Abstract
Long‐term oncological outcomes for primary renal cell carcinoma (RCC) treated with carbon‐ion radiotherapy (CIRT) are poorly understood. Patients with primary RCC were treated with 12 or 16‐fraction CIRT at The Hospital of the National Institute of Radiological Sciences outside of clinical trials. Outcome data were pooled and retrospectively analyzed for toxicity, local control, and disease‐free, cancer‐specific, and overall survival. From 1997 to 2014, 19 RCC patients (11 with T1aN0M0, 4 with T1bN0M0, and 4 with inoperable advanced stage [T4N0M0, T3aN1M0, and T1aN0M1]) were treated with CIRT and followed up for a median of 6.6 (range, 0.7‐16.5) years; 9 of these patients were inoperable because of comorbidities or advanced‐stage disease. Diagnoses were confirmed by imaging in 11 patients and by biopsy in the remaining 8. In 4 of 5 patients with definitive renal comorbidities, including diabetic nephropathy, sclerotic kidney or solitary kidney pre‐CIRT progressed to grade 4 chronic kidney disease (CKD). In contrast, the remaining 14 patients without definitive renal comorbidities did not progress to grade 3 or higher CKD. Furthermore, although 1 case of grade 4 dermatitis was observed, there were no other grade 3 or higher non‐renal adverse events. Local control rate, and disease‐free, cancer‐specific, and overall survival rates at 5 years of all 19 patients were 94.1%, 68.9%, 100%, and 89.2%, respectively. This updated retrospective analysis based on long‐term follow‐up data suggests that CIRT is a safe treatment for primary RCC patients without definitive renal comorbidities pre‐CIRT, and yield favorable treatment outcomes, even in inoperable cases.
Keywords: adverse event, carbon‐ion radiotherapy, local control, renal cell carcinoma, survival
1. INTRODUCTION
Standard therapy for patients with primary renal cell carcinoma (RCC) is surgery; in particular, partial nephrectomy has shown high efficacy with low complication rates, compared with radical nephrectomy, for localized RCC.1 Alternative treatment options for stage Ia RCC patients include ablation therapies such as cryoablation and radiofrequency ablation when active surveillance is not selected.1, 2, 3, 4, 5 In contrast, there is no standard radical therapy for primary RCC patients who are ineligible for surgery or ablation.
Until recently, radiotherapy has not been used to treat primary RCC, because this cancer is thought to be poorly sensitive to radiation. As a result of recent improvements in radiotherapy techniques, X‐ray stereotactic body radiotherapy (SBRT) or stereotactic ablative radiotherapy (SABR) provide highly focused hypofractionated irradiation of tumors. These novel radiotherapy techniques have shown favorable local control rates (97.8%‐100% at 2 years) and few severe adverse events.6, 7, 8, 9 However, to the best of our knowledge, there are no long‐term follow‐up data of SBRT/SABR for primary RCC.
Carbon‐ion radiotherapy (CIRT) has notable characteristics in both physics and biology: it limits irradiation to the target volume with little effect on surrounding healthy tissue, and shows strong cytotoxicity as a result of high linear energy transfer, unlike the X‐ray beam.10, 11 The Hospital of the National Institute of Radiological Sciences began using CIRT to treat primary RCC in 1997. We previously reported a pilot study involving 10 primary RCC patients treated with CIRT, and the results showed high local control rates (100% at 5 years) with few adverse events, even in the inoperable patients.12 However, one limitation of our previous study was that only 4 patients were followed up for 5 years or longer, limiting the amount of information on late adverse events and RCC recurrence/mortality rates. The purpose of the current study is to clarify the late adverse events of CIRT, including renal function, and the secondary objective is to evaluate long‐term treatment effects, based on updated data on the long‐term outcomes of CIRT carried out at our institution.
2. MATERIALS AND METHODS
2.1. Study design
This study consisted of 15 patients with RCC treated with 16‐fraction CIRT from April 1997 to March 2013, and 4 additional patients who were deemed ineligible for the 12‐fraction phase I/II trial that started in April 2013 at our institution. Therefore, a total of 19 RCC patients were retrospectively analyzed in this study. For 16‐fraction CIRT, the total dose used was generally 72 Gy (relative biological effectiveness [RBE]), and one time, the dose was escalated to 80 Gy (RBE) until severe skin adverse effects occurred. In addition, a total dose of 64 Gy (RBE) in 16 fractions was used when the tumor was close to the gastrointestinal tract. For 12‐fraction CIRT, 66 Gy (RBE) was set as the starting dose. Diagnosis was confirmed using computed tomography (CT), magnetic resonance imaging (MRI), ultrasonography, angiography, and needle biopsy; however, biopsy was not carried out if the radiographical findings were typical of RCC. The TNM Classification of Malignant Tumors (seventh edition) was used for tumor staging.13 These 19 treatments were conducted in accordance with the ethical standards set forth by the Declaration of Helsinki,14 and all patients satisfying the enrollment criteria were approved by the ethics committee.
