Abstract
Mangroves are amongst the most productive marine ecosystems on Earth, providing a unique habitat opportunity for many species and key goods and services for human beings. Mangrove habitats are regressing at an alarming rate, due to direct anthropogenic impacts and global change. Here, in order to assess the effects of mangrove habitat degradation on benthic biodiversity and ecosystem functioning, we investigated meiofaunal biodiversity (as proxy of benthic biodiversity), benthic biomass and prokaryotic heterotrophic production (as proxies of ecosystem functioning) and trophic state in a disturbed and an undisturbed mangrove forests. We report here that disturbed mangrove area showed a loss of 20% of benthic biodiversity, with the local extinction of four Phyla (Cladocera, Kynorincha, Priapulida, Tanaidacea), a loss of 80% of microbial-mediated decomposition rates, of the benthic biomass and of the trophic resources. The results of this study strengthen the need to preserve mangrove forests and to restore those degraded to guarantee the provision of goods and services needed to support the biodiversity and functioning of wide portions of tropical ecosystems.
Introduction
Mangrove ecosystems are of great ecological and economic importance1. They cover 15,000,000 ha2. with high biomass and economic values3. These forests, at the land-sea interface, provide food, breeding grounds and nursery sites for a variety of terrestrial and marine organisms, including many commercial species and juvenile reef fish4,5. Mangrove forests are highly productive ecosystems with rates of primary production equal to those of tropical humid evergreen forests6. They accumulate carbon in tree biomass, and most of this carbon is lost by decomposition and export to adjacent ecosystems7. Mangroves play also a key role in human sustainability and livelihoods, being heavily used for food, timber, fuel and medicine8,9. They offer protection from catastrophic events, such as tsunami, tropical cyclones and tidal bores and can dampen shoreline erosion6,10.
Despite their importance, mangroves are disappearing at a global loss rate of 1–2% per year11, and the loss rate reached 35% during the last 20 years4,12. Climate changes (sea level rise and altered rainfalls) and human activities (urban development, aquaculture, mining, and overexploitation of timber, fish, crustaceans and shellfish) represent major threats for mangrove habitats13–16.
Habitat loss is typically associated with a loss in terms of biodiversity12. Theoretical ecology predicts that biodiversity can influence ecosystems’ functioning, although outputs of correlative investigations and manipulative experiments have provided contrasting results17. The relationships between biodiversity and functioning of marine ecosystems are most often positive18, so that biodiversity loss could result in a reduction of the ecosystem functioning and, consequently, of the ecosystems’ capacity to provide goods and services to humans19–22. This is particularly evident in tropical ecosystems, such as mangroves, which host an important fraction of coastal biodiversity and are among those that will experience the earliest emergence of the impacts of global changes23. Sea level rise represents the main concern considering their tidal nature, but also changes in temperature, salinity, and increases in greenhouse gas concentrations need to be considered3,10. It has been reported that also changes in precipitations and thus in soil water content and salinity, can lead to variations in mangrove species composition and growth10.
In mangrove systems, a large proportion of the algal and leaf biomass are processed by searmid crabs, important keystone engineers in many forests24,25. In addition, in both sediments and tidal waters, organic matter and energy flow is funnelled through a highly diverse, actively growing, microbial loop and subsequently transferred to higher trophic levels through detritivorous, bacterivorous, and deposit feeders inhabiting the benthos25,26. Thus, a biodiversity loss in marine benthic biodiversity, whatever the phylum considered, could cause a variably reduction of ecosystem functions26.
Meiofauna are characterized by high abundance, species richness, short generation time and sensitivity to variations in environmental conditions26,27. In mangrove ecosystem, meiofaunal organisms play key ecological roles: i) accelerating re-mineralization of organic matter and thus nutrient regeneration, ii) stimulating prokaryotic activity and iii) sustaining mangrove food web28–30. All these characteristics, along with their direct contact with sediments as permanent members of the benthos, make them a potential tool for detecting rapid and unequivocal reaction of benthic assemblages to environmental changes.
In the present study, we investigated the effects of mangrove habitat degradation on trophic state and food availability, on biodiversity and on ecosystem processes by comparing an undisturbed with a disturbed mangrove forests (Fig. 1). We used meiofaunal biodiversity as a proxy of the overall benthic biodiversity, and benthic biomass and prokaryotic heterotrophic production (i.e., prokaryotic C incorporation) as proxies of ecosystem functioning. We hypothesised that disturbed mangrove area displays a lower biodiversity and altered ecosystem processes when compared to the undisturbed one.
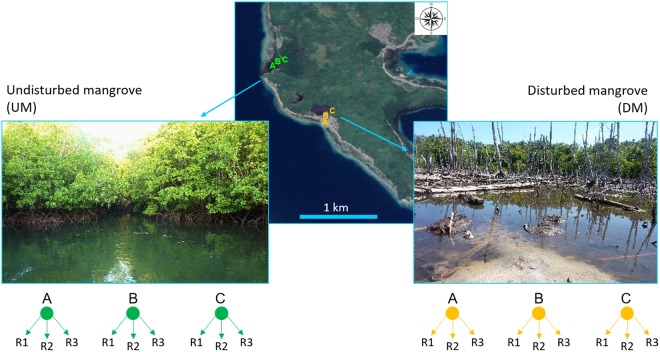
Figure 1.
Sampling area and the location of the two investigated mangroves: Undisturbed Mangrove (UM) and Disturbed Mangrove (DM). Reported are sites (A–C) sampled within each mangrove area. The map was generated using Google Earth Pro (version 7.3.0.3832, 32-bit), https://earth.google.com (Map Data: Google, 2017 DigitalGlobe; Google, 2017 TerraMetrics; Google, 2017 CNES/Airbus), and modified using Microsoft Power Point (version 16.0.8201.2200, 32-bit).
Results
Data on environmental variables (salinity, grain size) and on meiofaunal richness of taxa are reported in Table 1. In both mangrove systems, the redox potential discontinuity (RPD) level is ca. 2 cm below the sediment surface. The results of the PERMANOVA tests revealed the presence of significant differences between disturbed and undisturbed mangroves in most investigated variables (Tables 2–4).
Table 1.
Area, site, salinity, grain size, meiofaunal richness of taxa in the sediments of the undisturbed and disturbed mangroves.
| Area | Site | Salinity | Grain size | Meiofaunal taxa richness |
|---|---|---|---|---|
| n | ||||
| Undisturbed | A | 32 | Sand-mud | 12 |
| B | 30 | Mud-sand | 7 | |
| C | 28 | Mud-sand | 8 | |
| Disturbed | A | 33 | Sand-mud | 8 |
| B | 30 | Mud-sand | 7 | |
| C | 25 | Very fine sand | 6 |
Table 2.
