Abstract
Background
Despite lower cancer screening rates and survival rates in the Medicaid population compared to those with private insurance, there is a dearth of population-based evidence-based interventions targeting Medicaid clients to address this problem.
Methods
This study reports results of a population-based randomized controlled trial (RCT) among all individuals enrolled in Minnesota’s Medicaid program who were overdue for breast cancer (BC; n=22,113) and/or colorectal cancer (CRC; n=94,294) screening. Individuals were randomized to intervention or control groups. The intervention group received persuasive direct and innovative mail materials coupled with a $20 incentive for using their Medicaid benefit to get screened. Direct mail materials provided a phone number to a call center staffed by patient navigators who addressed barriers and scheduled appointments via three-way calls. The control group received the intervention 15 months later. Primary outcomes were completion of mammography or colonoscopy within 12 weeks of the intervention. Billing claims served as evidence of screening.
Results
Multivariate logistic regression showed significant differences for both BC (p<.001) and CRC (p<.01). The odds of receiving a mammogram for the treatment group were significantly higher than the control group (OR = 1.30; CI = 1.16-1.46), and the treatment group was more likely to receive a colonoscopy than the control group (OR = 1.12; CI = 1.04-1.21).
Conclusions
This population-based intervention increased BC and CRC screening in a Medicaid population overdue for screening
Impact
These findings may have broad application for reaching individuals who generally remain outside the health care system despite having public health insurance.
Keywords: randomized controlled trial, incentives, screening mammography, screening colonoscopy, Medicaid
Introduction
Despite progress in early detection and treatment, breast cancer (BC) remains a leading cause of death among women in the United States (1,2). Significant disparities in stage at diagnosis and survival are associated with insurance status. Women with private health insurance fare best: about 13% of women with private insurance present with Stage III or IV BC compared with 28% of women on Medicaid. Correspondingly, the adjusted mean overall years of survival is 30% higher (3), and five-year BC survival more than 14% higher for privately insured compared to Medicaid-insured women (4). In fact, women insured by Medicaid often fare as poorly as or worse than uninsured women (5). Later stage of cancer and poorer cancer survival among Medicaid patients are not recent phenomena -- these trends were well-documented over a decade ago (5).
Colorectal cancer (CRC) shows remarkably similar patterns of disparity to those found for BC. Medicaid patients have more than a 40% increased mortality risk for CRC compared to those with private insurance (6), and there is a long-standing parallel of CRC to BC that can also be seen in the disparities in screening rates, stage and survival between privately insured and Medicaid-insured populations (5). Furthermore, compared to patients with private health insurance, the stage at diagnosis and survival for CRC patients on Medicaid are substantially poorer (5).
Screening rate differences may partially explain these disparities. In 2013, rates of mammography among age-appropriate women were significantly higher for those with private insurance (73.4%) compared to those insured by Medicaid (63.5%) (7). Although poverty alone does not qualify individuals for Medicaid, having limited financial assets is one of the principal requirements for eligibility, and there is a direct, virtually linear relationship between screening rates and gradations of the federal poverty level (FPL). For women ages 50-64, 80.9% of women at 400% of FPL had a mammogram in 2013 compared to 55% for women below 100% of FPL. Screening for CRC shows the same pattern: the colonoscopy rate among people living at 400% of FPL is more than 50% higher than those living below 100% of FPL (7). Whether these lower screening rates are attributable to Medicaid insurance, poverty, disability status, or other factors for which these measures are proxies is unknown. Competing health and life priorities, geographic distance to providers, and disability status are among the myriad barriers to screening among Medicaid recipients (8-11).
As these barriers to screening are not readily malleable and some are potentially immutable, an approach that facilitates screening despite these barriers is needed. Public health is challenged to find population-based strategies and evidence-based interventions that can reach low-socioeconomic status (SES) populations, especially Medicaid beneficiaries, in order to encourage the uptake of preventive services, and particularly cancer screening, to decrease cancer-related and other health disparities (12,13). In light of Medicaid expansion resulting from the Affordable Care Act, improving access to preventive services among Medicaid recipients and other low-SES populations has clear potential to reduce health disparities in the U.S. (12,14,15).
Health care setting-based and managed care organization-based strategies (hereafter grouped together as “clinic-based strategies”) have generally shown promising results (16-20), with some exceptions (21,22). However, clinic-based approaches tend to systematically miss individuals who remain outside the health care system despite having insurance. Furthermore, low-SES populations such as the Medicaid population are less likely to use preventive services when they are asymptomatic and more likely to present for acute care, a less propitious time for offering mammograms or other preventive services which require scheduling (8,23,24).
We have previously demonstrated that a multicomponent intervention (25,26) composed of direct mail coupled with financial incentives and a centralized patient navigator-staffed call center (27,28) can increase mammography and is scalable (25,26,29). Questions remain about the feasibility and effectiveness of this approach for an entire state Medicaid population, and whether it can be effectively used to promote screening for other cancers. To this end, we conducted a randomized controlled trial among all age- and gender-appropriate Medicaid beneficiaries in Minnesota to test whether this multicomponent strategy could increase their uptake of mammography and colonoscopy. The primary outcome was evidence of screening mammography or colonoscopy in medical claims data.
Methods
Study Context
This trial was conducted between April 2014 and July 2015 and implemented through Sage, Minnesota’s National Breast and Cervical Cancer Early Detection Program (NBCCEDP) (30), housed within the Minnesota Department of Health (MDH). Sage provides free breast and cervical cancer screening services to inadequately insured women with household incomes at or below 250% of the FPL. Unique among NBCCEDPs, Sage has a patient navigator-staffed call center. In order to reach Medicaid enrollees, MDH established a partnership with the Minnesota Department of Human Services (MDHS) which houses all State public health insurance programs. A brief description of the study protocol, its context, and its potential implications for programs such as NBCCEDPs and Medicaid have been briefly discussed in a previous publication (30).