2.2. Treatment
The treatment method as well as the physical and biological characteristics of CIRT have been described in previous studies.12 Briefly, the irradiation fields were established using a 3‐D planning system based on 2.5‐5‐mm‐thick CT images. Gross tumor volume was defined as the macroscopic tumor, and the clinical tumor volume was defined as the gross tumor volume +5 mm to account for microscopic invasion. The planning target volume was defined as the clinical tumor volume +10 mm in the cranial and caudal directions and +5 mm in the other directions, including the internal and set‐up margins. For accurate reproduction of the target position, an immobilization device, insertion of fiducial markers and respiratory gating at the end of the expiratory phase were used.
2.3. Follow up
Each patient was examined using blood tests, ultrasonography, dynamic contrast‐enhanced CT, and MRI according to the European Society of Urogenital Radiology guidelines,15 at least once every 3 months for the first 6 months and then usually every 6 months thereafter.
To evaluate kidney toxicity, estimated glomerular filtration rate (eGFR) was calculated in all cases according to the formula reported by Matsuo et al.16 Kidney function as well as other adverse events were evaluated according to the National Cancer Institute's Common Terminology Criteria for Adverse Events version 4.0,17 and the definition of chronic kidney disease (CKD) is given in Table 1. Because the lower limit of normal for eGFR is 60 mL/min/1.73 m2 at our institution, grade 0 CKD was defined as an eGFR ≥60 mL/min/1.73 m2 without proteinuria 2+ or a urine protein/creatinine level >0.5, and grade 1 CKD was defined as an eGFR ≥60 mL/min/1.73 m2 with proteinuria 2+ or a urine protein/creatinine level >0.5.
Table 1.
Definition of chronic kidney disease according to CTCAE ver. 4.017
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
| eGFR or CrCl <LLN‐60 mL/min/1.73 m2 or proteinuria 2+; urine protein/creatinine >0.5 | eGFR or CrCl 30‐59 mL/min/1.73 m2 | eGFR or CrCl 15‐29 mL/min/1.73 m2 | eGFR or CrCl <15 mL/min/1.73 m2; dialysis or renal transplant indicated | Death |
CrCl, creatinine clearance; CTCAE, Common Terminology Criteria for Adverse Events; eGFR, estimated glomerular filtration rate; LLN, lower limit of normal.
2.4. Evaluation
Evaluation of eGFR was conducted from pre‐CIRT to the end of follow up or to the start of dialysis. Local failure was defined as either progressive disease according to the modified response evaluation criteria in solid tumors18 or as the new appearance of lesions within the target tumor. Distant failure was defined as the development of metastatic lesions outside of the kidney. Time to failure was defined as the interval between the start of CIRT and the date of diagnosis of recurrence. Survival time was defined as the interval between the start of CIRT and the date of death or the last follow up. The cutoff date for analysis was March 2018.
2.5. Statistics
Cumulative local control rate and disease‐free, cancer‐specific, and overall survival rates were calculated using the Kaplan‐Meier method. Log‐rank test was used to compare the cancer‐specific survival rates. P‐value <0.05 was considered significant. All statistical analyses were carried out using IBM SPSS Statistics, version 20 (IBM Japan, Ltd, Tokyo, Japan).
3. RESULTS
Patient, tumor, and treatment characteristics of the 19 patients are shown in Table 2. The first 10 patients (#1‐10) are the same as those reported previously.12 Median tumor size was 36 (range, 24‐120) mm. Of the 19 patients, 11 were diagnosed by radiographic findings. Biopsy was carried out in 8 patients, all of whom were diagnosed with clear cell carcinoma. Of the treatments received prior to CIRT, 2 advanced‐stage patients (#3 and #10) received interferon‐α (IFN‐α), but a treatment response was not observed.12 CIRT was delivered to the thoracic vertebra (64 Gy [RBE] in 16 fractions) in 1 metastatic case (#12), and the lesion was controlled until the patient died as a result of recurrent RCC at 5.8 years post‐CIRT. All 19 patients completed treatment for primary RCC, and the median (range) and mean (±SD) follow‐up times were 6.6 (interquartile range, 4.6‐10.7, range, 0.7‐16.5) and 7.7 (±4.3) years, respectively. One patient (#3) was lost to follow up at 6.1 years without any recurrence and died of RCC at 11.0 years post‐CIRT; thus, for patient #3, the recurrence status and late adverse events after 6.1 years are unknown. Except for this case, the remaining 18 patients were evaluated until the end of follow up.