Output of the PERMANOVA analysis carried out to test for differences in total phytopigments, biopolymeric carbon, percentage of chlorophyll-a to biopolymeric carbon and to phytopigments, percentage of proteins to biopolymeric carbon, protein to carbohydrate ratio and biochemical composition of organic matter between undisturbed and disturbed mangrove areas (df = degrees of freedom; MS = mean square; Pseudo-F = F statistic; P(MC) = probability levels obtained from Monte Carlo asymptotic distributions).
| Variable | Source | df | MS | Pseudo-F | P(MC) |
|---|---|---|---|---|---|
| Phytopigments | Area | 1 | 9,05 | 21,15 | ** |
| Site (Area) | 4 | 0,70 | 1,65 | ns | |
| Residual | 12 | 0,43 | |||
| Biopolymeric C | Area | 1 | 12,42 | 93,43 | *** |
| Site (Area) | 4 | 0,75 | 5,62 | ** | |
| Residual | 12 | 0,13 | |||
| Chlorophyll-a to biopolymeric C % | Area | 1 | 5,91 | 81,05 | *** |
| Site (Area) | 4 | 2,55 | 35,01 | *** | |
| Residual | 12 | 0,07 | |||
| Chlorophyll-a to phytopigments % | Area | 1 | 4,05 | 10,70 | ** |
| Site (Area) | 4 | 2,10 | 5,57 | ** | |
| Residual | 12 | 0,38 | |||
| Protein to biopolymeric C % | Area | 1 | 7,65 | 67,36 | *** |
| Site (Area) | 4 | 2,00 | 17,60 | *** | |
| Residual | 12 | 0,11 | |||
| Protein to carbohydrate ratio | Area | 1 | 5,90 | 96,43 | *** |
| Site (Area) | 4 | 2,59 | 42,37 | *** | |
| Residual | 12 | 0,06 | |||
| Biochemical composition | Area | 1 | 43,77 | 28,80 | *** |
| Site (Area) | 4 | 5,75 | 3,78 | ** | |
| Residual | 12 | 1,52 | |||
| Total | 17 |
***P < 0.001; **P < 0.01; ns = not significant.
Table 4.
Output of the PERMANOVA analysis carried out to test for differences in prokaryotic biomass and heterotrophic production between undisturbed and disturbed mangrove areas (df = degrees of freedom; MS = mean square; Pseudo-F = F statistic; P(MC) = probability levels obtained from Monte Carlo asymptotic distributions).
| Variable | Source | df | MS | Pseudo-F | P(MC) |
|---|---|---|---|---|---|
| Prokaryotic biomass | Area | 1 | 13,38 | 824,49 | *** |
| Site (Area) | 4 | 0,86 | 52,72 | *** | |
| Residual | 12 | 0,02 | |||
| Heterotrophic production | Area | 1 | 10,12 | 135,72 | *** |
| Site (Area) | 4 | 1,50 | 20,04 | *** | |
| Residual | 12 | 0,07 | |||
| Total | 17 |
***P < 0.001.
Sedimentary variables
The results of the PERMANOVA carried out between the two mangroves revealed the presence of significant differences for quantity and quality of organic matter (OM) (Table 2). The sedimentary concentrations of chlorophyll-a and total phytopigments were significantly higher in the undisturbed mangrove than in the disturbed one (PERMANOVA, P < 0.01; Fig. 2; Table 2). Chlorophyll-a was four times lower in the disturbed forest (3 ± 1 µg g−1) than in the undisturbed one (12 ± 2 µg g−1), whereas phytopigments were five times higher in the sediments of the undisturbed area (58 ± 11 µg g−1) than in the sediments of the disturbed one (11 ± 7 µg g−1). In the undisturbed mangrove, total phytopigments picked at site B (80 ± 36 µg g−1) and were lower at site C (44 ± 30 µg g−1). In the sediments of disturbed forest, concentration of phytopigments ranged from 3 ± 1 µg g−1 at site A to 26 ± 15 µg g−1 at site C.
Figure 2.
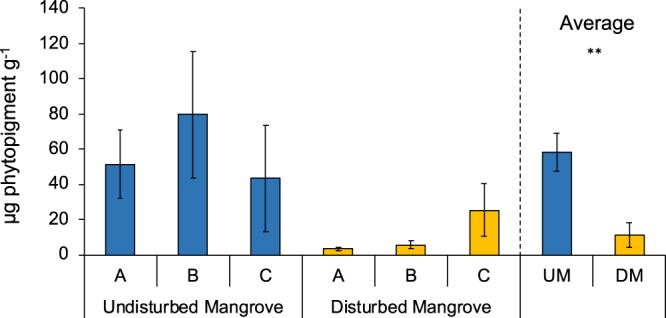
Total phytopigments. Reported are the concentrations of phytopigments in undisturbed and disturbed mangrove areas. Reported are also average values of Undisturbed Mangrove (UM) and Disturbed Mangrove (DM) ± standard error.
The quantity of sedimentary organic matter, in terms of proteins, carbohydrates, lipids, were significantly higher in the sediments of undisturbed mangrove than in the disturbed one (PERMANOVA P < 0.001; Supplementary Figure S1). The concentrations of biopolymeric C was five times higher in the undisturbed (26 ± 1 mg g−1) than in the disturbed forest (6 ± 4 mg g−1) (PERMANOVA, P < 0.001; Fig. 3; Table 2). In the undisturbed area, biopolymeric C ranged from 28 ± 3 mg g−1 at site A to 24 ± 10 mg g−1 at site C. Whereas, in the disturbed area, sedimentary concentrations of biopolymeric C varied from 0.4 ± 0.1 mg g−1 at site A to 15 ± 4 mg g−1 at site C (Supplementary Table S1).
Figure 3.
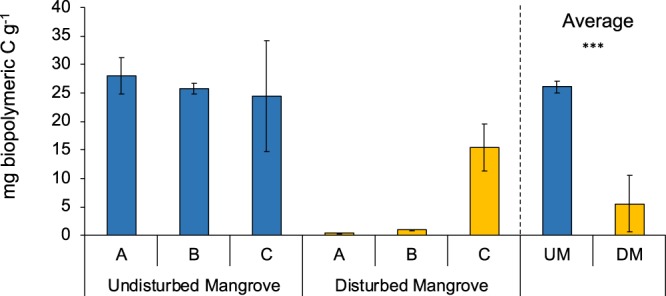
Biopolymeric carbon. Reported are the concentrations of biopolymeric carbon in undisturbed and disturbed mangrove areas. Reported are also average values of Undisturbed Mangrove (UM) and Disturbed Mangrove (DM) ± standard error.
In both the undisturbed and disturbed mangroves, carbohydrate carbon represented the major fraction of biopolymeric C, but at different extend, accounting on average for 68 and 42%, in undisturbed and disturbed mangroves, respectively. Protein carbon represented on average 21% in the undisturbed forest and 42% in the disturbed one. Lipids accounted at a similar percentage in both the areas, representing on average 9 and 8% of biopolymeric C, in the undisturbed and disturbed forests, respectively. Protein fraction of biopolymeric C was double in the disturbed than undisturbed mangrove area and values of the protein to carbohydrate ratio were four times significantly higher in the sediments of disturbed mangrove than in those of the undisturbed one (PERMANOVA, P < 0.001; Table 2).