Study Participants
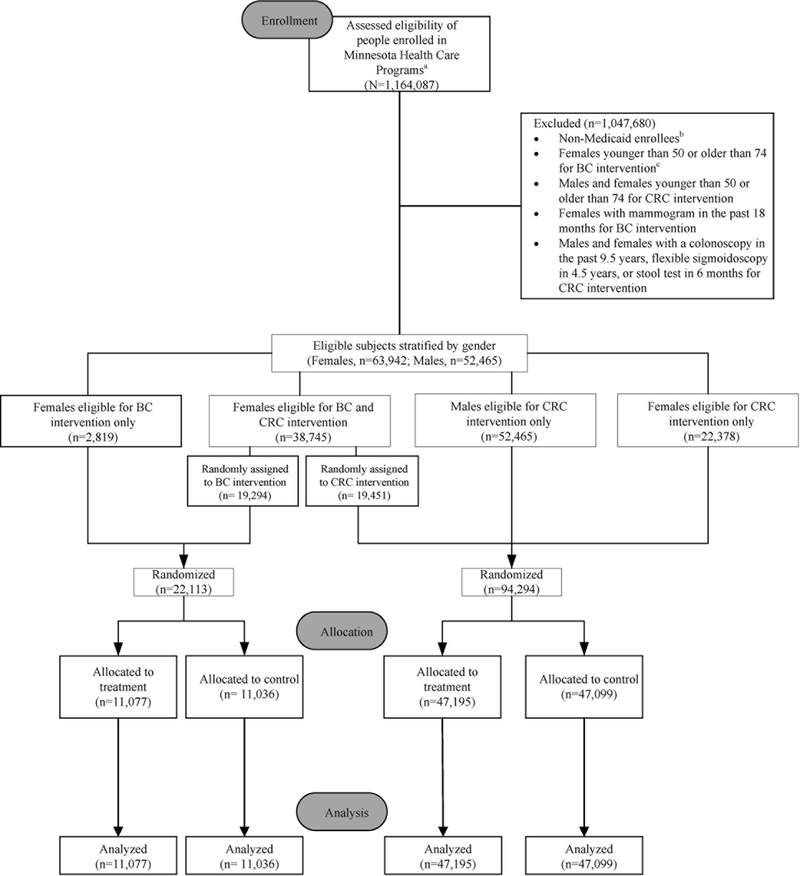
The target population was all Minnesota Medicaid beneficiaries ages 50-74 overdue for BC and CRC screening, according to current US Preventive Services Task Force guidelines (31). Thus “overdue” was operationalized as women with no record of a mammogram in the prior 18 months and women and men with no evidence of colonoscopy in the past 9.5 years, flexible sigmoidoscopy in the past 4.5 years, or fecal immunochemical or fecal occult blood test in the past six months based on claims. Enrollment and claims data, housed at MDHS, were used to identify the study population and to determine patient characteristics and outcomes. Figure 1 shows that 1,164,087 people enrolled in all Minnesota Health Care Programs (MHCP) were assessed for eligibility. (MHCP includes Medicaid as well as an array of other Minnesota-specific programs for low-income residents.) Individuals were excluded from the analytic sample if they were not enrolled in Medicaid, not overdue for BC or CRC screening, or not in the 50-74 age range, leaving 116,407 eligible for randomization.
Figure 1. CONSORT flow diagram depicting study recruitment and retention.
a. Includes all persons in Minnesota Health Care Programs (MHCP) that serve adults with low incomes. Programs include Medicaid (MA), MinnesotaCare, Minnesota Family Planning Program, and others.
b. The target population was restricted to MA enrollees. As a condition of being allowed to conduct this research, the MN Department of Human Services’ (MDHS) IRB stipulated that we could not discriminate among MHCP enrollees on the basis of MA status in terms of their having an equal chance of being offered the intervention. Consequently, all age- and gender- eligible individuals in the MHCPs had to be randomized to treatment or delayed treatment even though they were excluded a priori from the study. The n’s reported are MA enrollees only.
c. Although originally intended to be in the study, women ages 40-49 became ineligible prior to randomization at the request of the MN Medicaid Medical Director / IRB Chair and therefore were excluded. He felt that the lack of national consensus on promoting screening to women in this age group paralleled a lack of consensus among MN health care providers and health plans. Under these circumstances, he did not want it to appear that MDHS was endorsing screening in this age group.
This study was in accordance with the Declaration of Helsinki and the U.S. Common Rule. The intervention was designed so that the primary outcome was whether any of the participants engaged in the intervention after receipt of direct mail materials. Participants who called in response to the mailing were read the Tennessen Warning which informed them of the purpose and intended use of requested data and the consequences arising from refusal to supply the data. This Tennessen Warning enables individuals to make an informed decision about whether to give data about her/himself to the MDH. Willingness of the recipients of the direct mail invitation to call the MDH or to schedule a cancer screening appointment was indicative of consent, and the program offer was not withheld from any eligible Medicaid recipients. (The control group also received the program at a later date). Therefore, this study was approved with a waiver of informed consent by MDHS’ Institutional Review Board and is registered on clinicaltrials.gov (NCT03275987).
Stratification and Randomization
The 116,407 eligible Medicaid beneficiaries were stratified by gender. The 38,745 women eligible for both BC and CRC interventions were randomly assigned to one of the two interventions. A two-group posttest-only randomized design was used where all eligible beneficiaries were randomly assigned to a treatment or delayed treatment (control) group. A total of 22,113 women were randomized into treatment and delayed treatment for the BC intervention, and 41,829 women and 52,465 men were randomly assigned to treatment and delayed treatment for the CRC intervention.
Intervention Components
Beginning in April, 2014, the BC and CRC treatment groups were each sent three unique mailers (tailored to promote either BC or CRC screening) approximately three weeks apart. The BC mailers only mentioned mammography; the CRC mailers prominently featured colonoscopy but subsequently mentioned that there are other screening options. The delayed treatment group received identical mailers on the same schedule roughly 15 months later. Mailers were designed based on previous research (25,26). Multiple health behavior theories and health communications theories informed the design of the mailers and the use of the financial incentive. The mailers used loss-frame messaging which is effective for promoting cancer screenings (32) and was designed to inform individuals that a certain behavior will lead to an undesirable outcome. Loss-frame messages are most effective for promoting preventive behaviors when they are coupled with a clear articulation of achievable behavioral steps (i.e., a high-efficacy message), and therefore mailers included a high-efficacy message as well (33). A $20 incentive offer, included with every mailer, was presented via a small card affixed (with removable adhesive) to the inside of the mailer. Receipt of the incentive was contingent upon the recipient completing screening that was verified in the Medicaid claims file that we received quarterly. Incentives were mailed to beneficiaries immediately after verification. The presentation of the incentive offer was intended to influence the decisional balance by reducing perceived barriers, providing cues to action, and making the perceived benefits of behavior change outweigh the perceived costs (34,35). The eight mailers are provided in Supplementary Figures 1-8.
