Abstract
Clinical experience of ceritinib in patients who progressed on alectinib is limited. In this prospective phase II study, we evaluated the activity of ceritinib in alectinib‐pretreated patients with anaplastic lymphoma kinase (ALK)‐rearranged metastatic (stage IIIB/IV) non‐small‐cell lung cancer (NSCLC) in Japan. All patients were required to have ≥1 measurable lesion per RECIST, 1.1, and a World Health Organization Performance Status (WHO PS) of 0‐1. Prior crizotinib and/or up to 1 chemotherapy regimen was allowed. Primary endpoint was investigator‐assessed overall response rate (ORR) per RECIST 1.1. Ceritinib was given at a dose of 750 mg/day fasted. A total of 20 patients were enrolled from August 2015 to March 2017. All patients received prior alectinib (100%), 13 (65.0%) patients received prior platinum‐based chemotherapy, and 4 (20%) patients received prior crizotinib. Median duration of exposure and the follow‐up time with ceritinib were 3.7 months (range: 0.4‐15.1) and 11.6 months (range: 4.8‐23.0), respectively. Investigator‐assessed ORR was 25% (95% CI: 8.7‐49.1). Key secondary endpoints, all investigator assessed, included disease control rate (70.0%; 95% CI: 45.7‐88.1), time to response (median, 1.8 months; range: 1.8‐2.0), and duration of response (median, 6.3 months; 95% CI: 3.5‐9.2). Median progression‐free survival was 3.7 months (95% CI: 1.9‐5.3). The most common adverse events reported were diarrhea (85.0%), nausea (80.0%), and vomiting (65.0%). Based on our findings, ceritinib could be considered as one of the treatment options for patients with ALK‐positive NSCLC who progressed on alectinib. (Trial registration no. NCT02450903)
Keywords: ALK, anaplastic lymphoma kinase, ceritinib, Japan, NSCLC
1. INTRODUCTION
Anaplastic lymphoma kinase (ALK)‐rearrangements serve as a key oncogenic driver and occur in approximately 3%‐7% of patients with non‐small‐cell lung cancer (NSCLC).1, 2 Crizotinib, the first ALK inhibitor approved by the US Food and Drug Administration (FDA) (August 2011),3 significantly prolonged progression‐free survival (PFS) compared with chemotherapy in patients with untreated and chemotherapy‐treated advanced ALK‐positive NSCLC.4, 5 Crizotinib received approval in Japan in March 2012 in any line of treatment and was approved in the European Union in October 2012, in the first‐line setting.6, 7 Despite the efficacy of crizotinib, emergence of resistance to crizotinib is almost inevitable after a median of 10.9 months,4 which could be due to secondary mutations or amplification of ALK, activation of downstream molecules, or poor penetration to the brain.4, 8 To overcome crizotinib resistance, several next‐generation ALK inhibitors have been developed, including ceritinib, alectinib, brigatinib, and lorlatinib.9
Alectinib, a next‐generation ALK inhibitor was first approved in Japan in July 2014 for ALK‐inhibitor‐naïve patients with ALK‐rearranged (ALK‐positive) NSCLC, based on the results from the AF‐001JP study.10 Furthermore, the results from two phase III trials (J‐ALEX and ALEX) showed superior efficacy and lower toxicity of alectinib versus crizotinib in primary treatment of ALK‐positive NSCLC.11, 12 However, treatment options are lacking in patients who develop resistance to alectinib and thus post‐alectinib ALK inhibitor therapy is of high clinical interest.
Ceritinib, a selective oral ALK inhibitor, is approved for the treatment of patients with metastatic ALK‐positive NSCLC.13 In randomized, global phase III trials, ceritinib showed a statistically significant and clinically meaningful improvement in PFS versus chemotherapy in patients with advanced ALK‐positive NSCLC who were treatment‐naïve (ASCEND‐4 study)14 or previously treated with crizotinib and with 1 or 2 prior chemotherapy regimens (ASCEND‐5 study).15 Although the original approvals were at a dose of 750 mg/day under fasted conditions, the revised recommended dose of 450 mg/day with food was approved in the USA in December 201713 and in the European Union in May 2018.
Each ALK inhibitor (eg, ceritinib, alectinib, and crizotinib) has different IC50 for each mutation. Preclinical data have shown that ceritinib is active against mutations that are resistant to alectinib.16 In the phase I study of ceritinib in Japanese patients with ALK‐positive NSCLC, 2 of 4 patients pretreated with alectinib showed a partial response (PR) with ceritinib.17 These preliminary preclinical and clinical data of ceritinib indicate the possibility of expanding therapeutic options for patients with ALK‐positive NSCLC who have failed prior alectinib. However, none of the studies prospectively assessed the efficacy and safety of ceritinib in patients with ALK‐positive NSCLC who progressed on alectinib. Here, we present the results from the phase II ASCEND‐9 study, which investigated the activity of ceritinib in these patients.
2. MATERIALS AND METHODS
2.1. Study population
Adult patients (aged ≥18 years) were eligible if they had histologically or cytologically confirmed stage IIIB or IV ALK‐positive NSCLC (as determined locally by Vysis ALK Break Apart fluorescence in situ hybridization Probe Kit [Abbott Molecular Inc., Des Plaines, IL, USA] test), progressed on prior therapy with alectinib, at least 1 measurable lesion as defined by RECIST 1.1, and a World Health Organization performance status (WHO PS) of 0‐1. Patients could have asymptomatic untreated or treated brain metastases with no steroid therapy within 2 weeks before study enrollment. Prior cytotoxic chemotherapy up to 1 regimen was allowed and, for regimens given every 21 or 28 days, a minimum of 2 cycles was required to qualify as a prior chemotherapy regimen (unless chemotherapy was discontinued due to progressive disease [PD] after 1 cycle). Following protocol amendment, prior crizotinib was allowed, as a vast majority of patients in Japan had received crizotinib as first‐line therapy and alectinib as next line before approval of alectinib. However, alectinib was not required to be the last therapy prior to study enrollment, and no particular sequence of prior alectinib and crizotinib was required for enrollment. Patients must have recovered from all toxicities related to prior anticancer therapies and treatment‐related toxicities of grade ≤1 (Common Terminology Criteria for Adverse Events [CTCAE] version 4.03). However, patients with grade ≤2 peripheral neuropathy or any grade of alopecia, fatigue, nail changes or skin changes were eligible.
