Abstract
Backgrounds/Aims
It is important to point out that the identification of inflammation is an essential component of the pathogenesis and the progression of cancer. In this study, we analysed the neutrophil-to-lymphocyte ratio (NLR) and the platelets-to-lymphocyte ratio (PLR), with an overall survival (OS) in patients with pancreatic ductal adenocarcinoma (PDAC), who were treated with a resection following or not following a procedure of neoadjuvant chemotherapy/chemoradiation. We intended to identify the significance of the role of NLR and PLR, as prognostic markers in patients undergoing surgery for PDAC.
Methods
There were 127 patients enrolled in the study. The NLR and PLR were calculated on the basis of the pre-treatment blood cell count. An NLR>4 and a PLR >120 were considered to be elevated as measured. OS was analysed in relation to the NLR and PLR values, by using both the Kaplan-Meier and multivariate Cox-regression methods.
Results
Both high the NLR and high PLR were associated with a decreased OS in the univariate analysis. In the multivariate analysis, the high NLR, but not the high PLR, was an independent predictor of a decreased OS. When we divided patients into three groups (group 1: normal both NLR and PLR, group 2: high NLR or high PLR, group 3: high both NLR and PLR), the three-years OS rates for these groups were 48%, 32%, 7% (p=0.001) respectively.
Conclusions
It is noted that the pre-treatment NLR is an independent adverse prognostic factor, and considered to be superior to the PLR, in patients who undergo a resection for PDAC following or not neoadjuvant chemotherapy/chemoradiation.
Keywords: Pancreatic ductal adenocarcinoma, Inflammation, NLR, PLR, Prognostic factor
INTRODUCTION
Pancreatic cancer remains the fourth leading cause of death in relation to cancer worldwide, and it is noted with an overall 5-year survival rate that doesn't usually exceed 5%.1 A high percentage of patients are diagnosed at a late stage with a median survival of five to six months in patients with the advanced stages of the disease.2,3 On the other hand, there is a variation in the biological behaviour of pancreatic cancer and a difference in survival rates among these patients, indicating the need for the identification of predictive markers to assist in the patient risk stratification and prognosis. This could lead to an improvement in patient selection for surgery and delineation of chemotherapeutic approach, which may improve patient outcomes going forward.
The inflammatory response in the patient plays a key role in the carcinogenesis process and tumour microenvironment, as initially described by Virchow in 1876.4,5,6,7,8 A number of markers of inflammation and several ratios have been investigated in regards to their potential importance in prognosis and diagnosis in pancreatic cancer, as well as noted in other hepatobiliary malignancies including: C-reactive protein (CRP), the Modified Glasgow Prognostic Score, tumour necrosis factor (TNF)-α, T lymphocytes recruitment and also neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR).9,10,11,12,13,14,15,16,17,18,19 As international collaborative studies have previously addressed the development of an ‘immunoscore’ as a predictive form of a tumour's behaviour,20,21 the important role that prognostic markers and inflammation-based scores could potentially play is effortlessly highlighted for review below.
The aim of this study was to investigate the role of the pre-treatment NLR and PLR as well as their combination in the prediction of overall survival (OS) in patients with pancreatic ductal adenocarcinoma (PDAC), who are undergoing a resection with or without neoadjuvant chemotherapy/chemoradiation.
MATERIALS AND METHODS
From a prospectively collected surgical database of a single surgeon, the identified patients who underwent a pancreatectomy for PDAC as between the timeframes of January 2000 and June 2014 were identified. All of the patients were operated on by the same surgeon (SM) in two institutions. Patients who died because of postoperative complications, patients without follow up, and patients with metastatic disease were excluded. Additionally patients for whom there were no data available in order to calculate the pre-treatment NLR and PLR were also excluded from this study.
For the exclusion of distal metastases all patients underwent a computed tomography of the chest, abdomen and pelvis. The majority of the patients also underwent a fludeoxyglucose-positron emission tomography (FDG-PET). The resectability appropriateness for each patient was evaluated by an interdisciplinary panel review, which included a surgical expert and all resections were initiated with a curative intention. The patients who received the neoadjuvant chemotherapy/chemoradiation were diagnosed with borderline resectable tumours at presentation.
