Abstract
Plant defence responses to various biotic stresses via systemic acquired resistance (SAR) are induced by avirulent pathogens and chemical compounds, including certain plant hormones in volatile form, such as methyl salicylate and methyl jasmonate. SAR refers to the observation that, when a local part of a plant is exposed to elicitors, the entire plant exhibits a resistance response. In the natural environment, plants are continuously exposed to avirulent pathogens that induce SAR and volatile emissions affecting neighbouring plants as well as the plant itself. However, the underlying mechanism has not been intensively studied. In this study, we evaluated whether plants “memorise” the previous activation of plant immunity when exposed repeatedly to plant defensive volatiles such as methyl salicylate and methyl jasmonate. We hypothesised that stronger SAR responses would occur in plants treated with repeated applications of the volatile plant defence compound MeSA than in those exposed to a single or no treatment. Nicotiana benthamiana seedlings subjected to repeated applications of MeSA exhibited greater protection against Pseudomonas syringae pv. tabaci and Pectobacterium carotovorum subsp. carotovorum than the control. The increase in SAR capacity in response to repeated MeSA treatment was confirmed by analysing the defence priming of the expression of N. benthamiana Pathogenesis-Related 1a (NbPR1a) and NbPR2 by quantitative reverse-transcription PCR compared with the control. We propose the concept of plant memory of plant defence volatiles and suggest that SAR is strengthened by the repeated perception of volatile compounds in plants.
Keywords: methyl salicylate, plant immunity, plant memory, SAR, VOC
INTRODUCTION
Plants respond to pathogen attack by activating both local and systemic defences aimed at inhibiting the growth and spreading of the pathogen (Fu and Dong, 2013; Shah and Zeier, 2013). The defence response, systemic acquired resistance (SAR), develops following localised foliar infection by diverse avirulent pathogens and is expressed systemically (Fu and Dong, 2013; Hammond-Kosack and Jones, 1996; Shah and Zeier, 2013). During this process, leaves become primed to activate more rapid and/or stronger defence responses following attack by pathogens or insects or in response to abiotic stress upon secondary infection (Conrath et al., 2015; Martinez-Medina et al., 2016). SAR provides the benefits of enhanced protection without the costs associated with constitutive expression of stress-related genes (Bruce et al., 2007; Heil and Baldwin, 2002; Jung et al., 2009). This plant defence priming mechanism can be elicited by the exogenous application of chemicals as well as exposure to stress cues (Lyon, 2007). This plant response was recently attributed to “plant memory” (Crisp et al., 2016), a theory that has also been referred to as “plant stress memory” and “defence priming” (Crisp et al., 2016). In the natural state, plants are continuously exposed to avirulent pathogens that cause SAR and volatile emissions that affect neighbouring plants as well as the plant itself. However, little is known about the underlying mechanism.
Leaves infected with SAR-inducing bacteria produce modified compounds that confer disease resistance to systemic tissues (previously unexposed plants) (Fu and Dong, 2013; Van Bel and Gaupels, 2004), which indicates that a mobile systemic signal(s) is involved in SAR (Gao et al., 2015; Park et al., 2007; Shah et al., 2014). Previously, salicylic acid (SA) was postulated to be this mobile signal because it induces defence responses when applied to plants, moves systemically, is found in phloem exudates of infected leaves and is required in systemic tissue for SAR (Jung et al., 2009; Park et al., 2007). However, later grafting studies showed that infected, SA-deficient rootstocks can trigger SAR in wild-type scions, implying that SA is not a mobile SAR signal (Vernooij et al., 1994). SA is synthesised via the shikimic acid pathway, which bifurcates into two branches after the biosynthesis of chorismic acid. Both branches contribute to SA biosynthesis and are required for SAR (Wildermuth et al., 2001). SA accumulation alone is insufficient to induce SAR (Cameron et al., 1999). For instance, exogenous application of the 3C sugar alcohol glycerol-3-phosphate (G3P) or dicarboxylic acid azelaic acid (AzA) to induce SAR in wild-type plants does not induce SA accumulation. However, G3P or AzA cannot confer SAR in ics1 (sid2) mutant plants, which exhibit a significant reduction in basal- and pathogen-induced SA. Therefore, while SA is clearly important for SAR, the accumulation of SA is not sufficient to establish SAR. Moreover, SA accumulates to various levels in the distal tissues of SAR-induced plants, but there is no evidence that this accumulation is essential for SAR. Finally, SAR development is curbed when the expression of SA methyl transferase (which converts SA to methyl salicylate [MeSA]) is prevented (Ludwig-Müller et al., 2015), and MeSA treatment of lower leaves directly induces SAR in upper, untreated leaves (Park et al., 2007).
Since 2007, when MeSA was found to function as a mobile SAR signal, up to 13 compounds have been shown to move throughout the plant as SAR signals via the vascular system. Representative molecules include the abietane diterpenoid dehydroabietinal (DA), the lysine catabolite pipecolic acid (Pip), G3P and AzA (Chanda et al., 2011; Chaturvedi et al., 2012; Jung et al., 2009; Mandal et al., 2012; Návarová et al., 2012). These four signalling molecules are related to SA accumulation/signalling. Long-distance signalling by specific volatile organic compounds (VOCs) such as the SA-derivative MeSA has also been reported. Notably, green-leaf volatiles and herbivore-induced VOCs induce resistance from locally damaged leaves to the entire plant (Kost and Heil, 2006; Yi et al., 2009). These VOCs can also affect other plants by moving through the air. This so-called “plant–plant communication” can occur between taxonomically unrelated plants. For instance, lima bean (Phaseolus lunatus) and tobacco (Nicotiana tabacum) plants release modified VOCs upon SAR induction (Shulaev et al., 1997; Yi et al., 2009). Among the modified VOCs, nonanal and MeSA were detected in the headspaces of plants treated with the chemical SAR trigger benzothiadiazole (BTH) and Tobacco mosaic virus-challenged plants, respectively, resulting in the reduced appearance of symptoms in exposed neighbouring plants (Shulaev et al., 1997; Yi et al., 2009). The reduction of symptoms in neighbouring plants exposed to plants that release modified VOCs upon SAR induction is effective within a distance of 50 cm under field conditions (Heil and Adame-Álvarez, 2010). Pharmaceutical application of 1 mg/L nonanal and 4 mg/L MeSA to plants after an exposure of over 24 h increases plant defence against Pseudomonas syringae (Girón-Calva et al., 2012). However, in nature, mixed VOCs from “emitter” plants continuously affect “receiver” plants. A few studies have focused on these two issues, i.e., the effectiveness of continuous and mixed VOC treatments. However, no studies have investigated the effects of repeated application of plant volatile compounds on defence priming nor whether the repeated application of plant defensive volatiles such as nonanal and MeSA elicits stronger SAR than single treatments. This phenomenon would resemble memory responses to the previous activation of plant immune responses by biological and chemical triggers (Crisp et al., 2016).
