Abstract
Background
An analytic-decision model was built to estimate the cost-effectiveness of using ferric carboxymaltose for pre-operative haemoglobin optimisation in patients with iron deficiency anaemia undergoing primary knee arthroplasty.
Materials and methods
We simulated 20,000 patients who were randomly assigned to the haemoglobin optimisation arm or the non-optimisation control arm in a strict 1:1 ratio. The main outcomes were cost per patient transfusion avoided and red blood cell units spared. The analyses were performed from the hospital perspective with length of stay as the time horizon.
Results
In the reference case scenario, pre-operative haemoglobin optimisation led to fewer patients being exposed to allogeneic red blood cell transfusion (2,212 vs 6,595 out of 10,000 patients) and a relevant decrease in the number of red blood cell units transfused (4.342 vs 13.336). The costs of avoiding one patient transfusion and sparing one red blood cell unit were € 831 and € 405, respectively. Increased costs in the optimisation arm were mostly associated with the outpatient day hospital visit (54%) and ferric carboxymaltose treatment (40%).
Discussion
In primary knee arthroplasty, pre-operative haemoglobin optimisation with intravenous ferric carboxymaltose is less expensive than other reported patient blood management modalities and must be considered in patients with iron deficiency anaemia.
Keywords: anaemia, iron-deficiency, cost-effectiveness, knee replacement, erythrocyte transfusion
Introduction
Major orthopaedic surgery, especially hip and knee arthroplasties, result in significant peri-operative bleeding and the need for allogeneic red blood cell (RBC) transfusion, although criteria for transfusion vary widely from centre to centre1. Since pre-operative haemoglobin concentration is a major predictor of peri-operative transfusion, haemoglobin optimisation before surgery is a cornerstone in patient blood management (PBM) in this setting2. Intravenous iron is an essential drug to optimise pre-operative haemoglobin concentration, and consequently reduce anaemia and transfusion, in patients with iron-deficiency anaemia3.
The multimodal PBM approach has been shown to be more cost-effective than the traditional transfusion approach4. However, the modalities used in the various programmes described in the literature differ from one another with regard to efficacy and costs. Several studies have analysed the economic impact of multimodal PBM modalities that were efficacious in reducing the issuance of red blood cells (RBC), as reported by the Ontario5, NHS Blood and Transplant6 and Western Australia7 experiences. Nevertheless, the cost-effectiveness of haemoglobin optimisation as a single PBM modality has not been evaluated in patients undergoing orthopaedic surgery. In order to assign resources to the most efficient health technologies, it is important to conduct cost-effectiveness analyses of PBM modalities. This information may help to support recommendations and provide resources to implement PBM programmes effectively8.
This study was aimed at evaluating the cost-effectiveness of a pre-operative haemoglobin optimisation protocol based on the administration of intravenous ferric carboxymaltose (FCM) to reduce RBC transfusion in patients undergoing primary knee arthroplasty.
The hypothesis was that haemoglobin optimisation in iron-deficient patients undergoing knee arthroplasty with intravenous ferric carboxymaltose is cost-effective.
Materials and methods
The study consisted in a computer simulation that modelled a hypothetical trial comparing pre-operative haemoglobin optimisation with no optimisation in patients with real or functional iron-deficiency anaemia submitted to primary knee arthroplasty. Model parameters on the probability of transfusion according to pre-operative haemoglobin concentration were derived from a study previously published by our group9. A more recent cohort of 52 patients who underwent haemoglobin optimisation with FCM prior to knee arthroplasty was used to estimate data on the effectiveness of the optimisation.
