Abstract
Chemotherapy‐induced nausea and vomiting (CINV) remains a major adverse event in cancer chemotherapy. Although aprepitant is effective in preventing CINV, an increment in financial burden for uniform use of aprepitant is a concern. The aim of the present study was to define the cost‐effectiveness of aprepitant from the perspective of the Japanese National Health Insurance system. Based on the results of a randomized phase II trial comparing an aprepitant‐containing regimen versus a nonaprepitant regimen in Japanese patients who received cisplatin‐containing highly emetogenic chemotherapy, a decision analytic model was developed. The incremental cost‐effectiveness ratio (ICER) was calculated both in the outpatient care setting (OCS) and in the inpatient care setting (ICS). The use of the aprepitant‐containing regimen was associated with improved quality of life compared with the nonaprepitant regimen, with an increment in quality‐adjusted life years (QALY) of 0.0016. The incremental total medical costs associated with the use of the aprepitant regimen were lower in the OCS than in the ICS, 6192 JPY (56.92 USD) and 9820 JPY (90.27 USD), respectively. The ICER was calculated as 3 906 698 JPY (35 910 USD) per QALY gained in the OCS and 6 195 781 JPY (56 952 USD) per QALY gained in the ICS. Cost‐effectiveness of the aprepitant‐containing antiemetic therapy was limited to the OCS, considering the threshold of willingness‐to‐pay commonly accepted (5 million JPY [45 960 USD] in Japan and 50 000 USD in the USA). The efficacy of aprepitant offsets the costs for revisiting clinics or rehospitalization added with rescue medications in the OCS.
Keywords: chemotherapy‐induced nausea and vomiting, cost‐effectiveness, highly emetogenic chemotherapy, quality‐adjusted life year incremental cost‐effectiveness ratio
1. INTRODUCTION
Chemotherapy‐induced nausea and vomiting (CINV) remains one of the distressing events for patients who receive cancer chemotherapy. It causes reduced oral intake1 and has negative impacts on quality of life (QOL).2, 3 It may even reduce patients’ willingness to continue anticancer treatment.4 Clinical practice guidelines on antiemetics in Japan Society of Clinical Oncology5 classified cisplatin‐based chemotherapies as highly emetogenic chemotherapies (HEC) and recommend aprepitant‐containing 3‐drug regimens, including a 5‐hydroxytryptamine 3 receptor antagonist (5‐HT3RA) and dexamethasone, the same as those of major international organizations, the National Comprehensive Cancer Network (NCCN),6 the Multinational Association of Supportive Care in Cancer (MASCC)/European Society for Medical Oncology (ESMO),7 and the American Society of Clinical Oncology (ASCO).8 Aprepitant, a selective neurokinin‐1 receptor antagonist (NK1RA), inhibits nausea and vomiting signals by blocking the interaction of substance P with the NK‐1 receptor.9, 10, 11 The efficacy of aprepitant for CINV was confirmed in clinical trials globally12, 13, 14 and in Japan,15 and it was approved by the FDA in the USA in 2003 and by the Ministry of Health, Labor, and Welfare in Japan in 2009. Although optimal antiemetic prophylaxis according to emetogenic risk of chemotherapy is important for patients to continue their cancer treatment, the increased financial burden is a concern for aprepitant, which is a costly antiemetic agent. Gomez et al16 reported that socioeconomic barriers associated with NK1RA therapy affected suboptimal adherence to guideline recommendations for antiemetic prophylaxis. Cost‐effectiveness analyses of aprepitant have been reported from 6 countries. Five of them showed a positive result with aprepitant,17, 18, 19, 20, 21 whereas Moore et al22 from the USA reported that aprepitant provides only modest benefits. However, as far as we could ascertain, there are no reports on the cost‐effectiveness of aprepitant in Japan. The objective of the present study was to define, from the Japanese National Health Insurance payer perspective, the cost‐effectiveness of aprepitant for preventing CINV in patients who received cisplatin‐based HEC.