In this study, we evaluated whether plants memorise the previous activation of plant immunity when exposed repeatedly to plant defensive volatiles such as MeSA and methyl jasmonate (MeJA). To investigate volatile-induced plant memory, we designed a new experimental system and obtained phenotypic evidence for VOC-mediated defence priming in wild tobacco (Nicotiana benthamiana) and Arabidopsis thaliana by the repeated application of plant defence volatiles. MeSA and MeJA were selected as representative plant defence VOCs. To investigate the effects of repeated VOC exposure on plant memory, we assessed SAR after zero, one and two applications of MeSA. Plants treated twice with MeSA showed greater SAR against the biotrophic wildfire pathogen, P. syringae pv. tabaci (Pta), and higher expression of defence-related genes (N. benthamiana Pathogenesis-Related 1a (NbPR1a) and NbPR2) than those in the other treatment groups, while this treatment had no effect against the necrotrophic pathogen Pectobacterium carotovorum subsp. carotovorum (Pcc). In addition, subsequent MeJA treatment reduced the SAR capacity induced by pre-treatment with MeSA, indicating the existence of crosstalk between SA and jasmonic acid (JA) signalling in this system. To validate VOC-mediated SAR genetically, we employed the null Arabidopsis SAR mutant non-expresser of PR1 (npr1), which exhibited a lack of any SAR response. The npr1 mutant plant did not enhance the SAR when treated with plant VOCs twice indicating that SAR occurs though the plant recognition of treated VOC. Overall, our findings demonstrate that plants memorise the first application of a SAR trigger. In addition, our results lay the foundation for the use of plant volatile memory VOCs to protect plant health.
MATERIALS AND METHODS
Plant preparation and treatments
Seeds of Arabidopsis npr1-1 (which does not express PR proteins and SAR) were obtained from Dr. Xinnian Dong, Duke University, USA (Cao et al., 1997). Seeds of Nicotiana benthamiana, wild-type Arabidopsis thaliana Col-0 and the SAR mutant npr1 were surface sterilised with 3% sodium hypochlorite, washed four times with sterilised distilled water and maintained at 23°C under a 16 h:8 h light:dark cycle for 3 days until germination on half-strength Murashige and Skoog salt (MS) medium containing 0.6% agar and 1.5% sucrose, pH 5.8. 1. Transplantation: Four N. benthamiana and Arabidopsis seedlings were transferred to Incu Tissue culture vessels (72 × 72 × 100 mm, SPL). 2. First VOC treatment: Two weeks after transplantation, filter paper discs (8 mm, LOT no. 31210691, ADVATEC) that had been soaked in 100 μl of 1 mM MeSA were placed on the caps of the e-tubes in the Incu Tissue vessels (Song et al., 2013). 3. Removal of the first VOC application: One week after the first exposure, the filter paper discs were removed from the vessels. Residual VOC was removed by opening the vessels for 10 min. 4. Second VOC treatment: Fourteen days after the first treatment, N. benthamiana and Arabidopsis plants (5 weeks old) were exposed to a second treatment with 100 μl of 1 mM MeSA, 1 mM MeJA or water (Song et al., 2013; Yi et al., 2009). 5. Pathogen challenge following VOC treatment: Pseudomonas syringae pv. tabaci (Pta) and Pectobacterium carotovorum subsp. carotovorum Pcc (SCC1) (for N. benthamiana infection) were grown on solid King’s B and LB medium containing 100 μg/ml rifampicin at 30°C for 2 days, scraped off the plates, re-suspended in 10 mM MgCl2 and adjusted to the proper concentration for further experiments (Song et al., 2013). Pseudomonas syringae pv. tomato Pto (DC3000) (for Arabidopsis infection) was grown on solid King’s B medium containing 100 μg/mL rifampicin at 30°C for 2 days. To challenge the plants with pathogen, five leaves from each of four plants (6 weeks old; treatment 1: MeSA-MeSA; treatment 2: Water-MeSA; treatment 3: MeSA-MeJA; treatment 4: Water-Water) were treated with 20 μl drops containing a 1 × 108 cfu/ml suspension of Pta and Pcc or Pto. The disease severity of Pcc symptoms (0, no symptoms; 1, mild yellowing of the inoculated leaf; 2, partial softening or collapse of the leaf at the inoculation site; 3, almost complete softening or collapse of the leaf at the inoculation site; 4, intensification of leaf soft rot on other leaves; and 5, complete plant collapse) was measured 1 day after pathogen inoculation (Song et al., 2013). The severity of Pta symptoms was scored from 0 to 5 as follows: 0, no symptoms; 1, yellowish colour; 2, chlorosis only; 3, partial necrosis and chlorosis; 4, necrosis of the inoculated area and expanded chlorosis; and 5, complete necrosis of the inoculated area. To re-isolate pathogenic bacteria from the infection site, Pta and Pto were cultured in LB medium containing 100 μg/ml rifampicin, and Pcc was cultured in King’s B medium containing 100 μg/ml rifampicin. The total number of Pta and Pcc cells in leaves was counted at 7 and 1 days after drop application, respectively. The severity of Pto symptoms was scored as described previously (Song et al., 2013).