Patient Navigation
Mailers prompted recipients to call a toll-free number to reach Sage’s call center that was open Monday-Friday 7:30-5:30 as well as some weekends and evenings. Mailers promoting colonoscopy also suggested (in a footnote) that recipients could talk with their doctor directly about screening test options. Patient navigators provided callers with support and guidance related to barriers to cancer screening and care (28). Interested callers were encouraged to schedule a screening appointment immediately through a three-way call to the clinic of their choosing. Hispanic ethnicity and all races other than Native Hawaiian / Pacific Islander were represented among the call center’s multilingual patient navigators. Extensive caller information and process data were collected through a computer-assisted telephone data collection system containing scripts specific to study protocol.
Outcomes and Measures
The primary outcomes were completion of mammography or colonoscopy within the study period which was a 10-week interval starting two weeks after implementation of the intervention. Current Procedural Technology (CPT) codes were used to identify claims for mammography or colonoscopy in Medicaid administrative data. CPT codes used for mammography were conventional mammography (77055-77057), digital mammography (G0202, G0204, G0206), and computer-aided detection mammography (77051, 77052). CPT codes used for colonoscopy were G0105, G0121, 45378, and 45380-45385. These CPT codes were collapsed into dichotomous measures for both mammography and colonoscopy. Since multiple forms of CRC screening are available, we ran supplementary analyses that combined CPT codes for colonoscopy, stool tests, and flexible sigmoidoscopy.
In order to account for cost-sharing arrangements between Medicare and Medicaid for dually enrolled beneficiaries, both Medicare and Medicaid administrative data were used to identify claims for the dually enrolled. Medicaid and Medicare claims can provide accurate information on whether a patient has undergone colorectal endoscopy, but these claims are not able to reliably distinguish screening from diagnostic tests; consequently, it is recommended that researchers use these claims to assess rates of colorectal testing by including both screening and diagnostic endoscopy procedures in the analyses (36).
Reflecting previous research (37,38), outcomes were based on the presence of screening mammography and colonoscopy claims for services rendered during the aforementioned the study period. Claims from the first two weeks after the intervention were excluded from the study period in order to reduce measurement error, as call data and previous research demonstrated that participants set up appointments for screening within the first two weeks but are not screened in response to the intervention within those first two weeks. Previous research demonstrated individuals call in response to direct mail within two weeks and that mammography and colonoscopy appointment wait-times vary by clinic and over 95% of appointments can be arranged within three months (37,38). Additionally, interventions of this nature are most effective within the 90-day interval post-implementation (37,38). Therefore, we employ this 10-week study period for both mammography and colonoscopy arms. Since wait-times vary by clinic, we ran supplementary analyses that added four weeks to the study period for the BC arm and 12 weeks for CRC arm.
We also report the percent of the treatment groups that called within the study period and compared the screening rates of callers to non-callers within the treatment groups.
Covariates (obtained from Medicaid enrollment files) were: a continuous measure of age; categorical measures of Medicare enrollment, disability status, provider payment system, education, income, marital status, primary language, rural versus urban residence, and race/ethnicity.
For the BC arm, we controlled for previous mammography within the five years prior to the study, using four dummy variables that captured year (2009-2012) and previous screening mammograms. We did not include 2013 mammograms because participants were selected based on being unscreened in the 18 months prior to the intervention. We adjusted for whether participants were eligible for both CRC arms of the intervention or only the BC arm. We were unable to control for previous colonoscopy screening because we did not have access to the necessary 10 years of claims data prior to the intervention. Individuals who were not age-eligible to receive colonoscopy prior to the intervention were included because they had no prior colonoscopy.
Data Analysis
The mammography and colonoscopy interventions were examined separately. Across treatment and control, study sample characteristics were compared and absolute differences assessed using t-test and χ2 statistics. We used χ2 statistics to compare call center outcomes. Main outcome analyses consisted of logistic regression to compute odds ratios for receiving mammography or colonoscopy and to adjust for covariates. Both interventions were examined using two separate logistic regression models: 1) a bivariate model that examined treatment versus control, and 2) a multivariate model that adjusted for covariates. As noted, we also analyzed supplementary models that used extended study periods as well as different cancer testing options for CRC; any differences are reported within the text.
Some Medicaid beneficiaries had inaccurate mailing addresses (< 2% of each study sample) and thus did not receive intervention materials as intended. Others lost Medicaid coverage during the study period and so may not have had Medicaid claims available post-intervention. We conducted intent-to-treat analyses that included all randomized individuals whether they had an inaccurate mailing address or lost Medicaid coverage post-randomization, making our analyses more conservative. Analyses were conducted using Stata, version 13.
Results
Tables 1 and 2 display descriptive statistics for the mammography and colonoscopy interventions. Demographic and background characteristics were equivalent across treatment and control for both interventions.
Table 1. Select characteristics of women unscreened for breast cancer (N=22,113) in intervention groups among Minnesota Medicaid beneficiaries, 2014.