For each patient the following data was collected regarding: standard demographics, preoperative chemotherapy/chemoradiation, tumour characteristics, histopathology characteristics, type of operation, postoperative chemotherapy, and OS. OS was calculated from the date of treatment start (date that the neoadjuvant chemotherapy/chemoradiation started for the patients who received preoperative chemotherapy/chemoradiation, and the date of operation for patients who did not receive preoperative chemotherapy/chemoradiation), to the date of death and was censored at the time of the last follow up for the patients who were still alive. The follow-up data for all patients were available and noted.
The NLR and PLR were calculated by dividing the absolute number of neutrophils or platelets respectively by the absolute number of lymphocytes measured within 10 days prior to neoadjuvant chemotherapy/chemoradiation, or within 10 days prior to the surgery for the patients who did not receive neoadjuvant chemotherapy/chemoradiation, as part of the routine preoperative work up of the patients. All patients who started the treatment (neoadjuvant chemotherapy/chemoradiation or surgery) showed no signs of systemic inflammation or infection as noted at the time of laboratory testing, as this would have been an indication to postpone the proposed treatment. The study was approved by the the local ethics committee and the Institutional Review Board.
Statistical analyses
The primary end point of the study was OS. A NLR>4 and a PLR >120 were considered to be elevated. Thhe Chi-square test was used for calculating the association between patient's and the tumor's categorical characteristics and dichotomized NLR and PLR. The impact of these features on the OS was analysed using the Kaplan-Meier method. The survival outcomes between the groups were compared with the log-rank test. A p-value of less than 0.05 was considered statistically significant in this case. The factors who associated with OS (p>0.1) in univariate analysis were used for the performance of the multivariate Cox-regression analysis. The statistical analyses were performed with the Statistical Package of the Social Sciences (SPSS), version 17.0.
RESULTS
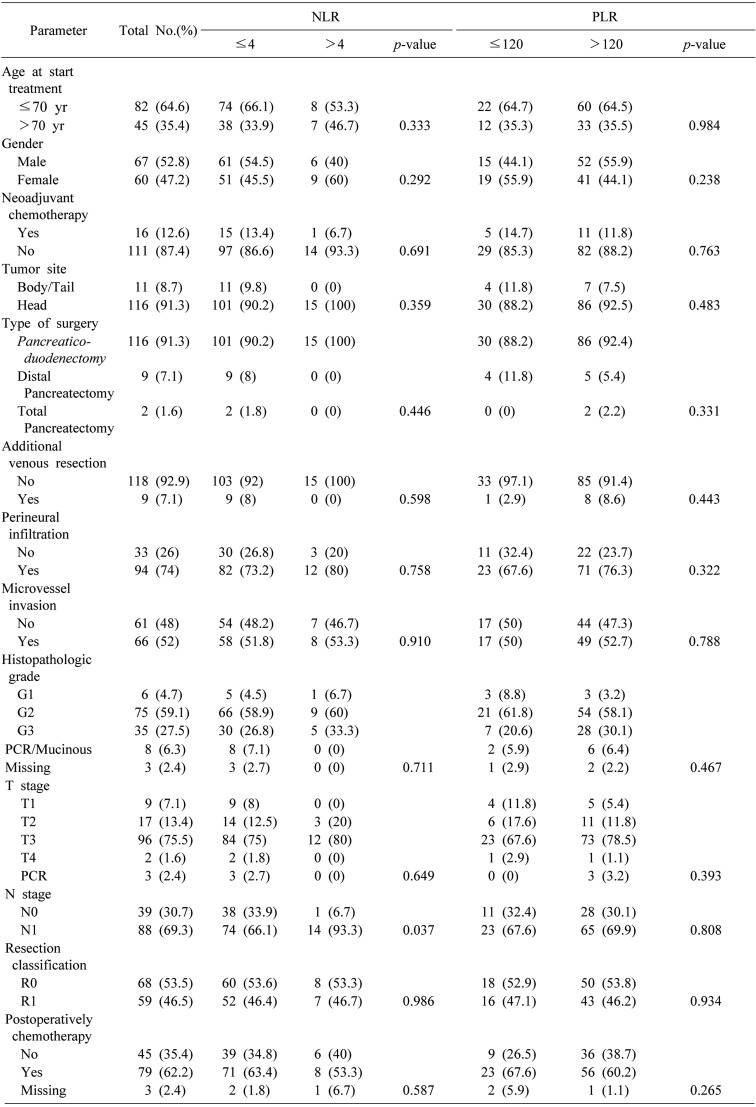
A total of 127 patients were eligible for inclusion in the study. The consort diagram is presented in Fig. 1. The patient demographics, characteristics of tumours and histopathology, details of treatment (neoadjuvant chemotherapy/chemoradiation plus surgery vs surgery alone, postoperative chemotherapy) and correlation of these characteristics with NLR and PLR are shown in Table 1. The median age of the patients was 68 years old (39 to 82 years old). There were sixteen patients (12.6%) who received neoadjuvant chemotherapy/chemoradiation. Twelve of these patients received only chemotherapy, while the rest received chemotherapy and radiotherapy. The pre-treatment NLR was high (>4) in 15 patients (11.8%) and it is noted that the preoperative PLR was high (>120) in 93 patients (73.2%). The high NLR was associated with positive lymph nodes (p=0.037).