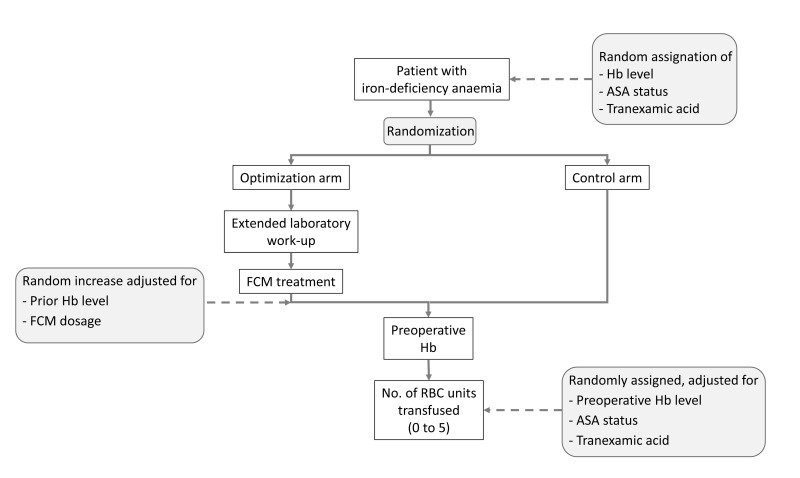
In brief, we simulated 20,000 patients who were randomly assigned to the haemoglobin optimisation arm or the non-optimisation, control arm in a strict 1:1 ratio. The 20,000 figure was arbitrarily selected to be large enough to stabilise the model’s outputs. In the optimisation arm, patients were initially submitted to an extensive laboratory work-up for anaemia and were sent from the pre-operative anaesthesiology visit to the outpatient clinic in order to receive a dose of intravenous FCM adjusted for the degree of anaemia. There had no further visits until they were admitted for surgery. In the control arm, patients underwent the standard pre-operative work-up only and did not receive FCM. Haemoglobin concentration was assayed in the patients in both study arms just before surgery and reflected the haemoglobin increase in the experimental arm but was similar to the presenting haemoglobin in the control arm. The haemoglobin level at this time determined the probability of peri-operative transfusion and the number of RBC units transfused. Figure 1 shows a general outline of both study arms.
Figure 1.
Flow-chart showing the simulated randomised controlled trial comparing preoperative haemoglobin optimisation with no optimisation. Factors subjected to probability distributions mimicking hazard are highlighted in grey.
The distribution of haemoglobin levels at the first visit, FCM dosage and the subsequent haemoglobin increase, as well as the probability of RBC transfusion at surgery are summarised in Tables I–III. Presenting haemoglobin levels at first visit, derived from the aforementioned data, were adjusted to a logistic distribution (mean: 10.9 g/dL; standard deviation: 0.5 g/dL) and truncated at 7.2 and 12.5 g/dL (no case outside this range was simulated; Table I). The increase in haemoglobin concentration in response to the administration of FCM was modelled as a triangular distribution. This kind of distribution is defined by three parameters: lowest value, mode, and highest value and is used when no other, more common distribution fits the data. We had previously found that patients with haemoglobin levels <11 g/dL have a steeper increase haemoglobin concentration after FCM than those with higher levels, so that two patterns of response to FCM were modelled according to the presenting level (Table II). The probabilities of transfusion and the number of RBC units transfused according to the immediate pre-operative haemoglobin level, the patient’s American Society of Anesthiology (ASA) category, and whether or not he/she received tranexamic acid were derived from previous studies by our group9 and are summarised in Tables III and IV.
Table I.
Characteristics of the hypothetical patients at entry into the clinical simulation.
| Characteristic | Frequency |
|---|---|
|
| |
| Presenting Hb (g/dL) | Adjusted to a logistic distribution (mean: 10.9; SD: 0.5; range: 7.2–12.5) |
| ASA category | |
| I/II | 80% |
| III/IV | 20% |
|
| |
| Tranexamic acid | |
| Contraindicated | 15% |
| Not contraindicated | 85% |
Hb: haemoglobin; SD: standard deviation; ASA: American Society of Anesthesiology.
Table III.