Patients were excluded if they had known hypersensitivity to any of the excipients of ceritinib; history of carcinomatous meningitis; history of interstitial lung disease or interstitial pneumonitis; received thoracic radiotherapy to lung fields ≤4 weeks prior to starting the study treatment or had not recovered from radiotherapy‐related toxicities; major surgery within 4 weeks prior to the first dose of study drug or had recovered from side‐effects of such procedure; a concurrent malignancy or history of a malignant disease other than NSCLC that has been diagnosed and/or required therapy within the past 3 years (except completely resected basal cell and squamous cell skin cancers, and completely resected carcinoma in situ of any type); a history of pancreatitis or history of increased amylase or lipase as a result of pancreatic disease; clinically significant, uncontrolled heart disease and/or recent cardiac event (within 6 months), impairment of gastrointestinal function or gastrointestinal disease that may significantly alter the absorption of ceritinib.
The study protocol and all amendments were reviewed by the independent ethics committee or institutional review board for each center. This study was conducted according to the ethical principles of the Declaration of Helsinki and the Good Clinical Practice guidelines. Written informed consent was obtained from each patient before enrollment.
2.2. Study design
This was a single‐arm, open‐label, multicenter, phase II study conducted at 9 centers in Japan (NCT02450903). Eligible patients were treated with ceritinib 750 mg/day orally (given in a fasted state). A treatment cycle was defined as 28 days of once‐daily ceritinib treatment. Treatment with ceritinib continued until the patient experienced unacceptable toxicity, pregnancy, start of new cancer therapy, disease progression as determined by RECIST 1.1, or withdrawal of consent and/or at the discretion of the investigator. Treatment beyond progression was permitted in patients who (according to investigators’ judgment) continued to have clinical benefit. Patients were permitted a maximum of 3 dose reductions (of 150 mg/day per reduction), after which they were required to discontinue treatment. Re‐escalation after dose reduction was not permitted.
Primary endpoint was overall response rate (ORR), defined as the proportion of patients with a best overall confirmed response of complete response (CR) or PR, as assessed per RECIST 1.1 by the investigator. Both CR and PR had to be confirmed by repeat assessments carried out not <4 weeks after the criteria for response were first met. Key secondary endpoints were disease control rate (DCR; defined as the proportion of patients with a best overall response of CR, PR, or stable disease), time to response (TTR; defined as the time from the start date of study drug to the date of first documented CR/PR among patients with a confirmed CR/PR), and duration of response (DOR; defined as the time from the date of first documented CR/PR to the date of first documented PD or death as a result of any cause among patients with a confirmed CR/PR) by investigator assessment. Other secondary endpoints were PFS (defined as the time from the start date of study drug to the date of first documented PD or death as a result of any cause) by investigator assessment, overall survival (OS; defined as the time from the start date of study drug to the date of death as a result of any cause), overall intracranial response rate (OIRR; defined as the proportion of patients with a best overall confirmed response of CR or PR in the brain, based on target, non‐target, and new lesions in the brain, among patients having measurable brain metastases at baseline) by investigator assessment, and safety outcomes such as adverse events (AE), electrocardiograms, and laboratory abnormalities.
2.3. Assessments
Tumor assessments were carried out by local investigators using computed tomography (CT) or magnetic resonance imaging (MRI) according to RECIST 1.1 at baseline; cycle 3 day 1, then every 8 weeks, and end of treatment (EOT) visit during treatment phase; and every 8 weeks following EOT until disease progression. Routine follow‐up brain MRI or CT scans were carried out at cycle 3 and then every 8 weeks only in patients with brain metastases at baseline.
All AE reported were recorded and graded according to CTCAE, version 4.03. All patients were followed up for AE and serious AE (SAE) for at least 30 days following discontinuation of ceritinib treatment.
2.4. Molecular tumor analyses
All patients were required to provide archival tumor tissue and plasma samples for central analysis of molecular status. Exploratory biomarker analysis was carried out at Novartis Institutes for BioMedical Research (NIBR; Cambridge, MA, USA) where the samples were tested for mutations, copy number alterations, and rearrangements in 570 known cancer‐related genes using next‐generation sequencing (NGS). We used the Promega Maxwell system for tissue extractions and Qiagen Circulating Nucleic Acid kit for plasma DNA extractions, library construction uses the Illumina TruSeq Nano kit. Tissue DNA was quantified by PicoGreen and integrity was assessed using the Agilent TapeStation. NGS libraries constructed from tissue DNA and circulating tumor DNA (ctDNA) were enriched for regions of interest using Agilent SureSelect hybrid capture. The captured DNA was subsequently sequenced using an Illumina HiSeq 2500s instrument. Detected genomic lesions were subsequently annotated and filtered based on state‐of‐the‐art public resources, such as Catalogue of Somatic Mutations in Cancer (COSMIC). The subsequent analyses focused only on the subset of functionally known and likely alterations in COSMIC.