Direct inhibition assay
To investigate whether MeSA and MeJA have a direct inhibitory effect against Pta and Pcc, a bioassay was carried out using an I-plate (SPL Lifesciences Co.). Solutions (1 mM) of MeSA and MeJA (100 μl each) were dropped onto paper discs in one compartment of the I-plate. Pta and Pcc suspensions (20 μl each; serial dilution) were dropped onto the surface of the LB medium in the other compartment of the I-plate. Two days later, the number of colonies was counted. At least three replicate plates were produced per assay.
Plant RNA extraction, cDNA synthesis and quantitative reverse-transcription PCR (qRT-PCR)
Following inoculation with pathogen, leaf tissue was harvested 0, 12, 24, 36 and 48 h after inoculation with Pta and used for total RNA isolation. Total RNA was isolated using an RNeasy® Plus Mini kit according to the manufacturer’s protocol (Qiagen, USA). First-strand cDNA was synthesised using 2 μg of RNA, oligo dT primer, dNTP and Moloney murine leukaemia virus reverse transcriptase (M-MLV RT; Enzynomics, Korea). Quantitative reverse-transcription PCR (qRT-PCR) was carried out using a Chromo4 real-time PCR system (Bio-Rad, USA). The reaction mixture contained 2× Brilliant SYBR Green qRT-PCR Supermix (Bio-Rad), cDNA and 0.5 μM of each gene-specific primer. The expression of candidate priming genes was analysed using the following primers: 5′-AATATCCCACTCTTGCCG-3′ (NbPR1a-F), 5′-CCTGGAGGATC ATAGTTG-3′ (NbPR1a-R), 5′-ACCATCAGACCAAGATGT-3′ (NbPR2-F) and 5′-TGGCTAAGAGTGGAAGGT-3′ (NbPR2-R) (Kim et al., 2003). RNA levels were calibrated and normalised relative to the level of NbACT mRNA (GenBank accession no. U60489). The sequences were amplified using the following thermocycler parameters: 10 min at 95°C, followed by 44 cycles of 30 s at 95°C, 30 s at 60°C and 42 s at 72°C.
Measurement of plant growth parameters
Plant growth parameters such as shoot weight, chlorophyll content and leaf senescence were measured at 7 days after the second exposure in 6-week-old plants. Chlorophyll concentration was measured using a SPAD-502 meter (Konica-Minolta, Japan) (Ling et al., 2011). This meter is used to determine the amount of chlorophyll in a leaf as the SPAD (single-photon avalanche diode) value (value indicating chlorophyll content), which is used as a measure of plant health. Leaf tissues were harvested for these measurements using a circular punch cork borer, yielding 1 cm diameter leaf discs with an area of 0.785 cm2. SPAD values were recorded using exactly the same leaves from the same plants and 12 independent plants. The fresh weight, i.e., shoot weight per treatment per plant, was measured using 12 plants.
Statistical analysis
Analysis of variance for the experimental datasets was performed using JMP software version 5.0 (SAS Institute Inc., USA; www.Sas.com). Significant effects of treatment were determined based on the magnitude of the F-value (P = 0.05). When a significant F-test was obtained, separation of means was accomplished by Fisher’s protected LSD at P = 0.05.
RESULTS
Repeated applications of MeSA boost SAR against Pseudomonas syringae pv. tabaci
We utilised in vitro-grown N. benthamiana plants (Fig. 1A) to evaluate the effect of repeated applications of MeSA on SAR against the biotrophic pathogen Pseudomonas syringae pv. tabaci (Pta). No direct inhibition was detected between the defence hormones (MeSA and MeJA) and pathogens (Pta and Pectobacterium carotovorum subsp. carotovorum (Pcc)), indicating that the reductions in pathogen population were caused by the elicitation of induced resistance (SAR) (Fig. 1B). Exposure to 1 mM MeSA-MeSA treatment significantly reduced disease severity compared with Water-MeSA and Water-Water (control) (Fig. 2A). MeSA-MeSA treatment was more effective than Water-MeSA for inducing resistance against Pta, and a concentration of 1 mM was sufficient to significantly reduce disease severity (Fig. 2A). The number of bacterial cells in leaves collected 3 and 5 days after inoculation was significantly reduced in plants exposed to MeSA-MeSA and Water-MeSA treatment, whereas bacterial growth was not significantly altered in plants exposed to MeSA-MeJA treatment (Fig. 2B). Specifically, at 3 dpi, the bacterial population size in plants treated with MeSA-MeSA was log 7.8 cfu/leaf disc, while at 6 dpi, the bacterial population size was reduced to log 6.3 cfu/leaf disc (Fig. 2B). These results suggest that the repeated application of MeSA triggers enhanced SAR against Pta.
Fig. 1. Schematic diagram of the experimental design used in this study.
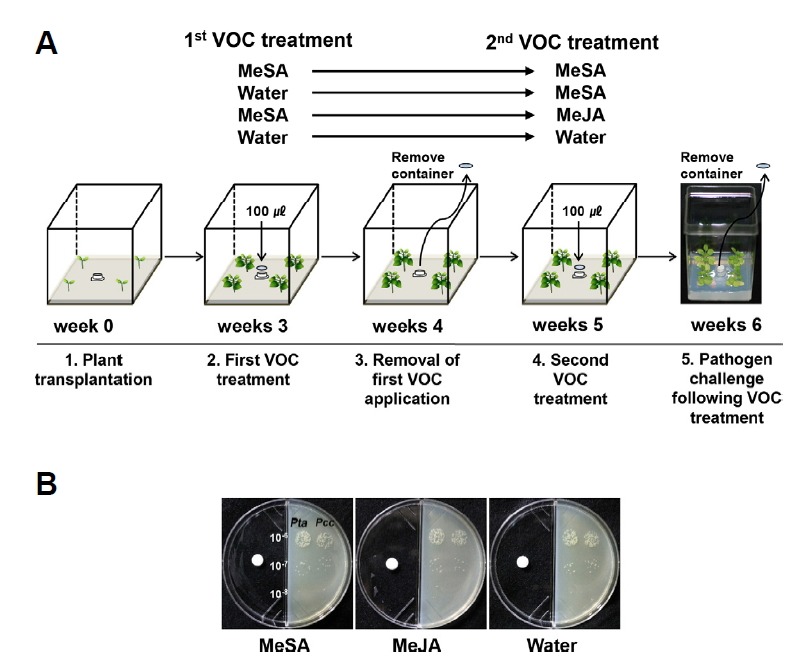
Signalling molecules were tested using the Incu Tissue (77 × 77 × 97 mm) system to assess their capacity for inducing SAR against bio- and necrotrophic bacterial pathogens (A). One hundred microlitres each of 1 mM MeSA and MeJA were dropped onto paper discs in one compartment of an I-plate. Twenty microlitres each of serial dilutions (10−6, 10−7 and 10−8) of Pseudomonas syringae pv. tabaci (Pta) and Pectobacterium carotovorum subsp. carotovorum (Pcc) suspensions (OD600 = 1) were dropped onto the surface of the LB medium in the other compartment of an I-plate. Two days later, the number of colonies was counted (B). The experiment was repeated three times with similar results.