Table 2.
Patient and tumor characteristics, treatments, and prognoses
| Patient characteristics | Tumor characteristics | Treatment | Follow up, y | Prognosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt no. | Age, y | M/F | Operability | Reason for no surgery | TNM stage (UICC v7)13 | Size, mm | Diagnosis | Total dose, Gy (RBE) | Fr. | LF, y | DM, y (site) | Vital status (cause of death) | |
| 1 | 67 | M | No | Comorbidity (low renal function) | T1bN0M0 | 50 | Biopsy | 72 | 16 | 10.1 | None | None | Dead (pneumonia) |
| 2 | 70 | M | No | Comorbidity (asthma) | T1aN0M0 | 32 | Biopsy | 72 | 16 | 3.5 | None | None | Dead (cardiac infarction) |
| 3 | 59 | M | No | Advanced RCC | T4N0M0 (muscle invasion) | 65 | Imaging | 80 | 16 | 11.0a | NAa | NAa | Dead (RCC) |
| 4 | 55 | M | Yes | Refusal | T1aN0M0 | 24 | Biopsy | 80 | 16 | 16.5 | None | None | Alive |
| 5 | 71 | M | No | Comorbidity (asthma) | T1aN0M0 | 30 | Biopsy | 80 | 16 | 15.4 | None | None | Alive |
| 6 | 69 | M | No | Advanced RCC | T4N0M0 (muscle invasion) | 120 | Biopsy | 72 | 16 | 6.2 | None | 2.0 (Lung, Bone) | Dead (RCC) |
| 7 | 82 | M | Yes | Refusal | T1aN0M0 | 30 | Biopsy | 72 | 16 | 0.7 | None | None | Dead (encephalitis) |
| 8 | 55 | M | Yes | Refusal | T1aN0M0 | 36 | Imaging | 72 | 16 | 11.6 | None | None | Alive |
| 9 | 61 | M | Yes | Refusal | T1bN0M0 | 50 | Biopsy | 72 | 16 | 9.4 | None | 3.5 (Lung) | Dead (RCC) |
| 10 | 52 | F | No | Advanced RCC | T3aN1M0 | 65 | Biopsy | 64 | 16 | 10.8 | 9.5 | 3.7 (Lung) | Alive |
| 11 | 68 | M | No | Comorbidity (COPD) | T1aN0M0 | 32 | Imaging | 72 | 16 | 9.8 | None | None | Alive |
| 12 | 75 | M | No | Advanced RCC | T1aN0M1 | 39 | Imaging | 64 | 16 | 5.8 | None | 3.9 (Lung) | Dead (RCC) |
| 13 | 80 | M | Yes | Refusal | T1aN0M0 | 30 | Imaging | 72 | 16 | 7.8 | None | None | Dead (cardiac infarction) |
| 14 | 75 | M | Yes | Refusal | T1aN0M0 | 38 | Imaging | 72 | 16 | 6.4 | 3.3 | 3.3 (Lung, Bone) | Alive |
| 15 | 47 | M | Yes | Refusal | T1aN0M0 | 32 | Imaging | 72 | 16 | 6.6 | None | None | Alive |
| 16 | 62 | M | Yes | Refusal | T1bN0M0 | 65 | Imaging | 66 | 12 | 5.1 | None | None | Alive |
| 17 | 65 | M | Yes | Refusal | T1aN0M0 | 30 | Imaging | 66 | 12 | 4.6 | None | None | Alive |
| 18 | 70 | M | Yes | Refusal | T1aN0M0 | 36 | Imaging | 66 | 12 | 4.1 | None | None | Alive |
| 19 | 57 | M | No | Comorbidity (low cardiac function) | T1bN0M0 | 54 | Imaging | 66 | 12 | 2.9 | None | None | Alive |
| Total: n = 19 | T1aN0M0: 11 T1bN0M0: 4 | 80 Gy (RBE)/16 fr.: 3 | Median follow–up time: 6.6 y | ||||||||||
| Median age 67 (range, 47–82) y | Advanced stage: 4 | 72 Gy (RBE)/16 fr.: 10 | LF: 2, DM: 5 | ||||||||||
| 18 M/1 F | Median tumor size: 36 mm | 64 Gy (RBE)/16 fr.: 2 | Alive: 11 | ||||||||||
| Inoperable due to comorbidities: 5 | Biopsy‐proven RCC: 8b | 66 Gy (RBE)/12 fr.: 4 | Death: 8 (due to RCC: 4, other causes: 4) | ||||||||||
| Inoperable due to advanced RCC: 4 | Imaging diagnosis: 11 | ||||||||||||
Pt., patient; M, male; F, female; CIRT, carbon‐ion radiotherapy; RBE, relative biological effectiveness; fr., fraction; RCC, renal cell carcinoma; COPD, chronic obstructive pulmonary disease.