2. METHODS
2.1. Model building
The cost‐effectiveness of the aprepitant‐containing antiemetic regimen was analyzed by comparison with the regimen without aprepitant in patients who received cisplatin‐based HEC. A decision analytic model was developed based on the phase II clinical trial, which verified the effect of aprepitant on CINV for Japanese patients who received cisplatin‐based chemotherapy, as a company sponsored trial for the new drug application in Japan with compliance with Good Clinical Practice.15 Patients who were enrolled in the cited trial were planned to receive chemotherapy including cisplatin (≥70 mg/m2). The aprepitant regimen consisted of the 3‐drug combination of aprepitant, a 5‐HT3RA and dexamethasone (Table 1), which corresponded to antiemetic regimens recommended in current major antiemetic guidelines. The antiemetic regimen without aprepitant consisted of the 2‐drug combination of a 5‐HT3RA and dexamethasone. The decision analytic model was designed to track health outcomes and costs related to episodes of nausea and vomiting. Nine health states were applied in the model and represent all possible pairings from 3 clinical outcomes in the acute and delayed phases of CINV (Figure 1). The clinical outcomes used to classify patients in the model were defined as: complete response (CR) with no emesis and no rescue antiemetic therapy, and incomplete response (IR) with some emesis or use of rescue therapy. CR was further subdivided into 2 mutually exclusive health outcomes: (a) complete protection (CP) with no emesis, no rescue therapy and no significant nausea; (b) complete response at best (CRB) with no emesis and no rescue therapy excluding CP.17, 18, 19, 20, 21
Table 1.
Antiemetic regimens for prevention of CINV used in the model, based on the Japanese phase II trial of aprepitant15 and clinical practice
| Antiemetic regimen | Drugs | Day 1 | Day 2 | Day 3 |
|---|---|---|---|---|
| Aprepitant regimen | Aprepitant p.o. | 125 mg | 80 mg | 80 mg |
| Granisetron i.v. | 40 μg/kg | NA | NA | |
| Dexamethasone i.v. | 6 mg | 4 mg | 4 mg | |
| Nonaprepitant regimen | Granisetron i.v. | 40 μg/kg | NA | NA |
| Dexamethasone i.v. | 12 mg | 8 mg | 8 mg |
CINV, chemotherapy‐induced nausea and vomiting; i.v., intravenous; p.o., oral; NA, not applicable.
Figure 1.
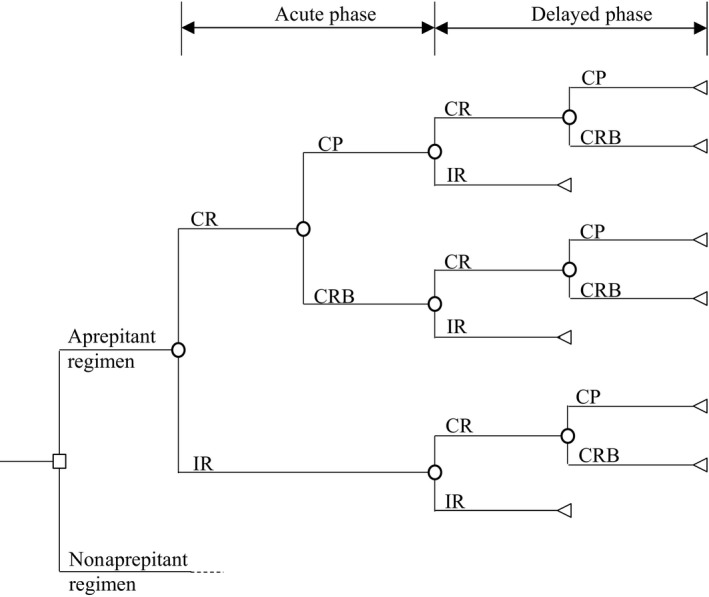
Decision analytic model. The model decision tree depicts 9 possible health states, marked by a triangle, to compare costs and clinical outcomes associated with an aprepitant‐containing antiemetic regimen vs a no aprepitant regimen. CP, complete protection; CR, complete response; CRB, complete response at best; IR, incomplete response
The probabilities of predicting the outcomes from one health state to another were determined using the data from the clinical trial results (Table 2).15 Duration of aprepitant administration was assumed to be 3 days according to the common practical use of aprepitant derived from Protocol 052.13 The dose of dexamethasone in the aprepitant‐containing regimen was assumed to be half of that in the nonaprepitant regimen considering the area under the blood concentration‐time curve (AUC) of dexamethasone, increasing to 2.2‐fold higher as a result of the inhibition of cytochrome P450 (CYP) 3A4 by aprepitant.23 The cost‐effectiveness of the aprepitant‐containing antiemetic regimen compared with that of the nonaprepitant regimen was assessed using the incremental cost‐effectiveness ratio (ICER) at a time horizon of 5 days according to the cited trial15 and duration of CINV.24, 25 The analysis was conducted both in the outpatient care setting (OCS) and in the inpatient care setting (ICS) from the Japanese National Health Insurance system payer perspective.
Table 2.