Fig. 2. Repeated applications of MeSA lead to stronger induction of systemic resistance against a biotrophic pathogen.
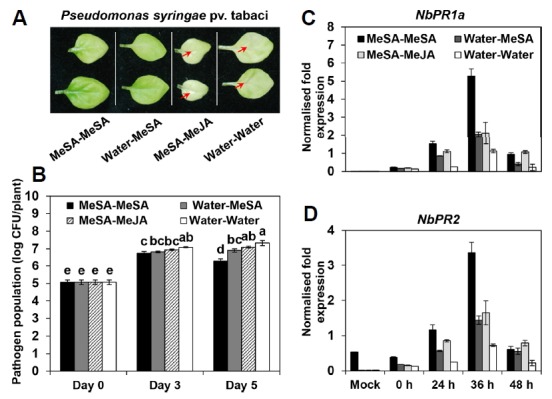
Representative photographs of N. benthamiana leaves inoculated with Pseudomonas syringae pv. tabaci (Pta) taken at 7 days post-inoculation (dpi) (A). Bacterial cell count measured 0, 3 and 5 days after pathogen inoculation with 108 cfu/mL Pta. Bars represent the mean ± SE (sample size, n = 16 replications per treatment) (B). Expression levels of SA-dependent resistance genes NbPR1a (C) and NbPR2 (D) assessed by qRT-PCR in N. benthamiana plants at 7 days after treatment (MeSA-MeSA, Water-MeSA, MeSA-MeJA or Water-Water) and again at 0, 24, 36 and 48 h after Pta inoculation. The housekeeping gene NbActin was used as a control. Error bars represent means ± SEM, n = 8 plants per treatment. Different letters indicate significant differences between treatments (P = 0.05 according to least significant difference). The experiment was repeated three times with similar results.
NbPR1a and NbPR2 expression increases after repeated applications of MeSA
To examine whether SAR-related defence genes were more strongly upregulated in response to MeSA-MeSA than Water-MeSA treatment, we measured the transcript levels of SA signalling-related genes, i.e., NbPR1a and NbPR2, after 0, 24, 36 and 48 h of pathogen challenge using qRT-PCR. NbPR1a and NbPR2 transcript levels were highest at 36 h post-inoculation (hpi) for all treatments (Figs. 2C and 2D). In plants exposed to MeSA-MeSA, Water-MeSA and MeSA-MeJA, NbPR1a transcript levels increased 26.5-, 20- and 21-fold from 0 to 36 h, respectively, whereas 8.4-fold increases were detected in control plants (Fig. 2C). NbPR2 transcript levels in plants treated with MeSA-MeSA, Water-MeSA and MeSA-MeJA increased 4.9-, 1.9- and 2.2-fold at 36 hpi, respectively, compared with the control (Fig. 2D). These results suggest that MeSA-MeSA treatment induces enhanced SAR through upregulating the expression of SA-dependent defence genes.
The effects of repeated applications of MeSA on plant physiological parameters
Previous studies suggest that the induction of SAR results in the inhibition of plant growth via a mechanism referred to as “allocation fitness cost” (Heil and Baldwin, 2002). In the current study, we measured fresh shoot weight at 6 weeks in plants under the conditions shown in Fig. 1. There were no differences in fresh shoot weight among MeSA-MeSA, Water-MeSA and Water-Water (control) plants. However, MeSA-MeJA significantly reduced fresh shoot weight (Figs. 3A and 3B). The total shoot weight per plant was 65.4 mg for MeSA-MeSA, 50.9 mg for MeSA-MeJA, 67 mg for Water-MeSA and 68.3 mg for Water-Water treatment (Figs. 3A and 3B). However, under MeSA-MeSA treatment, the SPAD value decreased by 2 compared with the control. For MeSA-MeJA treatment, which inhibited plant growth, the SPAD value decreased by 8 compared with the control (Figs. 3A and 3C). In addition, the number of senescent leaves in the MeSA-MeSA treatment group was 1.5-fold higher than that in the Water-MeSA treatment group. Under MeSA-MeJA treatment, the number of senescent leaves was approximately 16-fold that of the control (Figs. 3A and 3D). These results indicate that MeSA-MeSA treatment did not affect plant vegetative growth, as reflected by shoot length, but it did increase leaf senescence.
Fig. 3. Measurement of plant physiological parameters after repeated applications of MeSA.
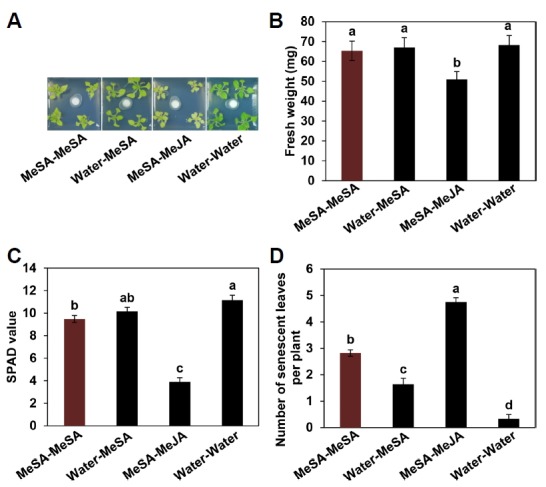
Representative photographs of plants taken at 7 days after the second application of MeSA (A). Plant physiological parameters such as weight (B), chlorophyll content (SPAD value measured with chlorophyll meter) (C) and leaf senescence (D) were measured at 7 days after the second exposure to MeSA (6 weeks). Bars represent the mean ± SE (sample size, n = 16 replications per treatment). Different letters indicate significant differences between treatments (P = 0.05 according to least significant difference). The experiment was repeated three times with similar results.
