Abstract
Background
In Europe, hepatitis E virus (HEV) infection is mainly a food-borne zoonosis, but it can also be transmitted by blood transfusion. It is usually a mild and self-limited infection. However, immunocompromised persons, who are also those more likely to undergo blood transfusions, may develop chronic hepatitis and often cirrhosis. Since this is a potential threat to blood safety, we aimed to investigate HEV prevalence in Italian blood donors.
Materials and methods
We used plasma donations collected during 2015–2016 by blood services (BS) scattered throughout the Italian regions and intended for the production of plasma-derived medicines. Plasma samples were tested for IgG and IgM anti-HEV and for HEV RNA using validated assays. Data concerning donor’s age and sex, and the location of the BS were collected.
Results
A total of 10,011 plasma samples were tested. Overall IgG and IgM prevalence rates were 8.7 and 0.4%, respectively. No sample was HEV RNA-positive. IgG prevalence was significantly higher in males and in donors aged 44 years and over. IgG prevalence differed greatly according to region. Overall regional rates over 15% were found in Abruzzo and in Sardinia, and rates of 10–15% were found in Lazio, Umbria and the Marche. Considering IgG prevalence according to the province where the BS was located, rates over 30% were found in Sardinia and Abruzzo. Age, sex and donor’s region of residence were independently associated with IgG positivity. BS location produced significant heterogeneity on prevalence rates within the regions.
Discussion
The detected IgG rate of 8.7% in this study represents one of the lowest seroprevalence rates reported among blood donors in Europe. Particularly high prevalence rates in some regions and provinces may be explained by local eating habits and/or intensive environmental HEV contamination. Before considering the introduction of HEV RNA screening for blood donations in Italy, further important issues should be addressed and prospective incidence and reliable cost-benefit studies are needed.
Keywords: hepatitis, prevalence, transfusion, zoonosis, Italy
Introduction
Hepatitis E virus (HEV) is a major cause of viral hepatitis in humans worldwide1. It is a non-enveloped single-stranded RNA virus that, according to a recent classification, belongs to the Hepeviridae family, Orthohepevirus genus; the type species is Orthohepevirus A (the taxonomic name of HEV). Within this species, at least four genotypes (from HEV-1 to HEV-4) can cause hepatitis in humans2 and they have been further subdivided into numerous subtypes3. So far, only one HEV serotype has been identified.
HEV-1 and HEV-2 infect only humans and are endemic in developing countries; HEV-1 is common in Asia, while HEV-2 is prevalent in Africa, Mexico and Central-South America. These genotypes are usually transmitted feco-orally by contaminated water, causing both outbreaks and sporadic cases. Their infection is often (80%) asymptomatic and clinical disease mainly affects young adults, being particularly severe in pregnant women and in patients with underlying chronic liver disease4–6. HEV-1 and HEV-2 travel-associated cases have also been observed in developed countries4–7.
HEV-3 and HEV-4 infect humans and various domestic and wild mammals, particularly domestic pigs, wild boars, deer and rabbits, which represent the main virus reservoirs for humans. HEV-3 has been reported with increasing frequency among autochthonous sporadic hepatitis E cases in developed countries of Europe, North America, Asia (Republic of China, Japan), and Oceania; HEV-4 is prevalent in Asia (Republic of China, Japan and Indonesia), although some cases have also been reported in Europe (Belgium, Germany, France and Italy1–8). These genotypes are zoonotically transmitted to humans mainly through consumption of contaminated raw or undercooked meat and meat products, or by contact with infected animals1–7. Transmission may also occur through consumption of vegetables, fruit, shellfish and drinking water contaminated by waste from infected animals and humans4,9; however, there is no firm evidence of these transmission modes. Finally, inter-human HEV-3 and HEV-4 transmission by transfusion of blood or blood products and via solid organ transplantation have also been reported10–14. Most HEV-3 or HEV-4 infections are asymptomatic; clinical disease mainly affects middle-aged and elderly individuals with co-existing illness. Both HEV-3 and HEV-4 may cause chronic hepatitis in immunocompromised individuals which, in approximately 60% of cases, may develop liver cirrhosis1,4–6.
Seroprevalence studies carried out in various European countries have reported variable, and sometimes unexpectedly high rates of anti-HEV immunoglobulin G (IgG) antibodies among the general population and blood donors9,15–33. A recent meta-analysis of HEV seroprevalence studies conducted in Europe from 2003 to 2015 reported estimates of prevalence ranging from 0.6 to 52.5%34. The wide variation in HEV prevalence among different European countries, and even within the same country, has mainly been attributed to the performance characteristics of the anti-HEV assays used, some geographical-environmental factors (including the natural environment, people’s behaviour and life-style, regional dietary habits, presence of animal reservoirs), and the cohort under investigation5–7,34.
The risk of HEV-3 and HEV-4 transmission by transfusion of blood or blood products (linked to transient viremia in asymptomatic blood donors) has raised particular concerns about the consequences for immunocompromised patients (e.g. patients with HIV infection, lymphoproliferative disorders, solid organ transplant, and malignancy under immunosuppressive therapy)19, which, given their underlying diseases, are also often exposed to transfusion of blood or blood products. The threat posed by HEV to transfusion safety has led many European countries to undertake studies to determine HEV seroprevalence in blood donors and also HEV RNA prevalence in blood donation with the aim of implementing universal or selective HEV RNA blood donor screening nationwide28.
The present retrospective study aimed to perform a nationwide study of HEV prevalence in Italian blood donors by using highly sensitive and specific validated assays to detect infection markers.
Materials and Methods
Study characteristics
The study was selected and approved by the Italian Ministry of Health from among those applying for the annual official grant of the Italian National Centre for Disease Prevention and Control (CCM, grant 2015). Therefore, the study received dedicated funds thanks to the relevance of the topic for public health.
Considering the high number of tested donors (approximately 10,000), and given that their individual health has not been impacted, and that the blood samples were intended to ensure the safety of blood products, it was not deemed necessary to receive the donors’ consent in compliance with the Authorisation n. 9/2014 of 11th December 2014 concerning personal data protection for scientific research purposes35.
Rationale for sample collection
The study was a collaborative effort among researchers from Centres and Departments of the Istituto Superiore di Sanità (ISS) (including the National Blood Centre, the National Centre for the Control and Evaluation of Medicines, and the Department of Infectious Disease) and the Kedrion Biopharma (Kedrion SpA, Castelvecchio Pascoli, LU, Italy), an international biopharmaceutical company specialising in the development, production and distribution of plasma-derived medicinal products (PDMPs).
