals were computed using standard error (SE) estimates obtained from an analysis of variance model with three sources of variance (σ2Lab = among-laboratory variance, σ2Run = among-run within-lab variance, and σ2Error = within-run variance), where SE = √(σ2Lab/NL + σ2Run/NR + σ2Error/N), and where N is the total number of measurements of the Pool, NR is the number of runs per lab, and NL is the number of labs.
Detailed results for the analysis of these five blended pools are given in Supplementary Table S3, subdivided by pool, laboratory and analyte. The ten analytes in that table included the free, glucuronide and total values for each of the three main nicotine metabolites (nicotine, cotinine and hydroxycotinine), plus the calculated TNE. All values are reported in ng/mL as the free base, including the concentrations for the glucuronides, except for the TNE results which are in nmol/mL. The methods used for interconversion of values and their underlying calculations are described in Supplementary Table S1. Each participating laboratory reported results for those analytes that they normally measure and not all laboratories measured each of the analytes included in this study, so the number of participant laboratories varies by analyte in Table S3. However, there were results from at least 7 laboratories for all analytes, with the greatest number of laboratories (n=10) reporting results for free cotinine.
Supplementary Figures S1–S10 present Mandel h and k plots for each of the analytes in Table S3. For the h plots, a positive value indicates that the lab measured a higher average value than the aggregate mean, whereas a negative value indicates a lower mean estimate for that laboratory relative to the group result. Mandel k plots summarize variability, a value > 1 indicates relatively greater than average inter-replicate variability, and a value < 1 is found for labs that had less than average variability for that analyte. The horizontal lines represent the 5% probability levels in each case. In general, most laboratories showed relatively limited deviations from the center line and most results were within 5% limits although a few laboratories exceeded the 5% probability level for certain analytes including the calculated TNE value.
The coefficients of variation (CVs) for analyte measurements by pool across all laboratories generally were higher for the glucuronide relative to the free forms, and the greatest variation among glucuronides was seen with hydroxycotinine (Table 2) . Hydroxycotinine glucuronide CVs ranged from 26 to 52% over the five pools. By contrast, the lowest CVs were seen for free cotinine with values ranging from 5.2 to a maximum of 12.6% in Pool A. Concentrations for the total value for nicotine, cotinine and hydroxycotinine had CVs ranging from approximately 9% to 16% in all cases except for the lowest pool A in which they were all greater than 19%. The highest CV overall was 57.6% for free nicotine in pool A, possibly reflecting background influence in some cases for this low pool.