2.5. Statistical analysis
The primary analysis of study data was conducted at the time when all patients had either completed ≥6 cycles of treatment or discontinued earlier, as per study protocol. Data cutoff for primary analysis was July 31, 2017. The study did not include any formal statistical hypothesis testing and the sample size (N = 20) was not derived based on power considerations. Efficacy and safety analysis was carried out in all patients who received at least 1 dose of ceritinib, unless otherwise specified. ORR, DCR, and OIRR were estimated and reported along with the exact binomial 95% confidence interval (CI). Median DOR, median PFS, and the associated 95% CI were estimated using Kaplan‐Meier method. For DOR and PFS analysis, if a patient had not had an event at the date of analysis cutoff or had received any further anticancer therapy in the absence of PD, DOR or PFS was censored at the date of the last adequate tumor assessment before the earlier of the cutoff date or the start date of new anticancer therapy. TTR was summarized using descriptive statistics for patients with a confirmed response (CR or PR). The OS rate with 95% CI at 12 months was estimated using the Kaplan‐Meier method. If a patient was alive at the date of analysis cutoff or lost to follow up, OS was censored at the last contact date prior to the cutoff date. Safety outcomes were summarized descriptively.
3. RESULTS
3.1. Patient disposition
From August 2015 to March 2017, a total of 20 patients were enrolled. The cutoff date for this analysis was July 31, 2017 when all patients had either completed at least 6 cycles (24 weeks) of treatment or discontinued earlier. Median duration of follow up (from start date of study drug to data cutoff date) was 11.6 months (range: 4.8‐23.0). At the time of data cutoff, 18 (90.0%) patients discontinued study treatment with disease progression as the most frequently reported primary reason for discontinuation in 14 (70.0%) patients; AE was reported as the primary reason for discontinuation in 3 (15.0%) patients. Of note, 3 patients continued treatment with ceritinib beyond RECIST‐defined progressive disease.
3.2. Patient demographics and disease characteristics
Median age of patients was 51 years (range: 29‐79), and the majority were female (60.0%). Most patients (95.0%) had stage IV disease, and all patients (100%) had adenocarcinoma histology. Of the 20 patients enrolled, 12 (60.0%) had brain metastasis at study entry. Median time from initial diagnosis to start of ceritinib treatment was 25.8 months (range: 6.5‐137.5) (Table 1).
Table 1.
Patient characteristics at baseline
| Characteristic | N = 20 |
|---|---|
| Median age (range), y | 51.0 (29‐79) |
| Gender, n (%) | |
| Female | 12 (60.0) |
| Male | 8 (40.0) |
| Race, n (%) | |
| Asiana | 20 (100) |
| WHO PS, n (%) | |
| 0 | 11 (55.0) |
| 1 | 9 (45.0) |
| Smoking history, n (%) | |
| Never smoked | 8 (40.0) |
| Ex‐smoker | 11 (55.0) |
| Current smoker | 1 (5.0) |
| Histology/cytology, n (%) | |
| Adenocarcinoma | 20 (100) |
| Stage at time of study entry, n (%) | |
| Stage IIIB | 1 (5.0) |
| Stage IV | 19 (95.0) |
| Brain metastases, n (%) | 12 (60.0) |
| Prior lines of anticancer regimens, n (%) | |
| 1 | 6 (30.0) |
| 2 | 9 (45.0) |
| 3 | 5 (25.0) |
| Prior radiotherapy, n (%) | |
| Yes | 11 (55.0) |
| No | 9 (45.0) |
| Therapy type at last treatment, n (%) | |
| Chemotherapy | 4 (20.0) |
| Targeted therapyb | 17 (85.0) |
| Prior ALK inhibitor, n (%) | |
| Alectinib only | 16 (80.0) |
| Alectinib and crizotinibc | 4 (20.0) |
| Best response to prior alectinib, n (%) | |
| CR | 3 (15.0) |
| PR | 14 (70.0) |
| SD | 1 (5.0) |
| Unknown | 2 (10.0) |
| Reason for alectinib discontinuation, n (%) | |
| PD | 20 (100) |
| Time since initial diagnosis of primary site to start of ceritinib treatment (months), median (range) | 25.82 (6.5‐137.5) |
ALK, anaplastic lymphoma kinase; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; WHO PS, World Health Organization performance status.
One patient was not Japanese.
Targeted therapy includes alectinib and bevacizumab. One patient who previously received bevacizumab and chemotherapy as a last regimen was counted in each category.
All 4 patients received crizotinib before prior alectinib.
All 20 (100.0%) patients were treated with alectinib prior to study entry. Of these patients, 6 (30.0%) were treated with 1 line of prior regimen (alectinib only), 9 (45.0%) were treated with 2 lines of prior regimen (1 patient with crizotinib and 8 patients with chemotherapy, in addition to alectinib) and 5 (25.0%) were treated with 3 lines of prior regimen (3 patients with crizotinib plus chemotherapy, and 2 patients with 2 regimens of chemotherapy, in addition to alectinib). Of the 20 patients, 12 (60.0%) were treated with prior platinum doublet chemotherapy in a therapeutic setting (with or without bevacizumab) and 2 (10.0%) in an adjuvant setting. All 20 patients in the study discontinued prior alectinib as a result of disease progression. Of those, 16 patients received alectinib as the last treatment and the remaining 4 patients received chemotherapy as the last treatment prior to study entry. Best response to prior alectinib was CR in 3 (15%) patients, PR in 14 (70.0%) patients, and stable disease in 1 (5.0%) patient (Table 1). Among 17 patients with best response of CR or PR, DOR was available from 14 patients and the median was 9.3 months (range: 2.1‐50.0).
3.3. Efficacy
The best overall responses are reported in Table 2. ORR by investigator review was 25.0% (95% CI: 8.7‐49.1), whereas DCR was 70.0% (95% CI: 45.7‐88.1). Among patients with measurable disease at baseline and ≥1 valid post‐baseline assessment, 70.6% (12/17) had decrease in tumor burden from baseline by investigator review (Figure 1A). Among the responders, median time to first response by investigator assessment was 1.8 months (range: 1.8‐2.0), and median DOR by investigator assessment was 6.3 months (95% CI: 3.5‐9.2), with 3 of 5 (60.0%) patients having an event (PD for all 3 patients). For each patient, treatment exposure and doses of ceritinib received, onset of first documented response (CR or PR) confirmed subsequently, and/or first documented PD on ceritinib are depicted in Figure 1B. One patient showed prolonged stable disease for 12.4 months. Among 4 patients who received chemotherapy as the last treatment prior to study entry, 1 patient experienced PR. In patients with brain metastases at baseline, regardless of target lesions (n = 12), the best overall response based on investigator assessment was CR in 1 patient (8.3%) and PR in 2 patients (16.7%); ORR was 25.0% (95% CI: 5.5‐57.2).
