Abstract
Myeloid‐derived suppressor cells (MDSC) are a heterogeneous population of immature myeloid cells which consist of 2 subsets: granulocytic MDSC (G‐MDSC) and monocytic MDSC (M‐MDSC). MDSC expand in tumor‐bearing hosts and contribute to immunotherapeutic resistance by remarkably blocking effector T‐cell activation via different mechanisms. Resveratrol (RSV) is a polyphenol and it has been widely used for its various health benefits. However, the underlying mechanism of its anti‐tumor properties remains unclear. In this study, a transplantable mouse model was used to investigate the effects of RSV on MDSC. The results showed that RSV ameliorated tumor development by decreasing G‐MDSC accumulation, impairing its suppressive ability on CD8+T cells and promoting M‐MDSC differentiation into CD11c+ and F4/80+ cells. Our results indicated that RSV should be considered as a modular of MDSC suppressive function and that RSV is a novel booster for tumor immunotherapy.
Keywords: HMGB1, macrophage, myeloid‐derived suppressor cells, resveratrol, tumor immunotherapy
1. INTRODUCTION
Cancer is a challenging disease to treat and is associated with infection and inflammation. The host immune system plays an important role in tumor growth and progression. It has become evident that tumor‐elicited immunosuppression is one of the main reasons for tumor evasion of immune surveillance.1 Tumor‐induced immunosuppressive factors that can suppress normal functions of effector T cells are thought to be one of the key reasons for limitations of cancer immunotherapy.2 Therefore, abolishing tumor‐induced immunosuppressive factors on effector T cells is a promising cancer immunotherapeutic strategy.
It has been reported that myeloid‐derived suppressor cells (MDSC), which expand in tumor‐bearing individuals, mediate immunosuppression through inhibiting NK and T cell functions.3 MDSC are defined by their ability to suppress innate and adaptive immunity. They are originated from myeloid progenitor cells and comprise a heterogeneous population of immature myeloid cells, in contrast to other fully differentiated myeloid cells. Their phenotype and functions may change with tumor progression4 and are classically divided into 2 major subsets in mice: monocytic (M‐MDSC) of the phenotype CD11b+Ly6G−Ly6Chi and granulocytic (G‐MDSC) with the expression profile CD11b+Ly6G+Ly6Clow. 5, 6 It is clear that human MDSC exhibit a great inconsistency in the phenotype of both M‐MDSC (CD11b+ CD14+ CD15−IL4Rα+ HLA‐DRlow CD33+) and G‐MDSC (CD11b+ CD14−CD15+ HLA−DRlow/−CD33+).7, 8 Accumulated evidence indicates that G‐MDSC are the main subset of MDSC, which represent more than 80% of MDSC,9 and immune suppression is a main function of MDSC. The 2 subsets utilize different mechanisms to suppress T cell function. M‐MDSC use nitric oxide synthase 2 (NOS2) and reactive oxygen species (ROS); however, G‐MDSC use ROS and the enzyme arginase 1 (Arg‐1).10, 11 Therefore, it has been proposed that reducing the number or abrogating the suppressive activity of MDSC might have therapeutic effects for cancers.
Resveratrol (RSV) is a pleiotropic phytochemical found in peanuts and grapes, and has been indicated to provide a wide range of health benefits, such as reducing oxidative, inflammatory and apoptotic signals12 protecting against neurological decline,13 improving cardiovascular health,14 ameliorating diabetes15 and preventing cancers.16 The anti‐cancer properties of RSV through diverse molecular mechanisms have been investigated in a plethora of cellular and animal models but have still not been well elucidated.17 RSV has also been suggested to activate some immune cells, including macrophages and effector T cells, enhancing its anti‐tumor effects.18, 19 Whether RSV could regulate MDSC through direct cytotoxicity or by impairing its promoting‐tumor effects remains unclear. Therefore, the present work addresses the above questions. Our results showed that the administration of RSV to tumor‐bearing mice could reduce G‐MDSC accumulation in vivo. In vitro, RSV could contribute to the apoptosis of G‐MDSC, impair G‐MDSC immunosuppressive capacity and enhance CTL. Furthermore, RSV could boost the maturation of M‐MDSC and eventually delay tumor progression. These findings indicate that RSV might be a modular of MDSC suppressive function and that RSV could be beneficial for anti‐tumor immunity.
2. MATERIALS AND METHODS
2.1. Cell lines, mice and tumor models
The Lewis lung carcinoma (LLC) was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The LLC cell line was cultured with DMEM supplemented with 10% FBS (Hyclone, Logan, UT, USA) in an incubator maintained at 37°C and 5% CO2.
Specific pathogen‐free male C57BL/6 mice (6‐8 weeks old) were purchased from the Animal Research Center of Jiangsu University (Zhenjiang, China) and were maintained in compliance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No.85‐23, revised 1996). All experimental protocols were approved by the Institutional Committee on the Use of Animals for Research and Teaching.
To establish tumor models, C57BL/6 mice were inoculated subcutaneously in the flank with LLC cells (1 × 106/mouse) in 200 μL of PBS, respectively. After tumor cell injection, the mice were randomized into 2 groups. They were orally treated with 200 μL of RSV (5 mg/mL in PBS; total 1 mg) or 200 μL of PBS every day with an intragastric gavage needle for 3 weeks. Tumor growth was monitored with bidirectional tumor measurements using a caliper every 2 days, and tumor volumes were calculated using the formula V = 1/2ab2, where V is the volume, a is the length and b is the width. All tumors were weighed when mice were killed. Their spleens, tumors and draining lymph nodes (dLN) were collected. Subsequently, cell suspensions of these tissues were prepared for flow cytometry analysis of the MDSC population.