Patient no. 3 was lost to follow up at 6.1 y without recurrence, therefore, the recurrence data for this patient from 6.1 to 11.0 y (time to death) are missing.
All eight biopsy‐proven cases were diagnosed as clear cell carcinoma.
3.1. Adverse events
Chronic kidney disease grades of the 19 patients pre‐CIRT versus at the end of follow up are shown in Table 3. Of the 7 patients with grade 2 or higher CKD pre‐CIRT, 4 had progressed to grade 4 by the end of follow up. Table 4 lists the details of the 7 patients with grade 2 or higher CKD pre‐CIRT. All 4 patients who progressed to grade 4 CKD had definitive renal comorbidities, such as diabetic nephropathy, renal sclerosis, or solitary kidney, pre‐CIRT. Mean time to grade 4 CKD in these 4 patients was 5.6 (range, 3.6‐7.6) years post‐CIRT, and 2 of the 4 patients were required to start dialysis (Table 4). The CKD grade of 1 patient (#2) with definitive renal comorbidity (solitary kidney) who died of cardiac infarction relatively soon post‐CIRT (3.5 years) did not increase. However, of the remaining 14 patients without definitive renal comorbidities, grade 3 or higher CKD was not observed at the end of follow up, and the average decrease in eGFR was 6.1 (range, −11.8 to 22.6) mL/min/1.73 m2.
Table 3.
CKD grades pre‐CIRT vs at the end of follow up (n = 19)
| CKD grade pre‐CIRT | Total | CKD grade at end of follow up | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| 0 | 12 (0) | 10 (0) | 0 | 2 (0) | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 6 (4) | 0 | 0 | 3 (1) | 0 | 3 (3) | 0 |
| 3 | 1 (1) | 0 | 0 | 0 | 0 | 1 (1) | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Values in parentheses indicate the number of patients with definitive renal comorbidity such as diabetic nephropathy, sclerotic kidneys, or solitary kidneys pre‐CIRT.
CIRT, carbon‐ion radiotherapy; CKD, chronic kidney disease.
Table 4.
Renal function prognoses of 7 patients with grade 2 or higher CKD pre‐CIRT and after CIRT
| Pt no. | Definitive renal comorbidity | eGFR (mL/min/1.73 m2) [CKD grade] | Follow‐up time, y | Time after CIRT, y | ||
|---|---|---|---|---|---|---|
| pre‐CIRT | End of follow up | Grade 4 CKD | Start of dialysis | |||
| 1 | Diabetic nephropathy | 25 [3] | 6 [4]b | 10.1 | 4.2 | 6.1 |
| 2 | Solitary kidneya | 47 [2] | 35 [2] | 3.5 | None | None |
| 6 | None | 52 [2] | 56 [2] | 6.2 | None | None |
| 9 | Solitary kidneya | 41 [2] | 13 [4] | 9.4 | 7.2 | None |
| 13 | Sclerotic kidney | 35 [2] | 15 [4] | 7.8 | 7.6 | None |
| 16 | Diabetic nephropathy | 40 [2] | 8 [4]b | 5.1 | 3.6 | 4.1 |
| 17 | None | 59 [2] | 57 [2] | 4.6 | None | None |
CIRT, carbon‐ion radiotherapy; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; Pt, patient.
Post‐radical nephrectomy for contralateral renal cell carcinoma.
The values were obtained just before start of dialysis.
Regarding adverse events outside the kidney, there was 1 case (#3) of grade 4 dermatitis that we reported previously.12 This patient and 1 other patient with subcutaneous induration (#5) required analgesics, and both were treated with 80 Gy (RBE) in 16 fractions between 1998 and 2001. Except for these initial cases, there were no grade 2 or higher late adverse events in the skin, gastrointestinal tract, or lower urinary tract (Table 5).
Table 5.