| Variable | Control (n = 11,036) |
Direct Mail with Incentive (n = 11,077) |
p-value* | |||
|---|---|---|---|---|---|---|
| Age (yrs) | Mean (SD) | 58.9 (6.4) | 58.7 (6.3) | 0.109 | ||
| N | % | N | % | |||
| Enrollment | Medicaid only | 6727 | 60.9 | 6797 | 61.4 | |
| Medicare and Medicaid | 4309 | 39.1 | 4280 | 38.6 | 0.535 | |
| Program type | All other programs | 7835 | 71.0 | 7920 | 71.5 | |
| Fee-for-service | 3201 | 29.0 | 3157 | 28.5 | 0.407 | |
| Race/ethnicity | White | 7476 | 67.8 | 7552 | 68.3 | |
| Other races/ethnicities | 3544 | 32.2 | 3509 | 31.7 | 0.488 | |
| Disability status | Not disabled | 6621 | 60.1 | 6680 | 60.3 | |
| Disabled | 4413 | 39.9 | 4395 | 39.7 | 0.637 | |
| Income | Above low-income | 4525 | 41.0 | 4558 | 41.2 | |
| Low-income | 4780 | 43.3 | 4674 | 42.2 | ||
| Missing income | 1731 | 15.7 | 1845 | 16.7 | 0.088 | |
| Marital status | Not married | 8983 | 82.3 | 8975 | 81.8 | |
| Married | 1938 | 17.8 | 1996 | 18.2 | 0.388 | |
| Primary language | English | 9174 | 83.6 | 9187 | 83.4 | |
| Other | 1797 | 16.4 | 1829 | 16.6 | 0.655 | |
| Metro location | Urban | 9615 | 88.9 | 9644 | 88.9 | |
| Rural | 1198 | 11.1 | 1199 | 11.1 | 0.960 | |
| Education | High school or less | 9442 | 85.6 | 9465 | 85.5 | |
| More than high school | 1594 | 14.4 | 1612 | 14.6 | 0.818 | |
| Study eligibility | BC arm only | 1403 | 12.7 | 1416 | 12.8 | |
| CRC and BC arms | 9633 | 87.3 | 9661 | 87.0 | 0.875 | |
| Prior behavior | Mammogram 2012 | 936 | 8.5 | 960 | 8.7 | 0.623 |
| Mammogram 2011 | 1489 | 13.5 | 1500 | 13.5 | 0.915 | |
| Mammogram 2010 | 1354 | 12.3 | 1445 | 13.1 | 0.083 | |
| Mammogram 2009 | 1468 | 13.3 | 1475 | 13.3 | 0.976 | |
p value represents χ2 test for categorical variables and two-sample t-test for age variable
Table 2. Select characteristics of individuals unscreened for colorectal cancer (N = 94,294) in intervention groups among Minnesota Medicaid beneficiaries, 2014.
| Variable | Control (n = 47,099) |
Direct Mail with Incentive (n = 47,195) |
p-value* | |||
|---|---|---|---|---|---|---|
| Age (yrs) | Mean (SD) | 58.4 (6.1) | 58.3 (6.1) | 0.394 | ||
| N | % | N | % | |||
| Enrollment | Medicaid only | 29022 | 61.6 | 29161 | 61.8 | |
| Medicare and Medicaid | 18077 | 38.4 | 18034 | 38.2 | 0.593 | |
| Program | All other programs | 33159 | 70.4 | 33490 | 70.9 | |
| Fee-for- service |
13940 | 29.6 | 13705 | 29.1 | 0.060 | |
| Race/ethnicity | White | 32391 | 68.9 | 32547 | 69.1 | |
| Other races/ethnicities | 14568 | 31.1 | 14528 | 30.9 | 0.592 | |
| Sex | Male | 26225 | 55.7 | 26240 | 55.6 | |
| Female | 20874 | 44.3 | 20955 | 44.4 | 0.801 | |
| Disability status | Not disabled | 27246 | 57.9 | 27554 | 58.4 | |
| Disabled | 19838 | 42.1 | 19629 | 41.6 | 0.098 | |
| Income | Above low income | 9997 | 21.2 | 10069 | 21.3 | |
| Low income | 29780 | 63.2 | 29967 | 63.5 | ||
| Missing income | 7322 | 15.6 | 7159 | 15.2 | 0.275 | |
| Marital status | Not married | 32391 | 68.9 | 32547 | 69.1 | |
| Married | 14568 | 31.1 | 14528 | 30.9 | 0.592 | |
| Primary language |
English | 40753 | 87.2 | 40796 | 87.1 | |
| Other | 5991 | 12.8 | 6030 | 12.9 | 0.781 | |
| Metro location | Urban | 40972 | 89.3 | 41047 | 89.3 | |
| Rural | 4913 | 10.7 | 4924 | 10.7 | 0.985 | |
| Education | High school or less | 39618 | 85.7 | 39663 | 85.7 | |
| More than high school | 6605 | 14.3 | 6605 | 14.3 | 0.952 | |
p value represents χ2 test for categorical variables and two-sample t-test for age variable
Mammography Intervention
The treatment group exhibited a higher mammography rate than control at 12-week follow-up (absolute difference = 1.37%; χ2 = 19.85, p<.001). Table 3 shows the odds of receiving a mammogram for the treatment group were significantly higher than the control group (odds ratio [OR] = 1.30; 95% confidence interval [CI] = 1.16-1.46). The odds of mammography screening for women in the treatment group are 30% higher than in the control group. As shown in Model 2, results remained unchanged adjusting for all covariates displayed in Table 3. White individuals relative to non-white individuals were less likely to receive a mammogram, and older individuals were less likely to be screened. Beneficiaries whose primary language was English were more likely to be screened, and those living in rural areas were less likely to receive mammography. Previous screening behavior (for all four years included) was significantly and positively related to being screened within the study period. In supplementary analyses that extended the BC study period to 16 weeks, the treatment effect was statistically significant but the effect size was weaker (AOR = 1.16; 95% CI = 1.05-1.27).
Table 3. Unadjusted and adjusted* odds ratios of post-intervention mammography use at 12-week follow-up among Minnesota Medicaid beneficiaries, 2014.
| Model 1 | Model 2 | |
|---|---|---|
| Variable | Odds Ratio (CI) | Odds Ratio(CI) |
| Treatment vs. control (vs. Control) |
1.30 (1.16, 1.46) | 1.31 (1.16, 1.48) |
| Age (Continuous) |
~ | 0.98 (0.97, 0.99) |
| Dually enrolled (vs. Medicaid only) |
~ | 0.94 (0.81, 1.11) |
| Fee for service (vs. All other programs) |
~ | 1.08 (0.94, 1.25) |
| White (vs. Other races/ethnicities) |
~ | 0.76 (0.66, 0.88) |
| Disabled (vs. Not disabled) |
~ | 0.77 (0.66, 0.90) |
| Income | ||
| above low-income (ref.) | ~ | ~ |
| low-income | ~ | 0.93 (0.81, 1.06) |
| missing income | ~ | 0.99 (0.82, 1.20) |
| Married (vs. Unmarried) |
~ | 1.10 (0.94, 1.29) |
| English primary language (vs. Other languages) |
~ | 1.62 (1.32, 1.98) |
| Rural residence (vs. Urban) |
~ | 0.76 (0.62, 0.93) |
| Education HS or less (vs. more than HS) |
~ | 0.99 (0.85, 1.18) |
| Eligible for both BC and CRC (vs. only eligible for BC) |
~ | 1.02 (0.86, 1.21) |
| Mammogram in 2012 (vs. no mammogram in 2012) |
~ | 1.92 (1.62, 2.27) |
| Mammogram in 2011 (vs. no mammogram in 2011) |
~ | 1.52 (1.30, 1.77) |
| Mammogram in 2010 (vs. no mammogram in 2010) |
~ | 1.63 (1.39, 1.91) |
| Mammogram in 2009 (vs. no mammogram in 2009) |
~ | 1.51 (1.29, 1.77) |
During the study period, 3.0% of the BC treatment group called. The mammography rate for treatment group participants who called was 48.6% versus 5.0% for treatment group non-callers (χ2= 943.37; p<.001).