Health state probabilities used in the model, based on the Japanese phase II trial of aprepitant15
| Health state outcomes by phase | Aprepitant regimen (n = 146) | Nonaprepitant regimen (n = 149) | |
|---|---|---|---|
| Acute phase (day 1) | Delayed phase (days 2‐5) | ||
| % | % | ||
| CP | CP | 61.6 | 43.0 |
| CRB | 5.5 | 6.0 | |
| IR | 16.4 | 32.9 | |
| CRB | CP | 2.1 | 1.3 |
| CRB | 1.4 | 0 | |
| IR | 0 | 0 | |
| IR | CP | 1.4 | 0 |
| CRB | 0 | 1.3 | |
| IR | 11.0 | 15.4 | |
CP, complete protection; CRB, complete response at best; IR, incomplete response.
2.2. Health state outcomes
Health state outcomes were evaluated using quality‐adjusted life years (QALY), calculated for 9 patterns of health conditions according to an established decision analytic model for days 1‐5 on chemotherapy (Table 3). QALY in each treatment group was integrated according to the probability of the health state in the acute phase and in the delayed phase. A utility value of each health state was assigned according to the previous reports,17, 18, 19, 20 and utility values of 0.9, 0.7 and 0.2 were assigned to CP, CRB, and IR, respectively.
Table 3.
Utility values for CINV outcomes
| Health state outcomes by phase | 5‐d QALY | |||
|---|---|---|---|---|
| Acute phase (day 1) | Delayed phase (days 2‐5) | Base case | Lower bound | Upper bound |
| CP | CP | 0.0123 | 0.0096 | 0.0137 |
| CRB | 0.0101 | 0.0073 | 0.0126 | |
| IR | 0.0047 | 0.0035 | 0.0056 | |
| CRB | CP | 0.0118 | 0.0090 | 0.0134 |
| CRB | 0.0096 | 0.0067 | 0.0123 | |
| IR | 0.0041 | 0.0029 | 0.0053 | |
| IR | CP | 0.0104 | 0.0081 | 0.0117 |
| CRB | 0.0082 | 0.0058 | 0.0106 | |
| IR | 0.0027 | 0.0019 | 0.0036 | |
5‐d QALY = ([utility value (acute phase) × 1 d] + [utility value (delayed phase) × 4 d])/365 d. The utility values of 0.9, 0.7 and 0.2 were assigned to CP, CRB, and IR, respectively.
CINV, chemotherapy‐induced nausea and vomiting; CP, complete protection; CRB, complete response at best; IR, incomplete response; QALY, quality‐adjusted life years.
2.3. Cost variables
Drug costs for prophylactic antiemetic therapies and rescue treatments for CINV in each treatment group were determined according to the probability of the health state in the acute phase and in the delayed phase.
Costs for rescue treatments were assessed based on the retrospective review of consumed medical resources from clinical records of patients in Aichi Medical University Hospital who received cisplatin‐containing HEC and a prophylactic antiemetic regimen with or without aprepitant (Table 4). The extracted clinical records were in the time period between the approval of aprepitant by the Japanese government and general use of aprepitant in the oncology clinic of this hospital. During that period, some oncologists were prescribing aprepitant and some were not, and clinical records for both an antiemetic regimen with aprepitant and that without aprepitant were available. Ethics approval for the survey was obtained in advance from the institutional review board.
Table 4.
Estimated costs of medical resources
| Costs JPY (USD) | Min JPY (USD) | Max JPY (USD) | |
|---|---|---|---|
| Druga | |||
| Aprepitant 125 mg (p.o.) | 4972.7 (45.71) | NA | NA |
| Aprepitant 80 mg (p.o.) | 3393 (31.19) | NA | NA |
| Dexamethasone 1.65 mg (i.v.) | 103 (0.95) | NA | NA |
| Dexamethasone 3.3 mg (i.v.) | 181 (1.66) | NA | NA |
| Dexamethasone 6.6 mg (i.v.) | 335 (3.08) | NA | NA |
| Granisetron 1 mg (i.v.) | 1485 (13.65) | NA | NA |
| Aprepitant regimenb | 15 631.7 (145.24) | 10 492.2 (101.67) | 20 321.2 (188.81) |
| Nonaprepitant regimenb | 4516 (42.12) | 3161.2 (29.48) | 5870.8 (54.75) |
| Rescue medicationb | |||
| Aprepitant group | 849.8 (7.81) | 594.9 (5.47) | 1104.7 (10.15) |
| Nonaprepitant group | 2145.2 (19.72) | 1501.6 (13.80) | 2788.8 (25.63) |
| Medical feesc | |||
| Costs for hospitalization | 25 210 (231.73) | 17 647 (162.21) | 32 773 (301.25) |
| Costs for revisit outpatient | 720 (6.62) | 504 (4.63) | 936 (8.60) |
| Costs for drug prescription (p.o., no more than 6 drugs) | 420 (3.86) | 294 (2.70) | 546 (5.02) |
Exchange rate, 1 USD = 108.79 JPY, based on the Organization for Economic Cooperation and Development (OECD) 2016.27
i.v., intravenous; p.o., oral; NA, not applicable.