In Italy, Kedrion Biopharma is authorised to fractionate national plasma according to a toll fractionation agreement with the Italian regions and autonomous provinces for the achievement of nationwide self-sufficiency in PDPMs. Kedrion receives the plasma intended for the production of PDMPs from the blood services (BS) scattered throughout the country. In the industrial plants, each donated plasma unit undergoes virological testing by using a mini-pool testing strategy. Usually, during this testing strategy, plasma obtained from each pilot tube attached to the relevant donated plasma unit container is transferred to a well in a 96-well backup plate (i.e. each plate contains 96 donated unit samples). Each backup plate is identified by a unique barcode indicating the donor’s province of origin. Each plasma sample (associated with a donor) occupies a unique position within the plate; this is identified with a letter (from A to H) and a number (from 1 to 12). After completing the testing procedure, these backup plates are stored frozen; once finished products resulting from the fractionation of donated plasma units are released for commercialisation, the stored frozen backup plates are disposed of. In our collaborative study, from 2015 to 2016, instead of being finally discharged, these backup plates were sent to the ISS and were used for HEV serological and molecular testing. Along with the plates, the company sent the ISS the relevant electronic documents in which donation codes and plate positions were reported, also adding the sex and age of the related donor. All this has allowed us to perform an HEV infection seroprevalence study on a very large plasma donor sample size representative of the Italian blood donor population.
Participants and study design
This study involved BS operating in each of the 19 Italian administrative regions and in the 2 autonomous provinces of Trento and Bolzano (which together make up the autonomous region of Trentino-Alto Adige/South Tyrol). For each region, approximately 500 plasma samples were selected. Where possible, plates were selected from different provinces of the same region in such a way as to try to maintain a constant number of samples (i.e. 96) per province. In some cases, when necessary, more than one BS for each province was involved.
All selected plasma samples were firstly assayed for anti-HEV immunoglobulin G (IgG) antibodies. Subsequently, in provinces with an anti-HEV IgG prevalence of 15% or more, anti-HEV IgM antibodies were assessed in both anti-HEV IgG positive and negative samples, whereas in provinces with an anti-HEV IgG prevalence less than 15%, only anti-HEV IgG positive plasma samples were tested for anti-HEV IgM.
Anti-HEV IgG and IgM antibodies were assessed using a commercial enzyme-linked immunosorbent assay (ELISA) kits (Wantai, Biologic Pharmacy Enterprise, Beijing, Republic of China). Both the IgG and IgM anti-HEV assays use recombinant antigens expressed from the ORF2 region.
All samples were tested for HEV RNA; however, different methodological approaches were adopted in order to optimise the economic resources available. Anti-HEV IgG positive samples were tested for HEV genome by an in-house PCR method on mini pools of 2–3 samples, having a sensitivity (95% cut-off) of approximately 80–120 UI/mL of HEV RNA per analysed sample. In anti-HEV IgG negative samples, HEV RNA detection was performed using a commercial kit (RealStar HEV RT-PCR kit 1.0, Altona Diagnostics GmbH, Hamburg, Germany) on mini pools of a maximum 10 samples, having a sensitivity (95% cut off) of approximately 250–300 UI/mL of HEV RNA per analysed sample. Finally, on all anti-HEV IgM positive samples, HEV RNA detection was carried out by the same commercial kit with a sensitivity (95% cut off) of approximately 25–30 IU/mL of HEV RNA per analysed sample.
Statistical analysis
Demographic data associated with HEV infection markers were evaluated using χ2 and Fisher’s exact tests, Student’s t-test or the Mann-Whitney U-test as appropriate.
To evaluate simultaneously the independent association of several characteristics of the participants with the IgG positivity, a mixed logistic model was performed with the blood donor centre as the clustering variable. The analysis included age at blood donation, sex, and administrative region (i.e. NUTS2 of Italy as defined by Eurostat)36.
Results
In this study, a total of 10,011 plasma unit samples, corresponding to the same number of blood donors, were assessed for HEV infection. Data concerning age were available for 8,973 (89.6%) donors (median age 44 years, range 18–70 years), while data concerning sex were available for 9,141 (91.3%) donors (6,835 males and 2,306 females; a male:female ratio of nearly 3:1).
Overall, 869 donors were anti-HEV IgG positive (8.7%; 95% confidence interval [CI], 8.14–9.25), while 39 (0.5%) donor samples showed a repeated indeterminate anti-HEV IgG result on the basis of the optical density cut-off value chosen for the analysis (Table I). There were no significant differences in the percentage of indeterminate IgG results between the different regions.
Table I.
The number of donors resulting anti-HEV IgG positive on the basis of the optical density cut-off value chosen.
| Region | N. of donors | Anti-IgG+ donors | Anti-IgG prevalence | Anti-IgM+ donors | HEV RNA + | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | M | F | Total | M | F | Total | M | F | N. | % | IgG+ too | ||
| Abruzzo | 619 | 453 | 166 | 141 | 112 | 29 | 22.8 | 24.7 | 17.5 | 13 | 2.1 | 10 | 0 |
| Basilicata | 179 | 70 | 109 | 4 | 1 | 3 | 2.2 | 1.4 | 3.7 | 0 | 0 | 0 | 0 |
| Calabria | 504 | 419 | 85 | 30 | 25 | 5 | 5.9 | 6.0 | 5.9 | 1 | 0.2 | 1 | 0 |
| Campania | 504 | 343 | 161 | 28 | 22 | 6 | 5.6 | 6.4 | 3.7 | 0 | 0 | 0 | 0 |
| Emilia R.1 | 503 | 382 | 121 | 18 | 15 | 3 | 3.6 | 3.9 | 2.5 | 0 | 0 | 0 | 0 |
| Friuli Venezia G.2 | 288 | ND | ND | 7 | ND | ND | 2.4 | ND | ND | 2 | 0.7 | 2 | 0 |
| Lazio3 | 576 | 335 | 145 | 71 | 35 | 12 | 12.3 | 10.4 | 8.3 | 5 | 0.9 | 5 | 0 |
| Liguria | 480 | 344 | 136 | 39 | 25 | 14 | 8.1 | 7.3 | 10.3 | 4 | 0.8 | 4 | 0 |
| Lombardy4 | 544 | 45 | 13 | 33 | 6 | 0 | 6.1 | 13.3 | 0 | 1 | 0.2 | 1 | 0 |
| The Marche | 504 | 430 | 74 | 65 | 56 | 9 | 13.0 | 13.0 | 13.8 | 6 | 1.2 | 6 | 0 |
| Molise | 479 | 351 | 128 | 33 | 25 | 8 | 6.9 | 7.1 | 6.2 | 0 | 0 | 0 | 0 |
| Piedmont | 616 | 465 | 151 | 50 | 40 | 10 | 8.1 | 8.6 | 6.6 | 4 | 0.6 | 3 | 0 |
| Apulia | 504 | 390 | 114 | 18 | 15 | 3 | 3.6 | 3.8 | 2.6 | 0 | 0 | 0 | 0 |
| Sardinia | 672 | 458 | 214 | 134 | 108 | 26 | 19.9 | 23.6 | 12.1 | 2 | 0.3 | 2 | 0 |
| Sicily | 576 | 458 | 118 | 39 | 33 | 6 | 6.8 | 7.7 | 5.1 | 1 | 0.2 | 1 | 0 |
| Tuscany | 623 | 456 | 167 | 49 | 35 | 14 | 7.9 | 7.7 | 8.4 | 5 | 0.8 | 5 | 0 |
| Trentino-Alto A.5 | 488 | 380 | 108 | 22 | 18 | 4 | 4.5 | 4.7 | 3.7 | 0 | 0 | 0 | 0 |
| Umbria | 496 | 379 | 117 | 51 | 43 | 8 | 10.3 | 11.3 | 6.8 | 2 | 0.4 | 2 | 0 |
| Aosta Valley | 376 | 306 | 70 | 18 | 16 | 2 | 4.8 | 5.2 | 1.4 | 0 | 0 | 0 | 0 |
| Veneto | 480 | 371 | 109 | 19 | 17 | 2 | 4.0 | 5.1 | 1.8 | 0 | 0 | 0 | 0 |
| Total | 10,011 | 6,8356 | 2,3066 | 869 | 6476 | 1646 | 8.7 | 9.5 | 7.1 | 46 | 0.4 | 42 | 0 |
N: number; IgG+: anti-HEV IgG-positive; M: males; F: females; IgM+: anti-HEV IgM-positive; IgG+ too: anti-HEV IgM and IgG-positive; ND: no data.