Faunal diversity and assemblage structure
Data on meiofaunal abundance, richness of taxa and taxonomic composition are shown in Fig. 4a,b. Meiofaunal abundance was significantly higher in the sediments of undisturbed mangroves (2684 ± 1132 ind. 10 cm−2) than in the sediments of disturbed ones (1614 ± 441 ind. 10 cm−2) (PERMANOVA, P < 0.05; Fig. 4a; Table 3). In the undisturbed mangrove area, the total number of meiofaunal individuals was higher at site B (4893 ± 1572 ind. 10 cm−2) than at site A (1148 ± 401 ind. 10 cm−2) and C (2012 ± 389 ind. 10 cm−2). In the disturbed forest, the highest value of meiofaunal abundance was recorded in sediments at site C (2266 ± 1651 ind. 10 cm−2), whereas the lowest one was found at site B (775 ± 402 ind. 10 cm−2) (Supplementary Table S2).
Figure 4.
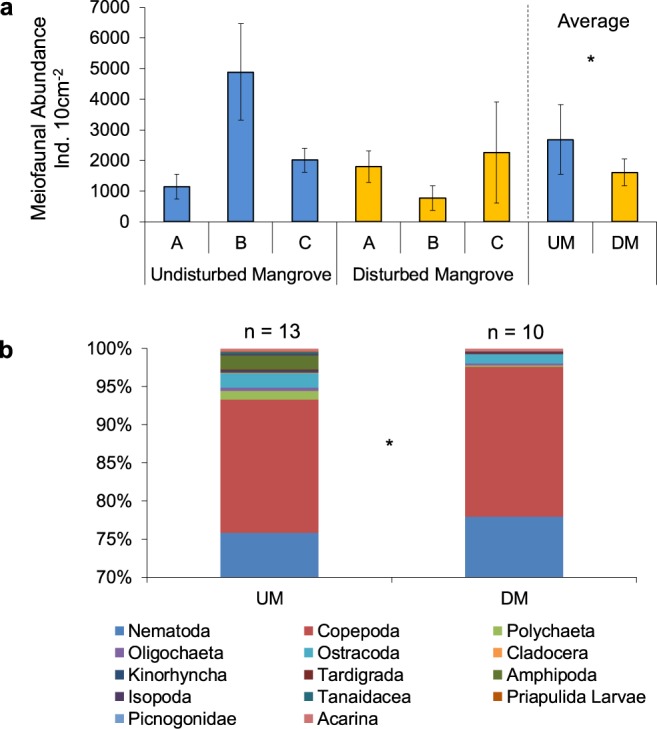
Meiofaunal assemblages. Illustrated are meiofaunal abundance (a) and taxonomic composition (b) with the number of higher taxa found in the sediments of undisturbed and disturbed mangroves. Reported are also average values of Undisturbed Mangrove (UM) and Disturbed Mangrove (DM) ± standard error.
Table 3.
Output of the PERMANOVA analysis carried out to test for differences in total meiofaunal abundance, richness of higher taxa, taxonomic composition between undisturbed and disturbed mangrove areas (df = degrees of freedom; MS = mean square; Pseudo-F = F statistic; P(MC) = probability levels obtained from Monte Carlo asymptotic distributions).
| Variable | Source | df | MS | Pseudo-F | P(MC) |
|---|---|---|---|---|---|
| Abundance | Area | 1 | 5,16E + 06 | 4,35 | * |
| Site (Area) | 4 | 6,64E + 06 | 5,60 | ** | |
| Residual | 12 | 1,19E + 06 | |||
| Richness of higher taxa | Area | 1 | 8,00 | 6,26 | * |
| Site (Area) | 4 | 4,94 | 3,87 | * | |
| Residual | 12 | 1,28 | |||
| Composition as higher taxa | Area | 1 | 1527,60 | 1,51 | ns |
| Site (Area) | 4 | 2768,10 | 2,74 | ** | |
| Residual | 12 | 1010,50 | |||
| Composition as rare taxa | Area | 1 | 9352,90 | 5,51 | ** |
| Site (Area) | 4 | 4453,10 | 2,62 | ** | |
| Residual | 12 | 1697,30 | |||
| Total | 17 |
**P < 0.01; *P < 0.05; ns = not significant.
Overall, 14 taxa have been identified in the two sampling areas, and PERMANOVA tests revealed that the richness of meiofaunal taxa was significantly higher in the sediments of undisturbed mangrove (13 taxa) than in those of disturbed area (10 taxa) (PERMANOVA, P < 0.05; Fig. 4b; Table 3). In both areas and at all sites, nematodes were the dominant taxon (76 and 78% in the undisturbed and disturbed mangroves, respectively), followed by copepods (18 and 20%) and ostracods (2% in both areas). The contribution of all other identified taxa (acarins, amphipods, cladocerans, isopods, kinorinchs, oligochaetes, tanaidaceans, tardigrades, priapulids larvae, pycnogonids, polychaetes) varied from 0 to 11% of the total meiofaunal abundance (Fig. 4b). Amphipods, isopods, oligochaetes, polychaetes, tardigrades were encountered in both sampling areas. Cladocerans, kinorinchs, priapulids larvae, tanaidaceans occurred exclusively in the undisturbed mangrove area, whereas pycnogonids were observed only in the sediments of disturbed one, at site B.
The taxonomic composition of meiofaunal higher taxa did not significantly vary between the two mangroves (PERMANOVA, ns; Table 3). Nevertheless, the results of the pairwise tests showed that the meiofaunal assemblages significantly changed between sites sampled in the undisturbed mangroves (Supplementary Table S2). The taxonomic composition of rare meiofaunal taxa (i.e., excluding nematodes and copepods) varied significantly between the sediments of the undisturbed and disturbed mangroves (PERMANOVA, P < 0.01). This has been confirmed also by the Multi-Dimensional Scaling (MDS) plot and the results of the Canonical Analysis of Principal Coordinates (CAP) analyses (Fig. 5a,b).
Figure 5.
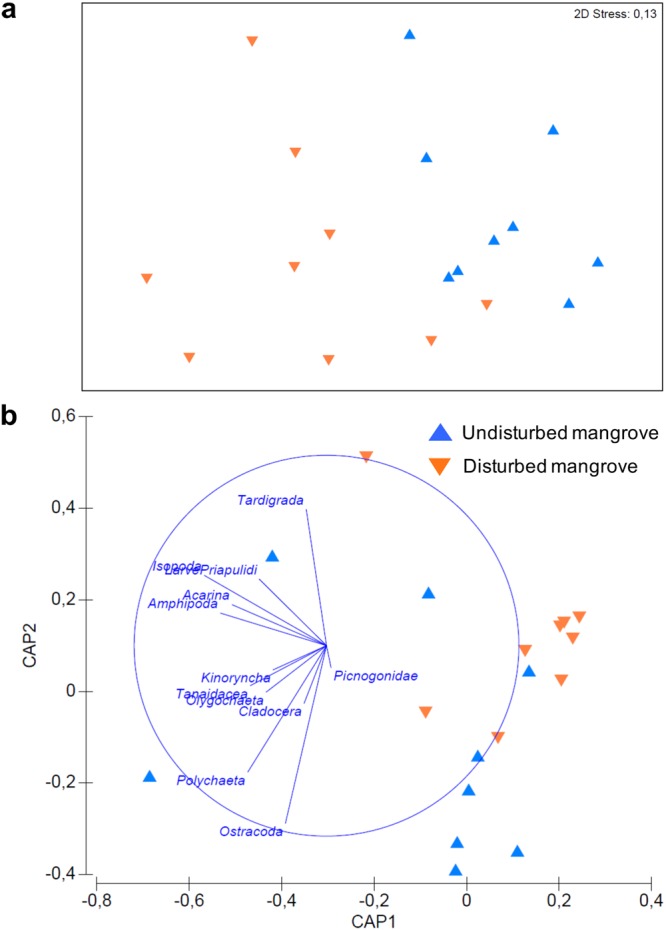
Taxonomic composition of rare meiofaunal taxa. MDS ordination plot (a) and output of canonical analysis of principal coordinates (CAP) (b) illustrating the differences in the composition of meiofaunal assemblages (excluding nematodes and copepods) in the sediments of the two investigated areas.