2.2. Drug preparation
For in vivo analysis, carboxymethylcellulose sodium (Aladdin, Shanghai, China) was used to promote the dissolution of RSV (Aladdin, Shanghai, China); then the drug was sequentially diluted to the appropriate concentration in PBS. For in vitro analysis, RSV was dissolved as a 100‐μmol/L stock solution in DMSO.
2.3. Spleen, tumor and draining lymph node cell isolation
After the tumor challenge, all mice were killed and their spleens, tumors and dLN were dissected immediately. All fresh tissues were minced in small pieces with scissors and washed in PBS. Then chopped neoplastic tissues were further digested in serum‐free medium contain Collagenase I, Deoxyribonuclease I and Hyaluronidase (Sigma Aldrich, St. Louis, MO, USA). The mixture was incubated at 37°C for 2 hours. After assimilation, all fragments of tissues, including spleens, tumors and dLN, were filtered through a 70‐mm nylon cell strainer and erythrocytes were removed by ACK lysis. Finally, all cell suspensions were pelleted and re‐suspended in PBS for flow cytometry analysis.
2.4. Myeloid‐derived suppressor cell isolation
According to the manufacturer's instructions, murine G‐MDSC and M‐MDSC were sorted from splenocytes of LLC‐bearing mice with a Mouse MDSC Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and the MS column. Their purity was confirmed by measuring the expression of CD11b, Ly6G and Ly6C with flow cytometry. The frequency of both CD11b+Ly6G+ cells and CD11b+Ly6C+ cells was >95% (Supplementary Figure S1).
2.5. Apoptotic assay
Isolated G‐MDSC (2 × 106 cells/well) were seeded in 24‐well plates and treated with RSV in various concentrations (0, 1, 10, 20, 50 and 100 μmol/L) for 6 and 12 hours, respectively. Cells were harvested and washed in PBS. The levels of apoptosis were examined using an Annexin‐V/7‐AAD apoptosis kit (MultiSciences [Lianke] Biotech, Hangzhou, China) following the manufacturer's protocol by flow cytometry.
2.6. Transwell migration assay
Following previous methods,20 G‐MDSC migration assays were performed using a 24‐well transwell chamber with polycarbonate filter (diameter, 10 mm; pore size, 8.0 μm; Costar, Cambridge, MA, USA). Freshly isolated G‐MDSC were seeded in the upper chamber. The lower chamber included the preconditioned tumor cell supernatant, which was used as a chemoattractant. To obtain chemotactic factors, LLC cells (2 × 105 cells per well) were first seeded in 24‐well plates and stimulated with or without RSV in indicated concentrations for 24 hours at 37°C in 5% CO2 humidified atmosphere. After incubation, LLC cells in different groups were collected and counted again. Then, equivalent amounts of cells were repeatedly added into the wells and sequentially cultured with serum‐free DMEM medium for 24 hours. After 6 hours of migration, the immigrated cells were wiped off with a wet cotton swab and the membranes were fixed, stained with crystal violet, counted and imaged. Images from 5 microscopic fields of each membrane were selected randomly to obtain the mean cell number. Experiments were independently repeated 3 times.
2.7. Flow cytometry analysis
For surface markers, cells from tissues, sorted G‐MDSC and CD8+T cells were stained with various fluorochrome‐conjugated CD11b (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), Gr‐1, Ly6G, Ly6C, CD11C and F4/80 (BioLegend, San Diego, CA, USA) in PBS for 30 minutes at 4°C. For detection of cytoplasmic cytokine expression of CD8+T cells, single‐cell suspensions were stimulated using the eBioscience Cell Stimulation Cocktail (500×) (a mixture of PMA and ionomycin; eBioscience, San Diego, CA, USA) according to the manufacturer's instructions. After 5 hours, cells were harvested and stained with PE‐conjugated CD8 (BioLegend). Cells were then fixed, permeabilized and stained with FITC‐conjugated IFN‐γ (BioLegend). All samples were analyzed using the FACSCalibur Flow Cytometer (Becton, Dickinson and Company).
2.8. Western blot
Lewis lung carcinoma cells and G‐MDSC were lysed in radioimmunoprecipitation (RIPA) buffer (Auragene Bioscience, Hunan, China). The extracted protein samples were separated by 10% or 12% SDS‐PAGE and transferred onto polyvinylidene fluoride (PVDF) transfer membranes (Bio‐Rad, Hercules, CA, USA). After blocking with 5% (w/v) skimmed milk for 2 hours on a shaker at room temperature, membranes were incubated overnight at 4°C with primary antibodies specific for HMGB1, Arginase‐1 (Arg‐1), total caspase 3, cleaved caspase‐3, total stat 3 (rabbit; CST, Danvers, MA, USA), Bax, Bcl‐2 (Santa Cruz Biotechnology, Shanghai, China) and β‐actin (Abcam, Shanghai, China). Then, membranes were washed with TBS supplemented 5% Tween 20 (TBST) (for 20 minutes, 3 times) and subjected to secondary HRP‐labeled goat anti‐rabbit/mouse antibodies (Abcam Cambridge, UK). Detection was carried out with Luminescent Image Analyzer (Image Quant LAS4000mini, GE Healthcare, China) and relevant blots were quantified by densitometry using the Gel‐Pro 32 software.
2.9. Reactive oxygen species staining
Granulocytic myeloid‐derived suppressor cells from RSV‐treated or untreated groups were incubated with PMA (30 ng/mL) and oxidation‐sensitive dye 2, 7‐dichlorofluorescin diacetate (DCFDA, 2.5 nmol/L, Invitrogen, Carlsbad, CA, USA) for 1 hour. After that, ROS produced by G‐MDSC were detected by flow cytometry and the fluorescence intensity was analyzed using FlowJo software.