Japanese National Health Insurance Drug Price Standard listed in 2016.
Average costs estimated from the clinical practice in our institution.
Japanese National Health Insurance Price listed in 2016.
In Japan, patients are covered by the national health insurance (NHI) system, and copayment of a patient is 10%‐30% of the total medical cost according to his/her age. Ministry of Health, Labor, and Welfare determines prescribing drug prices and expenses for medical treatment and care and registers them into the NHI standard list to which national insurance is applicable. All costs for drugs in this study were assigned from the NHI Drug Price Standard listed in 2016. Diagnosis Procedure Combination (DPC) system is a diagnosis group classification and the medical fee associated with any particular hospitalization is determined based on DPC assigned to that hospitalization.26 The current analysis was, however, carried out without considering the DPC system and copayment of a patient, which was to assess the interaction of health outcomes and total medical costs, focusing on the consumed medical resources.
The medical fees for revisiting the outpatient clinic, rehospitalization and drug prescriptions were assigned from the NHI price listed in 2016. The costs calculated in Japanese yen (JPY) were converted to US dollars (USD) using the exchange rate reported by the Organization for Economic Cooperation and Development (OECD) in 2016; 1 USD = 108.79 JPY.27
2.4. Valuing outcomes
The incremental cost‐effectiveness ratio (ICER) was calculated to verify the cost‐effectiveness of the aprepitant‐containing antiemetic regimen. A discount was not applied to this analysis because the values of drug costs and medical treatment were assumed not to change in the short 5‐day observational period in this study. A willingness‐to‐pay (WTP) threshold of 5 million JPY (45 960 USD/QALY) was used to define strategies that provide cost‐effective utilization of resources in the Japanese health‐care system, as defined by Shiroiwa et al.28
2.5. Sensitivity analysis
Deterministic and probabilistic sensitivity analyses were carried out to assess uncertainty and robustness of this model by evaluating the effects of differing model parameters. Probabilistic sensitivity analysis was conducted by 10 000 iterations of an automatic multiple random numbering method using Monte Carlo simulation. In the sensitivity analysis, the ranges of parameters varied were 95% confidence intervals for probability and ±30% for utility weights and drug costs.
All analyses in this study were conducted using TreeAge PRO 2014 (TreeAge Software, Inc., Williamstown, MA, USA).
3. RESULTS
3.1. Health state outcomes
Use of the aprepitant‐containing regimen was associated with improved QOL compared with the nonaprepitant regimen for CINV caused by cisplatin‐based highly emetogenic chemotherapy. The estimated gain in QALY with the aprepitant regimen was 0.00159 (Table 5).
Table 5.
Results of cost‐utility analysis
| Setting | Aprepitant regimen | Nonaprepitant regimen | Difference | ICER JPY/QALY (USD/QALY) | |
|---|---|---|---|---|---|
| QALY | – | 0.00948 | 0.00789 | 0.00159 | – |
| Costs JPY (USD) | Outpatient setting | 19 542 (179.63) | 13 349 (122.71) | 6192 (56.92) | 3 906 698 (35 910) |
| Inpatient setting | 16 482 (151.50) | 6661 (61.23) | 9820 (90.27) | 6 195 781 (56 952) |
3.2. Cost variables
Total medical costs associated with the use of the aprepitant regimen were higher than those of the nonaprepitant regimen both in the OCS and in the ICS. Estimated increment in total medical costs with the aprepitant regimen was lower in the OCS than in the ICS, 6192 JPY (56.92 USD) versus 9820 JPY (90.27 USD), respectively.
3.3. Outcomes in cost‐effectiveness
In the OCS, we calculated the ICER to be 3 906 698 JPY (converted to 35 910 USD) per QALY gained, indicating that the aprepitant regimen was more cost‐effective in the OCS. In contrast, in the ICS, the ICER was calculated as 6 195 781 JPY (converted to 56 952 USD) per QALY, which was over the WTP threshold (5 million JPY [45 960 USD] in Japan and 50 000 USD in the USA).