Data concerning age are not available for 168 donors from Pievesestina (Forlì-Cesena, Emilia Romagna).
Data concerning age and sex are not available for the 288 donors from San Vito al Tagliamento (Pordenone, Friuli Venezia Giulia).
Data concerning age and sex are not available for 96 donors from Rieti.
Data concerning age and sex are not available for 486 out of 584 donors from Lombardy.
Trentino-Alto Adige/South Tyrol includes the two autonomous provinces of Trento and Bolzano.
Complete data are not available for Friuli Venezia Giulia, and only partially available for Lazio and Lombardy.
The overall national anti-HEV IgG prevalence was significantly higher in male than in female donors (9.5% [647 of 6,835; 95% CI: 8.79–10.18] vs 7.1% [164 of 2,306; 95% CI: 6.13–8.23]; p<0.001). This prevalence was also significantly higher in donors aged 44 years or over than in younger donors (12% [552 of 4,603; 95% CI: 11.09–12.96] vs 5.8% [254 of 4,370; 95% CI: 5.16–6.55]; p<0.001).
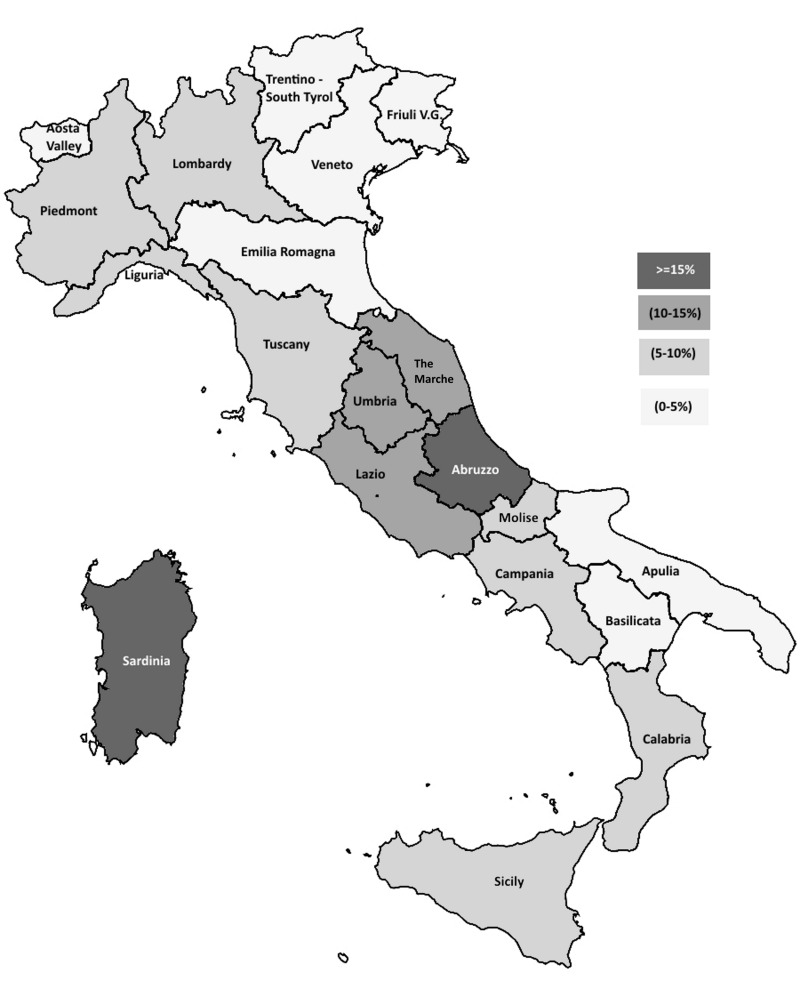
Table I and Figure 1 show the overall anti-HEV IgG prevalence found in blood donors from each region. There was a great variation in anti-HEV prevalence among them (from 22.8% in Abruzzo to 2.2% in Basilicata); overall regional prevalence rates over 15% were found not only in Abruzzo but also in Sardinia (19.9%). Overall rates between 10 and 15% were detected in several regions of Central Italy (Lazio, Umbria and the Marche). For all these regions, except Umbria, the differences with respect to the national prevalence rate reported herein (8.7%) were statistically significant (at least p<0.01). Overall prevalence rates of 5% and over and less than 10% were found in regions located in the North-West (Lombardy, Piedmont, Liguria and Tuscany) and South-West (Campania, Calabria and Sicily) and in the Eastern-Central area (Molise) of the Italian peninsula. Finally, overall low prevalence rates (<5%) were found in the North-East (Trentino- Alto Adige/South Tyrol, Friuli Venezia Giulia, Veneto and Emilia Romagna) and South-East (Apulia and Basilicata) regions of the peninsula, as well as in Aosta Valley, a tiny region in North-West Italy on the border with France (Figure 1).
Figure 1.
Prevalence of anti-HEV IgG in Italian regions.
HEV: Hepatitis E virus; IgG: immunoglobulin G.
The higher anti-HEV IgG prevalence in males compared to females was observed also when stratifying results according to region, but the opposite was seen in Basilicata, Liguria, the Marche and Tuscany. However, considering each region separately, the difference in sex was not statistically significant with the exception of Sardinia (p<0.001).
Median ages and ranges for donors from regions with an anti-HEV IgG prevalence of more than 10% and those from regions with a prevalence of 10% or under were nearly identical (44 years [range 19–70] and 44 years [range 18–70], respectively; p=0.612) (Table I). Also, there was no significant difference in the proportion of male donors in regions with anti-HEV IgG prevalence over 10% and in regions with a prevalence of 10% or under (2,055 of 2,867 [71.7%] vs 4,409 of 6,856 [64.3%], respectively; p=0.527).