Colonoscopy Intervention
The treatment group had a higher colonoscopy rate than control at 12-week follow-up (absolute difference = 0.30%; χ2 = 8.17, p<.01). In Table 4, results show that the odds of the treatment group receiving a colonoscopy were significantly higher than the control group (OR = 1.12; 95% CI = 1.04-1.21). The odds of being screened for the treatment group increased by 12% relative to control. Model 2 in Table 4 shows that the treatment effect was unchanged in the multivariate logistic regression analyses. Males compared to females were less likely to receive a colonoscopy, as were older individuals. Participants with low levels of income and missing income data were less likely to receive a colonoscopy compared to those with higher levels of income, and participants whose primary language was English were more likely to receive a colonoscopy.
Table 4. Unadjusted and adjusted* odds ratios of post-intervention colonoscopy use at 12-week follow-up among Minnesota Medicaid beneficiaries, 2014.
| Model 1 | Model 2 | |
|---|---|---|
| Variable | Odds Ratio(CI) | Odds Ratio(CI) |
| Treatment (vs. Control) |
1.12 (1.04, 1.21) | 1.11 (1.03, 1.21) |
| Age (Continuous) |
~ | 0.98 (0.97, 0.99) |
| Dually enrolled (vs. Medicaid only) |
~ | 1.03 (0.92, 1.15) |
| Fee for service (vs. All other programs) |
~ | 1.00 (0.91, 1.11) |
| White (vs. Other races ethnicities) |
~ | 0.99 (0.90, 1.10) |
| Male (vs. Female) |
0.88 (0.81, 0.96) | |
| Disabled (vs. Not disabled) |
~ | 0.95 (0.86, 1.05) |
| Income | ||
| above low-income (ref.) | ~ | ~ |
| low- income |
~ | 0.88 (0.79, 0.97) |
| missing income | ~ | 0.74 (0.64, 0.86) |
| Married (vs. Unmarried) |
~ | 1.03 (0.92, 1.14) |
| English primary language (vs. Other languages) |
~ | 1.31 (1.12, 1.52) |
| Rural residence (vs. Urban) |
~ | 1.01 (0.89, 1.16) |
| Education HS or less (vs. More than HS) |
~ | 0.95 (0.85, 1.06) |
Adjusted for variables shown; Model 1 does not include covariates; Model 1 N=94,294; Model 2 N=88,565; bolded odds ratios are statistically significant
A significant treatment effect was also observed in supplementary analyses that included all possible CRC tests in addition to colonoscopy (OR = 1.08; 95% CI = 1.01-1.15). Treatment effects were identical in the multivariate models. Supplementary analyses extending the study period to 24 months demonstrated an identical effect size (AOR = 1.12; 95% CI = 1.05, 1.19).
Within the treatment group, 1.7% of participants called within the study period. The colonoscopy rate for treatment group participants who called was 29.1% versus 2.4% for non-callers (p<.001).
We tested for interaction effects for both intervention arms and found that the intervention did not interact with any of key demographic variables, including gender for the CRC arm.
Discussion
This large population-based RCT demonstrated that direct mail coupled with an incentive offer and patient navigation can increase mammography and colonoscopy among Medicaid beneficiaries. It also showed that is feasible to implement this intervention statewide to all eligible beneficiaries.
The magnitude of the effect exceeded or matched the effect size observed in prior work (25,36) for a number of possible reasons. This is the lowest income population in which we have tried this approach, so the incentive’s perceived value could have been greater compared to previous iterations of the interventions. Furthermore, because the mailing addresses were extremely current, the mailings likely reached a higher proportion of the target population. Since this is our third iteration of this outreach strategy, the intervention has probably improved. We believe that the current messaging is more poignant and effective. Our evocative loss-frame messaging with a clear and simple call to action to schedule an appointment was based on extensive prior work (26), and we believe these materials are state-of-the art. Even though previous research has shown that direct mail’s efficacy is improved by adding incentives and/or patient navigation (18,25), it is important to note that this multicomponent study was not designed to determine the relative contribution of each component. Rather, this RCT was designed to determine whether this multifaceted program could be scaled to the population level in order to address cancer-related disparities in a Medicaid population.
Prospective patients were given the toll-free phone number of a call center that was staffed by multicultural patient navigators trained in motivational interviewing. They provided services to reduce structural barriers (such as transportation) and enhance patient access. Because navigators had access to clinic information, they could help beneficiaries who did not have a regular doctor or place of care make appointments at clinics where physicians were accepting new Medicaid clients. Roughly 30% of physicians in the U.S. are not willing to provide care to new Medicaid patients (39,40).
Patient navigation has been shown to improve access to BC and CRC screening among socioeconomically disadvantaged populations (41), and patient navigator race/ethnicity and language concordance have been shown to be beneficial (42). The use of dollar denominated major payment network gift cards rather than gift cards for specific merchants may have optimized the perceived value and utility of the incentive. Using first class postage and envelopes printed with the state health department logo and return address may have optimized the proportion of mail opened and read because it was less likely perceived as junk mail. Since we cannot disentangle the elements of the intervention to understand how each contributed to the success observed, future research could examine the added value of the most complex or expensive components of this intervention.
Our findings are consistent with the conclusions of systematic reviews of the literature regarding increasing mammography screening in low income populations (8,29). That is, access-enhancing interventions that used multiple strategies and included some type of person-to-person contact lead to the greatest increases in mammography. More specifically, there is strong evidence to support use of direct mail as a targeted small media strategy coupled with other strategies (43), especially patient navigation to reduce structural barriers (28). Although there is strong evidence in support of small media for increasing FOBT use, there has not been sufficient evidence to support its use for increasing colonoscopy (29).