The SIMPER analysis revealed that the highest dissimilarity in the meiofaunal assemblage occurred among sites in the undisturbed mangrove (52%) than that among the two forests (49%). Whereas, the meiofaunal beta diversity of rare taxa was higher between the two sampling forests (78%) and lower values were found comparing sites among the same sampling area (37% in the disturbed forest and 53% in the undisturbed one). Variations in ostracods and polychaetes abundance were responsible for the observed percentage dissimilarity, as also shown in the plots of canonical analysis of principal coordinates (Fig. 5b).
Biomasses and processes
Prokaryotic biomass was significantly higher in the undisturbed area (17 ± 3 µgC g−1) than in the disturbed one (5 ± 2 µgC g−1). In the undisturbed forest, prokaryotic biomass showed the highest value at site B (21.2 ± 0.6 µgC g−1) and the lowest at site A (12 ± 1 µgC g−1). In the disturbed mangrove area, prokaryotic biomass showed lower values in sediments at site A (2.6 ± 0.3 µgC g−1) and higher values in sediments at site C (8.1 ± 0.4 µgC g−1) (Supplementary Table S3). Prokaryotic heterotrophic production (PHP) were significantly higher in the undisturbed mangrove (7 ± 1 µgC g−1 d−1) than in the disturbed one (1.4 ± 0.4 µgC g−1 d−1) (PERMANOVA, P < 0.001; Fig. 6a,b; Table 4). In the undisturbed mangrove area, PHP values varied from 3.8 ± 0.8 to 10 ± 2 µgC g−1 d−1, at site C and A, respectively. In the disturbed mangrove, values of PHP ranged from 0.5 ± 0.2 to 2.2 ± 0.1 µgC g−1 d−1, at site A and C, respectively (Supplementary Table S3).
Figure 6.
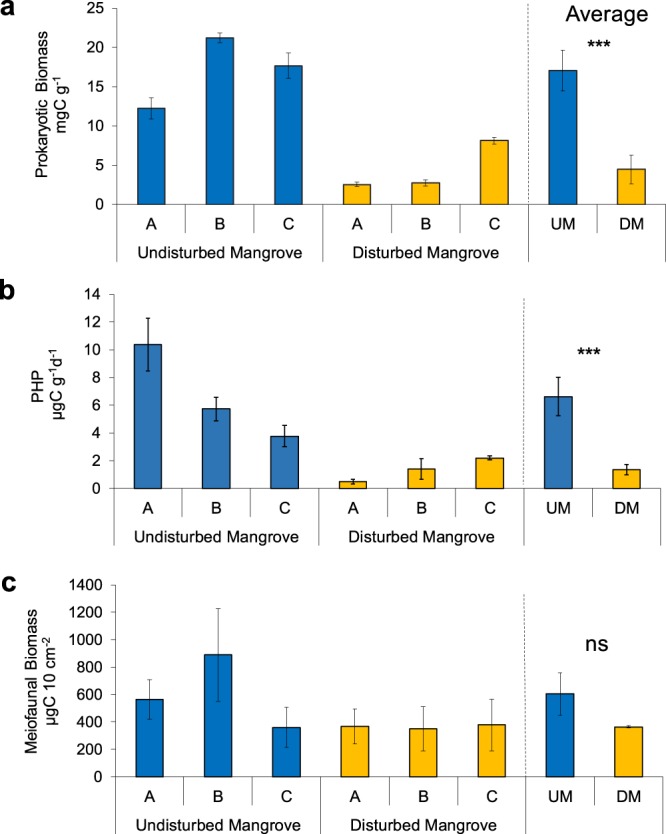
Ecosystem processes. Illustrated are prokaryotic biomass (a), prokaryotic heterotrophic production (µgC g−1 d−1) (b) and meiofaunal biomass (c) in undisturbed and disturbed mangrove areas. Reported are also average values of Undisturbed Mangrove (UM) and Disturbed Mangrove (DM) ± standard error.
Meiofaunal biomass showed double values in the sediments of the undisturbed forest (604 ± 154 µgC 10 cm−2), than in the disturbed one (364 ± 8 µgC 10 cm−2), but they did not significantly vary (Fig. 6c; PERMANOVA, ns). In undisturbed mangrove, values of meiofaunal biomass ranged from 360 ± 147 µgC 10 cm−2 in sediments at site C to 888 ± 339 µgC 10 cm−2 in sediments at site B. In the disturbed forest, meiofaunal biomass varied from 351 ± 161 µgC 10 cm−2 in sediments at site B, to 377 ± 189 µgC 10 cm−2 in sediments at site C.
Discussion
Effect of habitat degradation on trophic state and food availability
In the present study, we found significant differences between the undisturbed and disturbed mangrove areas in terms of quantity and quality of sedimentary organic matter. In the sediments of undisturbed mangrove, the concentration of biopolymeric carbon and total phytopigments, which fall within the range of previous studies26,31,32, were ca 5 times higher than those reported for the sediments of disturbed mangrove area. Our results provide evidence that the main component of OM in mangrove habitat was represented by carbohydrates that usually dominate in all vegetated systems, representing up to 66% of organic carbon in plants26,33. The values of components of organic matter (i.e., proteins, carbohydrates and lipids) as well as the indicators of freshly produced autotrophic biomass (i.e., chlorophyll-a and phaeopigments), which could be the basis of the benthic food webs and sustain the trophic guild of detritus feeders, were several times higher in the sediments of undisturbed mangrove than in those of the disturbed one. The higher proteins:carbohydrates ratio found in the disturbed area could be driven by complex interactions with environmental conditions and biological processes constraining the degradation of proteins. Indeed, it has been recently demonstrated that some labile compounds (i.e., proteins or sugars) can persist not for weeks but for decades because of the requirement of co-metabolism with missing compound, or the presence of microenvironmental conditions that restrict the access (or activity) of enzymes34. Our results clearly indicate that the degradation of the mangrove habitat determined a collapse of the ability of these systems to produce OM. Although this finding was expected, we are now in the position to provide direct evidence that the ability to store organic material in surface sediments was reduced by ca 80% in the disturbed forest when compared to the undisturbed one.