Successive treatments with MeSA and MeJA boost SAR against Pectobacterium carotovorum subsp. carotovorum
To evaluate the effect of repeated applications of MeSA on SAR against the necrotrophic pathogen Pcc, we measured the number of symptomatic leaves per plant after pathogen inoculation in five leaves per treatment. Exposure to Water-MeSA, MeSA-MeSA and MeSA-MeJA treatment reduced the number of symptomatic leaves compared with Water-Water treatment (Fig. 4A). Interestingly, unlike for the biotrophic pathogen, plants treated with MeSA-MeJA exhibited the most resistance against the necrotrophic pathogen (Fig. 4B). In addition, Water-MeSA treatment also reduced the number of symptomatic leaves (3.2) compared with MeSA-MeSA treatment (4.3; Fig. 4B).
Fig. 4. Exposing plants twice to MeSA emission induces systemic resistance against a necrotrophic pathogen.
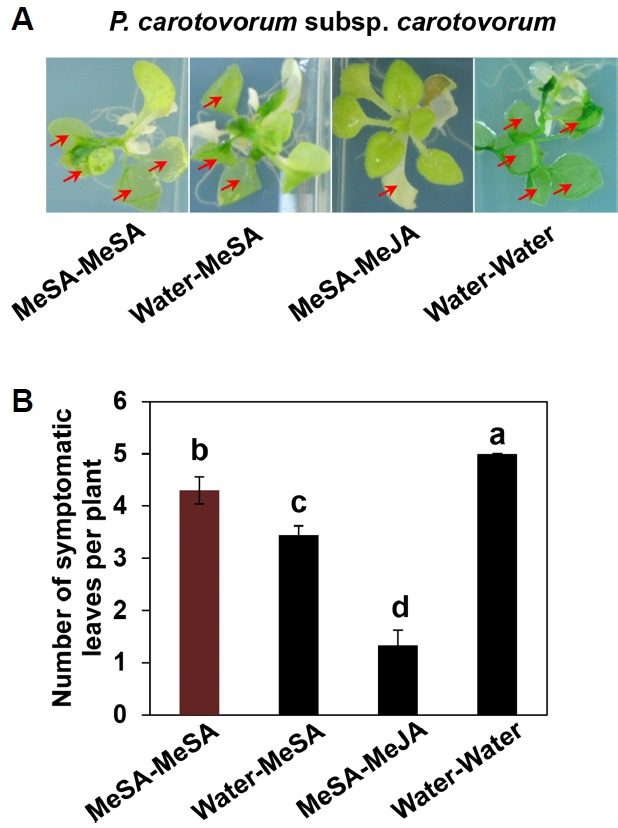
Representative photographs of plants taken at 24 h after challenge with 108 cfu/ml Pectobacterium carotovorum subsp. carotovorum (Pcc) (A). Number of symptomatic leaves per plant measured at 24 h (B). Bars represent the mean ± SE (sample size, n = 16 replications per treatment). Different letters indicate significant differences between treatments (P = 0.05 according to least significant difference). The experiment was repeated three times with similar results.
Genetic validation of plant memory using an Arabidopsis mutant
Next, we used the Incu Tissue system to test the effects of repeated applications of MeSA on plant defence pathway signalling in the Arabidopsis mutant npr1 challenged with Pto. Arabidopsis Col-0 consistently showed enhanced SAR after repeated applications of MeSA; MeSA-MeSA treatment was more effective than Water-MeSA at inducing resistance against Pto (Fig. 5A). The SA signalling-related mutant npr1 displayed severe disease symptoms after all treatments (Fig. 5B). There were no differences in fresh shoot weight among MeSA-MeSA, Water-MeSA and Water-Water (control) plants. However, MeSA-MeJA treatment significantly reduced fresh shoot weight (Figs. 5C and 5D). The total shoot weight per plant was 15.6 g for MeSA-MeSA, 15.6 g for Water-MeSA and 16.7 g for the Water-Water control (Fig. 5D). However, for MeSA-MeJA treatment, the total shoot weight per plant was only 11.6 g. There were no differences in fresh shoot weights among treated npr plants (Fig. 5E). Similar results were obtained from three independent experiments.
Fig. 5. Effect of repeated applications of MeSA on the induction of SAR and growth in Arabidopsis.
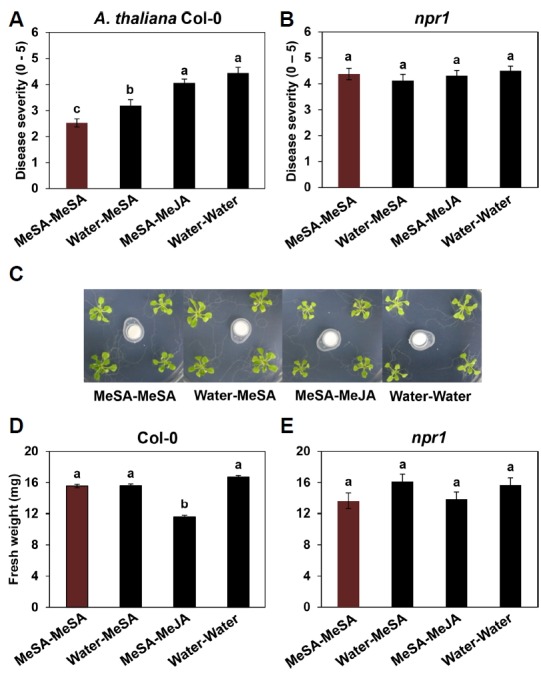
Disease severity (0–5) per plant was measured at 7 days after challenge with 108 cfu/mL P. syringae pv. tomato DC3000 in Arabidopsis Col-0 and the SA-deficient npr1 mutant (A, B). Representative photographs of plants taken at 7 days after the second application of MeSA (C). Plant physiological parameters such as weight were measured 7 days after the second exposure (6 weeks) in wild-type Col-0 and the mutant npr1 (D, E). Bars represent the mean ± SE (sample size, n = 15 replications per treatment). Different letters indicate significant differences between treatments (P = 0.05 according to least significant difference). The experiment was repeated three times with similar results.