Fig. 1. Consort diagram of the study.
Table 1. Relationships between clinicopathologic characteristics and NLR and PLR.
NLR, Neutrophil to Lymphocyte ratio; PLR, Platelet to Lymphocyte ratio; PCR, Pathologic Complete Response
The median follow-up period for all patients was 21 months (3 to 150 months). During the follow-up 93 patients (73.2%) died. For the entire study population, 1-, 3- and 5-years OS rates were 87%, 33% and 12% respectively.
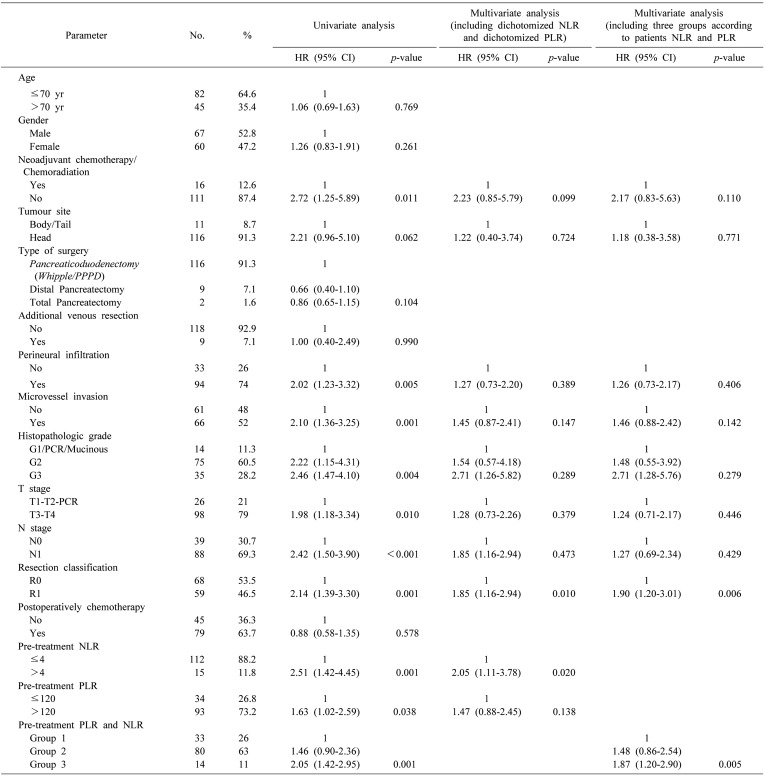
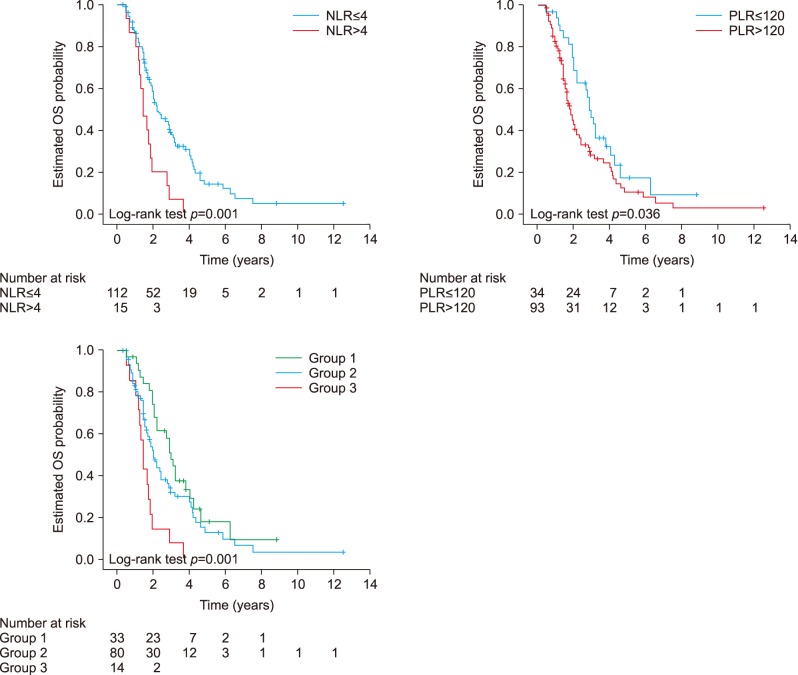
The univariate analyses (Table 2) demonstrated when there was no administration of neoadjuvant chemotherapy/chemoradiation (HR 2.72; 95% CI, 1.25–5.89; p=0.011), presence of perineural infiltration (HR 2.02; 95% CI, 1.23–3.32; p=0.005), presence of microvascular invasion (HR 2.10; 95% CI, 1.36–3.25; p=0.001), advanced histopathologic grade (p=000.4), advanced T stage (HR 1.98; 95% CI, 1.18–3.34; p=0.010), N1 stage (HR 2.42; 95% CI, 1.50–3.90; p<0.001), R1 resection (HR 2.14; 95% CI, 1.39–3.30; p=0.001), high (>4) NLR (HR 2.51; 95% CI, 1.42–4.45; p=0.001) and high (>120) PLR (HR 1.63; 95% CI, 1.02–2.59; p=0.038) were associated with a decreased OS (Fig. 2). Of note the site of the R1 disease was usually the margin along the superior mesenteric artery border. The patients with NLR>4 had a median OS of 17.2 months compared to an OS of 26.2 months for the patients with NLR≤4. The 3-year and 5-years OS rates were 7% and 0% respectively in patients with NLR>4 and 38% and 14% respectively in patients with NLR≤4. The patients with PLR>120 had a median OS of 21.8 months compared to an OS of 34.5 months for patients with a PLR≤120. The three and 5-years OS rates were 29% and 10% respectively in patients with PLR>120 and 46% and 17% respectively in patients with PLR≤120. In this case, it was analysed as a continuous variable, and the higher NLR was associated with a decreased OS (HR 1.10; 95% CI, 1.02–1.18; p=0.012), while the higher PLR was not associated with a decreased OS (HR 1.002; 95% CI, 1.000–1.005; p=0.055).