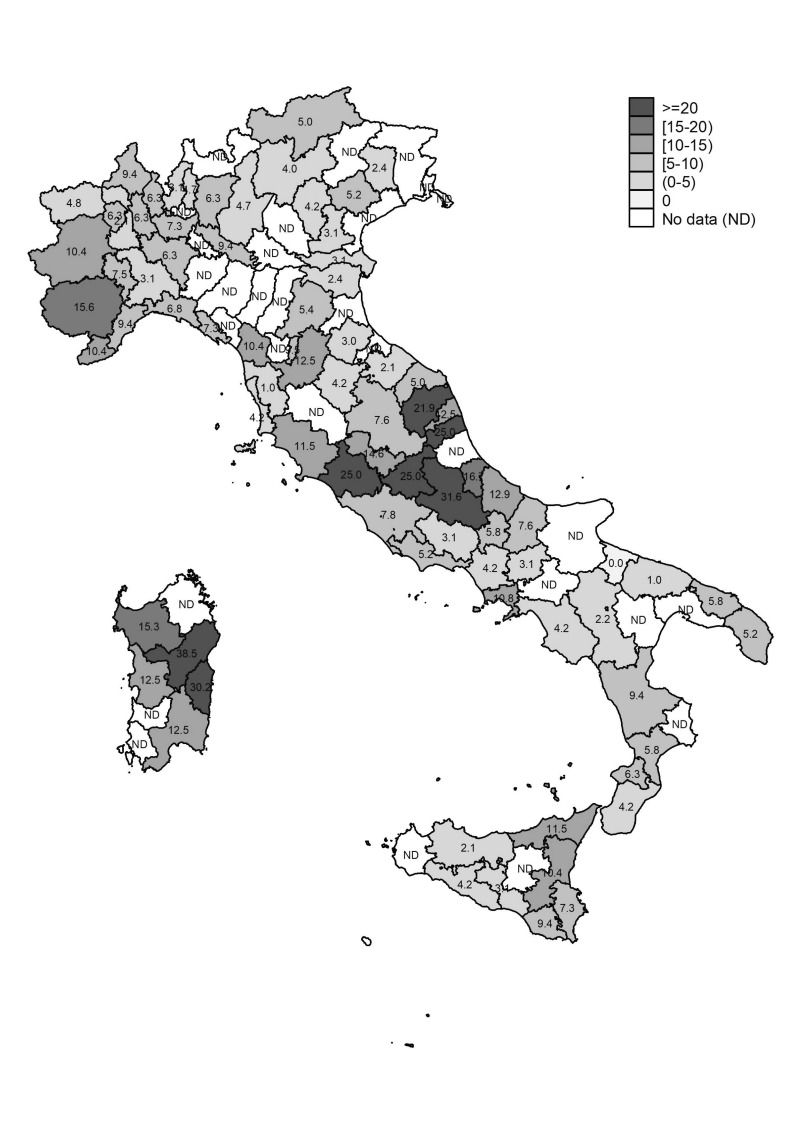
Figure 2 shows anti-HEV IgG prevalence according to province of BS location. As shown, some extremely high anti-HEV IgG prevalence rates were observed with values reaching and exceeding 30%, particularly in some towns of Sardinia and Abruzzo (e.g. Nuoro 38.5%, Lanusei 30.2%, and L’Aquila 31.6%).
Figure 2.
Prevalence of anti-HEV IgG according to location of blood transfusion service.
HEV: Hepatitis E virus; IgG: immunoglobulin G.
The results of the multivariable analysis of factors associated to the risk of being anti-HEV IgG positive are shown in Table II. We found that all three characteristics analysed (i.e. age, sex, and administrative region) were significantly associated with HEV-IgG positivity. In particular, blood donors aged 30–50 years and those aged 50 years or over had an adjusted odds ratio (AOR) between 2- and 3-fold and between 4- and 5-fold higher than those aged 30 years or under, respectively. Males had a statistically significant AOR of approximately 1.3 compared to female donors. In addition, there were statistically significant differences in AOR among the regions with lower AOR in northern and southern regions compared to central Italy. Finally, there was a significant residual effect of the BS location, confirming that, after controlling for age and sex, there was, on average, significant heterogeneity on HEV-IgG positivity within the regions.
Table II.
Adjusted odds ratio of being anti-HEV IgG-positive in blood donors in Italy, 2015–20161.
| AOR | 95% CI | p-value | ||
|---|---|---|---|---|
| Age (years) | ≤30 (ref) | 1.00 | - | - |
| 31–40 | 2.21 | 1.60–3.05 | <0.01 | |
| 41–50 | 2.62 | 1.93–3.55 | <0.01 | |
| 50+ | 4.76 | 3.55–6.39 | <0.01 | |
| Not indicated | 2.27 | 0.4910.58 | 0.30 | |
| Sex | Female (ref) | - | - | |
| Male | 1.28 | 1.06–154 | 0.01 | |
| Not indicated | 1.90 | 0.33–11.01 | 0.48 | |
| Region | Abruzzo (ref) | 1.00 | - | - |
| Basilicata | 0.09 | 0.02–0.38 | <0.01 | |
| Calabria | 0.25 | 0.11–0.55 | <0.01 | |
| Campania | 0.22 | 0.10–0.48 | <0.01 | |
| Emilia Romagna | 0.14 | 0.05–0.38 | <0.01 | |
| Friuli Venezia Giulia | 0.07 | 0.02–0.35 | <0.01 | |
| Lazio | 0.51 | 0.24–1.08 | 0.08 | |
| Liguria | 0.31 | 0.15–0.67 | <0.01 | |
| Lombardy | 0.19 | 0.06–0.56 | <0.01 | |
| The Marche | 0.52 | 0.25–1.08 | 0.08 | |
| Molise | 0.28 | 0.12–0.65 | <0.01 | |
| Piedmont | 0.31 | 0.16–0.63 | <0.01 | |
| Apulia | 0.13 | 0.06–0.30 | <0.01 | |
| Sardinia | 0.91 | 0.46–1.79 | 0.78 | |
| Sicily | 0.26 | 0.13–0.55 | <0.01 | |
| Tuscany | 0.31 | 0.15–0.63 | <0.01 | |
| Trentino-Alto Adige | 0.18 | 0.07–0.47 | <0.01 | |
| Umbria | 0.41 | 0.18–0.93 | 0.03 | |
| Aosta Valley | 0.18 | 0.06–0.56 | <0.01 | |
| Veneto | 0.15 | 0.06–0.34 | <0.01 | |
| Blood donation service residual effect | 0.20 | 0.12–0.34 | <0.01 | |
AOR: adjusted odds ratio; CI: confidence interval; HEV: Hepatitis E virus; IgG: immunoglobulin G.