DISCUSSION
Plant defence-related VOCs play an essential role in plant–plant communication (Heil and Bueno, 2007; Kost and Heil, 2006; Yi et al., 2009). Of the many studies investigating plant VOC emissions in response to herbivore damage and the occurrence of insect resistance in neighbouring plants, SAR against pathogenic microbes (such as viruses, bacteria and fungi) induced by plant VOCs has been intensively investigated only recently (Chen et al., 2008; Heil and Bueno, 2007; Karban et al., 2006). Furthermore, plants under natural conditions are continuously exposed to VOCs from neighbouring plants. In the current study, we developed a simplified system to test the concept of plant memory in which the repeated application of the plant defence VOC MeSA elicited higher plant protection capacity than the control and the expression of SAR marker genes NbPR1 and NbPR2 was also higher than in plants treated with zero or one application of MeSA, indicating that defence priming was boosted. These results provide indirect evidence that plants memorise the first application of this VOC. More intriguingly, this increased SAR occurred in a signalling molecule-dependent manner: 1) MeSA-MeJA treatment had an inhibitory effect on SAR capacity compared with MeSA-MeSA treatment in both tobacco and Arabidopsis; 2) repeated applications of MeSA were effective against a biotrophic pathogen but less effective against a necrotrophic pathogen, as plant resistance requires SA signalling for biotrophic pathogens and JA signalling for necrotrophic pathogens.
While the elicitation of SAR by extracellular application of plant defence-related VOCs (airborne signals) is well known (Heil and Adame-Álvarez, 2010; Yi et al., 2009), the increase in SAR in response to repeated applications of VOCs has not previously been demonstrated. The current study was designed to evaluate the plant memory effects of sequential releases of defensive airborne signals such as MeSA and MeJA on SAR. We previously reported that lima bean (Phaseolus lunatus) plants release the defensive VOCs MeSA and nonanal upon chemical SAR induction (Yi et al., 2009). Field trials revealed that emissions of MeSA and nonanal are sufficient to elicit SAR against P. syringae in plants within 50 cm of the emitter plant or a chemical paste (Girón-Calva et al., 2012; Heil and Adame-Álvarez, 2010). Since VOCs tend to spread rapidly in open spaces, it is not easy to release optimal levels of VOCs experimentally. Moreover, in closed spaces such as plates, the number of plants also has to be considered when investigating SAR because this number is related to O2 and CO2 emissions and the absorption of VOCs. To meet all of these requirements, we developed a new, VOC-based method not only by using specific MeSA concentrations and amounts but also by adjusting the number of plants placed in Incu Tissue vessels (data not shown; Fig. 1). In this miniaturised system, we observed clear SAR responses (P = 0.05) against P. syringae in tobacco and Arabidopsis under MeSA emissions (Figs. 2A and 5A).
Plants exhibit resistance to specific pathogens using different resistance signalling pathways, such as the SA and JA pathways (Kunkel and Brooks, 2002). SA signalling is generally effective against biotrophs, while JA signalling is generally effective against necrotrophs. Notably, in the current study, plants exposed to MeSA twice showed greater resistance against Pseudomonas syringae pv. tabaci (Pta) than those exposed to MeSA once (Figs. 2A and 2B). Moreover, SA target defence genes NbPR1 and NbPR2 were more highly expressed in plants exposed to MeSA twice than in plants exposed to MeSA once and the control (Figs. 2C and 2D). These plants were sensitised from previous pathogen attack, which helped induce stronger, more rapid resistance responses against pathogen challenge. When SAR is elicited, “defence priming”, i.e., the strong, rapid expression of defence genes (within 24 h of pathogen treatment), occurs (Conrath et al., 2015). Interestingly, in the current study, such expression was most noticeable at 36 h after pathogen inoculation. We attribute this to the plant’s perception of disease and the reduced expression of disease symptoms compared with the control, as shown in Fig. 2A. Furthermore, we obtained solid evidence for increased SAR by exposing the plants to repeated applications of MeSA at 1 week intervals in the same system (Figs. 2A and 5A). These results strongly suggest that plants possess a sophisticated mechanism for modulating defence signalling when the signalling molecules are repeatedly perceived.
Another interesting phenomenon is the occurrence of VOC-mediated signalling crosstalk in plant memory. Subsequent treatment with MeJA instead of a second MeSA treatment suppressed SA-dependent plant defence responses, such as resistance to the biotrophic pathogen P. syringae (Fig. 5). We hypothesize that this occurred because the crosstalk related to MeJA treatment blocked the MeSA pathway, resulting in attenuated expression of the SA signalling-related genes NbPR1 and NbPR2. The three treatments elicited different levels of disease resistance (MeSA-MeSA < Water-MeSA < MeSA-MeJA) against Pcc compared with the control (Fig. 4). These results may be due to the different levels of resistance elicited by plant memory of previous applications of the volatile SAR triggers MeSA and MeJA. Perhaps competition between the JA and SA signalling pathways (referred to as JA–SA crosstalk) may play an important role in fine-tuning defence responses. This notion was confirmed by evaluating the defence priming of SAR marker gene expression. For example, JA-induced PDF1.2 expression increases in Arabidopsis in response to apoplastic injection of SA at concentrations of up to 350 μM, whereas PDF1.2 expression is reduced at higher SA concentrations (Mur et al., 2006). Similarly, the upregulation of PR1 in response to 10 μM SA treatment increases in response to the application of JA at concentrations up to 125 μM, whereas JA concentrations above 125 μM reduce PR1 expression (Mur et al., 2006). These seemingly contradictory JA and SA responses may be indicators of the concentration-dependent interactions of the two signalling pathways. Perhaps the Pcc resistance observed under our experimental conditions is attributable to JA-dependent signalling, as MeS-MeJA treatment induced the lowest expression of SA signalling-dependent genes NbPR1a and NbPR2 and the strongest resistance to Pcc. To investigate the role of MeJA further, a MeJA-MeJA treatment group should be used. Nonetheless, using the Arabidopsis npr1 mutant, which lacks SA signalling, we were able to abolish the SAR-boosting and -induction effects of the two MeSA treatments (Figs. 5A and 5B).