Acute and late adverse events (excluding renal function) in 19 patients
| Grade | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Acute, n | ||||||
| Dermatitis | 7 | 11 | 1 | 0 | 0 | 0 |
| Gastrointestinal disorder | 19 | 0 | 0 | 0 | 0 | 0 |
| Lower urinary tract | 17 | 2 | 0 | 0 | 0 | 0 |
| Abdominal or flank/dorsal pain | 19 | 0 | 0 | 0 | 0 | 0 |
| Late, n | ||||||
| Dermatitis | 13 | 5 | 0 | 0 | 1 | 0 |
| Gastrointestinal disorder | 19 | 0 | 0 | 0 | 0 | 0 |
| Lower urinary tract | 19 | 0 | 0 | 0 | 0 | 0 |
| Abdominal or flank/dorsal pain | 17 | 0 | 2 | 0 | 0 | 0 |
3.2. Recurrence and survival
Other treatments for RCC were not carried out post‐CIRT until detection of recurrence, except for patient #3 with stage IV RCC at presentation who was treated with IFN‐α. As of March 2018, local failure was observed in 2 patients and distant failure in the lung and bone in 5 patients after CIRT (prognosis of patient #3 after 6.1 years post‐CIRT is unknown; Table 2). The following treatments were given after recurrence: IFN‐α in 2 patients (#6 and #12), interleukin‐2 in 1 (#9), molecular targeted therapy in 3 (#9, #10, and #14), and CIRT (60 Gy [RBE]/4 fractions for lung metastasis) in 1 patient (#10). By the end of follow up, 11 patients were alive and 8 patients were dead, including 4 deaths due to progressive RCC (Table 2).
Five‐year local control and progression‐free survival rates of all 19 patients were 94.1% (95% confidence interval [CI], 65.0‐99.1) and 68.9% (95% CI, 40.2‐85.8), respectively. Figure 1 shows the cancer‐specific survival rates of the patients with T1a/bN0M0 (n = 15) and advanced‐stage group (n = 4) disease, and a significant difference was observed between the 2 groups (P = 0.027). Cancer‐specific and overall survival rates of all 19 RCC patients were 100% (95% CI, 100‐100) and 89.2% (95% CI, 63.1‐97.2) at 5 years, and 74.0% (95% CI, 38.2‐91.0), and 58.7% (95% CI, 29.1‐79.5) at 10 years, respectively.
Figure 1.
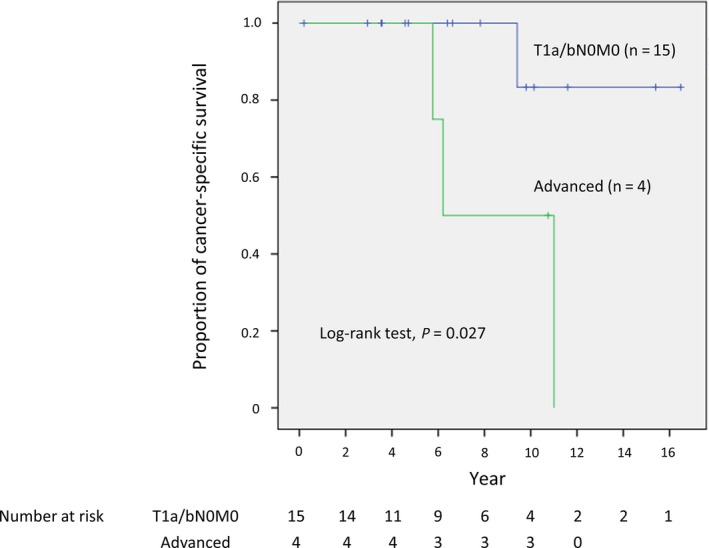
Cancer‐specific survival curves of 19 patients with renal cell carcinoma [T1a/bN0M0 (n = 15) and advanced stage (n = 4)] after carbon‐ion radiotherapy. A significant difference between the T1a/bN0M0 and advanced stage groups was observed (P = 0.027)
4. DISCUSSION
The 19 primary RCC patients treated with CIRT at our institution, including 9 inoperable patients with comorbidities and advanced stage, showed few severe adverse events including renal function effects, except for those with definitive renal comorbidities, and relatively favorable treatment effects after a median and mean follow up of 6.6 and 7.7 years, respectively. To the best of our knowledge, there have been no long‐term follow‐up data reported for primary RCC after radical radiotherapy, including CIRT and photon‐based series such as SBRT/SABR.
In the 14 patients without definitive renal comorbidities, average reduction in eGFR was 6.1 mL/min/1.73 m2. Siva et al19 showed a net change in GFR of −8.7 ± 13.4 mL/min at 1.1 years following treatment and in eGFR of −5.5 ± 13.3 mL/min at the end of follow up (median 2.6 years) after SABR.7 In a surgical series, mean eGFR decreases of 13 and 24 mL/min were noted after partial and radical nephrectomy with median follow‐up times of 44 and 57 months, respectively.20 These results suggest that the decrease in renal function in RCC patients without definitive renal comorbidities after CIRT is comparable with that after partial nephrectomy or SBRT/SABR, even after long‐term follow up. However, in the present study, except for 1 patient (#2) with a solitary kidney who died of cardiac infarction relatively soon after CIRT (3.5 years), all other patients with definitive renal comorbidities, such as diabetic nephropathy, sclerotic kidney, and solitary kidney, at the time of CIRT, progressed to grade 4 CKD. Therefore, patients with definitive renal comorbidities should be categorized as being at high risk of marked renal function deterioration after CIRT; however, progression to grade 4 CKD took a mean of 5.6 years following CIRT. Cause of the slow reduction in renal function may be attributed to the natural course of these renal diseases, especially diabetic nephropathy and sclerotic kidney, as well as to damage to healthy kidney tissue caused by CIRT.