Table 2.
Efficacy by investigator assessment
| Ceritinib 750 mg/d N = 20 | |
|---|---|
| ORR (CR + PR), n (%) (95% CI) | 5 (25.0) (8.7‐49.1) |
| CR | 1 (5.0) |
| PR | 4 (20.0) |
| SD | 9 (45.0) |
| PD | 6 (30.0) |
| DCR (CR + PR + SD), n (%) (95% CI) | 14 (70.0) (45.7‐88.1) |
| Median DOR (in responders), months (95% CI) | 6.3 (3.5‐9.2) |
| Median TTR (in responders), months (range) | 1.8 (1.8‐2.0) |
| Median PFS, months (95% CI) | 3.7 (1.9‐5.3) |
CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; ORR, overall response rate; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease; TTR, time to response.
Figure 1.
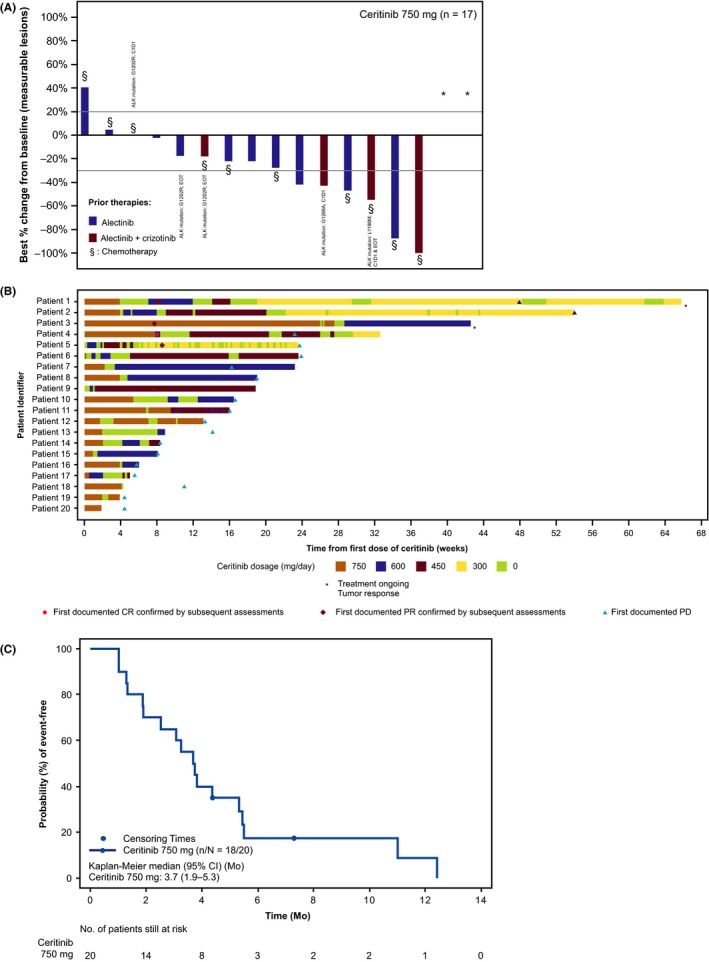
A, Waterfall plot of best percentage change from baseline by investigator review (includes 17 patients with measurable disease at baseline and at least 1 valid post‐baseline assessment). n is the number of patients with measurable disease at baseline and at least 1 valid post‐baseline assessment. * % change in target lesion available but contradicted by overall lesion response = progressive disease (contradicting assessment represents the only valid post‐baseline assessment). ALK mutations are reported for 5 patients; rest of the 12 patients did not have ALK mutations reported. B, Individual swimmer plots for all patients receiving ceritinib treatment. Only tumor responses assessed before start of new antineoplastic therapy are presented. ALK mutation in ctDNA at cycle 1 d 1 (C1D1) or end of treatment (EOT). Patient 5: L1196M (C1D1 and EOT), Patients 6 and 8: G1202R (EOT), Patient 10: G1202 (C1D1), Patient 13: G1269A (C1D1), Patient 14: G1202R (C1D1 and EOT), and Patient 19: G1202R, V1180L, and I1171N (C1D1). C, PFS by investigator assessment in patients with ALK‐rearranged NSCLC. n is the number of PFS events among patients included in the analysis (N). ALK, anaplastic lymphoma kinase; CI, confidence interval; CR, complete response; ctDNA, circulating tumor DNA; NSCLC, non‐small‐cell lung cancer; PD, progressive disease; PFS, progression‐free survival; PR, partial response
Median investigator‐assessed PFS was 3.7 months (95% CI: 1.9‐5.3) (Figure 1C), with 18 of 20 (90.0%) patients having an event (PD for all 18 patients). In 2 patients with measurable disease in the brain at baseline, best overall intracranial responses based on investigator assessment were stable disease and unknown (as a result of change in imaging modality). In a post‐hoc analysis, ORR by investigator review based on the number of prior regimens were 16.7% (1/6 patients), 22.2% (2/9 patients), and 40.0% (2/5 patients) for 1, 2, and 3 prior regimens, respectively. The 12‐month OS rate was 75.6% (95% CI: 44.8‐90.7), but it should be noted that OS data were not mature with 5 (25.0%) deaths. Among the 15 patients who were treated with antineoplastic regimens after ceritinib discontinuation, 6 patients were treated with platinum doublet.