Most prior research in the U.S. using direct mail to increase cancer screening has been on clinic-based reminder systems (44), or general population recruitment to mass screening programs in countries with national health care and call systems (45). Findings from these studies are not readily comparable to the present study. This intervention prompts people to screen, independent of their having a primary care doctor, their use of preventive services, or their contact with a health care system. This is a critical distinction since clinic-based reminders can only apply to existing patients, an important limitation for Medicaid patients in particular. Most population-based research using direct mail in the U.S. was published more than 15 years ago and showed mixed results (46-48). These studies differ in important ways from the present study in that they did not offer incentives or patient navigation, and the mailings did not target an unscreened population. More recent studies (25,26) have found small but statistically significant results and included incentives and patient navigation. Only one targeted an unscreened population (25), but the accuracy of screening status was limited because the time between determination of screening status and the mailed intervention was more than a year whereas in the present study, screening status was up-to-date within weeks of the mailing.
Community-based approaches have had mixed success (49,50) and rarely targeted the Medicaid population (51). Interventions to increase cancer screening in the Medicaid population have predominately been limited to clinic-based approaches (16). The significance of using a population-based approach that targets Medicaid enrollees is that it can reach those who remain outside the health care system despite their insurance coverage. To clarify, we use the term “population-based” to refer to approaches that emanate from outside clinic systems and seek to screen all eligible individuals within a defined target group as distinct from clinic- and community-based approaches (52). However, the distinction between approaches to screening in this taxonomy is not meant to imply that any given approach may not include elements of other approaches.
Based on our prior work, we believe that incentives played a crucial role in the intervention’s success. Incentives have been shown to be effective for improving a range of health behaviors (53) although their potential for influencing the behavior of Medicaid beneficiaries has been questioned, as has their value for maintaining healthy behavior (54, 55). Although there is some evidence about the effectiveness of incentives at the population level in non-Medicaid populations (25,26), understanding how these programs translate to Medicaid populations is a primary concern in public health research and practice (56). Unlike behaviors such as smoking cessation or weight loss, cancer screening only requires infrequent episodic behavior (potentially annually or even once per decade), so these concerns about incentives may not be pertinent to cancer screening. Whether individuals in the intervention groups continued to be screened routinely is beyond the scope of this study.
Colonoscopy was featured rather than equally promoting a menu of optional screening modalities because of the need to simplify the messaging (57) and recommendations that doctors suggest a "preferred” screening method to patients (58). Colonoscopy was emphasized over fecal occult immunochemical tests (FIT) because of concerns about compliance (59-61) with follow-up for abnormal FITs and lack of compliance with recommended annual FIT screening.
Because this intervention is not technically challenging, it has potential for wide-scale application. Replication would require a call center with patient navigators, adaptation of extant mailers, current mailing lists, capacity to offer and issue incentives, and (ideally) knowledge of screening status. It seems evident that these findings may be applicable to other state Medicaid programs. This intervention also may be successful in other settings, such as health plans and clinics, and it is already being used in these settings in Minnesota. However, there are notable differences between the approach used in the present study and typical reminder letters, such as the messaging (loss-frame and heavy reliance on evocative images and minimal text) and the use of incentives.
The intervention was more successful increasing mammography than colonoscopy. There is likely greater resistance to getting colonoscopy than mammography which would be consistent with the increase in mammography but not in colonoscopy observed after implementation of the Affordable Care Act (62). Furthermore, this low-intensity intervention may be less effective in getting people to overcome the greater logistical and psychological barriers for colonoscopy.
This research has some notable strengths. RCTs of this scale are rare, and because it encompassed Minnesota’s entire eligible Medicaid population, there was no possibility of an unrepresentative sample. Although claims data have limitations, they avoid some serious pitfalls of self-report, most notably telescoping and response bias due to demand characteristics, which could have been particularly threatening to the validity and reliability of this study’s measures.
Limitations of this study should be considered. Medicaid programs are heterogeneous; thus, whether the strategy presented here can adapted by other state Medicaid programs, and whether the target population in other states would respond as favorably, are important unanswered questions. In areas of the country where there is more widespread suspicion of government, a government agency mailing might suppress the response. Conversely, other locales might show even more favorable responses, especially where economic depression is greater (increasing the incentive’s relative value) or screening or health insurance rates are lower. (Minnesota’s health, economic, and insurance rate indices are more favorable than most states.) We did not use a factorial design which would have been optimal for isolating the effect of intervention components (63). Consequently, we were unable to experimentally assess the independent effects of direct mail, incentives, and patient navigation. Future research should seek to ascertain the relative contribution of the intervention components. Particular attention should be paid to determining the contribution of patient navigation because of its complexity.
This study demonstrated that a multicomponent intervention that uses persuasive direct mail coupled with incentives and patient navigation and targets a population overdue for screening can increase both BC and CRC screening among Medicaid beneficiaries. When considered in conjunction with prior research (25,26), this approach shows promise for increasing cancer screening in public health insurance programs.
Supplementary Material
Acknowledgments
The authors thank Mary Gothard, Bree Allen, and Laura Friedenberg for serving as project coordinators; Manjusha Pillai and Sage Call Center staff for providing patient navigation; Shelly Madigan and Sarah Diaz for providing administrative support; Laura Bombenger Jourdan from Triad Marketing for materials development assistance; and MDHS for their assistance and collaboration. Funding was provided by the HHS Centers for Disease Control and Prevention (#U58DP003922), received by Jonathan Slater, and supported all authors.
