Abstract
Cell-based approaches for musculoskeletal tissue repair are limited by poor cell survival and engraftment. Short-term hypoxic preconditioning of mesenchymal stem cells (MSCs) can prolong cell viability in vivo, while the aggregation of MSCs into spheroids increases cell survival, trophic factor secretion, and tissue formation in vivo. We hypothesized that preconditioning MSCs in hypoxic culture before spheroid formation would increase cell viability, proangiogenic potential, and resultant bone repair compared to that of individual MSCs. Human MSCs were preconditioned in 1% O2 in monolayer culture for 3 days (PC3) or kept in ambient air (PC0), formed into spheroids of increasing cell density, and then entrapped in alginate hydrogels. Hypoxia-preconditioned MSC spheroids were more resistant to apoptosis than ambient air controls, and this response correlated with duration of hypoxia exposure. Spheroids of the highest cell density exhibited the greatest osteogenic potential in vitro and VEGF secretion was greatest in PC3 spheroids. PC3 spheroids were then transplanted into rat critical-sized femoral segmental defects to evaluate their potential for bone healing. Spheroid-containing gels induced significantly more bone healing compared to gels containing preconditioned individual MSCs or acellular gels. These data demonstrate that hypoxic preconditioning represents a simple approach for enhancing the therapeutic potential of MSC spheroids when used for bone healing.
Keywords: spheroid, preconditioning, mesenchymal stem cell, bone, hydrogel
Graphical abstract
INTRODUCTION
Mesenchymal stem cells (MSCs) are a promising cell source in tissue engineering and regenerative medicine. Following expansion in culture to achieve sufficient numbers of cells, MSCs are enzymatically separated from their newly deposited endogenous extracellular matrix (ECM) and transplanted as individual cells into harsh microenvironments characterized by low oxygen or an inflammatory milieu. This widespread approach results in catastrophic cell death within a matter of days, vastly limiting the therapeutic potential of cell-based approaches.[1]
As an alternative to transplanting individual cells, the aggregation of cells into spheroids prior to transplantation is an exciting approach to combat rapid cell death. Compared to individual cells, spheroids better recapitulate the cell-cell interactions observed in native tissue while exhibiting enhanced viability, angiogenic potential, and immunomodulatory potential.[2–5] Furthermore, MSCs within spheroids continue to engage and interact with their endogenous ECM that can enhance viability and preserve differentiation towards osteogenic phenotypes, fulfilling multiple needs for bone tissue repair.[6] MSC spheroids have been successfully used to promote neovascularization in hindlimb ischemia and mineralized ectopic tissue formation.[2, 3, 7–9] However, the transplantation of MSC spheroids into a segmental bone defect required synergistic stimulation with the potent osteoinductive cue, Bone Morphogenetic Protein-2 (BMP-2), to induce appreciable bone formation.[10] These data suggest that the native repair mechanisms catalyzed by MSC spheroids are insufficient to achieve complete bone repair, necessitating alternative approaches when using MSC spheroids in bone healing.
When transplanted into large bone defects, MSCs expanded in standard culture conditions endure stark changes in their local oxygen microenvironment, suddenly shifting from hyperoxic (21% oxygen) to hypoxic sites in vivo. Such rapid changes in local oxygen conditions induce apoptosis in transplanted cells if they are not rapidly resolved by vascularization. Hypoxic preconditioning represents one approach to address these challenges that activates key signaling pathways, enhancing MSC viability and proangiogenic potential via hypoxia inducible factor-1α (HIF-1α).[11, 12] Indeed, the injection of individual MSCs preconditioned for at least 48 hours exhibited markedly increased cell survival over 3 weeks in vivo.[13] Hypoxic preconditioning of MSCs results in sustained downstream effects on cell survival, proangiogenic potential and associated neovascularization, and tissue regeneration.[14, 15] However, the effect of hypoxic preconditioning on the function of MSC spheroids is unknown.
We hypothesized that spheroids formed from hypoxic-preconditioned MSCs would exhibit increased survival, proangiogenic potential, and osteogenic potential compared to unconditioned MSCs. The function of preconditioned MSC spheroids in vitro and in vivo was evaluated when entrapped in RGD-modified alginate, a biomaterial platform presenting adhesion sites for entrapped cells that promotes MSC viability and trophic factor secretion.[16–18] The capacity of preconditioned MSC spheroids to promote bone repair was then evaluated in a rat critical-sized femoral segmental defect.
MATERIALS AND METHODS
Cell culture
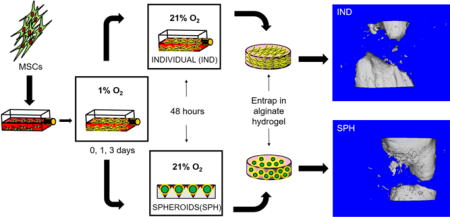
Human bone marrow-derived MSCs from a single donor were purchased from Lonza (Walkersville, MD) and used without further characterization. Cells were cultured in α-MEM (Invitrogen, Carlsbad, CA) supplemented with 10% v/v fetal bovine serum (JR Scientific, Woodland, CA), 100 units/mL penicillin, and 100 μg/mL streptomycin (Gemini Bio-Products, Sacramento, CA) under standard culture conditions until use at passage 5-6 (Fig. 1). At approximately 70% confluency, cells were moved from standard culture conditions to 1% O2 achieved using oxygen-controlled HERAcell 150i incubators (Thermo Scientific, Pittsburgh, PA) for 1 (PC1) or 3 days (PC3). MSCs maintained in standard culture conditions (PC0) and individual MSCs (not formed into spheroids, PC3-IND) served as controls. After preconditioning, MSCs were trypsinized (Corning, Manassas, VA) and immediately used to generate spheroids.
Figure 1. Schematic of experimental workflow.
Human MSCs are expanded flasks and then moved to oxygen-controlled incubators for hypoxic preconditioning for up to 3 days. After preconditioning, flasks for individual cells are either moved back to ambient air conditions (IND) or formed into spheroids for 48 hrs (SPH). Finally, individual cells or spheroids are entrapped in alginate hydrogels.
Fabrication of MSC spheroids
MSC spheroids were formed using a forced aggregation technique.[19] MSCs were seeded in nonadherent microwell plates formed of 1.5% agarose and centrifuged at 300×g for 5 min to yield spheroids of 3,000 (3K); 10,000 (10K); and 15,000 cells/spheroid (15K). Plates were kept static in the incubator under standard conditions for 48 hrs to enable full spheroid compaction.