Table 2.
Coefficient of Variation Across All Laboratories by Analyte
| Pool A | Pool B | Pool C | Pool D | Pool E | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | N | CV% | Mean | N | CV% | Mean | N | CV% | Mean | N | CV% | Mean | N | CV% | |
|
| |||||||||||||||
| Free Nicotine | 48.8 | 48 | 57.6 | 165.4 | 48 | 14.4 | 404.1 | 48 | 12.2 | 779.3 | 48 | 11.6 | 1039.6 | 48 | 13.6 |
| Nicotine Glucuronide | 10.2 | 42 | 38.1 | 32.7 | 42 | 17.6 | 86.0 | 42 | 15.7 | 158.9 | 42 | 20.3 | 439.5 | 42 | 9.4 |
| Total Nicotine | 61.2 | 48 | 48.7 | 206 | 48 | 15.0 | 504.7 | 48 | 11.6 | 950.1 | 48 | 9.1 | 1460.3 | 48 | 10.9 |
| Free Cotinine | 49.4 | 54 | 12.6 | 191.4 | 54 | 6.2 | 475.1 | 54 | 5.2 | 955.3 | 54 | 6.8 | 1782.0 | 48 | 6.3 |
| Cotinine Glucuronide | 45.2 | 42 | 28.7 | 177.6 | 42 | 18.1 | 443.3 | 42 | 14.3 | 878.7 | 42 | 17.9 | 2220.7 | 42 | 18.9 |
| Total Cotinine | 93.5 | 48 | 19.4 | 362.4 | 48 | 11.0 | 895.8 | 48 | 9.6 | 1763 | 48 | 13.5 | 3854.2 | 48 | 15.6 |
| Free Hydroxycotinine | 149.2 | 42 | 17.2 | 559.0 | 42 | 8.1 | 1433 | 42 | 7.4 | 2819 | 42 | 6.9 | 5478.8 | 42 | 9.2 |
| Hydroxycotinine Glucuronide | 50.4 | 42 | 52.0 | 184.5 | 42 | 26.0 | 463.7 | 42 | 28.4 | 939 | 42 | 29.2 | 1396.4 | 42 | 34.1 |
| Total Hydroxycotinine | 196.2 | 48 | 23.6 | 736.1 | 48 | 10.2 | 1871 | 48 | 10.1 | 3690 | 48 | 10.3 | 6711.2 | 48 | 11.9 |
| Total Nicotine Equivalents | 1.9 | 48 | 26.2 | 7.2 | 48 | 9.0 | 17.9 | 48 | 7.1 | 35.1 | 48 | 8.6 | 65.8 | 48 | 11.3 |
Laboratory #11 was omitted
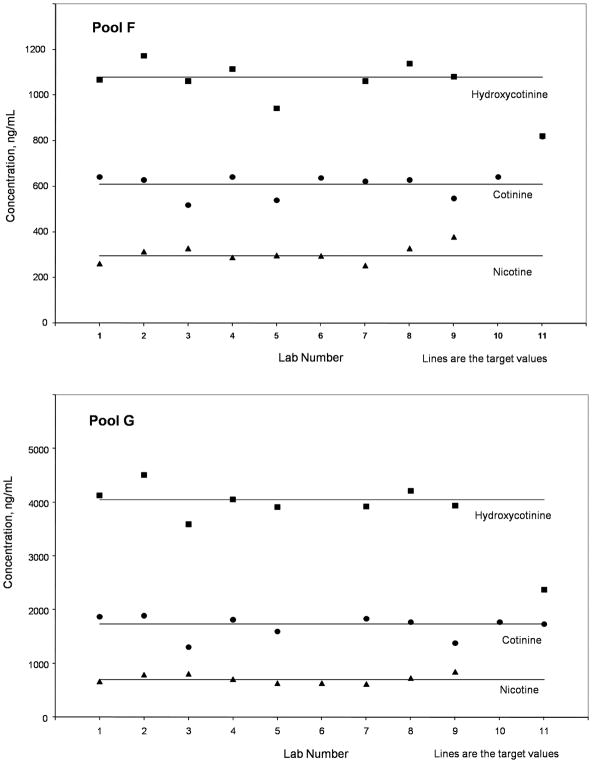
Pools F and G were the blank matrix pools fortified with known amounts of nicotine, cotinine and hydroxycotinine, which provided an estimate of accuracy as well as precision. Plots of mean values for pools F and G by laboratory are given in Fig 1, and the mean, 95% CI, and the estimated percentage mean bias values for the three analytes in those two pools together with the target values are given in Table 3. Since the pure salt forms were used as standards, only free analyte results were determined for these pools.
Figure 1.
Mean values by laboratory number for Pools F and G, spiked with known concentrations of Nicotine Salicylate, Cotinine Perchlorate and Hydroxycotinine Perchlorate. Target values (as the free base) are indicated by the horizontal lines. Nicotine (◆), Cotinine (●), Hydroxycotinine (■).
Table 3.