Table 2. Baseline clinicopathologic characteristics and their association with overall survival in univariate analysis and multivariate analysis.
HR, hazards ratio; CI, confidence interval; PCR, Pathologic Complete Response
Group 1, PLR≤120 and NLR≤4; Group 2, PLR>120 and NLR≤4 or PLR≤120 and NLR>4; Group 3, PLR>120 and NLR>4
Fig. 2. Pre treatment NLR, PLR and their combination and overall survival.
The multivariate analysis for OS was adjusted for the administration of neoadjuvant chemotherapy/chemoradiation, tumour site, perineural infiltration, microvessel invasion, histopathologic grade, T stage, N stage, R classification, pre-treatment NLR and pre-treatment PLR. In the multivariate analysis the only factors which remained statistically associated with OS was a R1 resection (HR 1.85; 95% CI, 1.16–2.94, p=0.010) and a high NLR (HR 2.05; 95% CI, 1.11–3.78, p=0.020), but not a high PLR (Table 2).
We further divided the patients into three groups according to their NLR and PLR. The patients with normal both NLR and PLR were assigned to group 1, with high NLR or PLR assigned to group 2, and with high both NLR and PLR to group 3. This categorization was associated with decreased OS both in univariate analysis (p=0.001) and in multivariate analysis (p=0.005) (Table 2, Fig. 2). The patients in group 1 have the best OS while group 3 the worst. The patients in group 1 had a median OS of 35.5 months compared to a OS of 24.1 months for patients in group 2, and 17.1 months for patients in group 3. The 3-years OS rates for these groups (group 1, group 2, group 3) were 48%, 32%, 7% (p=0.001) respectively.