Table 3 shows the demographic, disposition and efficacy results among the responders. Among the 5 responders, 4 patients had alectinib as the last prior therapy and 3 patients were reported to have baseline brain metastases. In addition to prior alectinib, 4 patients received prior chemotherapy and 2 patients received prior chemotherapy and prior crizotinib. Duration of response to prior alectinib ranged from 4.6 to 50.0 months. All the patients discontinued their last prior therapy due to PD. One patient with brain metastases at baseline and who never experienced CR to prior crizotinib and alectinib (Patient 4 in Table 3) showed CR. Figure S1 shows pre‐ceritinib and 2 months post‐ceritinib treatment scan images, illustrating regression of the target lesion in a patient (Patient 5 in Table 3) who was previously treated with first‐line chemotherapy, second‐line crizotinib (best response: PR), and third‐line alectinib (best response: PR). NGS analysis of tissue sample from the progressed liver lesion before ceritinib indicated an L1196M mutation.
Table 3.
Demographics and efficacy results among responders
| Patient number | 1 | 3 | 4 | 5 | 9 |
|---|---|---|---|---|---|
| Gender/Age in years | Female/67 | Male/35 | Female/46 | Female/41 | Female/29 |
| Brain metastases at baseline (measurable or non‐measurable) | Absent | Present | Present | Present | Absent |
| Total lines of prior therapy in the order received |
1. Chemotherapy 2. Alectinib |
1. Alectinib |
1. Chemotherapy 2. Crizotinib 3. Alectinib |
1. Chemotherapy 2. Crizotinib 3. Alectinib |
1. Alectinib 2. Chemotherapy |
| ALK mutation(s) in archival tumor sample (T) or ctDNA at C1D1 |
NA (T) None (ctDNA) |
NA (T) None (ctDNA) |
NA (T) None (ctDNA) |
L1196M (T and ctDNA) | None (T and ctDNA) |
| Alectinib therapy | |||||
| Duration of alectinib treatment (months) | 50.96 | 11.63 | 14.39 | 8.74 | 8.31 |
| Best response to alectinib | PR | PR | PR | PR | PR |
| Duration of response to alectinib (months) | 50.0 | 10.61 | 5.98 | 5.06 | 4.60 |
| Reason for alectinib discontinuation | PD | PD | PD | PD | PD |
| Ceritinib therapy | |||||
| Time from last dose of alectinib (days) | 3 | 1 | 1 | 1 | 345 |
| Best overall response | PR | PR | CR | PR | PR |
| Time to response (months) | 1.87 | 1.77 | 1.84 | 1.97 | 1.77 |
| DOR (months) | 9.17 | 5.55a | 3.52 | 3.52 | 2.63a |
ALK, anaplastic lymphoma kinase; CR; complete response; ctDNA at C1D1, circulating tumor DNA at cycle 1 day 1; DOR, duration of response; NA, not available; PD, progressive disease; PR, partial response.
Censored observations (Patient 9 due to addition of new cancer therapy and Patient 3 due to ongoing study treatment without an event).
To characterize genetic profile/mutations in NSCLC tumors and ctDNA, we carried out Illumina‐based Pan‐Cancer gene assay for archival tumor at screening visit and for ctDNA at cycle 1 day 1 (C1D1) and EOT. More gene rearrangements and mutations were found in plasma samples (ctDNA) than in archival tumor samples. Archival tumor samples were available from 6 patients for NGS analysis. One patient (Patient 5 in Figure 1B) had tissue sample available from the post‐alectinib treatment period (before the start of ceritinib treatment) and the remaining 5 patients (Patients 7, 9, 10, 12, and 14) had tissue samples from the pre‐alectinib treatment period. We identified ALK mutations from plasma at C1D1 in 5 of 20 patients, including G1202R (n = 2, Patients 10 and 14); G1269A (n = 1, Patient 13); L1196M (n = 1, Patient 5); and G1202R, V1180L, and I1171N (n = 1, Patient 19), but not from the archival tumor, except L1196M in Patient 5. Archival tissue showed ALK L1196M mutation in 1 patient only (Patient 5). The best response in the patient with ALK gene mutations from plasma (G1202R, V1180L, I1171N, or G1269A at C1D1) was stable disease or PD, whereas the best response in the patient who had ALK L1196M mutation was PR. At EOT, G1202R mutation was newly emerged in 2 patients (Patients 6 and 8).
3.4. Safety
Median duration of ceritinib exposure was 3.7 months (range: 0.4‐15.1), with a median relative dose intensity of 68.6% (range: 30.3%‐100%). All patients experienced at least 1 AE, with 10 (50.0%) patients reporting an SAE irrespective of relationship to study drug. The most frequently (>50%) reported all‐causality AE were diarrhea (85.0%), nausea (80.0%), and vomiting (65.0%) (Table 4). Grade 3/4 AE were reported in 70.0% of patients, with 60.0% of patients experiencing grade 3/4 AE suspected to be study drug‐related. The most common (>10%) drug‐related grade 3/4 AE were increased diarrhea (20.0%) and increase in alanine aminotransferase (ALT) (15.0%). Overall, 3 (15.0%) patients had AE that led to study discontinuation (anemia, acute kidney injury, and pleural effusion [non‐malignant] in 1 patient each). All 20 patients required at least 1 dose adjustment or interruption. AE requiring dose adjustment or interruption reported in 2 or more patients were: elevated ALT, elevated blood creatinine, diarrhea, nausea, pyrexia (20.0% each); vomiting (15.0%); and decreased appetite and metastases to the central nervous system (10.0% each).
Table 4.