The expression levels of defence genes in tobacco and Arabidopsis exposed to single MeSA treatments were not as high as those of plants exposed twice to MeSA, and the growth of these plants was noticeably inhibited (data not shown). This phenomenon can be attributed to “allocation fitness cost” or “trade-off”: the induction of SAR in response to chemical elicitors requires a substantial amount of energy, resulting in reduced plant growth (Heil and Baldwin, 2002). We found that the growth of both N. benthamiana and Arabidopsis plants treated twice with MeSA was no different from that of plants treated once with MeSA. Therefore, the disease resistance of plants exposed twice to MeSA differed from that of plants treated once due to the higher level of exposure to MeSA.
Previous investigations of chemical triggers eliciting plant immunity have involved the direct drenching of plants with compounds that induce resistance in a dose-dependent manner. This method has been successfully applied to pepper roots and cucumber seeds, leading to defence priming under field conditions for 4 consecutive years (Choi et al., 2014; Song and Ryu, 2013). In addition, 4-week-old pepper plants were dip-treated with 1 mM 3-pentanol solution before being transplanted into the field. This process elicited induced resistance in 2 year field trials without affecting fruit yield. Drench application of the volatiles 3-pentanol and 2-butanone upregulated the defence-related gene CsLOX in cucumber, leading to a decrease in the population of the sucking insect aphid (Myzus persicae) and significantly increasing the population of its natural enemy, ladybird beetle (Song and Ryu, 2013).
These findings demonstrate that, even when applied by drenching, water-soluble VOCs can help recruit a natural enemy of aphids due to the odours that they spread and may ultimately prevent plant disease and insect damage by eliciting induced resistance, even under open field conditions (Song et al., 2013). Successful cases of the use of VOCs for disease prevention in the field were recently reported (Choi et al., 2014). Nonetheless, the primary challenge to field application of VOCs is developing adequate chemical treatment methods. The application of volatiles has other drawbacks as well, including the high rate of volatile diffusion after application in the open field, inconsistent levels of effectiveness and negative effects on plant growth. However, with the increasing use of indoor cultivation systems, such as greenhouses and glasshouses, the chances for successfully applying VOCs to growing crops have increased (Kim et al., 2016). Eliciting induced resistance in plants through the use of VOCs without affecting plant growth may lead to the development of new biocontrol methods for future use in agriculture. Therefore, if we take advantage of the plant memory concept observed in this study, the agricultural application of VOCs to induce plant defence responses should be more feasible.
ACKNOWLEDGMENTS
This research was supported by grants from the Woo Jang-Choon Project (PJ01093904) of the Rural Development Administration (RDA) the Advanced Biomass R&D Center (ABC) of the Global Frontier Project funded by the Ministry of Science and ICT (ABC-2015M3A6A2065697), and the KRIBB Initiative Program, South Korea.
REFERENCES
- Bruce T.J., Matthes M.C., Napier J.A., Pickett J.A. Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci. 2007;173:603–608. [Google Scholar]
- Cameron R.K., Paiva N.L., Lamb C.J., Dixon R.A. Accumulation of salicylic acid and PR-1 gene transcripts in relation to the systemic acquired resistance (SAR). response induced by Pseudomonas syringae pv. tomato in Arabidopsis. Physiol Mol Plant Pathol. 1999;55:121–130. [Google Scholar]
- Cao H., Glazebrook J., Clarke J.D., Volko S., Dong X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997;88:57–63. doi: 10.1016/s0092-8674(00)81858-9. [DOI] [PubMed] [Google Scholar]
- Chanda B., Xia Y., Mandal M.K., Yu K., Sekine K.T., Gao Q.-m., Selote D., Hu Y., Stromberg A., Navarre D. Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat Genet. 2011;43:421–427. doi: 10.1038/ng.798. [DOI] [PubMed] [Google Scholar]
- Chaturvedi R., Venables B., Petros R.A., Nalam V., Li M., Wang X., Takemoto L.J., Shah J. An abietane diterpenoid is a potent activator of systemic acquired resistance. Plant J. 2012;71:161–172. doi: 10.1111/j.1365-313X.2012.04981.x. [DOI] [PubMed] [Google Scholar]
- Chen M.S. Inducible direct plant defense against insect herbivores: a review. Insect Sci. 2008;15:101–114. doi: 10.1111/1744-7917.12436. [DOI] [PubMed] [Google Scholar]
- Choi H.K., Song G.C., Yi H.-S., Ryu C.-M. Field evaluation of the bacterial volatile derivative 3-pentanol in priming for induced resistance in pepper. J Chem Ecol. 2014;40:882–892. doi: 10.1007/s10886-014-0488-z. [DOI] [PubMed] [Google Scholar]
- Conrath U., Beckers G.J., Langenbach C.J., Jaskiewicz M.R. Priming for enhanced defense. Annu Rev Phytopathol. 2015;53:97–119. doi: 10.1146/annurev-phyto-080614-120132. [DOI] [PubMed] [Google Scholar]
- Crisp P.A., Ganguly D., Eichten S.R., Borevitz J.O., Pogson B.J. Reconsidering plant memory: intersections between stress recovery, RNA turnover, and epigenetics. Sci Adv. 2016;2:e1501340. doi: 10.1126/sciadv.1501340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z.Q., Dong X. Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol. 2013;64:839–863. doi: 10.1146/annurev-arplant-042811-105606. [DOI] [PubMed] [Google Scholar]