Means, 95% Confidence Intervals, and Bias for Spiked Pools with Known Concentrations
| Pool | Number of Labs | N | Nicotine, ng/mL | 95% CI | Target | Mean % Bias | Range |
|---|---|---|---|---|---|---|---|
| F | 8 | 48 | 295.9 | 286.7 – 305 | 286 | 3.462 | − 8.5 to 14.8% |
| G | 8 | 48 | 699.2 | 676.2 – 722.4 | 714 | −2.07 | − 13.3 to 12.8% |
| Pool | Number of Labs | N | Cotinine, ng/mL | 95% CI | Target | Mean % Bias | Range |
|---|---|---|---|---|---|---|---|
| F | 9 | 54 | 610.2 | 596.1 – 624.3 | 605 | 0.860 | − 14.5 to 6.0% |
| G | 8 | 48 | 1729.3 | 1673.1 – 1785.5 | 1729 | 0.017 | − 24.5 to 9.1% |
| Pool | Number of Labs | N | Hydroxycotinine, ng/mL | 95% CI | Target | Mean % Bias | Range |
|---|---|---|---|---|---|---|---|
| F | 7 | 42 | 1079.2 | 1052.9 – 1105.1 | 1066 | 1.238 | − 11.7 to 9.9% |
| G | 7 | 42 | 4046.8 | 3948.1 – 4145.5 | 3999 | 1.195 | − 10.2 to 12.7% |
One outlier laboratory (#11) was omitted from these calculations
DISCUSSION
The objective of this study was to evaluate the current state of performance and reliability of urinary nicotine metabolite assays. It was designed as a collaborative effort addressing both method accuracy and precision, and according to the classification proposed by Vander-Heyden and Smeyers-Verbeke (18), might best be described as a method performance study rather than a laboratory performance (proficiency testing) type of study. Overall, the results from this trial demonstrated generally good agreement for most analytes and laboratories with only a few potential outliers. As expected, greater variation was seen with the lowest concentration samples.
The highest concentration pool (E) in this study was prepared from 15 urine samples pooled from smokers selected on the basis of the highest cotinine values found in preliminary analyses. In pool E the TNE was 65.8 nmol/mL. The most abundant analyte was free hydroxycotinine which was nearly 4-fold higher than its glucuronide form. Nicotine glucuronide was also lower than free nicotine whereas cotinine glucuronide was slightly more abundant than the free form. The relative contributions from these six major analytes in pool E were nicotine (8.4%), nicotine glucuronide (3.6%), cotinine (14.4%), cotinine glucuronide (18.0%), hydroxycotinine (44.3%) and hydroxycotinine glucuronide (11.3%). Thus, hydroxycotinine and its glucuronide represented more than 55% of the major metabolites of nicotine in this sample. These proportions are consistent with previously reported relative distributions in smoker’s urine samples (15,19,20).
The CVs for the five blended pools across both laboratory and analyte as summarized in Table 2 demonstrated excellent performance for all three forms of cotinine including free and total cotinine, the two most commonly measured tobacco markers. The results for TNE were also quite good with CVs mostly in single digits except for the highest pool (E) at 11.3%, and the lowest pool A at 26.2%.
Both free and total hydroxycotinine also showed good performance, but hydroxycotinine glucuronide measurements had much wider variation with CVs ranging from 26 – 52%. Although two different methods were used for measuring glucuronides in this study, that is unlikely to be a reason for this variation since both nicotine and cotinine glucuronides had lower CVs, and direct comparison between labs using the two alternative methods did not find a significant difference between them. Since all assays were by LC/MS/MS, the relatively high polarity of hydroxycotinine and especially its glucuronide may be a factor since they are typically the earliest to elute and thus potentially most susceptible to ion suppression, interference and related effects.
Reasonably low variation also was seen for nicotine measurements in this study, with the exception of Pool A. For that low pool the CVs were consistently high for all three forms including free, total and glucuronide. The result for free nicotine with a mean of 48.8 ng/mL and a CV of 57.6% were the most variable of all the measurements in this study. Nicotine is known to be difficult substance to measure due to its relative instability and ubiquity in the environment. Exceptional care is needed in measuring nicotine, especially at lower concentrations, to exclude all potential sources of environmental contamination. Interlaboratory variations, especially among relatively less experienced laboratories, were probably an important contributor to the relatively high CVs for these measurements in Pool A.
In the two spiked pools with known, target values, good agreement was seen between the overall mean laboratory estimates and the target values in each case with nicotine in pool F having the greatest mean bias overall of +3.5%, followed by nicotine in pool G with a negative bias of −2.1%. The aggregate mean value measured for cotinine had a bias < 1% in both pools which supports the excellent performance typically obtained with this marker, although the bias of −24.5% from one laboratory was the greatest difference found overall. With hydroxycotinine, both pools F and G had relatively low mean biases of about +1.2. Laboratories #1, 4 and 7 had the most consistent results overall for these analytes, whereas laboratory #11 had substantial deviations for many of the assays and was excluded from summary analyses as an outlier. In nearly all cases the results for the individual analytes and the calculated TNE were in good agreement with the target or consensus values for each pool.