2.10. T‐cell proliferation assay
Granulocytic myeloid‐derived suppressor cells isolated from spleens of tumor‐bearing mice were cultured with RSV in different concentrations overnight. After incubation, G‐MDSC in different groups were collected, washed, counted again and then used for co‐culture with CD8+T cells. CD8+T cells were sorted from the splenocytes of wild‐type C57BL/6 mice with a CD8+T Cell Isolation Kit (Miltenyi Biotec) according to the manufacturer's instructions. The purity of CD8+ T cells was >95%. For the T‐cell proliferation assay, CD8+T cells were incubated with CFSE (1 μmol/L, Thermo Fisher Scientific, Shanghai, China) for 20 minutes, and then equal volumes of serum‐free RPMI‐1640 medium were added to neutralize the combination. After 5 minutes, CD8+T cells were plated onto the U‐bottomed 96‐well plates (Costar) pre‐coated with anti‐CD3 (5 μg/mL; BioLegend) and anti‐CD28 mAbs (2 μg/mL; Biolegend) in triplicate and co‐cultured with different ratios of preconditioned G‐MDSC. After 2 days, cells were harvested and stained with PE‐conjugated antibodies against surface markers CD8 (BioLegend). Cell proliferation was evaluated by flow cytometry after gating on the CD8+T population.
2.11. ELISA assay
After co‐culture of G‐MDSC stimulated by RSV and activated CD8+T cells mentioned above, granzyme B concentration in the cell culture supernatants was detected using the Mouse Granzyme B ELISA kit (MultiSciences [Lianke] Biotech) following the manufacturer's instructions.
2.12. Statistical analysis
All statistical analyses were carried out using GraphPad Prism 5. Results were presented in this study as the mean ± SEM from at least 3 independent experiments. Paired t tests and one‐way ANOVA were performed to compare the differences between 2 or multiple groups. A log‐rank (Mantel‐Cox) statistical test was applied to analyze the survival of tumor‐bearing mice. P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Resveratrol decreased granulocytic‐myeloid‐derived suppressor cell accumulation and promoted CD8+IFN‐γ+ cell expansion in Lewis lung carcinoma‐bearing mice
Previous studies have demonstrated that RSV has direct antiproliferative effects, as well as the ability to inhibit the initiation and development of cancer in a wide variety of tumor models.21 To address RSV influencing MDSC in the present work, C57BL/6 mice were inoculated with LLC cells and treated with PBS or RSV every day for 3 weeks. As shown in Figure 1A, RSV significantly extended survival time and the tumor weights were significantly reduced compared with the untreated group (Figure 1B). The percentages of T‐MDSC (CD11b+Gr‐1+ cells) and G‐MDSC (CD11b+Ly6G+) were significantly decreased in cancer tissue compared with the untreated group; conversely, the proportions of M‐MDSC (CD11b+Ly6C+) showed no difference in cancer tissue (Figure 1C); similar phenomena were detected in spleens (Figure 1D). Furthermore, RSV could significantly increase the proportion of CD8+IFN‐γ+ cells priming in spleens, tumor tissues and dLN compared with the untreated group (Figure 1E). Similar data were obtained from CT26 and 4T1‐bearing mice (data not shown). Taken together, the results suggested that RSV ameliorated the cancer development, decreased the G‐MDSC population and promoted CD8+IFN‐γ+ cell expansion in tumor‐bearing mice.
Figure 1.

Resveratrol (RSV) delayed Lewis lung carcinoma (LLC)‐bearing mice progression, decreased granulocytic myeloid‐derived suppressor cells (G‐MDSC) population and promoted CD8+ IFN‐γ+ cells expansion. 1 × 106 LLC cells were subcutaneously (s.c.) inoculated into the flank of C57BL/6 mice to generate tumors. After cells inoculation, the mice were treated with 200 μL phosphate‐buffered saline or RSV (50 mg/kg) every day for 3 wk. All mice were killed and single‐cell suspensions from spleens, tumors, and draining lymph nodes (dLN) were collected and analyzed by flow cytometry. 6 mice were included in every group. A, Survival of mice in different groups (B) Endpoint tumor weight in grams was detected. C, The proportion of T‐MDSC (CD11b+Gr‐1+), G‐MDSC (CD11b+Ly6G+) and M‐MDSC (CD11b+Ly6C+) in cancer tissue. D, The proportion of T‐MDSC(CD11b+Gr‐1+) and G‐MDSC (CD11b+Ly6G+) in spleens. E, The frequencies of CD8+ IFN‐γ+ cells in spleens, tumors and dLN were examined. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01; NS, no significance
3.2. Resveratrol decreased granulocytic‐myeloid‐derived suppressor cell accumulation by triggering its apoptosis and decreased recruitment
Next, to determine how RSV decreased G‐MDSC accumulation, first, to confirm whether RSV directly affected the survival of G‐MDSC, RSV was used to treat G‐MDSC. As Figure 2A shows, RSV significantly induced G‐MDSC apoptosis at 100 μmol/L. For the 6‐hour treatment, RSV induced early apoptosis; conversely, for the 12‐hour treatment, RSV mainly induced late apoptosis. In line with these results, the expressions of Bax and cleaved caspase‐3 were markedly increased following RSV treatment, whereas Bcl‐2 was significantly decreased (Figure 2B). Furthermore, we confirmed that 50 or 100 μmol/L RSV did not have obvious effects on normal cells survival (Supplementary Figure S2).