Probabilities (%) of transfusion in the model according to the haemoglobin concentration immediately prior to surgery, the patient’s ASA score and the tranexamic acid category randomly assigned to each patient in both the optimised and non-optimised study arm.
| Preoperative Hb (g/dL) | Probabilities of transfusion | ||
|---|---|---|---|
| TA | No TA | ||
| ASA I/II | ASA III/IV | Any ASA category | |
| 7.2 – 8.0 | 80% | 95% | 100% |
| 8.1 – 9.0 | 70% | 80% | 95% |
| 9.1 – 10.0 | 63% | 85% | 80% |
| 10.1 – 10.5 | 45% | 75% | 70% |
| 10.6 – 11.0 | 35% | 6% | 60% |
| 11.1 – 11.5 | 18% | 45% | 55% |
| 11.6 – 12.0 | 10% | 30% | 50% |
| 12.1 – 12.5 | 7% | 18% | 45% |
Hb: haemoglobin; TA: tranexamic acid; ASA: American Society of Anesthesiology.
Table II.
Probability (%) distributions used in the model to simulate dosage of intravenous iron and subsequent increase in haemoglobin concentration, adjusted for the presenting level of haemoglobin level prior to optimisation.
| Iron dose | Presenting haemoglobin | |
|---|---|---|
| <11 g/dL | ≥11 g/dL | |
| 500 mg | 40% | 47% |
| 1,000 mg | 40% | 53% |
| 1,500 mg | 10% | 0% |
| 2,500 mg | 10% | 0% |
| Increase (g/dL) | Triangular distribution* (0.8; 3.0; 4.7) | Triangular distribution* (0; 1.3; 2.8) |
Triangular distributions are defined by three parameters: lowest value, mode, and highest value.
Table IV.
Probability (%) of number of RBC units transfused, according to preoperative haemoglobin concentration in the model, once the decision to transfuse was made.
| N. of RBC units if transfusion (according to preoperative) | Preoperative Hb | |
|---|---|---|
| <11 g/dL | ≥11 g/dL | |
| 1 unit | 90% | 62% |
| 2 units | 0% | 28% |
| 3 units | 5% | 5% |
| 4 units | 5% | 5% |
RBC: red blood cell; Hb: haemoglobin.
Costs were derived from the clinical accounting and pharmacy records of our hospital and included only direct costs attributable to the haemoglobin optimisation. Costs assigned to outpatient hospital day visits included the nursing workup, infusion sets and other ancillary material (€ 196.65 per patient). The cost of the specific laboratory work-up was estimated to be € 73.00 per case, while the cost of FCM was set at € 81.38 for every 500 mg dose.
The analysis was performed from the hospital perspective and a temporal horizon limited to the length of stay in hospital. The main outcomes were the cost per unit of RBC spared and patient transfusion avoided in the optimisation arm as compared with the control arm. The simulation model was built using Excel spreadsheets and analysed with the @Risk add-in (www.palisade.com). Parameters estimated with uncertainty (e.g. probability of transfusion) were submitted to sensitivity analysis within a range of plausible values. The study was approved by the Ethics Committee for Clinical Investigation of the Hospital Clinic of Barcelona.
Results
The main results for the reference case scenario are summarised in Table V. The simulated pre-operative optimisation protocol led to fewer patients being exposed to allogeneic RBC transfusion (2,212 vs 6,595 out of 10,000 patients) and a relevant decrease in the number of transfused RBC units (4,342 vs 13,336). Increased costs in the optimisation arm amounted to € 3,641,421 and were mostly associated with the outpatient day-hospital visit (54%) and the FCM treatment (40%). In the reference case scenario, the cost of one patient avoiding transfusion and one RBC unit being spared were € 831 and € 405, respectively.
Table V.
Outcomes of the simulated clinical trial on haemoglobin optimisation: 10,000 patients were randomly assigned to the haemoglobin optimisation or the control (no optimisation) arms.
| Control arm | Optimisation arm | ||
|---|---|---|---|
| Patients transfused | 6,595 | 2,212 | |
| RBC units transfused | 13,336 | 4,341 | |
| Cost* | - | € 3,641,421 | |
| Laboratory | - | € 210,000 | (6%) |
| Hospital outpatient clinic | - | € 1,966,500 | (54%) |
| Ferric carboxymaltose | - | € 1,464,921 | (40%) |
| Cost per patient transfused avoided | € 831 | ||
| Cost per RBC unit avoided | € 405 |
Incremental cost as compared with the control arm; RBC: red blood cell.