Otherwise, few grade 2 or higher adverse events were observed. Surely the skin and subcutaneous tissue are important organs at risk of late radiation‐induced morbidities after charged particle therapy because of lack of build‐up at the beam entrance.10 However, grade 2 or higher adverse events in the skin and subcutaneous tissue were observed only in those cases treated with 80 Gy (RBE)/16 fractions, which was the highest biologically effective dose (BED) in this study. Adjustment of the field number or irradiation angle may contribute to slight adverse events in superficial tissues.
Concerning the appropriate total dose, local recurrence was observed in patients treated with BED ≤72 Gy (RBE)/16 fractions, but in none of the patients treated with 80 Gy (RBE) in the present study. These results suggest that a higher BED ≥72 Gy (RBE)/16 fractions may be desirable to achieve sufficient local tumor control. Therefore, in the phase I/II dose escalation study of 12‐fraction CIRT that began at our institution in 2013, with the aim of reducing patient's burden, the starting dose was 66 Gy (RBE) as this results in a BED ≥72 Gy (RBE) in 16 fractions when an α/β ratio of 3 or 5 is applied. Treatment outcomes of that phase I/II study are forthcoming.
The present study showed relatively favorable 5‐year local control and cancer‐specific survival and overall survival rates, even in those 9 cases deemed inoperable because of advanced stage or comorbidities. Table 6 lists the treatment outcomes after CIRT in terms of the 5‐year rates according to stage Ia, stage Ib, and advanced stage, as well as the results of standard radical treatments such as partial/radical nephrectomy, cryoablation, and radiofrequency ablation. These results suggest that CIRT is a promising radical treatment for patients with primary RCC, including inoperable cases, and is potentially comparable with other standard treatments.
Table 6.
Review of treatment results for primary renal cell carcinoma following standard therapies and CIRT
| Stage | Author (year) | Treatment | Patient no. | Median follow up, y | Median size (range) cm | 5‐y rate (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC | DFS | CSS | OS | |||||||
| Ia | Davol et al (2006)21 | CA | 48 | 5.3 | 2.6 (1.1‐4.6) | 87.5a | 84.3 | 100 | 89.5 | |
| Olweny et al (2012)22 | RFA | 37 | 6.5 | 2.1 (1.8‐2.8) | 91.7 | 89.2 | 97.2 | 97.2 | ||
| PN | 37 | 6.1 | 2.5 (1.7‐3.1) | 94.6 | 89.2 | 100 | 100 | |||
| Lorber et al (2014)23 | RFA | 50 | 5.4 | 2.3 (0.3‐4.0) | 92.5a | 92.5 | 100 | 98 | ||
| Present study | CIRT | 11 | 6.6 | 3.1 (2.4‐3.8) | 90 | 90 | 100 | 82 | ||
| Ib | Leibovich et al (2004)24 | RN | 841 | 7.4 | 5.3 (4.0‐7.0) | 97.7a | 98 | 98 | NA | |
| PN | 91 | 4.5 (4.0‐7.0) | 94.5a | 94 | 86 | NA | ||||
| Carini et al (2006)25 | PN | 71 | 6 | 4.5 (4.0‐7.0) | 95.8a | NA | 85.1 | 87.2 | ||
| Peycelon et al (2009)26 | PN | 45 | 5.9 | 5.6b (4.1‐10) | 90.2a | 92 | 92 | 81 | ||
| Present study | CIRT | 4 | 7.1 | 5.2 (5.0‐6.5) | 100 | 66.7 | 100 | 100 | ||
| Advanced | Stage | |||||||||
| Margulis et al (2007)27 | T4 | RN | 18 | 2.7 | <pT4 | NA | 28 (3y) | 65 (3y) | NA | |
| 12 | pT4 | NA | 10 (3y) | 22 (3y) | NA | |||||
| Karellas et al (2009)28 | T3/4 | RN | 38 | 1.1 | 11.0 (8.0‐14.0) | NA | NA | NA | 2.6a | |
| Stewart et al (2012)29 | T3 | RN | 77 | 1.5 | 7.0 (2.5‐17.0) | NA | 44.6 | 62.6 | 54.2 | |
| Present study | CIRT | 4 | 8.5 | 6.5 (3.9‐12.0) | 100 | 25 | 100 | 100 | ||
CA, cryoablation; CIRT, carbon‐ion radiotherapy; CSS, cancer‐specific survival; DFS, disease‐free survival; LC, local control; NA, not available; OS, overall survival; PN, partial nephrectomy; RFA, radiofrequency ablation; RN, radical nephrectomy.