Figure 2.
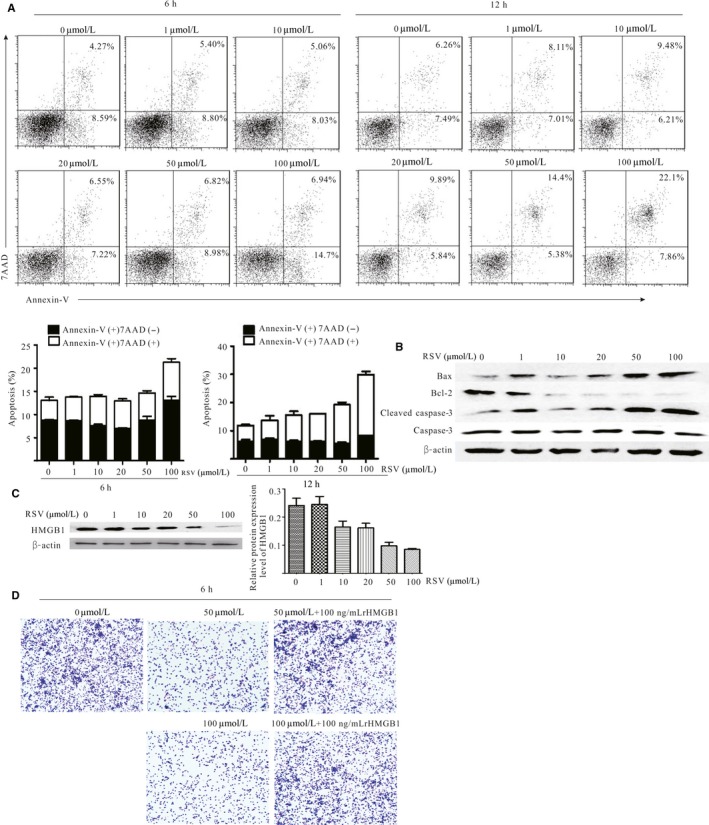
Resveratrol (RSV) decreased granulocytic myeloid‐derived suppressor cell (G‐MDSC) accumulation by triggering its apoptosis and decreased the recruitment. Freshly isolated G‐MDSC were treated with indicated doses of RSV for 6 and 12 h, respectively. The cells were subsequently stained with Annexin‐V and 7‐AAD to detect the apoptosis of G‐MDSC using flow cytometry. A, RSV induced G‐MDSC apoptosis. The average percentage of apoptotic cells (Annexin‐V+/7‐AAD − and Annexin‐V+/7‐AAD +) was calculated, as shown in the histogram. B, The expression of Bax, Bcl‐2, caspase 3 and cleaved caspase‐3 in G‐MDSC after RSV exposure for 6 h. C, The expression of HMGB1 in Lewis lung carcinoma (LLC) cells was determined by western blot; β‐actin was examined as a loading control. LLC cells were cultured with RSV in various concentrations (0, 1, 10, 20, 50 and 100 μmol/L). The representative blots showed left and densitometric analysis showed right. Data are means ± SD from 3 independent experiments. D, Migrated G‐MDSC were imaged as the average number of cells per field of view. G‐MDSC were sorted from spleens of LLC tumor‐bearing mice and co‐cultured with the preconditioned LLC cells culturing supernatant for 6 h using a Transwell insert. All the data were obtained from 3 independent experiments
Published studies also indicate that high mobility group box 1 (HMGB1) could promote MDSC expansion in different conditions22, 23, 24 and could also be downregulated by RSV in various diseases.25, 26, 27 Therefore, we speculated that the decrease in G‐MDSC accumulation might be associated with HMGB1 downregulation in RSV‐treated cancer‐bearing mice. To confirm the hypothesis, HMGB1 expression in LLC cells treated with RSV was detected. As Figure 2C shows, HMGB1 expression was obviously downregulated in a concentration‐dependent manner; furthermore, LLC cell culture supernatant following RSV treatment significantly inhibited the migration of G‐MDSC, and rHMGB1 partially reversed the inhibitory effect of supernatant from RSV‐treated cancer cells (Figure 2D). Taken together, RSV not only directly induced G‐MDSC apoptosis but also indirectly inhibited the migration of G‐MDSC, which may be associated with downregulating HMGB1 expression in cancer cells.
3.3. Resveratrol impaired the suppressive capability of granulocytic‐myeloid‐derived suppressor cell on CD8+T cells
The key characteristic of MDSC is their ability to suppress T‐cell‐induced anti‐tumor immune responses and the immunosuppressive activity of G‐MDSC has been linked to Arg‐1 expression and ROS production.28 Therefore, first, the levels of Arg‐1 and ROS were detected. As Figure 3A,B shows, the protein level of Arg‐1 and the production of ROS were strikingly decreased after RSV exposure at 50 and 100 μmol/L. Furthermore, to determine the direct suppressive function of G‐MDSC on CD8+T cells, CD8+T cells were isolated from the spleens of C57BL/6 mice and co‐cultured with G‐MDSC at 3 different ratios (CD8+T : G‐MDSC = 4:1, 2:1 and 1:1). As Figure 3C shows, CD8+T cell proliferation was increased in the RSV‐treated group compared with the untreated group (CD8+T : G‐MDSC = 4:1), which suggested that the suppressive ability of G‐MDSC on CD8+T cells was impaired after RSV treatment; the secretion of granzyme B produced by CD8+T cells was also significantly upregulated compared with the untreated group (Figure 3D).Taken together, RSV impaired the suppressive capability of G‐MDSC on CD8+T cells.