Preparation of RGD-modified alginate and entrapment of MSC spheroids
RGD-modified alginate was prepared as described previously.[16, 20, 21] Briefly, G4RGDSP (Commonwealth Biotechnologies, Richmond, VA) was covalently coupled to UltraPure VLVG sodium alginate (Pronova, Lysaker, Norway) using standard carbodiimide chemistry, yielding hydrogels with 0.8 mM RGD. The resulting RGD-alginate was sterile filtered and lyophilized for 4 days. Lyophilized alginate was reconstituted in serum-free α-MEM to obtain a 2% (w/v) solution. MSC spheroids or individual MSCs were then suspended in alginate at 5×106 cells/mL and crosslinked by dialyzing 200 mM CaCl2 for 10 min.[22]
Evaluation of MSC spheroid function within alginate gels
Gels were cultured in α-MEM under serum-deprived and hypoxic (1% O2) culture conditions for 4 days, collected, minced, and lysed in passive lysis buffer (Promega, Madison, WI). Apoptosis was measured by analyzing 100 μL lysate per sample using a Caspase-Glo 3/7 assay (Promega) as reported.[23] Luminescence was detected on a microplate reader and normalized to DNA content determined from the same lysate using the Quant-iT PicoGreen DNA Assay Kit (Invitrogen). To measure VEGF secretion, media was refreshed 24 hrs before collection, and VEGF concentration was determined using a human specific VEGF ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.[24]
To quantify osteogenic potential of entrapped spheroids, gels prepared as described above were cultured in α-MEM overnight and then moved to osteogenic media, which consisted of the above expansion medium containing standard osteogenic supplements (10 mM β-glycerophosphate, 50 μg/mL ascorbate-2-phosphate, and 100 nM dexamethasone; all from Sigma, St. Louis, MO). Osteogenic potential was assessed by quantifying intracellular alkaline phosphatase (ALP) activity from a p-nitrophenyl phosphate assay, cell number by DNA content, osteocalcin secretion via ELISA, and calcium deposition by o-cresolphthalein assay.[16, 25, 26] Constructs were embedded in OCT, cryosectioned at 5 μm, and underwent immunohistochemistry (IHC) using a primary antibody against osteocalcin (1:1000, ab13420, Abcam, Cambridge, MA) and a mouse specific HRP/AEC detection kit (ab93705, Abcam). Osteogenic assessments were performed up to 14 days in accordance with previous work.[27]
To assess cell viability within alginate gels, calcein AM and propidium iodide (Invitrogen) were added to α-MEM to create a 2 μM and 5 μM solution of each reagent, respectively. Gels were fully immersed for 30 min before imaging using an Olympus Fluoview FV1000 system (Olympus, Tokyo, Japan).
Determination of HIF-1α in MSC spheroids by Western blot
Human MSCs were preconditioned under 1% O2 for 3 days or maintained in ambient air as stated above. Spheroids were then formed for 48 hrs under ambient air and collected immediately. For comparison with MSCs in monolayer culture, MSCs were seeded at 5 × 104 cells/cm2 in well plates and allowed to attach overnight. Cells were then moved to 1% O2 for 3 days of preconditioning and then placed under ambient air for an additional 48 hrs before collection. Samples were collected in protein collection buffer (0.1% Triton-X, 1% Tris-EDTA, 1% protease inhibitor cocktail), and protein concentration was measured using the Pierce BCA protein assay (Thermo Fisher Scientific). 30 μg of protein per sample was resolved 10% Tris–HCl acrylamide gels and transferred onto 0.2 mm nitrocellulose. Blots were blocked in 5% nonfat milk in Tris-buffered saline with 0.05% Tween-20 (TBST) for 1 hr and probed overnight at 4°C with mouse anti-human polyclonal antibody to HIF-1α (1:500 in blocking buffer; BD Biosciences, San Jose, CA) and the loading control, rabbit anti-human β-tubulin (1:1000; #2128P; Cell Signaling, Danvers, MA). Membranes were washed and probed with horseradish peroxidase-conjugated secondary antibodies (1:1000; BD Biosciences and Cell Signaling) and reactive bands were visualized using enhanced chemiluminescence and the Bio-Rad Gel Imaging System.
Assessment of MSC spheroids to promote bone healing in vivo
Animals were treated in accordance with all UC Davis and National Institutes of Health (NIH) animal care and handling procedures. MSCs, either spheroids or individual cells, were entrapped in alginate hydrogels (2 mm diameter, 6 mm length) at 30 × 106 cells/mL and kept in complete media in standard culture conditions for 18 hrs before implantation. Male athymic rats (NIH/RNU, 10-12 weeks old, Taconic, Hudson, NY) were anesthetized and maintained under a 3-4% isoflurane/O2 mixture delivered through a nose cone. Six millimeter diaphyseal critical-size defects were created in the right femora of each animal and stabilized with a radiolucent polyetheretherketone (PEEK) plate and 6 angular stable bicortical titanium screws (RISystem AG, Davos, Switzerland) as described.[28] Defects were immediately filled with one of four RGD-modified alginate constructs: 1) MSC spheroids formed from PC3 preconditioned cells; 2) PC3 individual MSCs; 3) acellular gels containing 2 μg BMP-2 (Medtronic, Minneapolis, MN), previously reported to successfully promote union within 12 weeks[29], or 4) acellular gels.
Quantification of bone formation and assessment of mechanical properties of repair tissue
Bone formation was monitored noninvasively using contact high-resolution radiographs (20 kVp, 3 mA, 2 min exposure time, 61 cm source-film distance) taken in a cabinet radiograph unit (Faxitron 43805N, Field Emission Corporation, Tucson, AZ) with high-resolution mammography film (Oncology Film PPL-2, Kodak, Rochester, NY) and digitized using a high-resolution flatbed scanner (SilverFast 500 ppi, LaserSoft, Sarasota, FL). Radiographs at 4, 8, and 12 weeks were scored on a scale of 0 (implant failure) to 5 (homogeneous bone structure) by evaluation from 3 blinded, independent reviewers.
At 12 weeks post-surgery, animals were euthanized and femurs were explanted, wrapped in sterile gauze, submerged in PBS, and stored at −20°C until analysis. Excised femurs were imaged (45 kVp, 177 μA, 400 μs integration time, average of four images) at 6 μm resolution using a high-resolution μCT specimen scanner (μCT 35; Scanco Medical, Brüttisellen, Switzerland). Bone volume within the tissue defect and bone mineral density (BMD) were determined from resulting images. Explants were then analyzed via torsional testing to ascertain mechanical properties of repair tissue as we reported.[30] Prior to testing, all PEEK plates on the explants were cut to ensure stress was concentrated in the defect.