Abbreviations list
- breast cancer
(BC)
- colorectal cancer
(CRC)
- federal poverty level
(FPL)
- National Breast and Cervical Cancer Early Detection Program
(NBCCEDP)
- Minnesota Department of Health
(MDH)
- Minnesota Department of Human Services
(MDHS)
- Minnesota Health Care Programs
(MHCP)
- Current Procedural Technology
(CPT)
- fecal occult immunochemical tests
(FIT)
Footnotes
Conflict of interest disclosure statement: All authors declare no potential conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: Cancer J Clin. 2017;67:7-30. [DOI] [PubMed] [Google Scholar]
- 2.Use of Mammography among Women 40 Years of Age and Over, by Selected Characteristics: United Sates, Selected Years 1987-2010 (Table 83). Washington, DC: National Center for Health Statistics, National Health Interview Survey in Health; 2013. Retrieved from http://www.cdc.gov/nchs/data/hus/2013/083.pdf [Google Scholar]
- 3.Shi R, Taylor H, McLarty J, Liu L, Mills G, Burton G. Effects of payer status on breast cancer survival: a retrospective study. BMC Cancer. 2015;15:211-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward W, Halpern M, Scrag N, Cokkinides V, DeSatnis C, Bandi P et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9-31. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald TL, Lea CS, Atluri PM, Brinkley J, Zervos EE. Insurance payer status and race explains much of the variability in colorectal cancer survival. J of Cancer Therapy. 2014; 5:1223-1233. [Google Scholar]
- 7.Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics; 2016. http://www.cdc.gov/nchs/data/hus/hus15.pdf#070. [PubMed] [Google Scholar]
- 8.Garnder MP, Adams A, Jeffreys M. Interventions to increase the uptake of mammography amongst low income women: A systematic review and meta-analysis. PLOS ONE. 2013;8:e55574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamman MK, Kapinos KA. Mandated coverage of preventive care and reduction in disparities: Evidence from colorectal cancer screening. Am J Public Health. 2017;105:S508-S516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annual Rev Sociol. 2010;36:349-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deroche CB, McDermott SW, Mann JR, Hardin JW. Colorectal cancer screening adherence in selected disabilities over 10 years. Am J Prev Med. 2017;52:735-741. [DOI] [PubMed] [Google Scholar]
- 12.Cooper LA, Ortega AN, Ammerman AS, Buchwald D, Paskett ED, Powell LD, et al. Calling for a bold new vision of health disparities intervention research. Am J Public Health. 2015;105:S374-S376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkelstein A, Taubman S. Health care policy: randomize evaluations to improve health care delivery. Science. 2015;347:720-722. [DOI] [PubMed] [Google Scholar]
- 14.Decker SL, Kostova D, Kenney GM, Long SK. Health status, risk factors, and medical conditions among persons enrolled in Medicaid vs uninsured low-income adults potentially eligible for Medicaid under the Affordable Care Act. JAMA. 2013;309:2579-2586. [DOI] [PubMed] [Google Scholar]
- 15.Sommers DB, Baicker K, Epstein AM. Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367:1025-1034. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich AJ, Tobin JN, Robinson CM, Cassells A, Greene MA, Dunn VA, et al. Telephone outreach to increase colon cancer screening in Medicaid managed care organizations: A randomized controlled trial. Ann Fam Med. 2013;11(4):335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietrich AJ, Tobin JN, Cassells A, Robinson CM, Reh M, Romero KA, et al. Translation of an efficacious cancer-screening intervention to women enrolled in a Medicaid managed care organization. Ann Fam Med. 2007;5(4):320-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers RE, Bittner-Fagan H, Daskalakis C, Sifri R, Verson SW, Cocroft C, et a A randomized controlled trial of tailored navigation and standard intervention in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2013;22(1):109-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeanne S, Mandelblatt JS, Yabroff KR. Effectiveness of interventions designed to increase mammography use: A meta-analysis of provider-targeted strategies. Cancer Epidemiol Biomarkers Prev. 1999;8:759-767. [PubMed] [Google Scholar]
- 20.Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: Enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152(10):668-676. [DOI] [PubMed] [Google Scholar]
- 21.Vernon SW, Bartholomew LK, McQueen A, Bettenourt JL, Greisinger A, Coan SP, et al. A randomized controlled trial of a tailored interactive computer-delivered intervention to promote colorectal cancer screening: Sometimes more is just the same. Ann Behav Med. 2011;41(3):284-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner TH. The effectiveness of mailed patient reminders on mammography screening: A meta-analysis. Am J Public Health. 1998;14(1):64-70. [DOI] [PubMed] [Google Scholar]
- 23.Kangovi1 S, Bar FK, Carter T, Long JA, Shannon R, Grande D. Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff. 2013;32(7):1196-1203. [DOI] [PubMed] [Google Scholar]
- 24.Legler J, Meissner HI, Coyne C, Breen N, Chollette V, Rimer BK. The effectiveness of interventions to promote mammography among women with historically lower rates of screening. Cancer Epidemiol Biomarkers Prev. 2002;11(1):59-71. [PubMed] [Google Scholar]
- 25.Slater JS, Parks MJ, Malone ME, Henly GA, Nelson CL. Coupling financial incentives with direct mail in population-based practice: A randomized trial of mammography promotion. Health Edu Behav. 2017;44:165-174. [DOI] [PubMed] [Google Scholar]
- 26.Slater JS, Henly GA, Ha CN, Malone ME, Nyman JA, Diaz S, et al. Effect of direct mail as a population-based strategy to increase mammography use among low-income under-insured women ages 40 to 64 years. Cancer Epidemiol Biomarkers Prev. 2005;14:2346-2352. [DOI] [PubMed] [Google Scholar]
- 27.Freeman HP. The origin, evolution, and principles of patient navigation. Cancer Epidemiol Biomarkers Prev. 2012;21:1614-1617. [DOI] [PubMed] [Google Scholar]
- 28.Byers T Assessing the value of patient navigation for completing cancer screening. Cancer Epidemiol Biomarkers Prev. 2012;21:1618-1619. [DOI] [PubMed] [Google Scholar]
- 29.Sabatino SA, Lawrence B, Elder R, Mercer SL, Wilson KM, DeVinney B, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: Nine updates systematic reviews for the Guide to Community Preventive Services. Am J Prev Med. 2012;43:97-118. [DOI] [PubMed] [Google Scholar]
- 30.Plescia M, Wong FL, Pieters J, Joseph D. The National Breast and Cervical Cancer Early Detection Program in the era of health reform: A vision forward . Cancer. 2014;120:2620-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Preventive Task Force. Screening for colorectal cancer: U.S. Preventive Service Task Force Recommendation Statement. JAMA. 2016;315(23):2564-2575. [DOI] [PubMed] [Google Scholar]