The effects of mangrove habitat degradation on biodiversity
Mangrove sediments usually host a significantly lower meiofaunal abundance when compared to the adjacent soft bottoms systems26,30,35. These differences are generally related to the huge organic enrichment leading to the confinement of the fauna in the top few oxygenated mm of the sediments36. In the present study the lower meiofaunal abundance and diversity we found in the disturbed area cannot be explained by oxygen availability (since the sediments of disturbed mangrove displayed similar oxygen penetration in the sediments) and were likely linked to the extreme conditions (higher temperatures and irradiation) characterizing the disturbed area as well as to the lower organic matter availability.
Moreover, we here report that meiofaunal diversity (in terms of higher taxa) was significantly lower in the disturbed than in the undisturbed mangrove sediments. The dissimilarity between the undisturbed and disturbed sampling areas was related to the loss, in the latter, of Cladocera, Kynorincha, Priapulida and Tanaidacea, which are known to be sensitive to the changes determined by habitat loss37. Some of these taxa, indeed, display habitat preference for the vegetated systems and the colonization/utilization of vegetal debris37. Kynorincha have been also suggested as sentinel of impact, as they disappear in altered or contaminated sediments38,39.
In addition, the undisturbed mangrove area was characterised by a higher spatial variability (as indicated by higher beta diversity found among sites). This finding reflects the presence of several types of substrates, even at smaller spatial scale (tens of cm), such as bare sediments at different decomposition stages, leaf litter and biotic surfaces (e.g., aerial roots, pneumatophores), which lead to the presence of different microenvironments, supporting a more diverse fauna40,41. Such a variability at small spatial scale is common in soft bottom ecosystems, which are typically characterized by high variability in environmental variables, even at the scale of few centimetres42. Overall these findings suggest that habitat degradation led to an average reduction of ca 40% of the abundance of individuals and, at the level of higher taxa, a loss of biodiversity of ca 20%.
Effects of habitat degradation on ecosystem processes
In the present study, we utilised 3 main proxies of ecosystem functioning: prokaryotic biomass, heterotrophic production and meiofaunal biomass, which reflect the ability of the system to perform organic matter degradation and to convert primary production in biomass20. In the disturbed mangrove area, the values of prokaryotic biomass were three times lower than those observed in the undisturbed one. Similarly, prokaryotic heterotrophic production was 5 times lower in the sediments of disturbed mangroves. Meiofaunal biomass reflects the accumulation of organic detritus, the concentrations of labile organic compounds and of vegetal biomass (expressed as concentration of total phytopigments). Higher values of meiofaunal biomass were observed at all sites sampled in the undisturbed area.
Such differences suggest that disturbed sediments can loss ca 80% of their potential to degrade/utilise carbon resources and ca 40% of faunal biomass, when compared to undisturbed ones.
Conclusions
Overall, our results indicate that the sediments of disturbed mangroves, when compared to undisturbed ones, were characterized by altered biogeochemical cycles and a different diagenesis of the organic matter, as pointed out by the significant decrease of sedimentary organic carbon, the potential of OM degradation by microbial metabolism, biomass and biodiversity of meiobenthic assemblages. Since meiofaunal biomass is the main target for the feeding of juvenile reef fishes that are particularly abundant in all mangrove systems37,43, these findings indicate that mangrove degradation could have important consequences also on neighbouring ecosystems and functions. Our study highlights the need of further understanding the effects of anthropogenic and natural stressors on mangrove ecosystems. Additional efforts are also needed to manage human activities within mangrove catchment, to conserve and sustainably use mangroves and, in case of habitat loss, to restore such important ecosystems, in order to ensure the provision of goods and services, and related ecological and economic benefits they provide.
Methods
Study area
This study has been conducted in a small archipelago located at latitude 1°45’ N (Fig. 1; Table 1). The investigated equatorial region hosts different marine ecosystems spanning from mangrove forests to seagrass meadows. The archipelago is impacted by different anthropogenic activities including destructive fishing (e.g., blast fishing and poison fishing) and kind of exploitation of the natural resources. Human impacts in the last years have determined the rapid degradation of wide portions of the mangroves of the island, while other remain pristine and were selected for a comparison (Supplementary Study area).
Sampling strategy
Two sampling areas were compared in this study. The first one is represented by an undisturbed mangrove forest, located distant from human settlements. It was dominated by Rhizophora sp., while Sonneratia alba and Bruguiera spp. were less abundant. The undisturbed area of study was supplied with salt/brackish water from the tide. Some scuba diving and few fishing activities were observed, but there was no evidence of disturbance occurring and the mangroves were not affected. The disturbed area was located near to a local village and characterized by desiccated and dead mangroves. It was dominated by red mangroves, as the undisturbed forest. The disturbed area was affected by anthropogenic activities, i.e., tree cutting, housing settlement, sewages and fishing activities. In both sampling areas, three sites (A, B, C) were selected according to a stratified random sampling design (Fig. 1, Table 1). All sediment samples have been collected by using Plexiglas manual cores (inner diameter 3.6 cm). At each site in each mangrove area, three replicate sediment samples were collected for organic matter and prokaryotic analyses and three replicates were collected for meiofaunal analyses. Most of sampling sites presented comparable characteristics in terms of grain size (mud-sand and sand-mud; Table 1) and sedimentary vertical profile in terms of the depth of the RPD level (ca. 2–3 cm). All sediment samples for the determinations of OM, meiofaunal and prokaryotic assemblages were stored at −20 °C until the analyses in the laboratory, whereas samples for the determination of prokaryotic heterotrophic production were immediately incubated as described below. Despite the storage at −20 °C, all the identified organisms, including the soft-body individuals, resulted well-preserved. In addition, freezing did not damage the morphological features used to recognise organisms at the higher taxonomic levels (order, class or phylum) to which we identified them.
Sedimentary organic matter
Once at laboratory, sediment samples were analysed for OM biochemical composition in terms of phytopigment (chlorophyll-a and phaeopigments), protein, carbohydrate and lipid contents. Proxies of primary organic material associated with primary producers, namely chlorophyll-a and phaeopigments were analysed fluorometrically44. Chlorophyll-a and phaeopigment concentrations were summed up and reported as total phytopigment (CPE) concentrations. Total phytopigment contents were utilized as an estimate of the organic material of algal origin, including the living (chlorophyll-a) and senescent/detrital (i.e., phaeopigments) fractions and converted into C equivalents33,45. Protein, carbohydrate and lipid contents were determined spectrophotometrically33,45. The concentrations were converted to C equivalents and their sum referred as biopolymeric C, BPC33,45.
The percentage contributions of chlorophyll-a to biopolymeric C concentrations and the values of the protein to carbohydrate ratio were then used as descriptors of ageing and nutritional quality of OM in the sediment33. The percentage contribution of total chlorophyll-a to biopolymeric C is an estimate of the freshness of the organic material deposited in the sediment: since photosynthetic pigments and their degradation products are assumed to be labile compounds in a trophodynamic perspective, the lower their contribution to sediment organic C the more aged the organic material46. Since N is the most limiting factor for heterotrophic nutrition and proteins are N-rich products, the protein to biopolymeric C and the protein to carbohydrate ratios are indicative of the nutritional value of the organic matter33,46.