Since the cost of an outpatient day-hospital visit may vary largely among institutions, we recalculated the main outcomes for different cost scenarios. On the assumptions of a 50% reduction and a 50% increase in the costs of the outpatient visit, the average costs per patient not exposed to allogeneic RBC were € 606 and € 1,055, respectively. With the same assumptions, the cost per RBC unit spared ranged from € 296 and € 514.
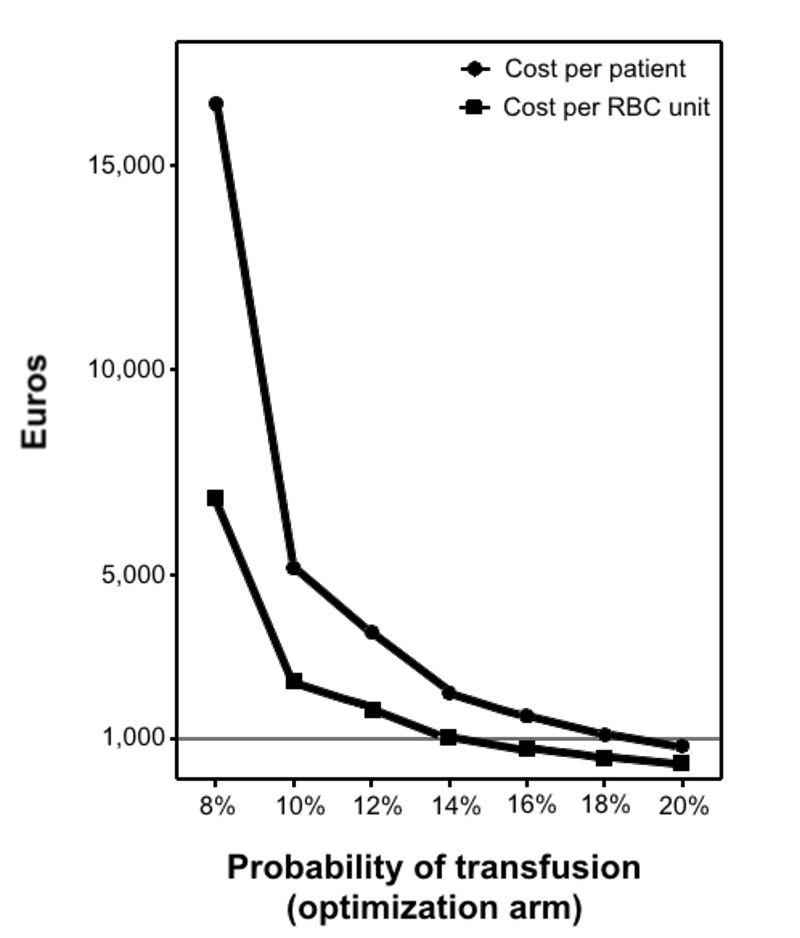
The probability of transfusion has been steadily decreasing because of improvements in surgical and anaesthesia procedures. We, therefore, submitted this variable to a sensitivity analysis in which such probability randomly ranged from × 0.1 to × 1.0 the basal value according to a uniform distribution. Figure 2 shows the cost per transfused patient avoided and RBC unit spared for different probabilities of transfusion in the optimisation arm. As expected, both costs increased exponentially as the probability of transfusion decreased. For probabilities of transfusion over 20%, there were no differences in transfusion requirements between the optimisation and control arms.
Figure 2.
Sensitivity analysis: cost per transfused patient avoided/RBC unit spared for different hypothetical probabilities of transfusion in the optimisation arm.
Discussion
The main outcomes in the present analysis were the cost of sparing a RBC unit and avoiding a patient’s exposure to allogeneic blood. We selected these yardsticks to evaluate the efficacy of pre-operative optimisation of haemoglobin concentration since the cost assigned to RBC transfusion varies widely from centre to centre, and the health and economic impact of avoiding allogeneic RBC transfusion is quite difficult to estimate and very sensitive to analytical variables.