Note: multilevel logistic models with blood donation service as clustering level.
Overall, 46 (0.4%; CI, 0.34–0.61) donors were anti-HEV IgM-reactive. Forty-two of them were also anti-HEV IgG- positive. No donors were HEV RNA positive. The highest anti-HEV IgM prevalence rates were found in Abruzzo (2.1%; CI: 1.2–3.5), the Marche (1.2; CI: 0.55–2.57) and Lazio (0.9%; 0.37–2.02) (Table I). Data concerning age and sex were available for 42 of 46 anti-HEV IgM reactive (31 males; median age 47.5 years [range 21–66]).
Discussion
This was the first large-scale, nationwide study of prevalence of HEV infection in Italian blood donors performed by using highly sensitive and specific serological and molecular assays to detect infection markers37. The study indicated that during 2015–2016 the prevalence of anti-HEV IgG and IgM among adult blood donors in Italy was 8.7 and 0.4%, respectively. None of 10,011 donors was HEV RNA-positive.
Since estimates of anti-HEV IgG prevalence for a given population sample mainly depend on the performance characteristic of the serological assay employed26,34,37–40, it is essential to compare the results of an HEV seroprevalence study on a given population with those of other similar studies using the same serological assay.
Over the past two decades, several HEV serosurveys have been carried out among blood donors in Italy, but most of them used non-validated assays with a poor sensitivity. In fact, only two such surveys have been performed using the Wantai anti-HEV assay32,41. One of these two was performed by some of our team in the period February-March 2014. An anti-HEV IgG prevalence of 49% was observed among 313 blood donors in L’Aquila (Abruzzo)32. Three donors had acute/recent infection, being positive for either anti-HEV IgM (n=2; 0.6%) or HEV RNA (HEV-3) (n=2; 0.6%); eating raw dried pig-liver sausage was the only independent predictor of infection. Comparing these previous results with those of this current study, it seems that there has been a significant decrease in anti-HEV IgG prevalence (from 49 to 31.6%; p<0.001) among blood donors in L’Aquila over a time-span of nearly two years. Similar temporal changes (increases or decreases) in anti-HEV IgG prevalence among blood donors in the same geographical area evaluated by using the same assay have already been documented in other countries, even if the time-span was longer26. It is possible that, in some geographical areas, in relation to particular dietary habits or to the introduction of new risk factors, the degree of exposure to HEV risk factors could change more quickly (perhaps even seasonally). For example, the above cited study conducted in L’Aquila in 2014 was carried out during February-March of that year, that is just after the Christmas and New Year’s holidays (note that the incubation period of HEV infection is 2–6 weeks5,6). During those particular festivities, there is likely to be a big increase in the consumption of local delicacies, such as pig liver sausages, often eaten raw (e.g. air dried or coated in oil). This hypothesis is also supported by the results of another anti-HEV IgG survey carried out by some of our team among 250 blood donors in L’Aquila during February-March 2013; 114 of them (46.2%) were found to be anti-HEV IgG positive using the Wantai assay (Ciccaglione et al., 2013, unpublished data).
The second Italian published HEV serosurvey employing the Wantai assay was carried out among blood donors attending a BS in Sondrio, a town in the Lombardy region41. In this study, the anti-HEV IgG prevalence detected by the Wantai assay was 9.8%. Unfortunately, the BS of Sondrio did not participate in our current survey, and a direct comparison of results could not, therefore, be made.
The anti-HEV IgG prevalence estimated by using the Wantai assay in blood donors of other European countries was: 27% in the Netherlands27, 22.4% in France26, 19.9% in Catalonia (Spain)38, 19.8% in Denmark30, 13.5% in Upper Austria31, 5.3% in the Republic of Ireland29; in United Kingdom rates of 16.2%39, 10% (England and North Welsh25) and 4.7% (Scotland21), have been reported. Finally, in order to have a complete picture of the situation in Europe, in Germany, the anti-HEV IgG prevalence estimated among blood donors was 6.8%, but a much less sensitive assay was used in that study23,40. Thus, Italy has one of the lowest seroprevalence rates in Europe.
As already reported by others in Europe, our study also found that older blood donors were significantly more likely to be anti-HEV IgG positive21,26,27,29,31–34,38. It is not clear if this was due to an evenly distributed exposure to HEV over their lifetime or to a cohort-dependent exposure in the past. Anti-HEV IgG prevalence was also significantly higher in males than in females. Such a difference in prevalence has already been observed in other countries26,27,38 and might be linked to sex differences in exposure to particular HEV risk factors (professional exposure, dietary habits and leisure activities, hunting, etc.).
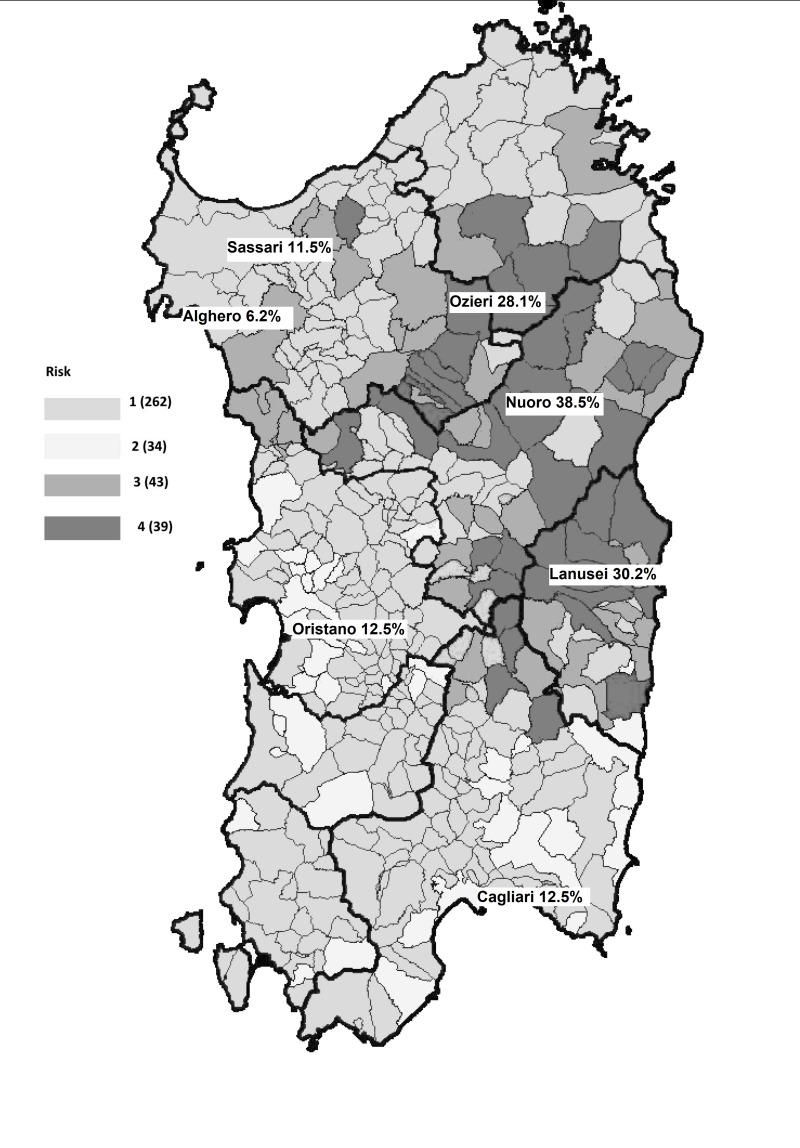
This nationwide study showed up to 10-fold variations in anti-HEV IgG prevalence rates among the different regions (e.g. 22.8% in Abruzzo vs 2.2% in Basilicata). Besides this, within some regions we identified hyperendemic areas with prevalence rates approaching or even higher than 30%, such as in Sardinia (e.g. Nuoro 38.5%, Lanusei 30.2%, Ozieri 28.1%) and in Abruzzo (L’Aquila 31.6%). The reasons for such variations and high prevalence rates in certain areas are not fully known, but may lie in local dietary habits, as well as in geographical and ecological/environmental factors. The influence of certain dietary habits on the spread of HEV infection has been well demonstrated by our previous study in L’Aquila and, even earlier, by several studies performed in France and Germany9,22,42–44. The role of some ecological/environmental factors in influencing HEV prevalence is well illustrated by the data from Sardinia. In this region, on the basis of the findings of this current study, it is possible to document an almost precise geographical correspondence between the areas of free-range pig production and those