Prokaryotic abundance and biomass
Total prokaryotic abundance was determined by epifluorescence microscopy47. Sediment samples were treated three times for 1 min by ultrasounds (Branson Sonifier 2200, 60 W) after addition of 0.2 µm pre-filtered tetrasodium pyrophosphate solution at a final concentration of 5 mM, then properly diluted before filtration onto 0.2 µm pore-size Nuclepore black filters (Whatman). Each filter was then stained with 20 µl of SYBR Green I (Sigma Chemicals, previously diluted 1:20 with 0.2 µm pre-filtered Milli-Q water), washed twice with 3 ml sterilized Milli-Q water and mounted onto microscope slide. Filters were analyzed using epifluorescence microscopy (Zeiss Axioskop 2MOT, magnification 1,000×). At least 20 microscope fields and 400 cells were respectively observed and counted for each filter48. Prokaryotic abundance was expressed as cells per g of dry sediment, after desiccation at 60 °C for 24 h45. Prokaryotic biomass was determined based on cell size, converted into bio-volume, assuming 310 fg C m3 as a conversion factor, following standard inter-calibration with Scanning Electron Microscope (SEM)45,48,49.
Prokaryotic Heterotrophic Production
3[H]–leucine incorporation method was used for the determination of PHP, according to the procedure previously described45,48,50. Sediment samples were added with 0.2-µm pre-filtered seawater, containing ³[H]-leucine (68 Ci mmol−1; final 0.5–1.0 µM), then incubated in the dark, at in-situ temperature. To define the linearity and the saturation level of the ³[H]-leucine incorporation, time-course experiments over 6 h and concentration-dependent incorporation experiments (from 0.05 μM to 5.0 μM leucine) were also carried out. Blanks (n = 3) for each sediment sample were added with ethanol immediately before3[H]-leucine addition. After incubation, samples were supplemented with ethanol (80%), centrifuged, washed again two times with ethanol (80%), and the sediment was re-suspended in ethanol (80%) and filtered onto polycarbonate filters (0.2 µm pore size; vacuum <100 mm Hg). Afterward, each filter was washed four times with 2 ml of 5% TCA, then transferred into a Pyrex tube containing 2 ml of NaOH (2 M) and incubated for 2 h at 100 °C. After centrifugation at 800 × g, 1 ml of supernatant fluid was transferred to vials containing the appropriate scintillation liquid. A liquid scintillation counter (PerkinElmer-Packard Tri-Carb 2100 TR) was used to measure the incorporated radioactivity in the sediment samples48,50. The prokaryotic heterotrophic production was calculated by equation (1):
| 1 |
where: LI is the leucine incorporation rate (mol g−1 h−1), 131.2 is the molecular weight of leucine, %Leu is the fraction of leucine in a protein (0.073), C/protein is the ratio of cellular carbon to protein (0.86), and ID is the isotope dilution, assuming a value of 2.
Meiofaunal abundance, taxon diversity and biomass
Each sediment sample was treated with ultrasound (for 1 min 3 times, with 30 s intervals) to detach organisms from the grain particle surface and, then, carefully and gently sieved through a 1000-µm and a 20-µm mesh net to retain the smallest organisms. The fraction remaining on the latter sieve was re-suspended and centrifuged three times with Ludox HS 40 (final density of 1.18 g cm−3)51. Subsequently, sediment samples have been carefully checked to search for remnant organisms. After staining with Rose Bengal (0.5 gL−1), all specimens were counted and classified per taxon, under a stereomicroscope, using a Delfuss cuvette26. Meiofaunal taxa representing <1% of the total meiofaunal abundance were defined as rare taxa52. Meiofaunal biomass was assessed by bio-volumetric measurements of all retrieved specimens. Nematode biomass was calculated from their biovolume, using the Andrassy’s53 formula (V = L × W2 × 0.063 × 10−5, in which body length, L, and width, W, are expressed in µm). Body volumes of all other taxa were derived from measurements of body length (L, in mm) and width (W, in mm), using the formula V = L × W2 × C, where C is the conversion factor specific for each meiofaunal taxon, used to convert L × W2 to body volume, according to models relating body dimensions and volume54. Each body volume was multiplied by an average density of 1.13 g cm−3 to obtain the biomass. The carbon content was considered to be 40% of the dry weight54.
Statistical analyses
To assess differences between the two mangrove areas and sites, we applied uni- and multivariate distance-based permutational analyses of variance (PERMANOVA). All the statistical analyses were carried out using the same sampling design, considering two factors as main sources of variance: Area (fixed, two levels: undisturbed and disturbed mangroves) and Site (fixed, three levels: A, B, C, nested in Area).
Univariate distance-based permutational analyses of variance (PERMANOVA) were used to assess the variability in the OM compounds contents, total meiofaunal abundance and biomass, prokaryotic biomass and heterotrophic production55,56. The variability in the biochemical composition and nutritional quality of OM, taxonomic composition of meiofaunal communities were assessed using distance-based permutational multivariate analyses of variance (PERMANOVA). The analyses were carried out on Euclidean distances (for organic matter, prokaryotic and meiofaunal abundance and biomass) or Bray–Curtis similarity matrices (for meiofaunal taxonomic composition) of previously normalized (OM) or untransformed (faunal) data, using 999 permutations of the residuals under a reduced model. Bray-Curtis distance matrix was used for meiofaunal taxonomic composition, because for differences in community structure and composition, the semi-metric Bray–Curtis measure57 of ecological distance is preferred over metric measure55, like Euclidean distance57–61. Significant differences were investigated using a posteriori pair-wise test. P values in the PERMANOVA and pairwise tests were obtained from Monte Carlo asymptotic distributions, because of the restricted number of unique permutations62.
To visualize differences between areas in the meiofaunal community, Multidimensional scaling (MDS) and bi-plots after a CAP were prepared63.
To assess the percentage of dissimilarity64 in the meiofaunal assemblage composition among the sampling areas for (i) higher taxa and (ii) rare taxa and to identify the meiofaunal taxa most responsible for the observed differences, SIMPER analyses were carried out. A ranked matrix of Bray–Curtis similarities, was used as input for the SIMPER tests.
The PERMANOVA, MDS, CAP, SIMPER analyses were performed using the routines included in the software PRIMER 6+65,66.
Electronic supplementary material
Acknowledgements
This study has been conducted in the framework of the Project MERCES funded from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 689518, and supported by the Fondi di Ateneo UNIVPM.
Author Contributions
R.D. conceived the idea; L.C. R.D. designed sampling design; L.C. and B.G. collected the samples; L.C., E.R., M.L.M., E.R., S.G. analysed the data; L.C. and R.D. drafted the manuscn. All authors contributed critically to the drafts and gave final approval for publication.
Data Availability
All data generated and analysed during this study are included in this published article and its Supplementary Information file.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Laura Carugati, Email: l.carugati@univpm.it.
Roberto Danovaro, Email: r.danovaro@univpm.it.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31683-0.