3.4. Sensitivity analysis
Univariate sensitivity analyses showed that factors that mainly affected these results were cost of the aprepitant regimen, CR rate of the delayed phase, utility weight of CP, and CR rate of the acute phase (Figure 2A,B). Costs for rescue treatment had less effect than those for rehospitalization. The probability that the aprepitant regimen was cost‐effective was higher in the OCS than in the ICS, 65% versus 35%, respectively (Figure 2C). In the incremental cost‐effectiveness scatterplot, the presence of a dot in the first quadrant means that the aprepitant regimen is both costlier and more effective than the nonaprepitant regimen. In the OCS, the first quadrant contained 85% of the samples, 60% of which had an ICER of <5 million JPY (45 960 USD)/QALY. In the ICS, the first quadrant contained 97% of the samples, 35% of which had an ICER of <5 million JPY/QALY. These results mean that more samples were included in the cost‐effective area in the OCS than in the ICS (Figure 2D).
Figure 2.
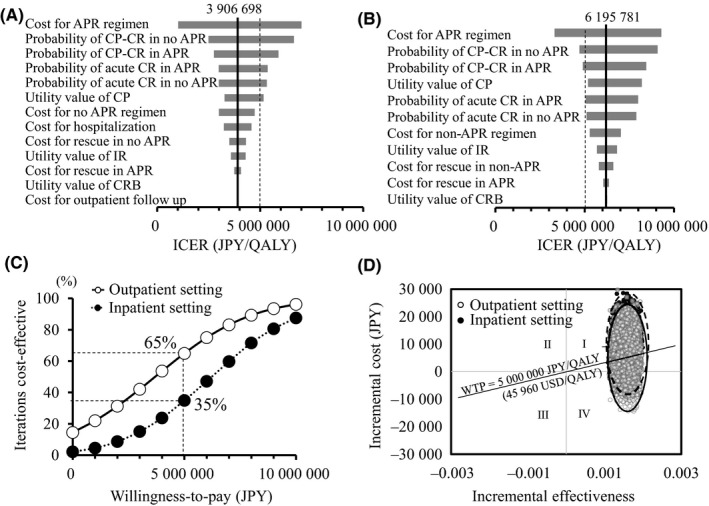
Sensitivity analysis. Tornado diagrams show the results of one‐way sensitivity analyses in the (A) outpatient care setting and (B) inpatient care setting. (C) Cost‐effectiveness acceptability curve and (D) scatter plot show the results of probabilistic sensitivity analysis by Monte Carlo simulation; the outpatient care setting is shown by white circles, and the inpatient care setting is shown by closed circles. In the scatter plot, dots in the outpatient setting are joined with a solid line, and those in the inpatient setting are joined with a broken line. APR, aprepitant; CP‐CR, complete protection in acute phase and complete response in delayed phase; CRB complete response at best; ICER, incremental cost‐effectiveness ratio; QALY, quality‐adjusted life year; WTP, willingness‐to‐pay which was assumed to be 5 million JPY as validated by Shiroiwa et al;28 Exchange rate, 1 USD = 108.79 JPY, based on the OECD 201627
4. DISCUSSION
This study showed the cost‐effectiveness of an aprepitant‐containing prophylactic antiemetic regimen for CINV in patients who received cisplatin‐based chemotherapy from the Japanese National Health insurance system payer perspective. Results of this study suggested that the cost‐effectiveness of aprepitant was higher in the OCS than in the ICS. Lordick et al17 showed the beneficial cost‐effectiveness of the aprepitant‐containing therapy for CINV in high‐dose cisplatin‐based chemotherapy based on protocols 052/054 from the German legal health insurance perspective. Humphreys et al18 showed that aprepitant was cost‐effective in anthracycline‐cyclophosphamide‐based chemotherapy from the British National Health Service (NHS) perspective. Annemans et al in Belgium, Chan et al in Hong Kong, and Lopes et al in Singapore reported consistently better results, with aprepitant cost‐effective in both cisplatin and anthracycline‐cyclophosphamide‐based chemotherapy in each national health system.19, 20, 21 Reports from Germany and Hong Kong noted that the analyses were conducted in the OCS.17, 20 Other reports from the UK, Belgium and Singapore21 did not clearly describe the setting, but it was implied that it was the OCS as they considered costs for reconsultation, rehospitalization, and visits of a home doctor or other health‐care professionals when adverse events as a result of chemotherapy occurred.18, 19, 21 These reports all concluded that aprepitant was superior in cost‐effectiveness in analyses using a decision analytic model similar to that of the present study. The results of the present study that aprepitant was superior in cost‐effectiveness in the OCS support these reports. One of the reasons why there was no cost‐effectiveness in the ICS would include the difference in costs related to rescue treatment as compared with that in the OCS. Prevention of CINV with aprepitant would reduce rescue treatment, and this would decrease opportunities for revisiting the hospital and rehospitalization in the OCS.