Recently, two different approaches have been used for measuring the glucuronide forms of urinary nicotine metabolites, and there are advantages and disadvantages to each of those methods. The more established difference method requires two separate analyses of each sample, both with and without a prior incubation with β-glucuronidase, and is therefore slower and more labor intensive than the direct assay. However, important advantages of this approach are that all native and labeled standards are commercially available, and effective sample extraction and preparation methods can be used to provide relatively clean extracts. Extracts into volatile solvents can be further concentrated to increase the sensitivity, so this method can provide better detection limits for measurements at relatively low exposure levels.
By contrast, the direct analysis of both free and glucuronide forms can be completed with a single assay, and is thus both simpler and quicker. However, the highly polar glucuronides provide restricted extraction options and have generally been limited to active tobacco users with higher metabolite concentrations, although a direct assay method using more extensive sample preparation and concentration procedures and resulting in lower detection limits has been reported (21). The high polarity and relatively poor chromatographic retention of intact glucuronides, especially hydroxycotinine glucuronide, also may lead to more challenging analytical resolution and short retention times with a greater susceptibility to ion suppression effects. Direct assays also require pure standards of all metabolites including the glucuronides in both native and isotopically-labeled forms, and currently there is limited availability of some of these standards, although labeled and unlabeled glucuronide standards may become more available over time.
This study provided the opportunity to evaluate and compare the results obtained for a set of common urine pools measured by these two methods as used by a subset of participating laboratories, and the results obtained from 6 laboratories with good overall performance, 3 which used the difference (indirect) method and 3 which used direct analysis are given in Table 4. With the exception of pools A and E for nicotine glucuronide, the direct assays had consistently higher mean glucuronide values compared to the indirect assays, although most differences were small. The most notable differences were seen for hydroxycotinine glucuronide. Whether this reflects possible losses in the indirect method, interferences in the direct method, or potential standards-related issues cannot be determined. However, these evaluations did show good agreement overall, with no significant differences found between the two methods for any of the analytes or pools. Because these evaluations involved multiple comparisons (i.e., 5 statistical tests for each of the three primary nicotine metabolites), we also evaluated each observed significance value using the false discovery rate (FDR) as described by Benjamini and Hochberg (22). This FDR adjustment further confirmed the absence of any statistically significant difference between the indirect and the direct measurements. The direct method resulted in notably better precision than did difference estimates for nicotine glucuronide measurements (Table 4), although both methods had comparable CVs for the other two glucuronides. We conclude that at present either method can provide good results for urinary assays at moderate to high metabolite levels, although direct measurements appear to be capable of greater precision for the nicotine glucuronide assays.
Table 4.
Analysis of Glucuronides by Difference or by Direct Assays
| A. Analysis by Difference | B. Direct Analysis | Analysis of Variance | ||||||
|---|---|---|---|---|---|---|---|---|
| Nicotine Glucuronide | ||||||||
| Mean | SD | %CV | Mean | SD | %CV | F | Prob_F | |
|
|
|
|
||||||
| Pool A | 10.9 | 5.2 | 47.8 | 8.2 | 0.7 | 8.9 | 1.42 | 0.2988 |
| Pool B | 30.1 | 7.2 | 23.9 | 33.3 | 3.2 | 9.7 | 0.97 | 0.3813 |
| Pool C | 83.0 | 20.0 | 24.1 | 88.8 | 8.4 | 9.4 | 1.37 | 0.3062 |
| Pool D | 144.3 | 46.6 | 32.3 | 171.1 | 10.2 | 6.0 | 1.85 | 0.2453 |