References
- 1.Lee SY, et al. Ecological role and services of tropical mangrove ecosystems: a reassessment. Global Ecol. Biogeogr. 2014;23:726–743. doi: 10.1111/geb.12155. [DOI] [Google Scholar]
- 2.Giri C. Status and distribution of mangrove forests of the world using earth observation satellite data. Global Ecol. Biogeogr. 2011;20:154–159. doi: 10.1111/j.1466-8238.2010.00584.x. [DOI] [Google Scholar]
- 3.Alongi DM. The Impact of Climate Change on Mangrove Forests. Curr. Clim. Change Rep. 2015;1:30–39. doi: 10.1007/s40641-015-0002-x. [DOI] [Google Scholar]
- 4.FAO (Food and Agriculture Organization of the United Nations) In The world’s mangroves 1980–2005. FAOForestry Paper 153. (Forest Resources Division, FAO, Rome, 2007).
- 5.Igulu MM, et al. Mangrove Habitat Use by Juvenile Reef Fish: Meta-Analysis Reveals that Tidal Regime Matters More than Biogeographic Region. PLoS ONE. 2014;9(12):e114715. doi: 10.1371/journal.pone.0114715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alongi DM. Carbon Cycling and Storage in Mangrove Forests. Annu. Rev. Mar. Sci. 2014;6:195–219. doi: 10.1146/annurev-marine-010213-135020. [DOI] [PubMed] [Google Scholar]
- 7.Alongi DM. Carbon sequestration in mangrove forests. Carbon Manag. 2012;3:313–22. doi: 10.4155/cmt.12.20. [DOI] [Google Scholar]
- 8.Alongi DM. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002;29:331–49. doi: 10.1017/S0376892902000231. [DOI] [Google Scholar]
- 9.Saenger, P. In Mangrove ecology, siviculture and conservation. (Kluwer Academic Publishers, Dordrecht, 2003).
- 10.Alongi DM. Mangrove Forests: Resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast. Shelf S. 2008;76:1–13. doi: 10.1016/j.ecss.2007.08.024. [DOI] [Google Scholar]
- 11.Spalding, M., Kainuma, M. & Collins, L. In World Atlas of Mangroves. A collaborative project of ITTO, ISME, FAO, UNEP-WCMC, UNESCO-MAB and UNU-INWEH. (Earthscan, London, 2010).
- 12.Polidoro BA, et al. The loss of species: mangrove extinction risk and geographic areas of global concern. PLoS ONE. 2010;5:e10095. doi: 10.1371/journal.pone.0010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLeod, E. & Salm, R. V. In Managing Mangroves for Resilience to Climate Change. (IUCN, Gland, Switzerland, 2006).
- 14.Gilman E, Ellison J, Duke N, Field C. Threats to mangroves from climate change and adaptation options: a review. Aquat. Bot. 2008;89:237–250. doi: 10.1016/j.aquabot.2007.12.009. [DOI] [Google Scholar]
- 15.Van Lavieren, H. et al. In Securing the future of mangroves. A Policy Brief. UNU-INWEH, UNESCO MAB with ISME, ITTO, FAO, UNEP WCMC and TNC. (UNU-INWEH, Canada, 2012).
- 16.Ellison JC, Zouh I. Vulnerability to Climate Change of Mangroves: Assessment from Cameroon. Cent. Afr. Biol. 2012;1:617–638. doi: 10.3390/biology1030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loreau M. Linking biodiversity and ecosystems: towards a unifying ecological theory. Proc. Trans. R. Soc. B. 2010;365:49–60. doi: 10.1098/rstb.2009.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardinale BJ, et al. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443:989–992. doi: 10.1038/nature05202. [DOI] [PubMed] [Google Scholar]
- 19.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 20.Danovaro R, et al. Exponential decline of deep-sea ecosystem functioning linked to benthic biodiversity loss. Curr. Biol. 2008;18:1–8. doi: 10.1016/j.cub.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 21.Bulling MT, et al. Marine biodiversity-ecosystem functions under uncertain environmental futures. Philos. T. Roy. Soc. B. 2010;365:2107–2116. doi: 10.1098/rstb.2010.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 23.Solan M, Raffaelli DG, Paterson DM, White PCL, Pierce GJ. Marine biodiversity and ecosystem function: empirical approaches and future research needs. Mar. Ecol. Progr. Ser. 2006;311:175–178. doi: 10.3354/meps311175. [DOI] [Google Scholar]
- 24.Cannicci SD, et al. Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquat. Bot. 2008;89:186–200. doi: 10.1016/j.aquabot.2008.01.009. [DOI] [Google Scholar]
- 25.Nagelkerken I, et al. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat. Bot. 2008;89:155–185. doi: 10.1016/j.aquabot.2007.12.007. [DOI] [Google Scholar]
- 26.Pusceddu A, Gambi C, Corinaldesi C, Scopa M, Danovaro R. Relationships between Meiofaunal Biodiversity and Prokaryotic Heterotrophic Production in Different Tropical Habitats and Oceanic Areas. PLoS One. 2014;9(3):e91056. doi: 10.1371/journal.pone.0091056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto TK, et al. Nematode diversity in different microhabitats in a mangrove region. Mar. Ecol. 2013;34(3):257–268. doi: 10.1111/maec.12011. [DOI] [Google Scholar]
- 28.Tietjen JH, Alongi DM. Population growth and effects of nematodes on nutrient regeneration and bacteria associated with mangrove detritus from northeastern Queensland (Australia) Mar. Ecol. Prog. Ser. 1990;68:169–179. doi: 10.3354/meps068169. [DOI] [Google Scholar]
- 29.Coull BC. Role of meiobenthos in estuarine soft-bottom habitats. Aust. J. Ecol. 1999;24:327–343. doi: 10.1046/j.1442-9993.1999.00979.x. [DOI] [Google Scholar]
- 30.Sahoo G, Suchiang SR, Anzari ZA. Meiofauna-Mangrove interaction: a pilot study from a tropical mangrove habitat. Cahiers Biol. Mar. 2013;54:349–358. [Google Scholar]
- 31.Joseph MM, Ratheesh Kumar CS, Gireesh Kumar TR, Renjith KR, Chandramohanakumar N. Biogeochemistry of surficial sediments in the intertidal systems of a tropical environment. Chem. Ecol. 2008;24(4):247–258. doi: 10.1080/02757540802119871. [DOI] [Google Scholar]
- 32.Fontana LF, et al. Geomicrobiology of cores from Suruí Mangrove - Guanabara Bay - Brazil. Mar. Pollut. Bull. 2010;60(10):1674–1681. doi: 10.1016/j.marpolbul.2010.06.049. [DOI] [PubMed] [Google Scholar]
- 33.Pusceddu A, Dell’Anno A, Fabiano M, Danovaro R. Quantity and bioavailability of sediment organic matter as signatures of benthic trophic status. Mar. Ecol. Progr. Ser. 2009;375:41–52. doi: 10.3354/meps07735. [DOI] [Google Scholar]
- 34.Schmidt MW, et al. Persistence of soil organic matter as an ecosystem property. Nature. 2011;478(7367):49. doi: 10.1038/nature10386. [DOI] [PubMed] [Google Scholar]
- 35.Alongi DM. The role of soft-bottom benthic communities in tropical mangrove and coral reef ecosystems. Rev. Aquat. Sci. 1989;1:243–280. [Google Scholar]
- 36.Somerfield P, Gee J, Aryuthaka C. Meiofaunal Communities in a Malaysian Mangrove Forest. J. Mar. Biol. Assoc. UK. 1998;78(3):717–732. doi: 10.1017/S0025315400044738. [DOI] [Google Scholar]
- 37.Giere, O., In Meiobenthology. The microscopic motile fauna of aquatic sediments (2nd edn. Springer-Verlag) (Berlin, 2009).