Figure 3.
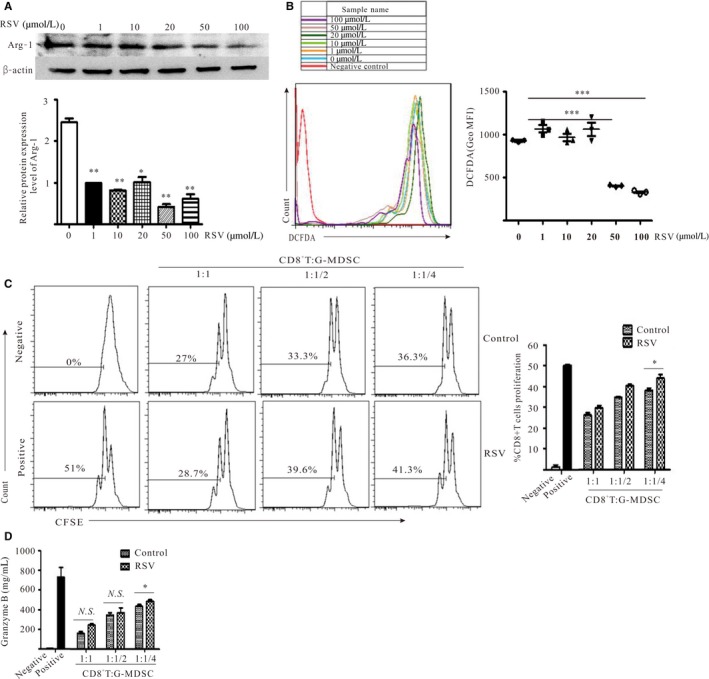
Resveratrol (RSV) impaired the suppressive capability of granulocytic myeloid‐derived suppressor cells (G‐MDSC) on CD8+T cells. G‐MDSC isolated from spleens of Lewis lung carcinoma‐bearing mice were treated with RSV at indicated concentrations for 12 h. A, Arg‐1 protein levels in G‐MDSC was assayed using western blot. B, Production of ROS in G‐MDSC was measured by flow cytometry. C, RSV ameliorated the suppressive ability of G‐MDSC on CD8+T cells. G‐MDSC treated with or without RSV (50 μmol/L) for 12 h were harvested and co‐cultured with CD8+T cells isolated from wild‐type C57BL/6 mice at different ratios (CD8+T : G‐MDSC = 4:1, 2:1 and 1:1) in the presence of anti‐CD3 mAb and anti‐CD8 mAb. After 48 h, CD8+T cell proliferation was determined by measuring the dilution of CFSE using flow cytometry. D, The levels of granzyme B in culture supernatants from the CD8+T cell proliferation system were detected using ELISA. *P < 0.05, **P < 0.01, ***P < 0.001. Geo MFI, geometric mean fluorescent intensity
3.4. Resveratrol facilitated monocytic‐myeloid‐derived suppressor cells isolated from Lewis lung carcinoma‐bearing mice differentiation into CD11c+ or F4/80+ cells
Although RSV mainly downregulated the G‐MDSC population in LLC‐bearing mice in vivo, our results also indicated that RSV downregulated HMGB1 of cancer cells, which contributed to M‐MDSC differentiation from bone marrow progenitor cells.24, 29 Published studies have also indicated that β‐glucan, a natural compound, can delay tumor progression by partially regulating M‐MDSC differentiation.30 To determine whether RSV also influenced M‐MDSC, M‐MDSC were isolated from splenocytes of LLC‐bearing mice and treated with RSV. Our results showed that the population of CD11c+ and F4/80+ cells was evidently increased following RSV treatment (Figure 4). These results supported that M‐MDSC treated with RSV could differentiate into more mature myeloid cells.
Figure 4.
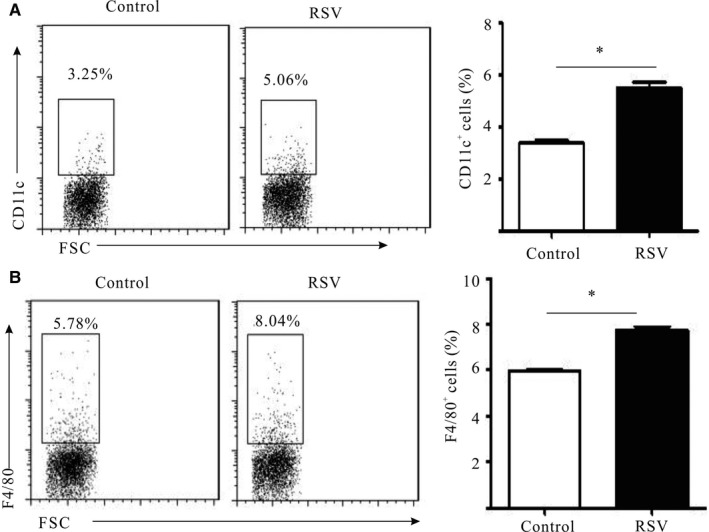
Resveratrol (RSV) facilitated granulocytic myeloid‐derived suppressor cell (G‐MDSC) isolated from Lewis lung carcinoma (LLC)‐bearing mice differentiation into CD11c+ and F4/80+ cells. Monocytic MDSC (M‐MDSC) isolated from splenocytes of LLC‐bearing mice were stimulated with or without RSV (50 μmol/L) for 48 h, and then, cells were collected to analyze the expression of CD11c (A) and F4/80 (B) by flow cytometry. *P < 0.05. The data represented 1 of the 3 experiments that were independently performed and analyzed
4. DISCUSSION
The tumor microenvironment (TME) consists of cancer cells, blood vessels, immune infiltrates, stromal cells and the extracellular matrix, and the complicated milieu promotes immune evasion, tolerance and tumor progression.31 Immune evasion is a hallmark of cancer. However, there are multiple different mechanisms used by cancer cells: for example, myeloid‐derived suppressor cells (MDSC) are among the key drivers of tumor‐mediated immune evasion. In healthy individuals, there are low numbers of MDSC in the circulation, which contribute to regulating the immune responses and tissue repair. However, in the context of cancer, MDSC expand rapidly and are recruited to the TME, where MDSC efficiently suppress T‐cell function and are involved in tumor immune escape. MDSC can be subdivided into M‐MDSC, which lack markers of DC (CD11c) and macrophage (F4/80), and G‐MDSC, which make up the majority of MDSC. Diverse therapeutic methods are being developed to abrogate MDSC‐mediated immune dysfunctions and then to re‐activate host immunity through different mechanisms, including reducing their presence, inhibiting their function, and affecting their development as well as their differentiation.32 Accumulated preclinical