Explants were subsequently demineralized in Calci-Clear Rapid (National Diagnostics, Atlanta, GA), processed, paraffin embedded, and sectioned at 5 μm thickness. Sections were stained with hematoxylin and eosin (H&E) and imaged using a Nikon Eclipse TE2000U microscope and Andor Zyla 5.5 sCMOS digital camera (Concord, MA). We performed IHC using a primary antibody against Lamin A/C (1:250, ab108595, Abcam), a human nuclear envelope marker, to visualize transplanted human cells and von Willebrand factor (1:200, F3520, Sigma-Aldrich) to visualize the basement membrane of capillaries on 2-week tissue explants. Explants from 12-weeks underwent Masson’s trichrome staining to visualize collagen deposition within repair tissue.
Statistical analysis
Data are presented as means ± standard deviation. Statistical analysis was performed using a two-way analysis of variance with Bonferroni correction for multiple comparisons in Prism 7 software (GraphPad, San Diego, CA); p-values less than 0.05 were considered statistically significant. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, while groups with significance do not share the same letters.
RESULTS
Osteogenic potential is greater in spheroids of higher cell density
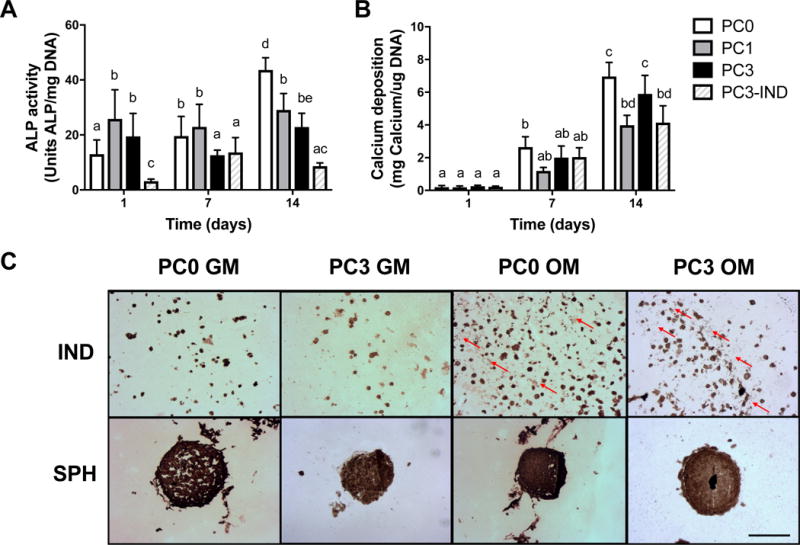
We evaluated MSC spheroids formed with increasing cell density prior to exposure to hypoxic preconditioning to select spheroids with the highest osteogenic potential. ALP activity increased with spheroid cell density (Fig. 2A), with greater activity in the 15K cell spheroids compared to the 3K cell spheroids at 7 and 14 days (2.1- and 2.4-fold increase, respectively). After 7 days, 15K spheroids contained significantly more calcium than did other spheroids, yet calcium levels remained flat over the next 7 days. Despite calcium levels comparable to baseline levels at 7 days, 10K spheroids exhibited the largest increase in calcium after 2 weeks (Fig. 2B).
Figure 2. Osteogenic potential is greater for MSC spheroids of higher cell density.
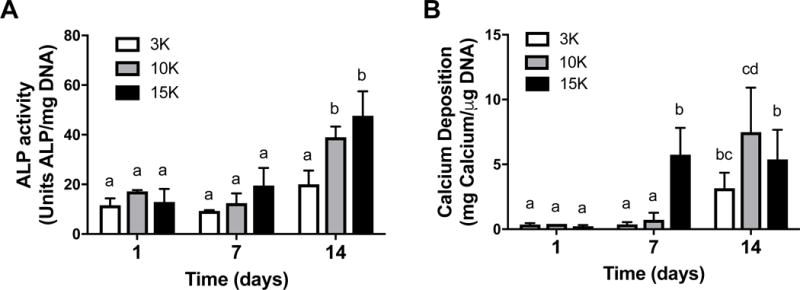
(A) ALP activity and (B) calcium deposition evaluated over 14 days; n=4. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, while groups with significance do not share the same letters.
Hypoxic preconditioning and spheroid cell density influence viability of MSC spheroids
We assessed cell viability of spheroids formed from increasing cell densities and hypoxic preconditioning durations. MSC spheroids formed from unconditioned or preconditioned cells exhibited similar diameters (approximately 400 μm) regardless of preconditioning duration (data not shown). Caspase 3/7 activity in MSC spheroids decreased with increasing cell density, with the lowest levels of caspase activity in the largest 15K cells/spheroid group (Fig. 3A–C). Additionally, PC1 and PC3 groups exhibited decreased caspase activity compared to unconditioned groups with sustained effects on cell viability through 4 days. DNA content was similar among groups (data not shown). Live/Dead staining revealed visibly greater cell death in spheroids formed from unconditioned MSCs compared to preconditioned spheroids (Fig. 3D).
Figure 3. Viability of entrapped MSCs is enhanced with hypoxic preconditioning and in larger spheroids.
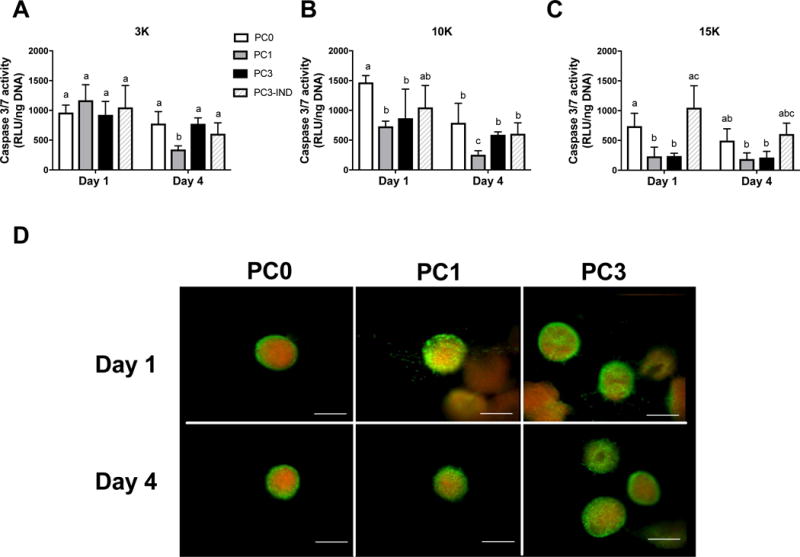
Caspase 3/7 activity as an indicator of apoptosis in (A) 3K cells/spheroid, (B) 10K cells/spheroid, and (C) 15K cells/spheroid; n=4. (D) Representative live/dead staining of 15K spheroids preconditioned for 0, 1, or 3 days and entrapped in RGD-modified alginate for 1 and 4 days. PCn indicates n days of hypoxic preconditioning and IND denotes individual (non-aggregated) cells. Live/dead images at 10× magnification; scale bar represents 200 μm. Different letters above bars indicate significance of at least p<0.05. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, while groups with significance do not share the same letters.