areas with the highest anti-HEV IgG rates (Figure 3)45. Pigs kept under all production systems can be the host of a variety of zoonotic and non-zoonotic pathogens (e.g. HEV, toxoplasmosis, African swine fever infection, etc.). On free-range pig farms, the risk of transmission of these infections to other pigs or other wild and domestic animals, and to humans, is increased. In particular, wild boars have been implicated in virus transmission when they come into contact with infected free-range domestic pigs. It has also been reported that wild boars, particularly those developing chronic or sub-acute HEV infection, given their high virus loads and long duration of viral shedding, can naturally and experimentally transmit HEV-3 to healthy pigs46,47. The uncontrolled expansion of the wild boar population and of the free-range pig population can cause widespread contamination of soil and watercourses. This could represent a serious health problem in some Italian regions, such as Sardinia and Abruzzo, but also elsewhere, where depopulation programmes have already been ongoing for several years.
Figure 3.
Free-range pig farming in Sardinia.
Areas at highest risk for African swine fever infection due to free-range pig production are indicated in dark grey (areas 3 and 4). These areas also perfectly correspond to those where, in this study, the highest anti-HEV IgG prevalence rates were found among Sardinian blood donors. (Modified from: Extraordinary program 2015–2017 for African Swine Fever infection eradication. Available at: n://www.regione.sardegna.it/documenti/1_73_20141230130127.pdf; up-dated 24 Nov 201745).
In Italy, the most intensive pig-breeding areas are located in: Lombardy (4.3 million [mln] pigs), Piedmont (1.2 mln pigs), Emilia-Romagna (1.1 mln pigs) and Veneto (824,000 pigs) (Table III)48. In these regions, the anti-HEV IgG prevalence detected by the current survey were 6.1, 8.1, 3.6, and 4.0%, respectively (Table I). These data are in agreement with the results of surveys carried out in France, in the Netherlands and also in Upper Austria which failed to demonstrate an association between living in areas of high pig density and frequency of HEV infection in humans26,27,31. The farming system adopted and the modes of treatment of contaminated farm animal waste and slaughterhouse waste could likely influence the geographical HEV spread more than the actual location of the pig farms26.
Table III.
Number of pigs and pig farms in Italy according to region.
| Region | N. of farms | N. of pigs |
|---|---|---|
| Piedmont | 915 | 1,208,377 |
| Aosta Valley | 9 | 43 |
| Liguria | 90 | 618 |
| Lombardy | 2,376 | 4,309,738 |
| Trentino-Alto Adige | 384 | 5,464 |
| Veneto | 2,675 | 82,445 |
| Friuli Venezia Giulia | 575 | 199,658 |
| Emilia Romagna | 1,107 | 1,085,506 |
| Tuscany | 1,121 | 18,279 |
| Umbria | 568 | 112,975 |
| The Marche | 1,126 | 113,014 |
| Lazio | 869 | 45,093 |
| Abruzzo | 1,907 | 81,053 |
| Molise | 272 | 22,898 |
| Campania | 3,694 | 110,197 |
| Apulia | 692 | 39,659 |
| Basilicata | 369 | 54,646 |
| Calabria | 896 | 40,551 |
| Sicily | 823 | 41,910 |
| Sardinia | 6,114 | 128,457 |
| Total | 26,582 | 8,607,093 |
Source: Italian National Institute of Statistics. Survey on the structure and production of farms (2013)49.
Geographical variations in prevalence within the same country have also been well documented in France26, the UK21,25,39, Austria31, the Netherlands27 and the Republic of Ireland29. Dietary habits, such as consumption of undercooked contaminated pork meat or products, and consumption of contaminated water have been, often only hypothetically, used to explain these variations. But instead, further and well-designed analytical/etiological studies, particularly in high prevalence areas, are needed to clearly identify all the possible sources and factors (geographic, zoological, geological, atmospheric, human) favouring HEV spread in these areas.
Our study has shown that Italian blood donors have one of the lowest HEV seroprevalence rates in Europe, even if there are important geographical variations with hyperendemic areas. A non-negligible number of blood donors in Italy have serological markers of recent infection (0.5% IgM positive) even though no donor enrolled in the present study has been found HEV RNA positive. However, as mentioned above, in a previous study in L’Aquila 0.64% (2 of 313) of blood donors were HEV RNA positive32. In any case, only a few transfusion-transmitted HEV infections have been reported in developed countries10–12 and no cases have ever been reported in Italy. It is possible that the vast majority of transfusion-transmitted infections remain asymptomatic and unrecognised, or low infectious dose and/or high anti-HEV titre in blood donation may hamper transmission49.
Because of its consequences in terms of morbidity and mortality, HEV infection represents a major threat for immunocompromised people. The main risks of HEV infection for such patients are the dietary exposure to undercooked contaminated food (meat, meat products, vegetables, water, shellfish) and the transfusion of HEV RNA-contaminated blood or blood products. While on the one hand most immunocompromised patients (e.g. transplanted patients or those under immunosuppressive treatment) are usually strongly and repeatedly advised by doctors to avoid eating any kind of raw or undercooked food, on the other hand, among all patients they are those more prone to receive blood and blood products21. Universal screening of blood donations has already been implemented in the Republic of Ireland since 2016; the UK switched from selective screening (on blood donation intended for transfusion to patients at risk) to universal screening in 2017, while the Netherlands started universal screening in 2017. Germany and France screen plasma donations intended for use in recipients at risk. In Australia, considering that the estimate seroprevalence is low and HEV viremia is rare, as well as the fact that the risk of adverse outcomes from transfusion-transmitted-HEV is negligible, HEV RNA donor screening has not yet been introduced50. Other countries, such as Italy, are currently evaluating the situation28.