studies indicate that multiple therapeutic strategies can be used alone or in combination to circumvent MDSC‐mediated immunosuppression: for example, monoclonal antibodies (anti‐Gr‐1 or anti‐Ly6G), immune checkpoint inhibitors (anti‐CTLA‐4 or anti‐PD‐1), gemcitabine, 5‐FU, ATRA and many other chemical compounds.33, 34 RSV (3, 5, 4′‐trihydroxystilbene) is a natural phytoalexin contained in the skin of grapes, different berries and nuts. It is a non‐flavonoid polyphenol that belongs to the stilbene family. RSV exists in 2 geometric isomers: cis‐RSV (Z) and trans‐RSV (E). RSV has many protective benefits in response to stress, infection and injury, and is a vital chemotherapeutic agent. Recently, an increasing number of studies have focused on the anti‐cancer biological activity of RSV. It has been extensively explored for its ability to both prevent and treat cancers through a variety of mechanisms, such as inhibiting oral squamous carcinoma cell lines by inducing apoptosis and cell cycle arrest during G2/M transition cell, in a time‐dependent and dose‐dependent inhibition A431 human epidermoid carcinoma cell growth.35 This drug has also been found to modulate the status and functions of tumor‐associated macrophages to suppress tumor growth.36, 37 In addition, RSV has the potentiality to influence the MDSC population and function in inflammatory diseases.38, 39
In the present work, we first demonstrated that RSV could decrease the accumulation of MDSC in vivo and in vitro, particularly G‐MDSC. In an LLC‐bearing mouse model, RSV implementation delayed cancer progression, and decreased the frequencies of G‐MDSC in spleens and tumor tissues. In vitro results indicated that RSV attenuated G‐MDSC expansion by selectively inducing apoptosis of G‐MDSC and decreasing the recruitment. Because we did not focus on M‐MDSC in the current study, it is still unclear whether RSV could induce selective apoptosis of M‐MDSC. However, there are controversial results: in inflammatory disease models, it was demonstrated that RSV could upregulate the proportion of MDSC, thereby inhibiting the occurrence of inflammation.38, 39 What led to the different conclusions (eg, different microenvironments) still needs further confirmation. Furthermore, RSV stimulation could inhibit the expression of Arg‐1 and the production of ROS, which led to impairment of G‐MDSC immunosuppressive function. In vitro, the results illustrated that RSV could promote the maturation and differentiation of M‐MDSC; however, there is no significant difference in the proportions of M‐MDSC after RSV administration in vivo. Therefore, we focused on G‐MDSC, which were formed the majority of MDSC in the present work, and found that G‐MDSC pre‐exposure to RSV could significantly improve the suppressive ability on CD8+T cells. Although G‐MDSC and M‐MDSC could both impede T cell responses, the latter was more suppressive.40 Except for immunosuppressive effects on cancer development, more evidence showed that MDSC could accelerate the development of cancers by inducing autophagy, reprogramming metabolic status and supporting cancer cell stemness.41, 42, 43 It remains unclear whether RSV also modulated M‐MDSC in other cancer‐bearing mice models. Therefore, in future, we will consider the influence of RSV on M‐MDSC in different cancer‐bearing models.
In conclusion, in the present work, we first demonstrated that RSV not only decreased the accumulation of G‐MDSC but also abrogated its immunosuppressive ability in LLC‐bearing mice; furthermore, RSV promoted the M‐MDSC differentiation into CD11c+ or macrophage cells (Figure 5). Our results indicated that RSV should be considered as a modulator of MDSC suppressive function and that RSV is a novel booster for tumor immunotherapy.
Figure 5.