DISCUSSION
The inflammation and cancer are two entities that have been associated with each other and these links are under investigation via the analysis of the tumor microenvironment, the host's response, the inflammatory pathways and the chain of systemic effects.8,12 The role of the NLR has attracted a lot of attention the last years, with the incidence of an elevated NLR at different cancer stages reported to possess an ability to serve as a possible prognostic factor in several malignancies including colorectal cancer, hepatocellular carcinoma and pancreatic cancer.22,23,24,25 Our group has previously reported its prognostic value in patients with colorectal liver metastases.26
On the other hand, the PLR is another ratio with potential prognostic values as demonstrated previously in different types of cancer including colorectal cancer, breast cancer and ovarian cancer.9,27,28 Our group has also reported its significance as a prognostic tool in colorectal liver metastases as well.29 The platelet's role in identifying the inflammatory processes is characterised by a review of the regulation of other types of cells such as neutrophils and facilitation of their adhesion to lymphocytes, and also by promoting the growth and spread of malignancies via onco-inflammatory mechanisms.30,31
We have demonstrated in this study that a pre-treatment NLR, but not a pre-treatment PLRm is an independent prognostic factor associated with OS in patients with PDAC undergoing resection following or not neoadjuvant chemotherapy/chemoradiation. The Arima et al.32 study demonstrated a potential diagnostic role of NLR>5 for PDAC in patients with pancreatic neoplastic disease. Additionally a Japanese group investigated the link between pre-treatment NLR and the pathological response to preoperative chemotherapy in pancreatic cancer patients, thereby concluding that the pre-treatment NLR is an independent predictive marker of the pathological response to preoperative therapy.33 Similarly the Luo et al.34 study reported that a baseline NLR and post-chemotherapy NLR change is appropriate to review to potentially serve as biomarkers for overall survival in patients who undergo chemotherapy having been diagnosed with pancreatic cancer. The conclusion that can be derived from our and other studies on the role of NLR in pancreatic cancer, is that as in other malignancies, it can potentially play a role in the delineation of therapy and surveillance in the era of multidisciplinary approach strategy, along with the highlighted importance of the variation of biological behaviour between patients with pancreatic cancer. These recommendations may help to promote better patient outcomes for patients who have presented with pancreatic cancer.
We also demonstrated that high NLR was statistically significantly associated with positive lymph nodes following pancreatic resection. Notably, the MD Anderson group have previously highlighted the significance of the number and ratio of positive lymph nodes on pancreatic cancer patient survival after neoadjuvant chemotherapy and pancreaticoduodenectomy.35 The significance of positive lymph nodes has also been emphasized by other authors, and we agree with these recommendations.36,37,38 We demonstrated a direct link between a high NLR and positive lymph nodes following a pancreatic resection. It has been proposed by the MD Anderson group that a subclassification of post-therapy node-positive group should be incorporated into the American Joint Committee on Cancer (AJCC) staging on PDAC patients. This is another indicator of the need for more prognostic factors in pancreatic cancer.
In the multivariate analysis, the only factors which remained statistically associated with OS was a R1 resection (HR 1.85; 95% CI, 1.16–2.94, p=0.010) and a high NLR (HR 2.05; 95% CI, 1.11–3.78, p=0.020), but not a high PLR. However we further divided patients into three groups according to their NLR and PLR. The patients with normal both NLR and PLR were assigned to group 1, and had the best OS of 35.5 months, with high NLR or PLR assigned to group 2 and an OS of 24.1 months, and with high both NLR and PLR to group 3 and an OS of 17.1 months. Although the NLR appears to be a stron ger prognostic factor for OS, there appears to be an interconnection between the NLR and PLR as one would expect from the biological link between neutrophils and platelets. This is in accordance with previous studies, where it was demonstrated that an elevated NLR is superior to a PLR for prognostication in patients with pancreatic cancer and with PLR not having independent prognostic value but elevated NLR being associated with higher PLR.39 Additionally the Garcea et al.40 study highlighted the characteristics of the preoperative NLR to be useful as a significant prognostic marker regarding disease-free survival following curative resection of pancreatic ductal adenocarcinoma in contradiction to the PLR alone. On the other hand, Spolverato et al.41 from the John Hopkins group reported that the elevated NLR and PLR were predictors of considerably worse long-term outcomes among patients with a HPB malignancy who are undergoing resection, including a proposed pancreatic ductal adenocarcinoma, however the ratios were calculated within 30 days prior to surgery, with a large percentage of the patient population having received neo-adjuvant chemotherapy though. As stated above, we therefore calculated the NLR and PLR within 10 days to the use of a neoadjuvant chemotherapy/chemoradiation, or within 10 days prior to surgery, for patients who did not receive the proposed neoadjuvant chemotherapy/chemoradiation.