Recently, a cost-effectiveness analysis concerning the introduction of HEV screening of blood donations was performed in the Netherlands51. This showed that HEV screening (especially universal screening) produces a small health gain considering the small number of chronic cases expected per year as well as the limited protection capacity, since most HEV infections are not transfusion related.
Some important issues require consideration before deciding whether to implement HEV blood donation screening: the rate of HEV transmission by transfusion, HEV viremia duration, minimal infectious dose, effect of anti-HEV antibodies in donors and recipients, frequency of chronic HEV infection in blood recipients. These have already been addressed by studies from other countries49,51,52. Thus, before considering the introduction of universal or selective HEV RNA screening of blood donations in Italy, in addition to carrying out a cost-effectiveness analysis, it would be essential to establish the true incidence of HEV infection and the related risk factors in a large representative sample of Italian blood donors. The Authors are conducting a prospective study to address these issues.
Acknowledgements
The Authors thank Mrs Daniela Adriani who took care of back-up plate storage.
Footnotes
Funding and resources
The study was funded by the Italian Centre for Disease Prevention and Control (CCM, grant 2015).
Authorship contributions
ES analysed the data and prepared the first draft of this manuscript. All Authors contributed to the study design, data interpretation, data analysis/consolidation and drafting/approval of the manuscript. GML is the study project scientific co-ordinator.
Disclosure of conflicts of interest
GML is the Editor-in-Chief of Blood Transfusion and this manuscript has undergone additional external review as a result. The other Authors declare no conflicts of interest.
References
- 1.Hepatitis WHO Fact Sheet E. [Accessed on 11/08/2017]. Available at: http://www.who.int/mediacentre/factsheets/fs280/en/
- 2.Smith DB, Simmonds P International Committee on Taxonomy of Viruses Hepeviridae Study Group. Consensus proposals for classification of the family Hepeviridae. J Gen Virol. 2014;95:2223–32. doi: 10.1099/vir.0.068429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16:5–36. doi: 10.1002/rmv.482. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly MC, Scobie L, Crossan CL, et al. Review article: hepatitis E-a concise review of virology, epidemiology, clinical presentation and therapy. Aliment Pharmacol Ther. 2017;46:126–41. doi: 10.1111/apt.14109. [DOI] [PubMed] [Google Scholar]
- 5.Kamar N, Dalton HR, Abravanel F, Izopet J. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27:116–38. doi: 10.1128/CMR.00057-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet. 2012;379:2477–88. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 7.Pavio N, Meng XJ, Doceul V. Zoonotic origin of hepatitis E. Curr Opin Virol. 2015;10:34–41. doi: 10.1016/j.coviro.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Garbuglia AR, Scognamiglio P, Petrosillo N, et al. Hepatitis E virus genotype 4 outbreak, Italy, 2011. Emerg Infect Dis. 2013;19:110–4. doi: 10.3201/eid1901.120983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansuy JM, Saune K, Rech H, et al. Seroprevalence in blood donors reveals widespread, multi-source exposure to hepatitis E virus, southern France, October 2011. Euro Surveill. 2015;20:27–34. [PubMed] [Google Scholar]
- 10.Boxall E, Herborn A, Kochethu G, et al. Transfusion- transmitted hepatitis E in a ‘nonhyperendemic’ country. Transfus Med. 2006;16:79–83. doi: 10.1111/j.1365-3148.2006.00652.x. [DOI] [PubMed] [Google Scholar]
- 11.Colson P, Coze C, Gallian P, et al. Transfusion-associated hepatitis E, France. Emerg Infect Dis. 2007;13:648–9. doi: 10.3201/eid1304.061387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsubayashi K, Kang JH, Sakata H, et al. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion. 2008;48:1368–75. doi: 10.1111/j.1537-2995.2008.01722.x. [DOI] [PubMed] [Google Scholar]
- 13.Schlosser B, Stein A, Neuhaus R, et al. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J Hepatol. 2012;56:500–2. doi: 10.1016/j.jhep.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Pourbaix A, Ouali N, Soussan P, et al. Evidence of hepatitis E virus transmission by renal graft. Transpl Infect Dis. 2017;19:e12624. doi: 10.1111/tid.12624. [DOI] [PubMed] [Google Scholar]
- 15.Buti M, Domínguez A, Plans P, et al. Community-based seroepidemiological survey of hepatitis E virus infection in Catalonia, Spain. Clin Vaccine Immunol. 2006;13:1328–32. doi: 10.1128/CVI.00255-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fogeda M, Avellon A, Echevarria JM. Prevalence of specific antibody to hepatitis E virus in the general population of the community of Madrid, Spain. J Med Virol. 2012;84:71–4. doi: 10.1002/jmv.22270. [DOI] [PubMed] [Google Scholar]
- 17.Wenzel JJ, Sichler M, Schemmerer M, et al. Decline in hepatitis E virus antibody prevalence in southeastern Germany, 1996–2011. Hepatology. 2014;60:1180–6. doi: 10.1002/hep.27244. [DOI] [PubMed] [Google Scholar]
- 18.Faber MS, Wenzel JJ, Jilg W, et al. Hepatitis E virus seroprevalence among adults, Germany. Emerg Infect Dis. 2012;18:1654–7. doi: 10.3201/eid1810.111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ijaz S, Vyse AJ, Morgan D, et al. Indigenous hepatitis E virus infection in England: more common than it seems. J Clin Virol. 2009;44:272–6. doi: 10.1016/j.jcv.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Dreier J, Juhl D. Autochthonous hepatitis e virus infections: a new transfusion-associated risk? Transfus Med Hemother. 2014;41:29–39. doi: 10.1159/000357098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleland A, Smith L, Crossan C, et al. Hepatitis E virus in Scottish blood donors. Vox Sang. 2013;105:283–9. doi: 10.1111/vox.12056. [DOI] [PubMed] [Google Scholar]
- 22.Mansuy JM, Bendall R, Legrand-Abravanel F, et al. Hepatitis E virus antibodies in blood donors, France. Emerg Infect Dis. 2011;17:2309–12. doi: 10.3201/eid1712.110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juhl D, Baylis SA, Blümel J, et al. Seroprevalence and incidence of hepatitis E virus infection in German blood donors. Transfusion. 2014;54:49–56. doi: 10.1111/trf.12121. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann A, Kenfak-Foguena A, André C, et al. Hepatitis E virus seroprevalence among blood donors in southwest Switzerland. PLoS One. 2011;6:e21150. doi: 10.1371/journal.pone.0021150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beale MA, Tettmar K, Szypulska R, et al. Is there evidence of recent hepatitis E virus infection in English and North Welsh blood donors? Vox Sang. 2011;100:340–2. doi: 10.1111/j.1423-0410.2010.01412.x. [DOI] [PubMed] [Google Scholar]