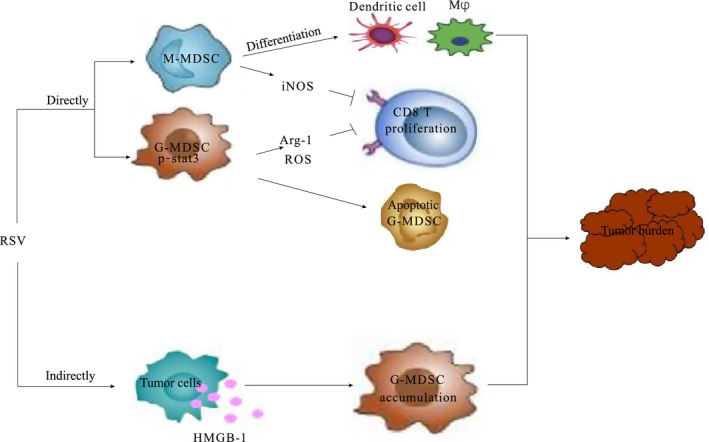
The schematic diagram of resveratrol (RSV) modulating myeloid‐derived suppressor cells (MDSC). RSV augments anti‐tumor activity by regulating development of MDSC. On the one hand, RSV directly triggered granulocytic MDSC (G‐MDSC) apoptotic program or indirectly decreased the accumulation of G‐MDSC by downregulation HMGB1 in TME; on the other hand, RSV delayed tumor progression by impeding the suppressive function of G‐MDSC. Moreover, RSV also could promote M‐MDSC differentiation into CD11c+ and F4/80+
FUNDING
This work was supported by the Key University Science Research Project of Jiangsu Province (Grant No. 16KJA320005); the Social Development Foundation of Jiangsu Province (Grant no. BE2016716); the Six Talent Peaks Project in Jiangsu Province (Grant no. 2015‐WSN‐005); the Maternal and Child Project in Jiangsu Province (Grant no. F201511); and the Medical Development Foundation of Jiangsu University (Grant no. JLY20160051).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Zhao Y, Shao Q, Zhu H, et al. Resveratrol ameliorates Lewis lung carcinoma‐bearing mice development, decreases granulocytic myeloid‐derived suppressor cell accumulation and impairs its suppressive ability. Cancer Sci. 2018;109:2677–2686. 10.1111/cas.13720
Zhao, Shao and Zhu contributed equally to this study
Contributor Information
Yan Wu, Email: jsdxwuyan@163.com.
Zhaoliang Su, Email: szl30@ujs.edu.cn.
REFERENCES
- 1. Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor‐induced immunosuppressive factors paralyze T cells. Oncoimmunology. 2015;4:e1016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Mitri D, Toso A, Alimonti A. Molecular pathways: targeting tumor‐infiltrating myeloid‐derived suppressor cells for cancer therapy. Clin Cancer Res. 2015;21:3108‐3112. [DOI] [PubMed] [Google Scholar]
- 3. Parker KH, Beury DW, Ostrand‐Rosenberg S. Myeloid‐derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res. 2015;128:95‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sato Y, Shimizu K, Shinga J, et al. Characterization of the myeloid‐derived suppressor cell subset regulated by NK cells in malignant lymphoma. Oncoimmunology. 2015;4:e995541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Z, Till B, Gao Q. Chemotherapeutic agent‐mediated elimination of myeloid‐derived suppressor cells. Oncoimmunology. 2017;6:e1331807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Su Z, Ni P, Zhou C, Wang J. Myeloid‐derived suppressor cells in cancers and inflammatory diseases: angel or demon? Scand J Immunol. 2016;84:255‐261. [DOI] [PubMed] [Google Scholar]
- 7. Elliott LA, Doherty GA, Sheahan K, Ryan EJ. Human tumor‐infiltrating myeloid cells: phenotypic and functional diversity. Front Immunol. 2017;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montero AJ, Diaz‐Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid‐derived suppressor cells in cancer patients: a clinical perspective. J Immunother. 2012;35:107‐115. [DOI] [PubMed] [Google Scholar]
- 9. Safarzadeh E, Orangi M, Mohammadi H, Babaie F, Baradaran B. Myeloid‐derived suppressor cells: important contributors to tumor progression and metastasis. J Cell Physiol. 2018;233(4):3024‐3036. [DOI] [PubMed] [Google Scholar]
- 10. Rui K, Tian J, Tang X, et al. Curdlan blocks the immune suppression by myeloid‐derived suppressor cells and reduces tumor burden. Immunol Res. 2016;64:931‐939. [DOI] [PubMed] [Google Scholar]
- 11. Alizadeh D, Trad M, Hanke NT, et al. Doxorubicin eliminates myeloid‐derived suppressor cells and enhances the efficacy of adoptive T‐cell transfer in breast cancer. Cancer Res. 2014;74:104‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J, Yu S, Ying J, Shi T, Wang P. Resveratrol prevents ROS‐induced apoptosis in high glucose‐treated retinal capillary endothelial cells via the activation of AMPK/Sirt1/PGC‐1alpha pathway. Oxid Med Cell Longev. 2017;2017:7584691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bastianetto S, Menard C, Quirion R. Neuroprotective action of resveratrol. Biochim Biophys Acta. 2015;1852:1195‐1201. [DOI] [PubMed] [Google Scholar]
- 14. Zordoky BN, Robertson IM, Dyck JR. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim Biophys Acta. 2015;1852:1155‐1177. [DOI] [PubMed] [Google Scholar]
- 15. Szkudelski T, Szkudelska K. Resveratrol and diabetes: from animal to human studies. Biochim Biophys Acta. 2015;1852:1145‐1154. [DOI] [PubMed] [Google Scholar]
- 16. Gwak H, Kim S, Dhanasekaran DN, Song YS. Resveratrol triggers ER stress‐mediated apoptosis by disrupting N‐linked glycosylation of proteins in ovarian cancer cells. Cancer Lett. 2016;371:347‐353. [DOI] [PubMed] [Google Scholar]
- 17. Guthrie AR, Chow HS, Martinez JA. Effects of resveratrol on drug‐ and carcinogen‐metabolizing enzymes, implications for cancer prevention. Pharmacol Res Perspect. 2017;5:e00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buttari B, Profumo E, Segoni L, et al. Resveratrol counteracts inflammation in human M1 and M2 macrophages upon challenge with 7‐oxo‐cholesterol: potential therapeutic implications in atherosclerosis. Oxid Med Cell Longev. 2014;2014:257543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P. Resveratrol (trans‐3,5,4′‐trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol. 2007;72:1508‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li ZL, Ye SB, OuYang LY, et al. COX‐2 promotes metastasis in nasopharyngeal carcinoma by mediating interactions between cancer cells and myeloid‐derived suppressor cells. Oncoimmunology. 2015;4:e1044712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siddiqui IA, Sanna V, Ahmad N, Sechi M, Mukhtar H. Resveratrol nanoformulation for cancer prevention and therapy. Ann N Y Acad Sci. 2015;1348:20‐31. [DOI] [PubMed] [Google Scholar]