Crude rate.
Mean.
There were limitations to the present study. First, this study was retrospectively conducted at a single institution and consisted of a limited number of patients; a multi‐institutional prospective study with long‐term follow‐up data is needed to definitively determine the provisional efficacy of CIRT for RCC, followed by randomized control trials. Second, imaging diagnoses were carried out without biopsy for 11 of the 19 patients in the pooled cohort. Although imaging diagnoses were conducted by more than 1 radiologist in this study, discrepancies between imaging and pathological results are possible30, 31 and, treatment effect therefore may have been overestimated.
In conclusion, this retrospective study with updated long‐term follow‐up data suggests that CIRT is a safe treatment option for RCC patients without definitive renal comorbidities, yielding favorable treatment outcomes even in inoperable cases.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
AUTHOR CONTRIBUTIONS
Goro Kasuya, Hiroshi Tsuji, Takuma Nomiya and Hirokazu Makishima designed the study, assembled the data, carried out the statistical analyses and interpretation, and wrote the manuscript. Yasuo Haruyama and Gen Kobashi carried out the statistical analyses and interpretation. Tokuhiko Omatsu and Riwa Kishimoto, who are radiologists, determined the imaging diagnosis and wrote the manuscript. Daniel K. Ebner, Kazuhiko Hayashi, Tatsuo Igarashi, Shigeo Yasuda, Mototsugu Oya, Koichiro Akakura, Hiroyoshi Suzuki, Tomohiko Ichikawa, and Jun Shimazaki interpreted the data and wrote the manuscript. Tadashi Kamada treated the patients and helped revise the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We wish to express our deep appreciation to the members of the working goup for Genitourinay Tumors.
Kasuya G, Tsuji H, Nomiya T, et al. Updated long‐term outcomes after carbon‐ion radiotherapy for primary renal cell carcinoma. Cancer Sci. 2018;109:2873–2880. 10.1111/cas.13727
Funding information
This work was supported by the Research Project for Heavy Ions at the National Institute of Radiological Sciences, Japan.
REFERENCES
- 1. Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:804‐834. [DOI] [PubMed] [Google Scholar]
- 2. Zini L, Perrotte P, Capitanio U, et al. Radical versus partial nephrectomy: effect on overall and noncancer mortality. Cancer. 2009;115:1465‐1471. [DOI] [PubMed] [Google Scholar]
- 3. Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron‐sparing surgery and radical nephrectomy for low‐stage renal cell carcinoma. Eur Urol. 2011;59:543‐552. [DOI] [PubMed] [Google Scholar]
- 4. Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271‐1279. [DOI] [PubMed] [Google Scholar]
- 5. Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta‐analysis. Cancer. 2008;113:2671‐2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siva S, Pham D, Kron T, et al. Stereotactic ablative body radiotherapy for inoperable primary kidney cancer: a prospective clinical trial. BJU Int. 2017;120:623‐630. [DOI] [PubMed] [Google Scholar]
- 7. Siva S, Louie AV, Warner A, et al. Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: A report from the International Radiosurgery Oncology Consortium for Kidney (IROCK). Cancer. 2018;124:934‐942. [DOI] [PubMed] [Google Scholar]
- 8. Pham D, Thompson A, Kron T, et al. Stereotactic ablative body radiation therapy for primary kidney cancer: a 3‐dimensional conformal technique associated with low rates of early toxicity. Int J Radiat Oncol Biol Phys. 2014;90:1061‐1068. [DOI] [PubMed] [Google Scholar]
- 9. Ponsky L, Lo SS, Zhang Y, et al. Phase I dose‐escalation study of stereotactic body radiotherapy (SBRT) for poor surgical candidates with localized renal cell carcinoma. Radiother Oncol. 2015;117:183‐187. [DOI] [PubMed] [Google Scholar]
- 10. Kanai T, Furusawa Y, Fukutsu K, et al. Irradiation of mixed beam and design of spread‐out Bragg peak for heavy‐ion radiotherapy. Radiat Res. 1997;147:78‐85. [PubMed] [Google Scholar]
- 11. Kanai T, Matsufuji N, Miyamoto T, et al. Examination of GyE system for HIMAC carbon therapy. Int J Radiat Oncol Biol Phys. 2006;64:650‐656. [DOI] [PubMed] [Google Scholar]
- 12. Nomiya T, Tsuji H, Hirasawa N, et al. Carbon ion radiation therapy for primary renal cell carcinoma: initial clinical experience. Int J Radiat Oncol Biol Phys. 2008;72:828‐833. [DOI] [PubMed] [Google Scholar]
- 13. International Union Against Cancer . TNM Classification of Malignant Tumours (7th ed.). New York, NY: Wiley‐Liss; 2009. [Google Scholar]
- 14. World Medical Association Declaration of Helsinki . Ethical principles for medical research involving human subjects. JAMA. 2000;284:3043‐3045. [PubMed] [Google Scholar]
- 15. European Society of Urogenital Radiology . Guidelines on Contrast Media. Available at http://www.esur.org/guidelines/. Accessed July 21, 2018.