This study carries the limitations of a prospectively accumulated database and the possible selection bias as noted in this study. On the other hand, the study involves patients who received surgical treatment by the same surgeon, and therefore we have tried to create a homogenous sample with all ratios calculated prior to the use of neoadjuvant chemotherapy/chemoradiation and surgery, or events noted as prior to surgery, for the patients who did not receive the associated neo-adjuvant chemotherapy.
In conclusion we have indicated that the pre-treatment NLR is an independent adverse prognostic factor in patients who undergo resection for PDAC, following or not the use of a neoadjuvant chemotherapy/chemoradiation. Further studies are needed to assess in more detail the exact role that the identified prognostic factors such as NLR or PLR can play in the strategy planning against pancreatic cancer. In this case however, it seems certain that we are entering into an era that the importance of inflammation in the progress of malignancy can't be overlooked and the use of prognostic tools, especially when easily calculated and at no extra cost, can prove to be useful allies in this case to aid the management of pancreatic cancer patients in disease management strategies going forward.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Cohen SJ, Pinover WH, Watson JC, Meropol NJ. Pancreatic cancer. Curr Treat Options Oncol. 2000;1:375–386. doi: 10.1007/s11864-000-0065-2. [DOI] [PubMed] [Google Scholar]
- 3.Geng Y, Qi Q, Sun M, Chen H, Wang P, Chen Z. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol. 2015;41:1508–1514. doi: 10.1016/j.ejso.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33(Suppl 1):S79–S84. doi: 10.1007/s10875-012-9847-0. [DOI] [PubMed] [Google Scholar]
- 5.Servais C, Erez N. From sentinel cells to inflammatory culprits: cancer-associated fibroblasts in tumour-related inflammation. J Pathol. 2013;229:198–207. doi: 10.1002/path.4103. [DOI] [PubMed] [Google Scholar]
- 6.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens L, Pathak S, Nunes QM, Pandanaboyana S, Macutkiewicz C, Smart N, et al. Prognostic significance of preoperative C-reactive protein and the neutrophil-lymphocyte ratio in resectable pancreatic cancer: a systematic review. HPB (Oxford) 2015;17:285–291. doi: 10.1111/hpb.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 9.Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Choi HJ, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17:216–222. doi: 10.3109/1354750X.2012.656705. [DOI] [PubMed] [Google Scholar]
- 10.Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012;107:695–699. doi: 10.1038/bjc.2012.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallappa S, Sinha A, Gupta S, Chadwick SJ. Preoperative neutrophil to lymphocyte ratio >5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15:323–328. doi: 10.1111/codi.12008. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 13.Jamieson NB, Denley SM, Logue J, MacKenzie DJ, Foulis AK, Dickson EJ, et al. A prospective comparison of the prognostic value of tumor- and patient-related factors in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2011;18:2318–2328. doi: 10.1245/s10434-011-1560-3. [DOI] [PubMed] [Google Scholar]
- 14.Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58–64. doi: 10.1016/j.jhep.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Sugiura T, Uesaka K, Kanemoto H, Mizuno T, Okamura Y. Elevated preoperative neutrophil-to-lymphocyte ratio as a predictor of survival after gastroenterostomy in patients with advanced pancreatic adenocarcinoma. Ann Surg Oncol. 2013;20:4330–4337. doi: 10.1245/s10434-013-3227-8. [DOI] [PubMed] [Google Scholar]