The aprepitant regimen not only had a positive impact on health outcomes and QOL for patients receiving cisplatin‐based chemotherapy, but also counterbalanced incremental total medical costs including indirect costs that would be wasted by CINV without it.29 However, the ICER slightly exceeded the WTP threshold in the ICS (6 195 781 JPY (56 952 USD)/QALY), but this does not negate the efficacy of aprepitant itself. Univariate sensitivity analysis indicated that factors that mainly affected these results included cost of aprepitant and CR rate. Recently, short hydration was shown to improve QOL for patients who received cisplatin‐based chemotherapy.30, 31, 32 Furthermore, aprepitant was used in an antiemetic regimen to prevent CINV in those studies. NK1RA and other newer generation antiemetics have markedly improved gastrointestinal toxicity induced by cisplatin,12, 13, 14, 15, 33, 34, 35 which may enable oral hydration. Moreover, the clinical use of aprepitant in the OCS may provide patients with better cost‐effectiveness.
Patients without nausea and vomiting in the acute phase are at reduced risk of nausea and vomiting in the delayed phase,36 and the success of CINV control in the first chemotherapy cycle is associated with a decrease in CINV in the subsequent chemotherapy cycles.37, 38 Considering the importance of CINV control in early phases of chemotherapy, aprepitant should be used from the onset of the first chemotherapy in accordance with antiemetic guidelines. In subsequent chemotherapy cycles, adjustment of antiemetic therapy may be considered depending on each patient's condition and taking into account the medical economic aspect.
This model analysis has some limitations. Parameters used in this model were estimates drawn from published sources. Although the probabilities of each health condition were estimated from the results of a domestic phase II study,15 the fixed utility values in each health condition were derived from reports on European and American patients.39, 40, 41 Biases arising from using utility values from different races and single clinical trial data cannot be excluded. To deal with the uncertainties associated with these potential biases, deterministic and probabilistic sensitivity analyses were carried out, and the effects of utility values on the results were modest. Health state probabilities in the present study were data from Japanese patients. Emetogenicity may differ depending on age, gender, alcohol consumption or other patient characteristics.42, 43 However, these factors were not considered in the current analysis, and our results may not directly be extrapolated to other HEC.
In conclusion, the aprepitant‐containing prophylactic antiemetic therapy was cost‐effective in the OCS, but not in the ICS, in Japanese patients who received cisplatin‐based HEC.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
Tsukiyama I, Hasegawa S, Ikeda Y, et al. Cost‐effectiveness of aprepitant in Japanese patients treated with cisplatin‐containing highly emetogenic chemotherapy. Cancer Sci. 2018;109:2881–2888. 10.1111/cas.13736
REFERENCES
- 1. Lorusso V, Giampaglia M, Petrucelli L, Saracino V, Perrone T, Gnoni A. Antiemetic efficacy of single‐dose palonosetron and dexamethasone in patients receiving multiple cycles of multiple day‐based chemotherapy. Support Care Cancer. 2012;20(12):3241‐3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haiderali A, Menditto L, Good M, Teitelbaum A, Wegner J. Impact on daily functioning and indirect/direct costs associated with chemotherapy‐induced nausea and vomiting (CINV) in a U.S. population. Support Care Cancer. 2011;19(6):843‐851. [DOI] [PubMed] [Google Scholar]
- 3. Bloechl‐Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24(27):4472‐4478. [DOI] [PubMed] [Google Scholar]
- 4. Herrstedt J. Nausea and emesis: still an unsolved problem in cancer patients? Support Care Cancer. 2002;10(2):85‐87. [DOI] [PubMed] [Google Scholar]
- 5. Takeuchi H, Saeki T, Aiba K, et al. Japanese Society of Clinical Oncology clinical practice guidelines 2010 for antiemesis in oncology: executive summary. Int J Clin Oncol. 2016;21(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. NCCN practice guidelines in oncology, Antiemesis 2017 ver. 2. 2017. https://www.nccn.org/professionals/physician_gls/pdf/ antiemesis.pdf. Accessed August 18, 2017.