VEGF secretion is dependent on hypoxic preconditioning but not spheroid cell density
Compared to preconditioning for 1 day, hypoxic preconditioning of MSCs for 3 days increased VEGF secretion in all spheroids, regardless of cell density (Fig. 4A–C). Additionally, all PC3 spheroids produced more VEGF compared to their PC3-individual cell controls, further confirming the benefits of spheroid formation on trophic factor secretion. No differences in VEGF secretion were observed in 3K cell spheroids at day 1, yet we detected a 2.25-fold increase in VEGF secretion for PC3 versus PC1 spheroids at day 4. The increase in VEGF secretion in preconditioned spheroids over unconditioned controls was greater in 3K spheroids than other cell densities. We observed significant increases in VEGF secretion in PC3 over PC1 spheroids at days 1 and 4 in both 10K (1.81- and 2.27-fold, respectively) and 15K cell spheroids (4.23- and 2.54-fold, respectively). No differences in VEGF secretion were detected between spheroid cell densities in alginate gels containing the same concentration of cells (data not shown).
Figure 4. Proangiogenic potential of MSC spheroids is enhanced with hypoxic preconditioning.
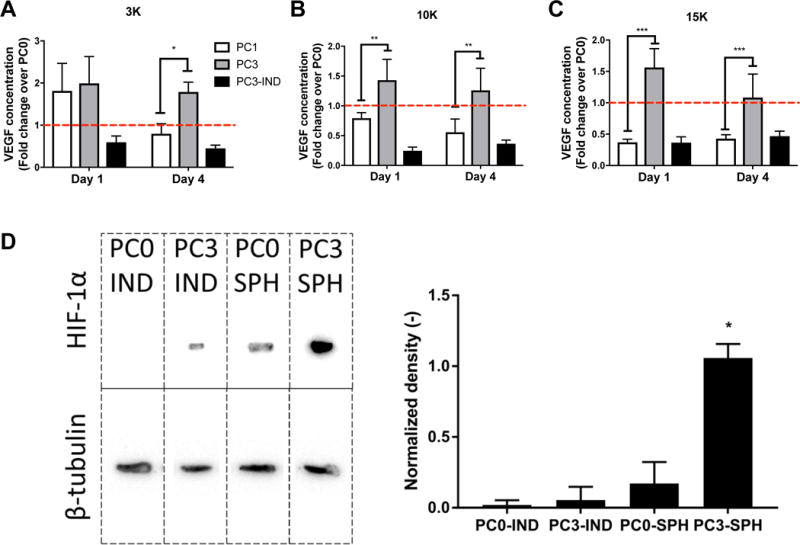
VEGF concentration in conditioned media from gels cultured for 1 or 4 days containing preconditioned MSCs as (A) 3K cells/spheroid, (B) 10K cells/spheroid, and (C) 15K cells/spheroid; n=4. *p<0.05, **p<0.01, ***p<0.001. Data are normalized to VEGF concentrations of unconditioned PC0 spheroids, and red dotted line indicates PC0 group. (D) Western blot for HIF-1α expression. Bands are (left to right) individual MSCs maintained in ambient air (PC0 IND) or preconditioned for 3 days (PC3 IND), spheroids from unconditioned MSCs (PC0 SPH), and spheroids formed from hypoxic preconditioned MSCs (PC3 SPH). β-tubulin served as the loading control. Density of bands was determined using ImageJ and normalized to housekeeping band density for quantification of protein (right, n=3). *p<0.05 vs. all groups.
HIF-1α expression is maintained in MSCs through spheroid formation
PC3 monolayer MSCs exhibited minimal HIF-1α expression, while HIF-1α was comparably expressed in unconditioned (PC0) spheroids (Fig. 4D). HIF-1α bands were appreciably heavier in PC3 spheroid groups, confirming that hypoxic preconditioned MSC spheroids maintain HIF-1α expression, even after spheroid formation in ambient air. We did not detect HIF-1α expression in unconditioned MSCs in monolayer culture (PC0).
MSC spheroids possess osteogenic potential after hypoxic preconditioning
We selected 15K spheroids to study the effect of hypoxic preconditioning duration on osteogenic potential in vitro due to increases in VEGF secretion as a function of preconditioning duration, together with reduced caspase 3/7 activity and robust osteogenic potential in ambient air. PC3 groups exhibited decreased ALP activity at days 7 and 14 compared to unconditioned (PC0) spheroids (Fig. 5A). However, regardless of preconditioning duration, MSC spheroids had greater ALP activity than did PC3-individual MSCs over 2 weeks. Calcium deposition increased in all groups over time with no differences among groups at day 7 (Fig. 5B). At day 14, PC3 spheroids secreted similar calcium mass as PC0 spheroids and more than PC3-individual cell controls. We did not detect any appreciable BMP-2 production in MSC spheroids or individual cells, regardless of preconditioning (data not shown). Gels containing individual cells exhibited faint positive staining for human osteocalcin when maintained in growth media and preconditioned for 0 or 3 days, but diffuse intra- and extracellular osteocalcin was evident in both individual cell gels in osteogenic media (Fig. 5C). Osteocalcin staining at Day 14 was comparable for all gels containing spheroids, regardless of preconditioning duration or media composition. These results indicate that hypoxic preconditioning of MSCs for 3 days does not inhibit subsequent MSC osteogenic differentiation.
Figure 5. Hypoxic preconditioning does not inhibit osteogenic differentiation in MSC spheroids.
(A) ALP activity in hypoxic preconditioned 15K spheroids. (B) Calcium deposition of hypoxic preconditioned 15K spheroids; n=4. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, while groups with significance do not share the same letters. (C) Osteocalcin staining in alginate gels containing individual MSCs or 15K spheroids at 14 days that were preconditioned for 3 (PC3) or 0 (PC0) days and maintained in growth (GM) or osteogenic media (OM). Red arrows indicate secreted extracellular osteocalcin. Scale bar = 200 μm.
Hypoxic preconditioning promotes angiogenesis and MSC persistence in vivo
Tissues were retrieved two weeks after implantation into femoral segmental bone defects to evaluate the capacity of MSC spheroids to promote vascularization. We observed more neovessels in defects treated with preconditioned MSC spheroids compared to all other groups (Fig. 6). Residual alginate remained in all groups but was more widely distributed in acellular gels. Immunohistochemistry for Lamin A/C revealed positive staining in defects treated with spheroids but only rare staining in defects treated with individual MSCs. As expected, no positive staining was observed in acellular gels with or without BMP-2.