- 16.Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55–60. doi: 10.1016/j.ejso.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Malietzis G, Giacometti M, Askari A, Nachiappan S, Kennedy RH, Faiz OD, et al. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann Surg. 2014;260:287–292. doi: 10.1097/SLA.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 18.Szkandera J, Pichler M, Absenger G, Stotz M, Arminger F, Weissmueller M, et al. The elevated preoperative platelet to lymphocyte ratio predicts decreased time to recurrence in colon cancer patients. Am J Surg. 2014;208:210–214. doi: 10.1016/j.amjsurg.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Stotz M, Pichler M, Absenger G, Szkandera J, Arminger F, Schaberl-Moser R, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110:435–440. doi: 10.1038/bjc.2013.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16:614–622. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 23.Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2008;97:513–518. doi: 10.1002/jso.21001. [DOI] [PubMed] [Google Scholar]
- 24.Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–1762. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 25.Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416–421. doi: 10.1038/bjc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giakoustidis A, Neofytou K, Khan AZ, Mudan S. Neutrophil to lymphocyte ratio predicts pattern of recurrence in patients undergoing liver resection for colorectal liver metastasis and thus the overall survival. J Surg Oncol. 2015;111:445–450. doi: 10.1002/jso.23845. [DOI] [PubMed] [Google Scholar]
- 27.Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol. 2011;13:499–503. doi: 10.1007/s12094-011-0687-9. [DOI] [PubMed] [Google Scholar]
- 28.Krenn-Pilko S, Langsenlehner U, Thurner EM, Stojakovic T, Pichler M, Gerger A, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110:2524–2530. doi: 10.1038/bjc.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neofytou K, Smyth EC, Giakoustidis A, Khan AZ, Cunningham D, Mudan S. Elevated platelet to lymphocyte ratio predicts poor prognosis after hepatectomy for liver-only colorectal metastases, and it is superior to neutrophil to lymphocyte ratio as an adverse prognostic factor. Med Oncol. 2014;31:239. doi: 10.1007/s12032-014-0239-6. [DOI] [PubMed] [Google Scholar]
- 30.Jenne CN, Urrutia R, Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol. 2013;35:254–261. doi: 10.1111/ijlh.12084. [DOI] [PubMed] [Google Scholar]
- 31.Egan K, Crowley D, Smyth P, O'Toole S, Spillane C, Martin C, et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PLoS One. 2011;6:e26125. doi: 10.1371/journal.pone.0026125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arima K, Okabe H, Hashimoto D, Chikamoto A, Tsuji A, Yamamura K, et al. The diagnostic role of the neutrophil-to-lymphocyte ratio in predicting pancreatic ductal adenocarcinoma in patients with pancreatic diseases. Int J Clin Oncol. 2016;21:940–945. doi: 10.1007/s10147-016-0975-z. [DOI] [PubMed] [Google Scholar]
- 33.Hasegawa S, Eguchi H, Tomokuni A, Tomimaru Y, Asaoka T, Wada H, et al. Pre-treatment neutrophil to lymphocyte ratio as a predictive marker for pathological response to preoperative chemoradiotherapy in pancreatic cancer. Oncol Lett. 2016;11:1560–1566. doi: 10.3892/ol.2015.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo G, Guo M, Liu Z, Xiao Z, Jin K, Long J, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22:670–676. doi: 10.1245/s10434-014-4021-y. [DOI] [PubMed] [Google Scholar]
- 35.Fischer LK, Katz MH, Lee SM, Liu L, Wang H, Varadhachary GR, et al. The number and ratio of positive lymph nodes affect pancreatic cancer patient survival after neoadjuvant therapy and pancreaticoduodenectomy. Histopathology. 2016;68:210–220. doi: 10.1111/his.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huebner M, Kendrick M, Reid-Lombardo KM, Que F, Therneau T, Qin R, et al. Number of lymph nodes evaluated: prognostic value in pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:920–926. doi: 10.1007/s11605-012-1853-2. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13:1189–1200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 38.Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 39.Wang DS, Luo HY, Qiu MZ, Wang ZQ, Zhang DS, Wang FH, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29:3092–3100. doi: 10.1007/s12032-012-0226-8. [DOI] [PubMed] [Google Scholar]
- 40.Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011;35:868–872. doi: 10.1007/s00268-011-0984-z. [DOI] [PubMed] [Google Scholar]
- 41.Spolverato G, Maqsood H, Kim Y, Margonis G, Luo T, Ejaz A, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol. 2015;111:868–874. doi: 10.1002/jso.23900. [DOI] [PubMed] [Google Scholar]