- 7. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119‐v133. [DOI] [PubMed] [Google Scholar]
- 8. Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(28):3240‐3261. [DOI] [PubMed] [Google Scholar]
- 9. Tattersall FD, Rycroft W, Cumberbatch M, et al. The novel NK1 receptor antagonist MK‐0869 (L‐754,030) and its water soluble phosphoryl prodrug, L‐758,298, inhibit acute and delayed cisplatin‐induced emesis in ferrets. Neuropharmacology. 2000;39(4):652‐663. [DOI] [PubMed] [Google Scholar]
- 10. Kramer MS, Cutler N, Feighner J, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281(5383):1640‐1645. [DOI] [PubMed] [Google Scholar]
- 11. Hale JJ, Mills SG, MacCoss M, et al. Structural optimization affording 2‐(R)‐(1‐(R)‐3,5‐bis(trifluoromethyl)phenylethoxy)‐3‐(S)‐(4‐fluoro) phenyl‐4‐(3‐oxo‐1,2,4‐triazol‐5‐yl)methylmorpholine, a potent, orally active, long‐acting morpholine acetal human NK‐1 receptor antagonist. J Med Chem. 1998;41(23):4607‐4614. [DOI] [PubMed] [Google Scholar]
- 12. Chawla SP, Grunberg SM, Gralla RJ, et al. Establishing the dose of the oral NK1 antagonist aprepitant for the prevention of chemotherapy‐induced nausea and vomiting. Cancer. 2003;97(9):2290‐2300. [DOI] [PubMed] [Google Scholar]
- 13. Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin‐1 antagonist aprepitant for the prevention of chemotherapy‐induced nausea and vomiting: a multinational, randomized, double‐blind, placebo‐controlled trial in patients receiving high‐dose cisplatin–the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21(22):4112‐4119. [DOI] [PubMed] [Google Scholar]
- 14. Poli‐Bigelli S, Rodrigues‐Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy‐induced nausea and vomiting. Results from a randomized, double‐blind, placebo‐controlled trial in Latin America. Cancer. 2003;97(12):3090‐3098. [DOI] [PubMed] [Google Scholar]
- 15. Takahashi T, Hoshi E, Takagi M, Katsumata N, Kawahara M, Eguchi K. Multicenter, phase II, placebo‐controlled, double‐blind, randomized study of aprepitant in Japanese patients receiving high‐dose cisplatin. Cancer Sci. 2010;101(11):2455‐2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gomez DR, Liao KP, Giordano S, Nguyen H, Smith BD, Elting LS. Adherence to national guidelines for antiemesis prophylaxis in patients undergoing chemotherapy for lung cancer: a population‐based study. Cancer. 2013;119(7):1428‐1436. [DOI] [PubMed] [Google Scholar]
- 17. Lordick F, Ehlken B, Ihbe‐Heffinger A, et al. Health outcomes and cost‐effectiveness of aprepitant in outpatients receiving antiemetic prophylaxis for highly emetogenic chemotherapy in Germany. Eur J Cancer. 2007;43(2):299‐307. [DOI] [PubMed] [Google Scholar]
- 18. Humphreys S, Pellissier J, Jones A. Cost‐effectiveness of an aprepitant regimen for prevention of chemotherapy‐induced nausea and vomiting in patients with breast cancer in the UK. Cancer Manag Res. 2013;5:215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Annemans L, Strens D, Lox E, Petit C, Malonne H. Cost‐effectiveness analysis of aprepitant in the prevention of chemotherapy‐induced nausea and vomiting in Belgium. Support Care Cancer. 2008;16(8):905‐915. [DOI] [PubMed] [Google Scholar]
- 20. Chan SL, Jen J, Burke T, Pellissier J. Economic analysis of aprepitant‐containing regimen to prevent chemotherapy‐induced nausea and vomiting in patients receiving highly emetogenic chemotherapy in Hong Kong. Asia Pac J Clin Oncol. 2014;10(1):80‐91. [DOI] [PubMed] [Google Scholar]
- 21. Lopes G, Burke T, Pellissier J, Zhang X‐H, Dedhiya S, Chan A. Aprepitant for patients receiving highly emetogenic chemotherapy: an economic analysis for Singapore. Value Health Reg. 2012;1(1):66‐74. [DOI] [PubMed] [Google Scholar]
- 22. Moore S, Tumeh J, Wojtanowski S, Flowers C. Cost‐effectiveness of aprepitant for the prevention of chemotherapy‐induced nausea and vomiting associated with highly emetogenic chemotherapy. Value Health. 2007;10(1):23‐31. [DOI] [PubMed] [Google Scholar]
- 23. McCrea J. Effects of the neurokinin1 receptor antagonist aprepitant on the pharmacokinetics of dexamethasone and methylprednisolone. Clin Pharmacol Ther. 2003;74(1):17‐24. [DOI] [PubMed] [Google Scholar]
- 24. Kris MG, Gralla RJ, Clark RA, et al. Incidence, course, and severity of delayed nausea and vomiting following the administration of high‐dose cisplatin. J Clin Oncol. 1985;3(10):1379‐1384. [DOI] [PubMed] [Google Scholar]
- 25. Martin M. The severity and pattern of emesis following different cytotoxic agents. Oncology. 1996;53(suppl 1):26‐31. [DOI] [PubMed] [Google Scholar]
- 26. Okamoto K, Uchiyama T, Takemura T, Kume N, Kuroda T, Yoshihara H. Automatic selection of diagnosis procedure combination codes based on partial treatment data relative to the number of hospitalization days. Eur J Biomed Inform. 2018;14(1):45‐51. [Google Scholar]
- 27. OECD Data . Exchange rates. https://data.oecd.org/conversion/exchange-rates.htm. Accessed August 17, 2017.