| Pool E | 447.0 | 48.4 | 10.8 | 447.8 | 26.3 | 5.9 | 0.00 | 0.9531 |
| Cotinine Glucuronide | ||||||||
| Mean | SD | %CV | Mean | SD | %CV | F | Prob_F | |
|
|
|
|
||||||
| Pool A | 40.3 | 5.3 | 13.2 | 41.4 | 5.3 | 12.7 | 0.19 | 0.6855 |
| Pool B | 160.4 | 14.7 | 9.2 | 176.1 | 24.9 | 14.1 | 1.62 | 0.2716 |
| Pool C | 409.9 | 33.0 | 8.0 | 439.3 | 44.3 | 9.9 | 1.81 | 0.2503 |
| Pool D | 803.3 | 66.9 | 8.3 | 853.0 | 105.0 | 12.3 | 1.11 | 0.3515 |
| Pool E | 2037.0 | 121.7 | 6.0 | 2118.3 | 248.3 | 11.7 | 0.4 | 0.5605 |
| trans-3-Hydroxycotinine Glucuronide | ||||||||
| Mean | SD | %CV | Mean | SD | %CV | F | Prob_F | |
|
|
|
|
||||||
| Pool A | 38.1 | 11.3 | 29.6 | 47.1 | 6.8 | 14.4 | 6.42 | 0.0644 |
| Pool B | 158.3 | 32.7 | 20.6 | 182.9 | 28.9 | 15.8 | 2.01 | 0.2291 |
| Pool C | 364.4 | 75.0 | 20.6 | 528.9 | 131.9 | 24.9 | 4.37 | 0.1048 |
| Pool D | 769.3 | 240.4 | 31.3 | 1014.3 | 249.0 | 24.6 | 2.97 | 0.1601 |
| Pool E | 1030.4 | 356.3 | 34.6 | 1597.9 | 358.9 | 22.5 | 5.19 | 0.0850 |
Results from 6 laboratories (#1,2,3,4,5,7): 3 which used the Difference method for measuring Glucuronides (A), and 3 which measured them directly (B).
Means are expressed as ng/mL as the free base in each case
In each case, the ANOVA degrees of freedom were Source=1 and Error=5
CONCLUSION
This inter-laboratory comparison provided generally good agreement for the main urinary nicotine analytes examined in the study, with a few exceptions. One laboratory appeared to diverge from the consensus results for several analytes, and both free nicotine and hydroxycotinine glucuronides were found to be more variable than most assays. Overall however, our results suggest that experienced laboratories active in this area can be expected to produce comparable results in the analysis of urinary nicotine metabolite profiles among those with significant exposure to nicotine. This should facilitate comparisons of studies in which different laboratories conduct the assays of these biomarkers. Although different methods were used by each laboratory, they all had a common basis involving sample preparation followed by analysis using liquid chromatography tandem mass spectrometry, and the general acceptance of this technique may have helped to facilitate reliable and comparable results. Nevertheless, these measurements are not simple and in particular, assays of nicotine at lower concentrations can be difficult, partly because of nicotine’s ubiquity in the environment. Estimates of TNE derived using data from the 8 laboratories that provided the necessary results for total nicotine, cotinine and hydroxycotinine were also in excellent agreement in this study, with relatively narrow 95% confidence intervals for the TNE estimate calculated for each pool. The relative expertise and experience of laboratories working in this area is likely an important factor in helping to assure good results.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the U.S. Centers for Disease Control and Prevention (L Wang, J.T. Bernert, J Feng, E McGahee, S.P. Caudill, B.C. Blount, J.L. Pirkle) and by grant number 1P50CA180890 from the U.S. National Cancer Institute and the U.S. Food and Drug Administration Center for Tobacco Products (N.L. Benowitz, P Jacob III). The content is solely the responsibility of the authors and does not necessarily represent the official views of the CDC, NIH or FDA.
We greatly appreciate the skillful assistance provided in this study by Vincent Pagnotti, Connie Sosnoff, Sharyn Miller, Stephen A. Arnstein, and Melissa Martinez at the Centers for Disease Control and Prevention, and by Lisa Yu and Trisha Mao at the University of California, San Francisco.
Footnotes
Contribution of the U.S. Government. Not subject to copyright.
Certain commercial equipment, instruments, or materials are identified in this report to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology or other governmental agency, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
N.L. Benowitz is a consultant/advisory board member for Pfizer and Achieve Life Sciences, and has provided expert testimony for tobacco litigation. No potential conflicts of interest were disclosed by the other authors.