All‐causality adverse events reported in ≥20.0% of patients
| Preferred term | Ceritinib 750 mg/d N = 20 | |
|---|---|---|
| Any grade, n (%) | Grade 3/4, n (%) | |
| Diarrhea | 17 (85.0) | 4 (20.0) |
| Nausea | 16 (80.0) | 1 (5.0) |
| Vomiting | 13 (65.0) | 0 |
| Alanine aminotransferase increase | 8 (40.0) | 3 (15.0) |
| Blood creatinine increase | 8 (40.0) | 0 |
| Decrease appetite | 8 (40.0) | 2 (10.0) |
| Aspartate aminotransferase increase | 7 (35.0) | 0 |
| Constipation | 6 (30.0) | 1 (5.0) |
| Electrocardiogram QT prolonged | 5 (25.0) | 1 (5.0) |
| Gamma‐glutamyltransferase increase | 5 (25.0) | 3 (15.0) |
| Pyrexia | 5 (25.0) | 0 |
Gastrointestinal AE were the most frequently reported all‐causality AE. Most gastrointestinal‐related AE were grade 1/2, with grade 3 diarrhea and nausea reported in 20.0% and 5.0% of patients, respectively. No grade 4 or serious gastrointestinal event was reported. Overall, 8 (40.0%) patients required a dose adjustment or interruption because of gastrointestinal‐related AE.
Corrected QT interval prolongation was reported in 5 (25.0%) patients and was suspected to be study drug‐related. Severity was grade 3 in 1 patient (who had electrocardiogram QT prolonged at baseline) and grade 1 in the other 4 patients. No events were reported as SAE and no patients discontinued the study drug as a result of the event. No cases of intestinal lung disease and Hy's law (aspartate aminotransferase [AST] and/or ALT >3.0× upper limit of normal [ULN] and total bilirubin >2.0× ULN in the absence of cholestasis [alkaline phosphatase (ALP) <2× ULN]) or Torsades de pointes were reported. As of the data cutoff date, 5 (25.0%) patients died during the study; all the deaths were considered to be due to study indication (NSCLC) and no on‐treatment deaths (only deaths occurring during treatment or within 30 days of the last dose of study drug) were reported.
4. DISCUSSION
In this phase II study, ceritinib showed rapid antitumor activity in ALK‐positive NSCLC patients who failed prior alectinib and/or other systemic therapies. Among the 20 patients, 16 patients received alectinib as the last treatment and had PD prior to study entry. Despite 70.0% of patients receiving ≥2 antineoplastic regimens prior to study initiation, and a high proportion of patients having baseline brain metastasis (60.0%), ceritinib indicated an ORR of 25% (95% CI: 8.7‐49.1) with reduction in tumor size in 70.6% of patients. DCR based on investigator assessment was 70.0% (95% CI: 45.7‐88.1). One patient had prolonged stable disease for 12.4 months. ORR by investigator review based on the number of prior regimens was 16.7% (1/6 patients), 22.2% (2/9 patients), and 40.0% (2/5 patients) at prior regimen 1, 2, and 3, respectively, which indicated clinical responses were observed regardless of the number of prior lines of therapy. Responses were rapid, with a median time to first response of 1.8 months (range: 1.8‐2.0 months). Median PFS and median DOR were 3.7 months (95% CI: 1.9‐5.3) and 6.3 months (95% CI: 3.5‐9.2), respectively. Three patients continued receiving ceritinib beyond PD as a result of continued clinical benefit.
Currently, in Japan, the standard treatment for advanced ALK‐positive NSCLC has changed from crizotinib to alectinib. There is no standard of care for patients who progress on first‐line alectinib. Clinical activity of available ALK tyrosine kinase inhibitors in patients pretreated with alectinib has been a clinical question of interest, as these inhibitors have different activities against various ALK mutations that cause resistance to the treatments. Although several phase I, II, and III studies have shown activity of ceritinib in patients with crizotinib resistance,15, 17, 18, 19 this is the first prospective study to show efficacy and safety of ceritinib in patients with ALK‐positive NSCLC who progressed on alectinib treatment. This study confirms the previous observations from a phase I study of ceritinib in Japanese patients, where clinical benefit was observed in 2 of 4 patients with recurrence or relapse after alectinib.17 Both preclinical and clinical studies have shown that alectinib is active in treating patients with brain metastasis.12, 20, 21 All the patients enrolled in our study were previously treated with alectinib, but 60% of these presented with brain metastasis at study entry. Best overall intracranial response by investigator in 2 patients with measurable brain metastasis at baseline was stable disease and unknown; however, in the predefined subgroup analysis, 3 of 12 patients who had measurable or non‐measurable brain metastases at baseline responded to ceritinib treatment, with 2 patients showing PR and 1 patient showing CR. Although the results of ORR subgroup analyses need to be interpreted with caution because of the small sample size of this study, clinically meaningful antitumor activity of ceritinib was observed regardless of the presence or absence of brain metastases (including the non‐target lesions) at baseline. ORR by the presence of brain metastases at baseline was 25.0% in both subgroups (3/12 patients with brain metastases, 2/8 patients without brain metastases), consistent with the ORR in the whole study population.
Among the 5 responders in the present study, 4 had been treated with 2 or more prior regimens of medications, including alectinib, and 4 had been treated with alectinib as the last therapy prior to study enrollment. Interestingly, among the responders, duration between discontinuing alectinib and start of ceritinib treatment was only 1‐3 days. Of note, 1 responder to ceritinib with a best overall response of PR had an L1196M ALK mutation known to be crizotinib resistant. Preclinical studies have shown that both ceritinib and alectinib are active against L1196 mutation with different IC50 values.22, 23 In non‐responders, based on biomarker data and patients’ demographics information, no specific characteristics of patients were identified. To better understand the activity of ceritinib in alectinib‐resistant patients, further biomarker analysis is warranted, but we can speculate that ceritinib efficacy in alectinib‐resistant patients could be a result of specific molecular alterations that impair alectinib binding to ALK.16
We determined the DNA gene profile in tumor tissue and ctDNA in NSCLC patients. The ALK gene mutations identified are consistent with the reported resistance mutations for patients treated with alectinib or crizotinib.24 From ctDNA we can detect mutations that are not in the formalin fixed paraffin embedded (FFPE) tumor because ctDNA is a collection of mutations that might come from different sites because of tumor heterogeneity.25 As the numbers of patients were small in the present study, further study will be required to investigate the relationship between the identified multiple gene alterations and clinical outcomes.