Figure 6. MSC spheroids stimulate vascularization and persist in vivo at 2 weeks in a critical sized femoral defect.
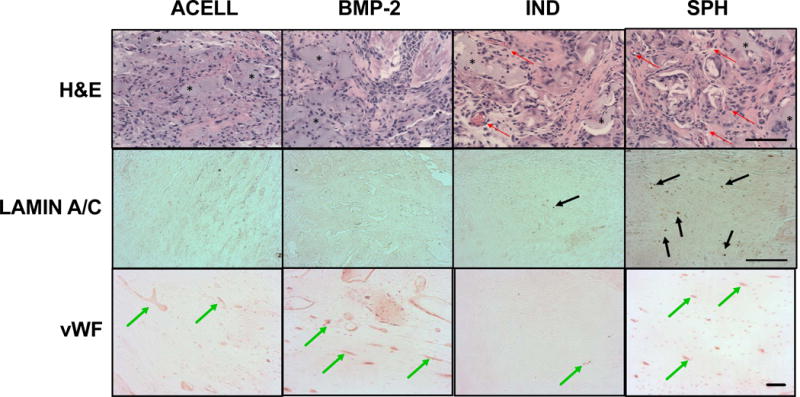
Representative H&E, Lamin A/C, and von Willebrand Factor (vWF) staining from acellular gels (ACELL), alginate gels containing BMP-2 (BMP-2), preconditioned individual MSCs (IND), or spheroids formed from preconditioned MSCs (SPH). Red arrows denote blood vessels, black arrows denote positive staining for human cells, and green arrows highlight positive staining for blood vessels. * indicates residual alginate. Images taken from the center of the defect. Scale bar = 100 μm for H&E; 200 μm for Lamin A/C and vWF.
Bone formation is accelerated when treated with spheroids of preconditioned MSCs
Radiography of bone defects over 12 weeks was evaluated by three blinded reviewers. Blinded scoring of radiographs (Supplementary Fig. 1) revealed significantly improved healing that continued over time in defects treated with spheroids, while defects treated with individual preconditioned MSCs did not heal. Defects treated with BMP-2 eluting alginate hydrogels demonstrated full radiographic bone bridging as early as 4 weeks that remained throughout the 12-week duration, while defects treated with acellular gels had minimal healing. Ex vivo microCT analysis after 12 weeks was in agreement with the radiographic results. More bone growth was visibly apparent in defects treated with spheroids compared to individual preconditioned MSCs (Fig. 7A). Quantification of microCT analysis revealed greater bone volume (Fig. 7B) and bone mineral density (Fig. 7C) compared to defects treated with individual MSCs. BMP-2 loaded hydrogels induced substantial bone formation over 12 weeks. Torsional testing revealed significantly greater stiffness in defects treated with spheroid-containing gels than either acellular gels or individual MSCs (Fig. 7D), and failure only occurred in the defect region. In agreement with increased bone volume and mineral density, the mechanical properties of repair tissue within BMP-2-treated defects were the most robust. Significant increases in collagen deposition were visually apparent in spheroid-treated defects compared to individual MSCs (Supplementary Fig. 2).
Figure 7. MSC spheroids formed from preconditioned cells promote bone formation in a critical-sized orthotopic bone defect.
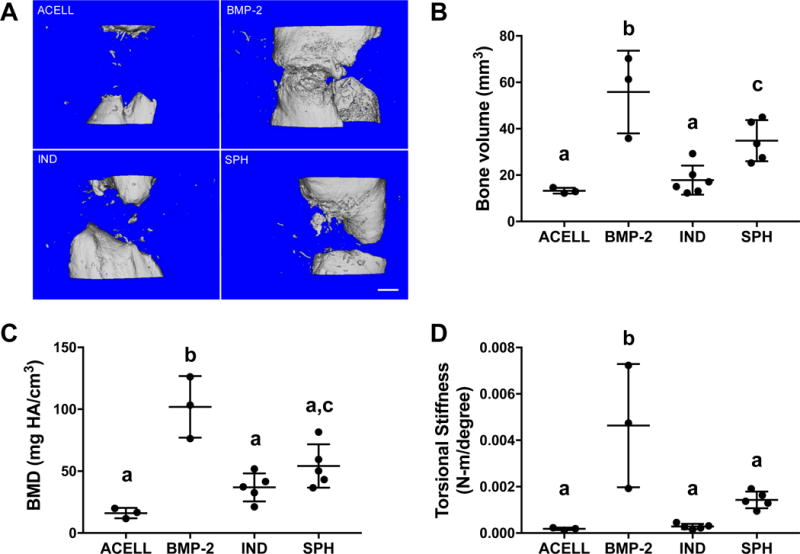
(A) Microcomputed tomography images at week 12 in the defect space with quantified (B) bone volume and (C) bone mineral density. (D) Torsional stiffness of explanted femurs at 12 weeks; n=3-5 per group. IND = individual (non-aggregated) MSCs; SPH = spheroids. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, while groups with significance do not share the same letters.
DISCUSSION
Cell therapies commonly involve direct injection or transplantation of individual cells into an injury site. Hypoxic preconditioning in monolayer culture enhances cell survival and proangiogenic potential both in vitro and in vivo through HIF-1α signaling pathway mechanisms.[13, 31, 32] We and others demonstrated the improved therapeutic potential of MSC spheroids compared to individual cells.[4, 24, 33] Moreover, we previously demonstrated that entrapment and transplantation of MSC spheroids in RGD-alginate hydrogels improves viability and function.[22] In light of the efficacy of each individual strategy, we anticipated a synergy between forming spheroids from MSCs preconditioned in hypoxia and transplanting MSC spheroids using instructive biomaterials. Therefore, the goal of our study was to determine the effect of hypoxic preconditioning and spheroid size on MSC contributions to bone formation when entrapped in RGD-alginate gels.