References
- 1.U.S. Department of Health and Human Services. The Health Consequences of Smoking – 50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, NCCDPHP, Office on Smoking and Health; 2014. [Google Scholar]
- 2.World Health Organization. [Accessed 2017 Sept 13];WHO report on the Global Tobacco Epidemic. 2011 http://www.who.int/tobacco/global_report/2011/en/
- 3.Anderson CL, Becher H, Winkler V. Tobacco Control Progress in Low and Middle Income Countries in Comparison to High Income Countries. Int J Environ Res Public Health. 2016;13:1039. doi: 10.3390/ijerph13101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. [Accessed 2017 Sept 13];WHO Report on the Global Tobacco Epidemic. 2017 http://www.who.int/tobacco/global_report/2017/en/
- 5.Stratton K, Pdma S, Wallace R, Bondurant S, editors. Clearing the Smoke. Assessing the Science Base for Tobacco Harm Reduction. Institute of Medicine, National Academy Press; Washington DC: 2001. [PubMed] [Google Scholar]
- 6.Zeller M, Hatsukami D the Strategic Dialogue on Tobacco Harm Reduction Group. The Strategic Dialogue on Tobacco Harm Reduction: a vision and blueprint for action in the U. S. Tob Control. 2009;18(4):324–32. doi: 10.1136/tc.2008.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagerström KO, Bridgman K. Tobacco harm reduction: The need for new products that can compete with cigarettes. Addictive Behaviors. 2014;39:507–11. doi: 10.1016/j.addbeh.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benowitz NL, Jacob P., III Individual differences in nicotine kinetics and metabolism in humans. NIDA Res Monogr. 1997;173(4864):48–64. [PubMed] [Google Scholar]
- 10.Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: Role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23(3):252–61. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benowitz NL, Swan GE, Jacob P, III, Lessov-Schlaggar L, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin Pharmacol Ther. 2006;80:457–67. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Murphy SE, Park S-SL, Thompson EF, Wilkens LR, Patel Y, Stram DO, et al. Nicotine-N-glucuronidation relative to N-oxidation and C-oxidation and UGBTB10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;35(11):2526–33. doi: 10.1093/carcin/bgu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu AZX, Renner CC, Hatsukami DK, Swan GE, Lerman C, Benowitz NL, et al. The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in CYP2A6: the influence of genetics, race and sex. Can Epidemiol Biomark Prev. 2013;22(4):708–18. doi: 10.1158/1055-9965.EPI-12-1234-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curvall M, Kazemi-Vala E, Englund G. Conjugation pathways in nicotine metabolism. In: Adlkofer F, Thurau K, editors. Effects of Nicotine on Biological Systems. Birkhauser-Verlag; Basel: 1991. pp. 69–75. [Google Scholar]
- 15.Benowitz NL, Jacob P, III, Fong I, Guota S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther. 1994;268:296–303. [PubMed] [Google Scholar]
- 16.Benowitz NL, Dains KM, Dempsey D, Yu L, Jacob P., III Estimation of nicotine dose after low-level exposure using plasma and urine nicotine metabolites. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1160–6. doi: 10.1158/1055-9965.EPI-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernert JT, Jacob P, III, Holiday DB, Benowitz NL, Sosnoff CS, Doig MV, et al. Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels: a round-robin study. Nicotine Tob Res. 2009;11(12):1458–66. doi: 10.1093/ntr/ntp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vander-Heyden Y, Smeyers-Verbeke J. Set-up and evaluation of interlaboratory studies. J Chromatogr A. 2007;1158:158–167. doi: 10.1016/j.chroma.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 19.McGuffey JE, Wei B, Bernert JT, Morrow JC, Xia B, Wang L, et al. Validation of a LC-MS/MS Method for Quantifying Urinary Nicotine, Six Nicotine Metabolites and the Minor Tobacco Alkaloids—Anatabine and Anabasine—in Smokers’ Urine. PLoS ONE. 2014;9(7):e101816. doi: 10.1371/journal.pone.0101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei B, Feng J, Rehmani IJ, Miller S, McGuffey JE, Blount BC, et al. A high-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin Chim Acta. 2014;436:290–7. doi: 10.1016/j.cca.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meger M, Meger-Kossien I, Schuler-Metz A, Janket D, Scherer G. Simultaneous determination of nicotine and eight nicotine metabolites in urine of smokers using liquid chromatography – tandem mass spectrometry. J Chromatogr B. 2002;778:251–61. doi: 10.1016/s0378-4347(01)00451-0. [DOI] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Society, Series B. 1995;57(1):289–3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.