- 32.Rothman AJ, Salovey P. Shaping perceptions to motivate healthy behavior: The role of message framing. Psychol Bull. 1997;121(1):3-19. [DOI] [PubMed] [Google Scholar]
- 33.Witte K, Allen M. A meta-analysis of fear appeals: Implications for effective public health campaigns. Health Edu Behav. 2000;27:591-615. [DOI] [PubMed] [Google Scholar]
- 34.Prochaska JO, Redding CA, Evers KE. The transtheoretical model and stages of change. In Glanz K, Rimer BK, & Viswanath K (Eds.), Health behavior and health education: Theory, research, and practice (pp. 97-121) (4th ed). San Francisco, CA: Jossey-Bass, 2008. [Google Scholar]
- 35.Weinstein ND, Sandman PM, Blalock SJ. The precaution adoption process model. In Glanz K, Rimer BK, & Viswanath K (Eds.), Health behavior and health education: Theory, research, and practice (pp. 123-147) (4th ed). San Francisco, CA: Jossey-Bass, 2008. [Google Scholar]
- 36.Schenck AP, Klabunde CN, Warren JL, Peacock S, Davis WW, Hawley ST, et al. Data sources for measuring colorectal endoscopy use among Medicare enrollees. Cancer Epidemiol Biomarkers Prev. 2007. ;16(10):211827. [DOI] [PubMed] [Google Scholar]
- 37.Elkin EB, Snow JG, Leoce NM, Atoria CL, Schrag D. Mammography capacity and appointment wait Times: Barriers to breast cancer screening. Cancer Causes Control. 2012;23:45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cameron KA, Persell SD, Brown T, Thompson J, Baker DW. Patient outreach to promote colorectal cancer screening among patients with an expired order for colonoscopy: A randomized controlled trial. Arch Intern Med. 2011;171:643-646. [DOI] [PubMed] [Google Scholar]
- 39.Hing E, Decker SL, Jamoom E. Acceptance of New Patients with Public and Private Insurance by Office-Based Physicians: United States, 2013. Washington, DC: National Center for Health Statistics; 2015. NCHS Data Brief 195. [PubMed] [Google Scholar]
- 40.Decker SL. In 2011 nearly one-third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff. 2012;31:1673-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nash D, Azeez S, Vlahov D, Schori M. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006;83(2):231-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charlot M, Santana MC, Chen CA, Bak S, Heeren TC, Battaglia TA, et al. Impact of patient and navigator race and language concordance on care after cancer screening abnormalities. Cancer. 2015;121(9):1477-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonfill X, Marzo M, Pladevall M, Marti J, Emparanza JI. Strategies for increasing women participation in community breast cancer screening. Cochrane Database Syst Rev. 2001;1:CD002943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baron RC, Melillo S, Rimer BK, Coates RJ, Kerner J, Habarta N, et al. Intervention to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers: a systematic review of provider reminders. Am J Prev Med. 2010;38(1):110-7. [DOI] [PubMed] [Google Scholar]
- 45.Camilloni L, Ferroni E, Cendales BJ, Pezzarossi A, Furnari G, Borgia P, et al. Methods to increase participation in organized screening programs: a systematic review. BMC Public Health. 2013;13:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bastani R, Marcus AC, Maxwell AE, Prabu I, Yan KX. Evaluation of an intervention to increase mammography screening in Los Angeles. Prev Med. 1994;23:83-90. [DOI] [PubMed] [Google Scholar]
- 47.Fox S, Stein J, Sockloskie RJ, Ory MG. Targeted mailed materials and the Medicare beneficiary: Increasing mammography screening among the elderly. Am J Public Health. AJPH. 2001;91(1):55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCaul KD, Wold KS. The effects of mailed reminders and tailored messages on mammography screening. J Community Health. 2002;27(3):181-189. [DOI] [PubMed] [Google Scholar]
- 49.Pasick RJ, Hiatt RA, Paskett ED. Lessons learned from community-based cancer screening intervention research. Cancer. 2004;101:1146-1164. [DOI] [PubMed] [Google Scholar]
- 50.Paskett ED, Tatum CM, D’Agostino Jr, Rushing J, Velez R, Michielutte R, et al. Community-based interventions to improve breast and cervical cancer screening: Results of the Forsyth County Cancer Screening (FoCaS). Cancer Epidemiol Biomarkers Prev. 1999;8:453-459. [PubMed] [Google Scholar]
- 51.Welsh AL,Sauaia A, Jacobellis J, Min SJ, Byers T. The effect of two church-based interventions on breast cancer screening rates among Medicaid-insured Latinas. Prev Chronic Dis. 2005;2(4):A07. [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. Increasing Population-based Breast and Cervical Cancer Screening: An Action Guide to Facilitate Evidence-based Strategies. Atlanta: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2014. [Google Scholar]
- 53.Giles EL, Robalino S, McColl E, Sniehotta FF, Adams J. The effectiveness of financial incentives for health behaviour change: systematic review and meta-analysis. PLOS ONE. 2014;9(3):e90347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katch H, Solomon. Are Medicaid Incentives an Effective Way to Improve Health Outcomes. Center on Budget and Policy Priorities, January 24, 2017. [Google Scholar]
- 55.Hand DJ, Heil SH, Sigmon SC, Higgins ST. Improving Medicaid health incentives programs: Lessons from substance abuse treatment research. Prev Med. 2014;63:87-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blumenthal KJ, Saulsgiver KA, Norton L, Troxel AB, Anarella JP, Gesten FC, et al. Medicaid incentive programs to encourage health behavior show mixed results to date and should be studied and improved. Health Aff. 2013;32:497-507. [DOI] [PubMed] [Google Scholar]
- 57.Vernon JA, Trujillo A, Rosenbaum S, DeBouno B. Low health literacy: implications for national health policy. Washington, DC: Department of Health Policy, School of Public Health and Health Services, The George Washington University; 2007. [Google Scholar]
- 58.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009. Am J Gastroenterol. 2009;104(3):739-50. [DOI] [PubMed] [Google Scholar]
- 59.Martin J, Halm EA, Tiro JA, Merchant Z, Balasubramanian B, McCallister K, et al. Reasons for lack of diagnostic colonoscopy after positive FIT in a safety-net health system. Am J Med. 2017;130(1):93.e1-93.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang PS, Wheat CL, Abhat A, Brenner AT, Fagerlin A, Hayward RA, et al. Adherence to competing strategies for colorectal cancer screening over 3 years. Am J Gastroenterol 2016;111:105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. multi-society task force on colorectal cancer. Am J Gasteroenterol. 2017;112:1016-1030. [DOI] [PubMed] [Google Scholar]
- 62.Cooper GS, Kou TD, Dor A, Koroukian SM, Schluchter MD. Cancer preventive services, socioeconomic status, and the Affordable Care Act. Cancer. 2017;123:1585-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collins LM, Dziak JJ, Kugler KC, Trail JB. Factorial experiments: efficient tools for evaluation of intervention components. Am J Prev Med. 2014;47(4):498-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.