- 26.Mansuy JM, Gallian P, Dimeglio C, et al. A nationwide survey of hepatitis E viral infection in French blood donors. Hepatology. 2016;63:1145–54. doi: 10.1002/hep.28436. [DOI] [PubMed] [Google Scholar]
- 27.Slot E, Hogema BM, Riezebos-Brilman A, et al. Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.31.20550. pii: 20550. [DOI] [PubMed] [Google Scholar]
- 28.Domanović D, Tedder R, Blümel J, et al. Hepatitis E and blood donation safety in selected European countries: a shift to Screening? Euro Surveill. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.16.30514. pii: 30514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Riordan J, Boland F, Williams P, et al. Hepatitis E virus infection in the Irish blood donor population. Transfusion. 2016;56:2868–76. doi: 10.1111/trf.13757. [DOI] [PubMed] [Google Scholar]
- 30.Holm DK, Moessner BK, Engle RE, et al. Declining prevalence of hepatitis E antibodies among Danish blood donors. Transfusion. 2015;55:1662–7. doi: 10.1111/trf.13028. [DOI] [PubMed] [Google Scholar]
- 31.Fischer C, Hofmann M, Danzer M, et al. Seroprevalence and Incidence of hepatitis E in blood donors in Upper Austria. PLoS One. 2015;10:e0119576. doi: 10.1371/journal.pone.0119576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucarelli C, Spada E, Taliani G, et al. High prevalence of anti-hepatitis E virus antibodies among blood donors in central Italy, February to March 2014. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.30.30299. pii: 30299. [DOI] [PubMed] [Google Scholar]
- 33.Marano G, Vaglio S, Pupella S, et al. Hepatitis E: an old infection with new implications. Blood Transfus. 2015;13:6–17. doi: 10.2450/2014.0063-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartl J, Otto B, Madden RG, et al. Hepatitis E seroprevalence in Europe: a meta-analysis. Viruses. 2016;8 doi: 10.3390/v8080211. pii: E211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Authorisation 9/2014 - General Authorisation to Process Personal Data for Scientific Research Purposes. [Accessed 03/11/2017]. Published in Italy’s Official Journal 301 of 30 December 2014 Available at: http://www.garanteprivacy.it/web/guest/home/docweb//docwebdisplay/docweb/3786078.
- 36.Eurostat. Official EU statistical data. [Accessed 03/11/2017]. Available at: http://ec.europa.eu/eurostat/documents/345175/7451602/nuts-map-IT.pdf.
- 37.Pas SD, Streefkerk RH, Pronk M, et al. Diagnostic performance of selected commercial HEV IgM and IgG ELISAs for immunocompromised and immunocompetent patients. J Clin Virol. 2013;58:629–34. doi: 10.1016/j.jcv.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Sauleda S, Ong E, Bes M, et al. Seroprevalence of hepatitis E virus (HEV) and detection of HEV RNA with a transcription-mediated amplification assay in blood donors from Catalonia (Spain) Transfusion. 2015;55:972–9. doi: 10.1111/trf.12929. [DOI] [PubMed] [Google Scholar]
- 39.Bendall R, Ellis V, Ijaz S, et al. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82:799–805. doi: 10.1002/jmv.21656. [DOI] [PubMed] [Google Scholar]
- 40.Wenzel JJ, Preiss J, Schemmerer M, et al. Test performance characteristics of Anti-HEV IgG assays strongly influence hepatitis E seroprevalence estimates. J Infect Dis. 2013;207:497–500. doi: 10.1093/infdis/jis688. [DOI] [PubMed] [Google Scholar]
- 41.Galli C, Fomiatti L, Tagliacarne C, et al. Seroprevalence of hepatitis E virus among blood donors in northern Italy (Sondrio, Lombardy) determined by three different assays. Blood Transfus. 2017;15:502–5. doi: 10.2450/2017.0089-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colson P, Borentain P, Queyriaux B, et al. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis. 2010;202:825–34. doi: 10.1086/655898. [DOI] [PubMed] [Google Scholar]
- 43.Legrand-Abravanel F, Kamar N, Sandres-Saune K, et al. Characteristics of autochthonous hepatitis E virus infection in solid-organ transplant recipients in France. J Infect Dis. 2010;202:835–44. doi: 10.1086/655899. [DOI] [PubMed] [Google Scholar]
- 44.Wichmann O, Schimanski S, Koch J, et al. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J Infect Dis. 2008;198:1732–41. doi: 10.1086/593211. [DOI] [PubMed] [Google Scholar]
- 45.Regione Sardegna. [Accessed on 24/11/2017]. Available at: https//www.regione.sardegna.it/documenti/1_73_20141230130127.pdf. In Italian.
- 46.Schlosser J, Eiden M, Vina-Rodriguez A, et al. Natural and experimental hepatitis E virus genotype 3-infection in European wild boar is transmissible to domestic pigs. Vet Res. 2014;45:121. doi: 10.1186/s13567-014-0121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlosser J, Vina-Rodriguez A, Fast C, et al. Chronically infected wild boar can transmit genotype 3 hepatitis E virus to domestic pigs. Vet Microbiol. 2015;180:15–21. doi: 10.1016/j.vetmic.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 48.ISTAT (Italian National Institute of Statistics) [Accessed on 24/11/2017]. Available at: http://www.istat.it/it/files/2016/12/C13.pdf. In Italian.
- 49.Hewitt PE, Ijaz S, Brailsford SR, et al. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384:1766–73. doi: 10.1016/S0140-6736(14)61034-5. [DOI] [PubMed] [Google Scholar]
- 50.Hoad VC, Seed CR, Fryk JJ, et al. Hepatitis E virus RNA in Australian blood donors: prevalence and risk assessment. Vox Sang. 2017;112:614–21. doi: 10.1111/vox.12559. [DOI] [PubMed] [Google Scholar]
- 51.de Vos AS, Janssen MP, Zaaijer HL, Hogema BM. Cost-effectiveness of the screening of blood donations for hepatitis E virus in the Netherlands. Transfusion. 2017;57:258–66. doi: 10.1111/trf.13978. [DOI] [PubMed] [Google Scholar]
- 52.Dreier J, Knabbe C, Vollmer T. Transfusion-transmitted Hepatitis E: NAT screening of blood donations and infectious dose. Front Med. 2018;5:5. doi: 10.3389/fmed.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Legrand-Abravanel F, Kamar N, Sandres-Saune K, et al. Hepatitis E virus infection without reactivation in solid-organ transplant recipients, France. Emerg Infect Dis. 2011;17:30–7. doi: 10.3201/eid1701.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]