The median duration of exposure was 3.7 months. All 20 patients required at least 1 dose adjustment or interruption. Median relative dose intensity in our study with ceritinib 750 mg fasted was 68.6% (range: 30.3‐100) which was consistent with that reported in the Japanese population in ASCEND‐2 (65.0% [range: 38.5‐100.0])26 and ASCEND‐5 (71.9% [range: 35.4%‐100.0%]).27 The safety profile of ceritinib was consistent with that previously reported,14, 15 with no new or unexpected AE identified. AE were manageable in most patients with dose adjustments/interruptions and/or additional therapies, resulting in a few discontinuations of the study drug. Overall, gastrointestinal AE (diarrhea, nausea, and vomiting) were the most frequently reported all‐causality drug‐related AE. Recent data from a phase I study of ceritinib (ASCEND‐8; NCT02299505) show that the frequency and severity of gastrointestinal toxicities was lower with a starting dose of 450 mg/day under fed condition compared to that with the currently approved recommended dose of 750 mg/day under fasted condition, with fewer patients requiring dose reduction or interruption, resulting in the higher median relative dose intensity and comparable efficacy.28, 29 Thus, ceritinib treatment with this new starting dose of 450 mg/day under fed condition is expected to improve the relative dose intensity on account of a better gastrointestinal safety profile and more favorable benefit/risk profile.
In the phase I study of lorlatinib, ORR in patients who received at least 1 prior line of alectinib (N = 74) was 35.1%.30 We acknowledge the activity of lorlatinib in alectinib‐pretreated patients with ALK‐positive NSCLC; however, comparison with our study is limited by different types of prior therapies (tyrosine kinase inhibitors and/or chemotherapy) received. Other limitations of our study include the small sample size and the fact that this was not a controlled trial comparing other ALK inhibitors or chemotherapy in this setting. As a result of the small sample size, it was also very difficult to identify specific patient characteristics for the responders and non‐responders.
In this phase II study, ceritinib showed rapid and meaningful antitumor activity in ALK‐rearranged, advanced NSCLC patients who progressed on prior therapy with alectinib including patients who experienced immediate progression on prior alectinib. Responders to ceritinib included patients who received prior crizotinib in addition to alectinib and who had 2 or more lines of prior therapies. Safety profile of ceritinib was consistent with that reported in previous studies, and no new or unexpected safety concerns were identified. Overall, ceritinib could be considered as one of the treatment options for patients with ALK‐positive NSCLC who progressed on alectinib.
CONFLICTS OF INTEREST
Annual value of remuneration received: Masayuki Ito from Novartis Pharma K.K. (employment). Koichi Ayukawa from Novartis Pharma K.K. (employment). Kota Tokushige from Novartis Pharma K.K. (employment). Masataka Yonemura from Novartis Pharma K.K. (employment). Total annual value of daily allowances/honoraria received: Takashi Seto from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd, Eli Lilly Japan K.K., MSD, Nippon Boehringer‐Ingelheim Co., Ltd, Ono Pharmaceutical Co. Ltd, Pfizer Japan Inc., and Taiho Pharmaceutical Co., Ltd. Hidehito Horinouchi from Eli Lilly. Katsuyuki Hotta from Astra Zeneca, Ono Pharmaceutical, Astellas, Novartis, BMS, MSD, Eli Lilly Japan, Daiichi‐Sankyo Pharmaceutical, Boehringer‐Ingelheim, Nihon Kayaku, Taiho Pharmaceutical, and Chugai Pharmaceutical. Shingo Matsumoto from Novartis Pharma, Pfizer Inc., Ono Pharmaceutical and Bristol‐Myers Squibb. Makoto Nishio from Ono Pharmaceutical, Bristol‐ Myers Squibb, Pfizer, Chugai Pharmaceutical, Eli Lilly, MSD, Novartis, Taiho Pharmaceutical, Astra Zeneca, and Boehringer‐Ingelheim. Total annual value of research funds, endowments, endowed chairs, and researcher‐employment costs received: Toyoaki Hida from Novartis, Chugai, Pfizer, Takeda, and Ignyta. Hidehito Horinouchi from Taiho, Merck Serono, Novartis, Astellas, Chugai, Genomic Health, Ono, and BMS. Katsuyuki Hotta from Astra Zeneca, Ono Pharmaceutical, Astellas, BMS, MSD, Eli Lilly Japan, Boehringer‐Ingelheim, and Chugai Pharmaceutical. Makoto Nishio reports from Ono Pharmaceutical, Bristol‐Myers Squibb, Pfizer, Chugai Pharmaceutical, Eli Lilly, MSD, Novartis, Taiho Pharmaceutical, Astra Zeneca, and Boehringer‐Ingelheim.
Supporting information
ACKNOWLEDGMENTS
This study was sponsored by Novartis Pharma K.K., Tokyo, Japan. We thank Pushkar Narvilkar and Shiva Krishna Rachamadugu, Novartis Healthcare Pvt. Ltd, for providing medical editorial assistance with this manuscript.
Hida T, Seto T, Horinouchi H, et al. Phase II study of ceritinib in alectinib‐pretreated patients with anaplastic lymphoma kinase‐rearranged metastatic non‐small‐cell lung cancer in Japan: ASCEND‐9. Cancer Sci. 2018;109:2863–2872. 10.1111/cas.13721
Dr Makoto Maemondo is currently an employee at Iwate Medical University, Iwate, Japan.
Dr Fumihiko Hirai is currently an employee at Kyushu University hospital, Fukuoka, Japan.