MSCs are typically expanded and maintained under standard culture conditions (21% O2, 5% CO2, 37˚C, complete culture medium), yet this environment is starkly different from the site of implantation. Oxygen concentrations in healthy bone range from 3-8% O2 and may be less than 1% in large bone defects where nutrient availability is markedly reduced.[34] Preconditioning of MSCs for 48-96 hours at 1% O2 increased cell viability over time when transplanted as individual cells in vivo, but differences in therapeutic potential were not reported.[13] In these studies, we preconditioned MSCs for 1 and 3 days in serum- and oxygen-deprived conditions prior to spheroid formation in ambient air to investigate downstream effects on cell viability and regenerative potential. We deprived MSCs of both serum and oxygen to better mimic implantation conditions compared to removal of oxygen alone. MSCs were returned to ambient air for spheroid formation after preconditioning to ensure adequate aggregation. Although, we did not observe differences in spheroid diameter with preconditioning duration, glioblastoma spheroids formed under 1% O2 exhibited smaller diameters compared to spheroids formed under normoxia.[35] Caspase 3/7 activity, an indicator of MSC apoptosis, was reduced in higher density spheroids, yet we did not observe differences between preconditioning durations. In contrast, VEGF secretion was increased from PC3 spheroids compared to PC1 spheroids or unconditioned MSC spheroids and consistently higher than from individual MSCs preconditioned for 3 days. The response of MSCs to hypoxic microenvironments is in agreement with previous studies of individual cells in monolayer culture reporting improved survival and increased VEGF secretion when preconditioned in low oxygen.[13, 36] These results suggest that short preconditioning in hypoxic conditions (≤ 1 day) is ineffective in upregulating VEGF secretion by MSC spheroids, and longer preconditioning durations are necessary to achieve therapeutic benefit.
The oxygen microenvironment plays a key role in osteogenic differentiation of MSCs in both 2- and 3-dimensions. Individual MSCs exposed to hypoxia exhibit increased transcription factors for stem cell plasticity including OCT4, NANOG, and REX-1.[37] Additionally, several studies report increased osteogenic differentiation of individual MSCs when maintained in low oxygen compared to ambient air conditions in monolayer culture.[38, 39] However, dimensionality may also affect timing of differentiation, as osteogenesis in 3-dimensional culture may be reduced or slowed.[38] Since OCT4 is regulated upstream by HIF-2α, a closely related HIF-1α isoform, the increase of stemness with hypoxia could contribute to the enhanced osteogenic differentiation of preconditioned MSCs.[40] With an eye toward transplanting MSC spheroids into large, hypoxic bone defects, we investigated the interplay between preconditioning duration and osteogenic potential. In agreement with others reporting impaired osteogenic differentiation of MSCs in reduced oxygen, we detected lower ALP activity in spheroids formed from preconditioned MSCs versus unconditioned MSCs at 14 days. ALP activity was higher in gels containing spheroids than in individual cells at 1 and 14 days. Similarly, calcium deposition per cell was greatest in gels containing PC0 spheroids, yet gels containing PC3 spheroids produced more calcium than did other preconditioned groups including PC3 individual MSCs. Thus, hypoxic preconditioning of MSCs for 3 days prior to spheroid formation in ambient air and entrapment in RGD-alginate hydrogels maintains the osteogenic potential of MSC spheroids. These studies represent the first analysis of osteogenic potential of MSCs that were preconditioned in low oxygen and then formed into spheroids. Overall, hypoxic preconditioning enhances the viability and therapeutic potential of MSC spheroids while enabling their osteogenic differentiation.
We investigated the mechanism of sustained hypoxic preconditioning effects on spheroid function by probing for HIF-1α expression due to its role in VEGF secretion. The diffusional limit of oxygen into tissues and aggregates is assumed to be approximately 200 μm,[41] similar to the radius of MSC spheroids used in this study. Although these boundaries may suggest an optimal cell size to activate hypoxic pathways that are anti-apoptotic and proangiogenic, we previously demonstrated that spheroids of this size and larger, up to 700 μm, had no evidence of a hypoxic region when cultured in ambient air.[42] However, our data are in agreement with previous work demonstrating spheroid formation alone activates HIF-1α expression.[43] Hypoxia upregulated recruitment of αvβ3 and αvβ5 integrins to the cell membrane of glioblastoma cells, while inhibition of these integrin dimers decreased intracellular HIF-1α.[14] In these studies, hypoxic preconditioning of MSCs prior to spheroid formation stabilizes and enhances HIF-1α expression, thereby sustaining VEGF secretion. Upon entrapment in RGD-modified alginate, MSCs in the spheroid engage with endogenous intraspheroid ECM and also bind to RGD ligands conjugated to alginate polymer chains. MSCs have numerous integrin dimers that will bind to the RGD motif including the αvβ3 and αvβ5 integrins implicated in HIF-1α expression.[44] Whether interacting with endogenous ECM or RGD ligands, integrins are activated that may sustain HIF-1α signaling and activate VEGF secretion. Although prolonged hypoxia can lead to caspase-dependent cell apoptosis,[45] HIF-1α is protective against apoptosis, likely through a p53-mediated pathway.[46] These data suggest an interplay between increased HIF-1α expression in MSC spheroids and integrin engagement to elements of the endogenous and engineered matrix which merits further investigation.
Bone repair of critical-sized bone defects represents a significant clinical challenge. Previous successful interventions to treat such large bone deficits in preclinical models require presentation of BMP-2 as a recombinant growth factor or via genetically modified MSCs that overexpress BMP-2 upon implantation.[47, 48] However, off-target effects or inefficient signaling, and undesirable effects due to high dosages limit the effectiveness of this approach.[49] To our knowledge, no studies have demonstrated significant repair of a critical-sized segmental defect strictly through transplantation of non-osteogenically induced cells, perhaps due to poor cell survival and long-term contributions to bone formation. In these studies, we observed increased cell persistence in tissues two weeks after implantation using spheroids formed from preconditioned MSCs compared to preconditioned individual MSCs. These data are in agreement with previous reports that transplantation of MSCs as spheroids increases cell viability in soft tissues in vivo.[2, 13] Moreover, bone formation was enhanced in vivo by increasing MSC survival when transplanted on engineered cell-secreted matrices[17] or with concomitant delivery of BMP-2 to protect MSCs against apoptosis.[30] Therefore, our results suggest a potentially synergistic effect of hypoxic preconditioning and spheroid formation to enhance cell survival in vivo that may contribute to continual bone growth. Beyond histological examination at each time point, we did not monitor cell survival in vivo in MSC spheroids versus individual cells to correlate extended viability with bone healing, representing a limitation of this study. This relationship merits further investigation.
The quantity and function of repair bone was increased in defects treated with spheroids formed from preconditioned MSCs compared to preconditioned individual MSCs or acellular gels. Although mineralization in tissues treated with preconditioned individual MSCs was greater than in acellular alginate, bone formation was minimal and apparent by low, unchanging radiographic healing scores over 12 weeks. These data are in agreement with other studies reporting little bone formation with individual MSCs,[30, 50] likely due to poor survival in vivo that limits paracrine-acting signals to recruit endogenous host cells or responsiveness to osteoinductive cues. Conversely, bone defects treated with MSC spheroids exhibited steadily increasing radiographic healing scores that were indistinguishable from BMP-2 treated defects at 12 weeks, indicating robust and continual bone formation throughout the study period. We did not assess the capacity of unconditioned MSCs, either as spheroids or individual cells, to promote bone healing in this defect, representing a limitation of this study. Although we previously reported that MSC spheroids resulted in improved tissue formation compared to an equal number of individual MSCs when transplanted into an ectopic site[22], the characteristics of the orthotopic bone defect differ markedly where bone healing naturally occurs via endochondral ossification. The focus of these studies was to test the capacity of preconditioned MSCs, either as spheroids or individual cells, to promote bone repair. Future studies will evaluate bone healing by MSC spheroids that have undergone preconditioning compared to unconditioned MSC spheroids in order to isolate the role of preconditioning from transplantation of MSCs as spheroids.