In the reference case scenario, the cost of sparing a RBC unit in the selected population as a result of pre-operative FCM-based haemoglobin optimisation amounted to € 405. This figure is much lower than that reported for others PBM modalities, i.e., € 7,300 for recombinant human erythropoietin10 and € 51,000 for autologous blood reinfusion modalities11.
In order to obtain the maximum therapeutic effect of intravenous iron, it should be administered a few weeks before surgery in an outpatient regimen. In our analysis, the cost of the outpatient visit, including personnel, infusion sets and other ancillary materials, was the greatest cost component (54% of total), even higher than the acquisition cost of FCM. In fact, the total cost per RBC unit spared was very sensitive to this factor, ranging from € 296 to € 514 under several realistic assumptions about the cost of outpatient visits.
Published reports on the cost-effectiveness of FCM to optimise pre-operative haemoglobin concentration are scarce and low quality because of heterogeneity and lack of statistical power. Based on the current evidence, a recent Cochrane review3 concluded that intravenous iron seems to be more effective that oral iron but that evidence on a significant reduction in RBC transfusion as a result of pre-operative haemoglobin optimisation is still lacking.
Muñoz et al.12 reported on PBM costs and cost savings in patients undergoing total hip or knee arthroplasty who were given iron sucrose (600 mg intravenous) or FCM after surgery. Post-operative administration of iron, while the patient was already in hospital, required substantially fewer resources than the pre-operative administration modelled in our study (i.e. outpatient visits) but is less effective in preventing RBC transfusion.
In a costs model of fixed dose FCM treatment of anaemia before knee and hip surgery, Luporsi et al. estimated an average annual cost savings of € 216 per patient undergoing knee and hip surgery13. The study was performed from a national health service perspective and a time horizon considerably larger than ours and the results cannot, therefore, be compared with those obtained in our study. As expected, cost savings were very sensitive to the proportion of patients who require transfusion and fall to zero when this proportion decreases to about 18%. In our case, not unexpectedly, costs per transfusion avoided and RBC unit spared were also very sensitive to the transfusion rate in the control arm. Costs increased exponentially with lineal reduction in the transfusion rate.
The PREVENTT study14, an undergoing phase III, double-blind randomised trial, will hopefully answer many of the current questions regarding the efficacy and effectiveness of pre-operative haemoglobin optimisation by FCM. Nevertheless, the studied population, patients submitted to open abdominal surgery, is quite different from that undergoing knee or hip arthroplasty since these latter patients are usually older, have significant comorbidities and probably a higher prevalence of functional iron-deficiency anaemia15.
The strength of this study is that the measure of the effectiveness evaluation was RBC unit transfusion avoided. We ruled out evaluating effectiveness through the cost of RBC unit, because of the high variability in the calculation of the transfusion cost that could imply a lack of applicability of the results. The estimated adjusted 2011 costs of transfusing two units of RBC ranged from € 672.38 to € 972.5616.
Given the great variability in transfusion practice2, the sensitivity analysis of the results, according to the transfusion rate, engenders applicability and external validity in the different centres adjusting for their own practice.
Our study has several limitations. It was based on a theoretical model rather than an empirical one. Despite all variables in the model having been derived from observed data, some of which have been previously published9, this may affect the representativeness of our results. In addition, we did not consider the costs incurred by patients, most of whom have reduced mobility, which is in accordance with the hospital perspective used in the model. On the other hand, our model did not capture the whole potential health benefits of FCM in elderly patients who may have subclinical iron deficiency. On the positive side, our study provides a sound estimate of the expenses incurred by hospitals to avoid allogeneic RBC transfusion. This will be useful for decision-makers whatever the costs and health effects assigned to transfusion.
Conclusions
In conclusion, our theoretical model suggests that FMC-based programs of pre-operative optimisation of haemoglobin concentration would be cost-effective in primary knee arthroplasty and should, therefore, be considered in patients with iron-deficiency anaemia.