REFERENCES
- 1. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature. 2007;448:561‐566. [DOI] [PubMed] [Google Scholar]
- 2. Shaw AT, Yeap BY, Mino‐Kenudson M, et al. Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol. 2009;27:4247‐4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. US Food and Drug Administration . Crizotinib US prescribing information. 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202570s011lbl.pdf. Accessed May 23, 2018.
- 4. Solomon BJ, Mok T, Kim D‐W, et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med. 2014;371:2167‐2177. [DOI] [PubMed] [Google Scholar]
- 5. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. N Engl J Med. 2013;368:2385‐2394. [DOI] [PubMed] [Google Scholar]
- 6. Pharmaceuticals and Medical Devices Agency . Report on the deliberation results (crizotinib). March, 2012. https://www.pmda.go.jp/files/000153949.pdf. Accessed March 23, 2018.
- 7. European Medicines Agency . Crizotinib EU prescribing information. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002489/WC500134759.pdf. Accessed March 23, 2018.
- 8. Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clinl Oncol. 2011;29:e443‐e445. [DOI] [PubMed] [Google Scholar]
- 9. Awad MM, Shaw AT. ALK inhibitors in non‐small cell lung cancer: crizotinib and beyond. Clin Adv Hematol Oncol. 2014;12:429‐439. [PMC free article] [PubMed] [Google Scholar]
- 10. Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK‐rearranged advanced non‐small‐cell lung cancer (AF‐001JP study): a single‐arm, open‐label, phase 1‐2 study. Lancet Oncol. 2013;14:590‐598. [DOI] [PubMed] [Google Scholar]
- 11. Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK‐positive non‐small‐cell lung cancer (J‐ALEX): an open‐label, randomised phase 3 trial. Lancet. 2017;390:29‐39. [DOI] [PubMed] [Google Scholar]
- 12. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK‐positive non‐small‐cell lung cancer. N Engl J Med. 2017;377:829‐838. [DOI] [PubMed] [Google Scholar]
- 13. US Food and Drug Administration . Ceritinib prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205755s010lbl.pdf. Accessed March 22, 2018.
- 14. Soria JC, Tan DSW, Chiari R, et al. First‐line ceritinib versus platinum‐based chemotherapy in advanced ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐4): a randomised, open‐label, phase 3 study. Lancet. 2017;389:917‐929. [DOI] [PubMed] [Google Scholar]
- 15. Shaw AT, Kim TM, Crino L, et al. Ceritinib versus chemotherapy in patients with ALK‐rearranged non‐small‐cell lung cancer previously given chemotherapy and crizotinib (ASCEND‐5): a randomised, controlled, open‐label, phase 3 trial. Lancet Oncol. 2017;18:874‐886. [DOI] [PubMed] [Google Scholar]
- 16. Katayama R, Friboulet L, Koike S, et al. Two novel ALK mutations mediate acquired resistance to the next‐generation ALK inhibitor alectinib. Clin Cancer Res. 2014;20:5686‐5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishio M, Murakami H, Horiike A, et al. Phase I study of ceritinib (LDK378) in Japanese patients with advanced, anaplastic lymphoma kinase‐rearranged non‐small‐cell lung cancer or other tumors. J Thorac Oncol. 2015;10:1058‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐1): updated results from the multicentre, open‐label, phase 1 trial. Lancet Oncol. 2016;17:452‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crinò L, Ahn M‐J, Marinis FD, et al. Multicenter phase II study of whole‐body and intracranial activity with ceritinib in patients with ALK‐rearranged non‐small‐cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND‐2. J Clin Oncol. 2016;34:2866‐2873. [DOI] [PubMed] [Google Scholar]
- 20. Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74:1023‐1028. [DOI] [PubMed] [Google Scholar]
- 21. Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib‐refractory ALK‐rearranged non‐small‐cell lung cancer: a phase II global study. J Clin Oncol. 2016;34:661‐668. [DOI] [PubMed] [Google Scholar]
- 22. Fontana D, Ceccon M, Gambacorti‐Passerini C, et al. Activity of second‐generation ALK inhibitors against crizotinib‐resistant mutants in an NPM‐ALK model compared to EML4‐ALK. Cancer Med. 2015;4:953‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non‐small cell lung cancer. Cancer Discov. 2014;4:662‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin JJ, Riely GJ, Shaw AT. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017;7:137‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hida T, Satouchi M, Nakagawa K, et al. Ceritinib in patients with advanced, crizotinib‐treated, anaplastic lymphoma kinase‐rearranged NSCLC: Japanese subset. Jpn J Clin Oncol. 2017;47:618‐624. [DOI] [PubMed] [Google Scholar]
- 27. Kiura K, Imamura F, Kagamu H, et al. Phase 3 study of ceritinib vs chemotherapy in ALK‐rearranged NSCLC patients previously treated with chemotherapy and crizotinib (ASCEND‐5): Japanese subset. Jpn J Clin Oncol. 2018;48(4):367‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cho BC, Kim DW, Bearz A, et al. ASCEND‐8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low‐fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)‐rearranged metastatic non‐small cell lung cancer (NSCLC). J Thorac Oncol. 2017;12:1357‐1367. [DOI] [PubMed] [Google Scholar]
- 29. Cho BC, Obermannová R, Bearz A, et al. Efficacy and updated safety of ceritinib (450 mg or 600 mg) with low‐fat meal vs 750 mg fasted in ALK+ metastatic NSCLC. In World Conference on Lung Cancer. 2017. Oral Presentation # OA 05.07. [Google Scholar]
- 30. Felip E, Shaw AT, Solomon BJ, et al. Efficacy and safety of Lorlatinib in patients With ALK+ non‐small cell lung cancer (NSCLC) previously treated with second‐generation ALK TKIs. In: European Society for Medical Oncology. 2017. Poster Presentation # 1343P. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