Previous attempts at treating femoral defects with MSC spheroids entrapped in alginate hydrogels were unsuccessful in forming bone and required synergistic presentation of BMP-2 to induce bone healing.[10] To our knowledge, this is the first demonstration of significant bone formation in a critical-sized femoral defect that is mediated by MSC spheroids alone without the use of BMP-2 or other exogenous growth factors. Although complete union was not achieved in this system, this approach suggests the benefit of following bone formation for longer periods or delivering minimal concentrations of osteoinductive factors. The supplementation of spheroids formed from preconditioned MSCs with low, functional dosages of BMP-2 to achieve complete healing may reduce complications associated with inflammation, ectopic bone formation, and prohibitive costs to treat large bone deficits.
CONCLUSION
These studies demonstrate that hypoxic preconditioning of MSCs prior to formation into spheroids and entrapment in RGD-alginate hydrogels is an effective strategy to enhance MSC therapeutic potential for bone formation and repair. Short-term exposure to low oxygen primes MSCs for survival and initiates angiogenesis by stabilizing HIF-1α signaling. Furthermore, these pathways are sustained through cell-cell signaling following spheroid formation. Hypoxic preconditioning of MSCs, in synergy with transplantation of cells as spheroids, should be considered for cell-based therapies to promote cell survival, angiogenesis, and bone formation.
Supplementary Material
Supplementary Figure 1: Representative radiographs of femoral defect at 4, 8, and 12 weeks. Quantitation of bone healing from radiographic evaluation of bone defects over 12 weeks. Bone healing was rated on a scale of 0 (implant failure) to 5 (massive bone healing and homogeneous bone structure) by 3 independent and blinded reviewers. ap<0.05 compared to individual MSCs, bp<0.05 compared to MSC spheroids at same time point.
Supplementary Figure 2: Representative Masson’s Trichrome staining of explants at 12 weeks taken from the center of the implant. Asterisks denote positive staining for collagen deposition.
Acknowledgments
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number R01DE025475 to JKL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. SH was supported by the T32 Animal Models of Infectious Disease Training Program Kirschstein-NRSA (T32 AI060555). The authors appreciate technical assistance from Blaine Christiansen for torsional testing of rat femurs and acknowledge Tanya Garcia-Nolan, Jennifer Fung, and Charles Smith for assistance in obtaining radiographs and microCT data.
Research Support: Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number R01 DE025475 to JKL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. SH was supported by the T32 Animal Models of Infectious Disease Training Program Kirschstein-NRSA (T32 AI060555).
Footnotes
Author contributions:
SSH: Study design, performed experiments, data analysis, manuscript preparation and editing
BPH: Study design, performed experiments, data analysis, manuscript preparation and editing
NH: Study design, performed experiments, data analysis, manuscript preparation
MAL: Study design, data analysis, and manuscript preparation
JKL: Study design, data analysis, manuscript preparation and editing, final manuscript approval
References
- 1.McGinley LM, McMahon J, Stocca A, et al. Mesenchymal stem cell survival in the infarcted heart Is enhanced by lentivirus vector-mediated heat shock protein 27 expression. Hum Gene Ther. 2013;24:840–851. doi: 10.1089/hum.2011.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhang SH, Lee S, Shin JY, et al. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng Pt A. 2012;18:2138–2147. doi: 10.1089/ten.tea.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhang SH, Lee S, Lee TJ, et al. Three-dimensional cell grafting enhances the angiogenic efficacy of human umbilical vein endothelial cells. Tissue Eng Pt A. 2012;18:310–319. doi: 10.1089/ten.tea.2011.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartosh TJ, Ylostalo JH, Mohammadipoor A, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci USA. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy KC, Fang SY, Leach JK. Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing. Cell Tissue Res. 2014;357:91–99. doi: 10.1007/s00441-014-1830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy KC, Hoch AI, Harvestine JN, et al. Mesenchymal stem cell spheroids retain osteogenic phenotype through alpha2beta1 signaling. Stem Cells Transl Med. 2016;5:1229–1237. doi: 10.5966/sctm.2015-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suenaga H, Furukawa KS, Suzuki Y, et al. Bone regeneration in calvarial defects in a rat model by implantation of human bone marrow-derived mesenchymal stromal cell spheroids. J Mater Sci Mater Med. 2015;26:254. doi: 10.1007/s10856-015-5591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaguchi Y, Ohno J, Sato A, et al. Mesenchymal stem cell spheroids exhibit enhanced in-vitro and in-vivo osteoregenerative potential. BMC Biotechnol. 2014;14:105. doi: 10.1186/s12896-014-0105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjea A, Yuan H, Fennema E, et al. Engineering new bone via a minimally invasive route using human bone marrow-derived stromal cell aggregates, microceramic particles, and human platelet-rich plasma gel. Tissue Eng Pt A. 2013;19:340–349. doi: 10.1089/ten.TEA.2012.0104. [DOI] [PubMed] [Google Scholar]
- 10.Allen AB, Zimmermann JA, Burnsed OA, et al. Environmental manipulation to promote stem cell survival in vivo: use of aggregation, oxygen carrier, and BMP-2 co-delivery strategies. J Mater Chem B. 2016;4:3594–3607. doi: 10.1039/c5tb02471d. [DOI] [PubMed] [Google Scholar]
- 11.Das R, Jahr H, van Osch GJ, et al. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Pt B Rev. 2010;16:159–168. doi: 10.1089/ten.TEB.2009.0296. [DOI] [PubMed] [Google Scholar]
- 12.Wei L, Fraser JL, Lu ZY, et al. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46:635–645. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beegle J, Lakatos K, Kalomoiris S, et al. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells. 2015;33:1818–1828. doi: 10.1002/stem.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skuli N, Monferran S, Delmas C, et al. Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: a novel pathway for hypoxia regulation in glioblastoma. Cancer Res. 2009;69:3308–3316. doi: 10.1158/0008-5472.CAN-08-2158. [DOI] [PubMed] [Google Scholar]
- 15.Chacko SM, Ahmed S, Selvendiran K, et al. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010;299:C1562–1570. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhat A, Hoch AI, Decaris ML, et al. Alginate hydrogels containing cell-interactive beads for bone formation. FASEB J. 2013;27:4844–4852. doi: 10.1096/fj.12-213611. [DOI] [PubMed] [Google Scholar]