Footnotes
Authorship contribution
MB and AP: substancial contribution to conception and study desing, data analysis, and writing up the paper. MC, MT and LL: data collection, data analysis, revising the draft critically, revising the draft critically, and final approval of the version.
Conflict of interests disclosure
The Authors did not receive honoraria for this project. MB has received industry-supplied funding for consultancies and travel from Vifor Pharma (Spain). MC, AP, LL, and MT declare that they have no potential conflicts of interest.
References
- 1.Franchini M, Marano G, Mengoli C, et al. Red blood cell transfusion policy: a critical literature review. Blood Transfus. 2017;15:307–17. doi: 10.2450/2017.0059-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gombotz H, Rehak PH, Shander A, Hofmann A. The second Austrian benchmark study for blood use in elective surgery: results and practice change. Transfusion. 2014;54:2646–57. doi: 10.1111/trf.12687. [DOI] [PubMed] [Google Scholar]
- 3.Ng O, Keeler A, Mishra BD, et al. Iron therapy for pre-operative anaemia. Cochrane Database Syst Revs. 2015;12:CD011588. doi: 10.1002/14651858.CD011588.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann A, Ozawa S, Farrugia A, et al. Economic considerations on transfusion medicine and patient blood management. Best Pract Res Clin Anaesthesiol. 2013;27:59–68. doi: 10.1016/j.bpa.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Freedman J, Luke K, Escobar M, et al. Experience of a network of transfusion coordinators for blood conservation (Ontario Transfusion Coordinators [ONTraC]) Transfusion. 2008;48:237–50. doi: 10.1111/j.1537-2995.2007.01515.x. [DOI] [PubMed] [Google Scholar]
- 6.Kotzé A, Carter LA, Scally AJ. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108:943–52. doi: 10.1093/bja/aes135. [DOI] [PubMed] [Google Scholar]
- 7.Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57:1347–58. doi: 10.1111/trf.14006. [DOI] [PubMed] [Google Scholar]
- 8.Franchini M, Muñoz M. Towards the implementation of patient blood management across Europe. Blood Transfus. 2017;15:292–3. doi: 10.2450/2017.0078-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basora M, Tió M, Martin N, et al. Should all patients be optimized to the same pre-operative hemoglobin level to avoid transfusion in primary knee arthroplasty? Vox Sang. 2014;107:148–52. doi: 10.1111/vox.12147. [DOI] [PubMed] [Google Scholar]
- 10.So-Osman C. Patient blood management in elective total hip- and knee-replacement surgery (part 2) Anesthesiology. 2014;120:852–60. doi: 10.1097/ALN.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 11.So-Osman C. RCT on patient blood management in orthopedic surgery. Anesthesiology. 2014;120:839–51. doi: 10.1097/ALN.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 12.Muñoz M, Gómez-Ramírez S, Martín-Montañez E, et al. Cost of post-operative intravenous iron therapy in total lower limb arthroplasty: a retrospective, matched cohort study. Blood Transfus. 2014;12:40–9. doi: 10.2450/2013.0088-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luporsi E, Mahi L, Morre C, et al. Evaluation of cost savings with ferric carboxymaltose in anemia treatment through its impact on erythropoiesis-stimulating agents and blood transfusion: French healthcare payer perspective. J Med Econ. 2012;15:225–32. doi: 10.3111/13696998.2011.639823. [DOI] [PubMed] [Google Scholar]
- 14.Richards T, Clevenger B, Keidan J, et al. PREVENTT: preoperative intravenous iron to treat anaemia in major surgery: study protocol for a randomised controlled trial. Trials. 2015;16:1–6. doi: 10.1186/s13063-015-0774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spahn DR. Anemia and patient blood management in hip and knee surgery. Anesthesiology. 2010;113:482–95. doi: 10.1097/ALN.0b013e3181e08e97. [DOI] [PubMed] [Google Scholar]
- 16.Abraham I, Sun D. The cost of blood transfusion in Western Europe as estimated from six studies. Transfusion. 2012;52:1983–8. doi: 10.1111/j.1537-2995.2011.03532.x. [DOI] [PubMed] [Google Scholar]