- 16. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982‐992. [DOI] [PubMed] [Google Scholar]
- 17. National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 2010. Available at: https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed July 21, 2018.
- 18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 19. Siva S, Jackson P, Kron T, et al. Impact of stereotactic radiotherapy on kidney function in primary renal cell carcinoma: Establishing a dose‐response relationship. Radiother Oncol. 2016;118:540‐546. [DOI] [PubMed] [Google Scholar]
- 20. Simmons MN, Weight CJ, Gill IS. Laparoscopic radical versus partial nephrectomy for tumors >4 cm: intermediate‐term oncologic and functional outcomes. Urology. 2009;73:1077‐1082. [DOI] [PubMed] [Google Scholar]
- 21. Davol PE, Fulmer BR, Rukstalis DB. Long‐term results of cryoablation for renal cancer and complex renal masses. Urology. 2006;68:2‐6. [DOI] [PubMed] [Google Scholar]
- 22. Olweny EO, Park SK, Tan YK, Best SL, Trimmer C, Cadeddu JA. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow‐up. Eur Urol. 2012;61:1156‐1161. [DOI] [PubMed] [Google Scholar]
- 23. Lorber G, Glamore M, Doshi M, Jorda M, Morillo‐Burgos G, Leveillee RJ. Long‐term oncologic outcomes following radiofrequency ablation with real‐time temperature monitoring for T1a renal cell cancer. Urol Oncol. 2014;32:1017‐1023. [DOI] [PubMed] [Google Scholar]
- 24. Leibovich BC, Blute M, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066‐1070. [DOI] [PubMed] [Google Scholar]
- 25. Carini M, Minervini A, Lapini A, Masieri L, Serni S. Simple enucleation for the treatment of renal cell carcinoma between 4 and 7 cm in greatest dimension: progression and long‐term survival. J Urol. 2006;175:2022‐2026. [DOI] [PubMed] [Google Scholar]
- 26. Peycelon M, Hupertan V, Comperat E, et al. Long‐term outcomes after nephron sparing surgery for renal cell carcinoma larger than 4 cm. J Urol. 2009;181:35‐41. [DOI] [PubMed] [Google Scholar]
- 27. Margulis V, Sánchez‐Ortiz RF, Tamboli P, Cohen DD, Swanson DA, Wood CG. Renal cell carcinoma clinically involving adjacent organs: experience with aggressive surgical management. Cancer. 2007;109:2025‐2030. [DOI] [PubMed] [Google Scholar]
- 28. Karellas ME, Jang TL, Kagiwada MA, Kinnaman MD, Jarnagin WR, Russo P. Advanced‐stage renal cell carcinoma treated by radical nephrectomy and adjacent organ or structure resection. BJU Int. 2009;103:160‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stewart GD, Ang WJ, Laird A, Tolley DA, Riddick AC, McNeill SA. The operative safety and oncological outcomes of laparoscopic nephrectomy for T3 renal cell cancer. BJU Int. 2012;110:884‐890. [DOI] [PubMed] [Google Scholar]
- 30. Lee‐Felker SA, Felker ER, Tan N, et al. Qualitative and quantitative MDCT features for differentiating clear cell renal cell carcinoma from other solid renal cortical masses. AJR Am J Roentgenol. 2014;203:W516‐W524. [DOI] [PubMed] [Google Scholar]
- 31. Young JR, Coy H, Kim HJ, et al. Performance of relative enhancement on multiphasic MRI for the differentiation of clear cell renal cell carcinoma (RCC) from papillary and chromophobe RCC subtypes and oncocytoma. Am J Roentgenol. 2017;208:812‐819. [DOI] [PubMed] [Google Scholar]