- Gao Q.-M., Zhu S., Kachroo P., Kachroo A. Signal regulators of systemic acquired resistance. Front Plant Sci. 2015;6:228. doi: 10.3389/fpls.2015.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón-Calva P.S., Molina-Torres J., Heil M. Volatile dose and exposure time impact perception in neighboring plants. J Chem Ecol. 2012;38:226–228. doi: 10.1007/s10886-012-0072-3. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack K.E., Jones J. Resistance gene-dependent plant defense responses. Plant Cell. 1996;8:1773. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M., Baldwin I.T. Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends Plant Sci. 2002;7:61–67. doi: 10.1016/s1360-1385(01)02186-0. [DOI] [PubMed] [Google Scholar]
- Heil M., Bueno J.C.S. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA. 2007;104:5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M., Adame-Álvarez R.M. Short signalling distances make plant communication a soliloquy. Biology Lett. 2010;6:843–845. doi: 10.1098/rsbl.2010.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H.W., Tschaplinski T.J., Wang L., Glazebrook J., Greenberg J.T. Priming in systemic plant immunity. Science. 2009;324:89–91. doi: 10.1126/science.1170025. [DOI] [PubMed] [Google Scholar]
- Karban R., Shiojiri K., Huntzinger M., McCall A.C. Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication. Ecology. 2006;87:922–930. doi: 10.1890/0012-9658(2006)87[922:drisva]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kim M., Ahn J.-W., Jin U.-H., Choi D., Paek K.-H., Pai H.-S. Activation of the programmed cell death pathway by inhibition of proteasome function in plants. J Biol Chem. 2003;278:19406–19415. doi: 10.1074/jbc.M210539200. [DOI] [PubMed] [Google Scholar]
- Kim H., Kojima M., Choi D., Park S., Matsui M., Sakakibara H., Hwang I. Overexpression of INCREASED CAMBIAL ACTIVITY, a putative methyltransferase, increases cambial activity and plant growth. J Inteqr Plant Biol. 2016;58:874–889. doi: 10.1111/jipb.12486. [DOI] [PubMed] [Google Scholar]
- Kost C., Heil M. Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J Ecol. 2006;94:619–628. [Google Scholar]
- Kunkel B.N., Brooks D.M. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol. 2002;5:325–331. doi: 10.1016/s1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Ling Q., Huang W., Jarvis P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth Res. 2011;107:209–214. doi: 10.1007/s11120-010-9606-0. [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J., Jülke S., Geiß K., Richter F., Mithöfer A.Šola IRusak G., Keenan S., Bulman S. A novel methyltransferase from the intracellular pathogen Plasmodiophora brassicae methylates salicylic acid. Mol Plant Pathol. 2015;16:349–364. doi: 10.1111/mpp.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon G. A sustainable approach to crop protection. Blackwell Publishing Ltd; Oxford: 2007. Agents that can elicit induced resistance. Induced resistance for plant defence; pp. 9–29. [Google Scholar]
- Mandal M.K., Chandra-Shekara A., Jeong R.-D., Yu K., Zhu S., Chanda B., Navarre D., Kachroo A., Kachroo P. Oleic acid–dependent modulation of NITRIC OXIDE ASSOCIATED1 protein levels regulates nitric oxide–mediated defense signaling in Arabidopsis. Plant Cell. 2012;24:1654–1674. doi: 10.1105/tpc.112.096768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Medina A., Flors V., Heil M., Mauch-Mani B., Pieterse C.M., Pozo M.J., Ton J., van Dam N.M., Conrath U. Recognizing plant defense priming. Trends Plant Sci. 2016;21:818–822. doi: 10.1016/j.tplants.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Mur L.A., Kenton P., Atzorn R., Miersch O., Wasternack C. The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 2006;140:249–262. doi: 10.1104/pp.105.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Návarová H., Bernsdorff F., Döring A.-C., Zeier J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell. 2012;24:5123–5141. doi: 10.1105/tpc.112.103564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-W., Kaimoyo E., Kumar D., Mosher S., Klessig D.F. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science. 2007;318:113–116. doi: 10.1126/science.1147113. [DOI] [PubMed] [Google Scholar]
- Shah J., Zeier J. Long-distance communication and signal amplification in systemic acquired resistance. Front Plant Sci. 2013;4:30. doi: 10.3389/fpls.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J., Chaturvedi R., Chowdhury Z., Venables B., Petros R.A. Signaling by small metabolites in systemic acquired resistance. Plant J. 2014;79:645–658. doi: 10.1111/tpj.12464. [DOI] [PubMed] [Google Scholar]
- Shulaev V., Silverman P., Raskin I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature. 1997;386:718–721. [Google Scholar]
- Song G.C., Ryu C.-M. Two volatile organic compounds trigger plant self-defense against a bacterial pathogen and a sucking insect in cucumber under open field conditions. Int J Mol Cell. 2013;14:9803–9819. doi: 10.3390/ijms14059803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G.C., Ryu S.Y., Kim Y.S., Lee J.Y., Choi J.S., Ryu C.-M. Elicitation of induced resistance against Pectobacterium carotovorum and Pseudomonas syringae by specific individual compounds derived from native Korean plant species. Molecules. 2013;18:12877–12895. doi: 10.3390/molecules181012877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bel A.J., Gaupels F. Pathogen-induced resistance and alarm signals in the phloem. Mol Plant Pathol. 2004;5:495–504. doi: 10.1111/j.1364-3703.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- Vernooij B., Friedrich L., Morse A., Reist R., Kolditz-Jawhar R., Ward E., Uknes S., Kessmann H., Ryals J. Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. Plant Cell. 1994;6:959–965. doi: 10.1105/tpc.6.7.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth M.C., Dewdney J., Wu G., Ausubel F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- Yi H.-S., Heil M., Adame-Álvarez R.M., Ballhorn D.J., Ryu C.-M. Airborne induction and priming of plant defenses against a bacterial pathogen. Plant Physiol. 2009;151:2152–2161. doi: 10.1104/pp.109.144782. [DOI] [PMC free article] [PubMed] [Google Scholar]