- 22. Ruan X, Darwiche SS, Cai C, Scott MJ, Pape HC, Billiar TR. Anti‐HMGB1 monoclonal antibody ameliorates immunosuppression after peripheral tissue trauma: attenuated T‐lymphocyte response and increased splenic CD11b+Gr‐1+ myeloid‐derived suppressor cells require HMGB1. Mediators Inflamm. 2015;2015:458626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li W, Wu K, Zhao E, et al. HMGB1 recruits myeloid derived suppressor cells to promote peritoneal dissemination of colon cancer after resection. Biochem Biophys Res Commun. 2013;436:156‐161. [DOI] [PubMed] [Google Scholar]
- 24. Su Z, Ni P, She P, et al. Bio‐HMGB1 from breast cancer contributes to M‐MDSC differentiation from bone marrow progenitor cells and facilitates conversion of monocytes into MDSC‐like cells. Cancer Immunol Immunother. 2017;66:391‐401. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25. Zainal N, Chang CP, Cheng YL, et al. Resveratrol treatment reveals a novel role for HMGB1 in regulation of the type 1 interferon response in dengue virus infection. Sci Rep. 2017;7:42998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu H, Sheng ZQ, Xie J, et al. Reduced HMGB 1‐mediated pathway and oxidative stress in resveratrol‐treated diabetic mice: a possible mechanism of cardioprotection of resveratrol in diabetes mellitus. Oxid Med Cell Longev. 2016;2016:9836860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karuppagounder V, Arumugam S, Thandavarayan RA, et al. Resveratrol attenuates HMGB1 signaling and inflammation in house dust mite‐induced atopic dermatitis in mice. Int Immunopharmacol. 2014;23:617‐623. [DOI] [PubMed] [Google Scholar]
- 28. Katoh H, Watanabe M. Myeloid‐derived suppressor cells and therapeutic strategies in cancer. Mediators Inflamm. 2015;2015:159269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parker KH, Sinha P, Horn LA, et al. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid‐derived suppressor cells. Cancer Res. 2014;74:5723‐5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Albeituni SH, Ding C, Liu M, et al. Yeast‐derived particulate beta‐glucan treatment subverts the suppression of myeloid‐derived suppressor cells (MDSC) by inducing polymorphonuclear MDSC apoptosis and monocytic MDSC differentiation to APC in cancer. J Immunol. 2016;196:2167‐2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pyzer AR, Cole L, Rosenblatt J, Avigan DE. Myeloid‐derived suppressor cells as effectors of immune suppression in cancer. Int J Cancer. 2016;139:1915‐1926. [DOI] [PubMed] [Google Scholar]
- 32. Marvel D, Gabrilovich DI. Myeloid‐derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356‐3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Srivastava MK, Zhu L, Harris‐White M, et al. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS ONE. 2012;7:e40677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chesney JA, Mitchell RA, Yaddanapudi K. Myeloid‐derived suppressor cells‐a new therapeutic target to overcome resistance to cancer immunotherapy. J Leukoc Biol. 2017;102:727‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Varoni EM, Lo Faro AF, Sharifi‐Rad J, Iriti M. Anticancer molecular mechanisms of resveratrol. Front Nutr. 2016;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kimura Y, Sumiyoshi M. Resveratrol prevents tumor growth and metastasis by inhibiting lymphangiogenesis and M2 macrophage activation and differentiation in tumor‐associated macrophages. Nutr Cancer. 2016;68:667‐678. [DOI] [PubMed] [Google Scholar]
- 37. Jeong SK, Yang K, Park YS, et al. Interferon gamma induced by resveratrol analog, HS‐1793, reverses the properties of tumor associated macrophages. Int Immunopharmacol. 2014;22:303‐310. [DOI] [PubMed] [Google Scholar]
- 38. Singh UP, Singh NP, Singh B, et al. Role of resveratrol‐induced CD11b+Gr‐1+ myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3+ T cells and amelioration of chronic colitis in IL‐10−/− mice. Brain Behav Immun. 2012;26:72‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rieder SA, Nagarkatti P, Nagarkatti M. Multiple anti‐inflammatory pathways triggered by resveratrol lead to amelioration of staphylococcal enterotoxin B‐induced lung injury. Br J Pharmacol. 2012;167:1244‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meirow Y, Kanterman J, Baniyash M. Paving the road to tumor development and spreading: myeloid‐derived suppressor cells are ruling the fate. Front Immunol. 2015;6:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parker KH, Horn LA, Ostrand‐Rosenberg S. High‐mobility group box protein 1 promotes the survival of myeloid‐derived suppressor cells by inducing autophagy. J Leukoc Biol. 2016;100:463‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jian SL, Chen WW, Su YC, et al. Glycolysis regulates the expansion of myeloid‐derived suppressor cells in tumor‐bearing hosts through prevention of ROS‐mediated apoptosis. Cell Death Dis. 2017;8:e2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sica A, Porta C, Amadori A, Pasto A. Tumor‐associated myeloid cells as guiding forces of cancer cell stemness. Cancer Immunol Immunother. 2017;66:1025‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