- 17.Hoch AI, Mittal V, Mitra D, et al. Cell-secreted matrices perpetuate the bone-forming phenotype of differentiated mesenchymal stem cells. Biomaterials. 2016;74:178–187. doi: 10.1016/j.biomaterials.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–521. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 19.Dahlmann J, Kensah G, Kempf H, et al. The use of agarose microwells for scalable embryoid body formation and cardiac differentiation of human and murine pluripotent stem cells. Biomaterials. 2013;34:2463–2471. doi: 10.1016/j.biomaterials.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 21.Jose S, Hughbanks ML, Binder BYK, et al. Enhanced trophic factor secretion by mesenchymal stem/stromal cells with Glycine-Histidine-Lysine (GHK)-modified alginate hydrogels. Acta Biomater. 2014;10:1955–1964. doi: 10.1016/j.actbio.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho SS, Murphy KC, Binder BY, et al. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl Med. 2016;5:773–781. doi: 10.5966/sctm.2015-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binder BY, Genetos DC, Leach JK. Lysophosphatidic acid protects human mesenchymal stromal cells from differentiation-dependent vulnerability to apoptosis. Tissue Eng Pt A. 2014;20:1156–1164. doi: 10.1089/ten.tea.2013.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy KC, Fang SY, Leach JK. Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing. Cell Tissue Res. 2014;357:91–99. doi: 10.1007/s00441-014-1830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis HE, Binder BY, Schaecher P, et al. Enhancing osteoconductivity of fibrin gels with apatite-coated polymer microspheres. Tissue Eng Pt A. 2013;19:1773–1782. doi: 10.1089/ten.tea.2012.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Decaris ML, Binder BY, Soicher MA, et al. Cell-derived matrix coatings for polymeric scaffolds. Tissue Eng Pt A. 2012;18:2148–2157. doi: 10.1089/ten.TEA.2011.0677. [DOI] [PubMed] [Google Scholar]
- 27.Mitra D, Whitehead J, Yasui OW, et al. Bioreactor culture duration of engineered constructs influences bone formation by mesenchymal stem cells. Biomaterials. 2017;146:29–39. doi: 10.1016/j.biomaterials.2017.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams JC, Maitra S, Anderson MJ, et al. BMP-7 and bone regeneration: Evaluation of dose-response in a rodent segmental defect model. J Orthop Trauma. 2015;29:e336–341. doi: 10.1097/BOT.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 29.Willett NJ, Li MT, Uhrig BA, et al. Attenuated human bone morphogenetic protein-2-mediated bone regeneration in a rat model of composite bone and muscle injury. Tissue Eng Pt C-Meth. 2013;19:316–325. doi: 10.1089/ten.tec.2012.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho SS, Vollmer NL, Refaat MI, et al. Bone morphogenetic protein-2 promotes human mesenchymal stem cell survival and resultant bone formation when entrapped in photocrosslinked alginate hydrogels. Adv Healthc Mater. 2016;5:2501–2509. doi: 10.1002/adhm.201600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okuyama H, Krishnamachary B, Zhou YF, et al. Expression of vascular endothelial growth factor receptor 1 in bone marrow-derived mesenchymal cells is dependent on hypoxia-inducible factor 1. J Biol Chem. 2006;281:15554–15563. doi: 10.1074/jbc.M602003200. [DOI] [PubMed] [Google Scholar]
- 32.Hung SC, Pochampally RR, Hsu SC, et al. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One. 2007;2:e416. doi: 10.1371/journal.pone.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baraniak PR, McDevitt TC. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res. 2012;347:701–711. doi: 10.1007/s00441-011-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Kolenda J, Jensen SS, Aaberg-Jessen C, et al. Effects of hypoxia on expression of a panel of stem cell and chemoresistance markers in glioblastoma-derived spheroids. J Neurooncol. 2011;103:43–58. doi: 10.1007/s11060-010-0357-8. [DOI] [PubMed] [Google Scholar]
- 36.Chang CP, Chio CC, Cheong CU, et al. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci. 2013;124:165–176. doi: 10.1042/CS20120226. [DOI] [PubMed] [Google Scholar]
- 37.Hung SP, Ho JH, Shih YR, et al. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J Orthop Res. 2012;30:260–266. doi: 10.1002/jor.21517. [DOI] [PubMed] [Google Scholar]
- 38.He J, Genetos DC, Yellowley CE, et al. Oxygen tension differentially influences osteogenic differentiation of human adipose stem cells in 2D and 3D cultures. J Cell Biochem. 2010;110:87–96. doi: 10.1002/jcb.22514. [DOI] [PubMed] [Google Scholar]
- 39.Grayson WL, Zhao F, Izadpanah R, et al. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207:331–339. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- 40.Han SM, Han SH, Coh YR, et al. Enhanced proliferation and differentiation of Oct4- and Sox2-overexpressing human adipose tissue mesenchymal stem cells. Exp Mol Med. 2014;46:e101. doi: 10.1038/emm.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 42.Murphy KC, Hung BP, Browne-Bourne S, et al. Measurement of oxygen tension within mesenchymal stem cell spheroids. J R Soc Interface. 2017;14 doi: 10.1098/rsif.2016.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhang SH, Cho SW, La WG, et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–2747. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 44.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lv B, Li F, Fang J, et al. Hypoxia inducible factor 1alpha promotes survival of mesenchymal stem cells under hypoxia. Am J Transl Res. 2017;9:1521–1529. [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson B, Zhang J, Iglesias R, et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120–129. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- 48.Kolambkar YM, Dupont KM, Boerckel JD, et al. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32:65–74. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.James AW, LaChaud G, Shen J, et al. A review of the clinical side effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev. 2016;22:284–297. doi: 10.1089/ten.teb.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harada N, Watanabe Y, Sato K, et al. Bone regeneration in a massive rat femur defect through endochondral ossification achieved with chondrogenically differentiated MSCs in a degradable scaffold. Biomaterials. 2014;35:7800–7810. doi: 10.1016/j.biomaterials.2014.05.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Representative radiographs of femoral defect at 4, 8, and 12 weeks. Quantitation of bone healing from radiographic evaluation of bone defects over 12 weeks. Bone healing was rated on a scale of 0 (implant failure) to 5 (massive bone healing and homogeneous bone structure) by 3 independent and blinded reviewers. ap<0.05 compared to individual MSCs, bp<0.05 compared to MSC spheroids at same time point.
Supplementary Figure 2: Representative Masson’s Trichrome staining of explants at 12 weeks taken from the center of the implant. Asterisks denote positive staining for collagen deposition.