- 28. Shiroiwa T, Sung YK, Fukuda T, Lang HC, Bae SC, Tsutani K. International survey on willingness‐to‐pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19(4):422‐437. [DOI] [PubMed] [Google Scholar]
- 29. Sommariva S, Pongiglione B, Tarricone R. Impact of chemotherapy‐induced nausea and vomiting on health‐related quality of life and resource utilization: a systematic review. Crit Rev Oncol Hematol. 2016;99:13‐36. [DOI] [PubMed] [Google Scholar]
- 30. Horinouchi H, Kubota K, Itani H, et al. Short hydration in chemotherapy containing cisplatin (≥75 mg/m2) for patients with lung cancer: a prospective study. Jpn J Clin Oncol. 2013;43(11):1105‐1109. [DOI] [PubMed] [Google Scholar]
- 31. Ninomiya K, Hotta K, Hisamoto‐Sato A, et al. Short‐term low‐volume hydration in cisplatin‐based chemotherapy for patients with lung cancer: the second prospective feasibility study in the Okayama Lung Cancer Study Group Trial 1201. Int J Clin Oncol. 2016;21(1):81‐87. [DOI] [PubMed] [Google Scholar]
- 32. Azuma T, Matayoshi Y, Sato Y, Sato Y, Nagase Y, Oshi M. The safety and effect of chemotherapy with short hydration for urothelial cancer on patients’ quality of life. Jpn J Clin Oncol. 2016;46(10):958‐963. [DOI] [PubMed] [Google Scholar]
- 33. Schmoll HJ, Aapro MS, Poli‐Bigelli S, et al. Comparison of an aprepitant regimen with a multiple‐day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high‐dose cisplatin treatment. Ann Oncol. 2006;17(6):1000‐1006. [DOI] [PubMed] [Google Scholar]
- 34. Rapoport BL, Jordan K, Boice JA, et al. Aprepitant for the prevention of chemotherapy‐induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double‐blind study. Support Care Cancer. 2010;18(4):423‐431. [DOI] [PubMed] [Google Scholar]
- 35. Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy‐induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23(12):2822‐2830. [DOI] [PubMed] [Google Scholar]
- 36. Warr DG, Grunberg SM, Gralla RJ, et al. The oral NK(1) antagonist aprepitant for the prevention of acute and delayed chemotherapy‐induced nausea and vomiting: pooled data from 2 randomised, double‐blind, placebo controlled trials. Eur J Cancer. 2005;41(9):1278‐1285. [DOI] [PubMed] [Google Scholar]
- 37. Burke TA, Wisniewski T, Ernst FR. Resource utilization and costs associated with chemotherapy‐induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Support Care Cancer. 2011;19(1):131‐140. [DOI] [PubMed] [Google Scholar]
- 38. Hesketh PJ. Chemotherapy‐induced nausea and vomiting. N EnglJ Med. 2008;358(23):2482‐2494. [DOI] [PubMed] [Google Scholar]
- 39. Grunberg SM, Boutin N, Ireland A, Miner S, Silveira J, Ashikaga T. Impact of nausea/vomiting on quality of life as a visual analogue scale‐derived utility score. Support Care Cancer. 1996;4(6):435‐439. [DOI] [PubMed] [Google Scholar]
- 40. Borjeson S, Hursti TJ, Peterson C, et al. Similarities and differences in assessing nausea on a verbal category scale and a visual analogue scale. Cancer Nurs. 1997;20(4):260‐266. [DOI] [PubMed] [Google Scholar]
- 41. Sun CC, Bodurka DC, Donato ML, et al. Patient preferences regarding side effects of chemotherapy for ovarian cancer: do they change over time? Gynecol Oncol. 2002;87(1):118‐128. [DOI] [PubMed] [Google Scholar]
- 42. Hesketh PJ, Aapro M, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard‐of‐care antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin‐based chemotherapy. Support Care Cancer. 2010;18(9):1171‐1177. [DOI] [PubMed] [Google Scholar]
- 43. Sekine I, Segawa Y, Kubota K, Saeki T. Risk factors of chemotherapy‐induced nausea and vomiting: index for personalized antiemetic prophylaxis. Cancer Sci. 2013;104(6):711‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]