- 38.Grego M, De Troch M, Forte J, Malej A. Main meiofauna taxa as an indicator for assessing the spatial and seasonal impact of fish farming. Mar. Pollut. Bull. 2009;58(8):1178–1186. doi: 10.1016/j.marpolbul.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Mirto S, et al. Fish farm impact on metazoan meiofauna in the Mediterranean Sea: analysis of regional vs. habitat effects. Mar. Environ. Res. 2010;69(1):38–47. doi: 10.1016/j.marenvres.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Netto SA, Gallucci F. Meiofauna and macrofauna communities in a mangrove from the Island of Santa Catarina, South Brazil. Hydrobiologia. 2003;505:159–170. doi: 10.1023/B:HYDR.0000007304.22992.b2. [DOI] [Google Scholar]
- 41.Armenteros M, et al. Spatial and temporal variations of meiofaunal communities from the western sector of the Gulf of Batabano, Cuba. I. Mangrove systems. Estuar. Coasts. 2006;29(1):124–132. doi: 10.1007/BF02784704. [DOI] [Google Scholar]
- 42.Danovaro R, et al. Small-scale distribution of bacteria, enzymatic activities and organic matter in coastal sediments. Microbial Ecol. 2001;42(2):177–185. doi: 10.1007/s002480000109. [DOI] [PubMed] [Google Scholar]
- 43.Leguerrier D, et al. Numerical analysis of the food web of an intertidal mudflat ecosystem on the Atlantic coast of France. Mar. Ecol. Progr. Ser. 2003;246:17–37. doi: 10.3354/meps246017. [DOI] [Google Scholar]
- 44.Lorenzen CJ, Jeffrey SW. Determination of chlorophyll and phaeopigments spectrophotometric equations. Limnol. Oceanogr. 1980;12:343–346. doi: 10.4319/lo.1967.12.2.0343. [DOI] [Google Scholar]
- 45.Danovaro, R. In Methods for the study of deep-sea sediments, their functioning and biodiversity (eds CRC Press, Taylor & Francis Group, Boca Raton, 2010).
- 46.Pusceddu A, et al. Organic matter in sediments of canyons and open slopes of the Portuguese, Catalan, Southern Adriatic and Cretan Sea margins. Deep-Sea Res. I. 2010;57(3):441–457. doi: 10.1016/j.dsr.2009.11.008. [DOI] [Google Scholar]
- 47.Danovaro R, Dell’Anno A, Trucco A, Vannucci S. Determination of virus abundance in marine sediments. Appl. Environ. Microb. 2001;67:1384–1387. doi: 10.1128/AEM.67.3.1384-1387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rastelli E, et al. Impact of CO2 leakage from sub-seabed carbon dioxide capture and storage (CCS) reservoirs on benthic virus–prokaryote interactions and functions. Front. Microbiol. 2015;6:935. doi: 10.3389/fmicb.2015.00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fry, J. C. Determination of biomass in Methods in Aquatic Bacteriology (ed. Austin, B.), 27–72 (John Wiley and Sons LTD, Chichester, 1990).
- 50.Van Duyl FC, Kop AJ. Bacterial production in north sea sediment: clues to seasonal and spatial variations. Mar. Biol. 1994;120:323–337. doi: 10.1007/BF00349694. [DOI] [Google Scholar]
- 51.Heip C, Vincx M, Vranken G. The ecology of marine nematodes. Oceanogr. Mar. Biol. Ann. Rev. 1985;23:399–489. [Google Scholar]
- 52.Bianchelli S, Gambi C, Zeppilli D, Danovaro R. Metazoan meiofauna in deep-sea canyons and adjacent open slopes: a large-scale comparison with focus on the rare taxa. Deep-Sea Res. I. 2010;57:420–433. doi: 10.1016/j.dsr.2009.12.001. [DOI] [Google Scholar]
- 53.Andrassy I. The determination of volume and weight of nematodes. Acta Zool. 1956;2:1–15. [Google Scholar]
- 54.Feller, R. J. & Warwick, R. M. In Energetics. Introduction to the study of meiofauna (ed. Higgins R. P. & Thiel H.) 181–196 (Smithsonian Institute Press, London, 1988).
- 55.Anderson MJ. Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 2001;58:626–639. doi: 10.1139/f01-004. [DOI] [Google Scholar]
- 56.McArdle BH, Anderson MJ. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology. 2001;82:290–297. doi: 10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2. [DOI] [Google Scholar]
- 57.Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957;27:325–49. doi: 10.2307/1942268. [DOI] [Google Scholar]
- 58.Odum EP. Bird populations of the Highlands (North Carolina) Plateau in relation to plant succession and avian invasion. Ecology. 1950;31:587–605. doi: 10.2307/1931577. [DOI] [Google Scholar]
- 59.Hajdu LJ. Graphical comparison of resemblance measures in phytosociology. Vegetatio. 1981;48:47–59. doi: 10.1007/BF00117360. [DOI] [Google Scholar]
- 60.Faith DP, Minchin PR, Belbin L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio. 1987;69:57–68. doi: 10.1007/BF00038687. [DOI] [Google Scholar]
- 61.Clarke KR. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993;18:117–43. doi: 10.1111/j.1442-9993.1993.tb00438.x. [DOI] [Google Scholar]
- 62.Anderson MJ, Robinson J. Generalised discriminant analysis based on distances. Aust. New Zeal. J. Stat. 2003;45:301–318. doi: 10.1111/1467-842X.00285. [DOI] [Google Scholar]
- 63.Anderson MJ, Willis TJ. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology. 2003;84:511–525. doi: 10.1890/0012-9658(2003)084[0511:CAOPCA]2.0.CO;2. [DOI] [Google Scholar]
- 64.Gray JS. The measurement of marine species diversity, with an application to the benthic fauna of the Norwegian continental shelf. J. Exp. Mar. Biol. Ecol. 2000;250:23–49. doi: 10.1016/S0022-0981(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 65.Clarke, K. R. & Gorley, R. N. In PRIMER v6: User Manual/Tutorial (PRIMER-E, Plymouth, 2006).
- 66.Anderson, M. J. Gorley, R. N. & Clarke, K. R. In PERMANOVA+ for PRIMER: guide to software and statistical methods. (PRIMER-E, Plymouth, UK., 2008).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analysed during this study are included in this published article and its Supplementary Information file.