Abstract
Postmarketing surveillance is useful to collect safety data in real‐world clinical settings. In this study, we applied postmarketing real‐world data on a mechanistic model analysis for neutropenic profiles of eribulin in patients with recurrent or metastatic breast cancer. Demographic and safety data were collected using an active surveillance method from eribulin‐treated recurrent or metastatic breast cancer patients. Changes in neutrophil counts over time were analyzed using a mechanistic pharmacodynamic model. Pathophysiological factors that might affect the severity of neutropenia were investigated, and neutropenic patterns were simulated for different treatment schedules. Clinical and laboratory data were collected from 401 patients (5199 neutrophil count measurements) who had not received granulocyte colony‐stimulating factor and were eligible for pharmacodynamic analysis. The estimated mean parameters were as follows: mean transit time = 104.5 h, neutrophil proliferation rate constant = 0.0377 h−1, neutrophil elimination rate constant = 0.0295 h−1, and linear coefficient of drug effect = 0.0413 mL/ng. Low serum albumin levels and low baseline neutrophil counts were associated with severe neutropenia. The probability of grade ≥3 neutropenia was predicted to be 69%, 27%, and 27% for patients on standard, biweekly, and triweekly treatment scenarios, respectively, based on virtual simulations using the developed pharmacodynamic model. In conclusion, this is the first application of postmarketing surveillance data to a model‐based safety analysis. This analysis of safety data reflecting authentic clinical settings will provide useful information on the safe use and potential risk factors of eribulin.
Keywords: eribulin, neutropenia, pharmacodynamic, pharmacometric, postmarketing surveillance
1. INTRODUCTION
Breast cancer is the most common cancer in women; in 2012, there were an estimated 1.67 million new cases and 0.52 million deaths from breast cancer worldwide.1 Cytotoxic chemotherapies based on anthracyclines and taxanes are the primary therapeutic options for recurrent or metastatic breast cancer (RBC/MBC). However, the disease often progresses due to primary or acquired resistance to these treatment regimens, and there are few subsequent therapeutic options for patients with refractory disease.2 Over the past several years, eribulin mesilate, a microtubule inhibitor, has shown reasonable efficacy with acceptable toxicity in patients with RBC/MBC.3, 4, 5 The phase III EMBRACE trial of eribulin mesilate for women with pretreated metastatic breast cancer showed promising results, showing a significant improvement in median overall survival of 2.5 months compared with the treatment of physician's choice.3
Eribulin mesilate was approved for the treatment of RBC/MBC in Japan in April 2011 based on a phase II domestic trial of 81 patients and premarketing clinical studies of only a small number of Japanese patients.6 Grade ≥3 neutropenia occurring during eribulin treatment appears to be more frequent in studies of East Asian populations (85%‐95%) than of global populations (20%‐65%).7, 8 Therefore, it is important that more information is obtained on the safety and toxicity of eribulin treatment, especially in Japanese patients.
Japanese regulations require postmarketing surveillance studies of new chemical entities and biological products to confirm their safety. A considerable amount of data has been reported by physicians who prescribe eribulin mesilate; however, the data generally only document and confirm the frequency of toxicities.9 Here, we used observational safety data to carry out a model‐based pharmacodynamic analysis of the safety profile of eribulin in the clinical setting of patients with RBC/MBC. The major reported adverse events and dose‐limiting toxicities associated with eribulin treatment include neuropathy and neutropenia.10 As severe neutropenia often requires changes in treatment schedules in the clinical setting, we focused on analysis of neutropenia as the most common toxicity related to eribulin treatment.
2. MATERIALS AND METHODS
2.1. Patients
Between July 2011 and December 2011, demographic and safety data were collected by the surveillance method from eribulin‐naïve RBC/MBC patients who were treated with eribulin mesilate in 325 centers in Japan. Patients with contraindications to treatment (high myelosuppression, known hypersensitivity to eribulin mesilate, pregnancy, or the possibility of pregnancy) were excluded from the postmarketing surveillance. The postmarketing surveillance of eribulin was carried out in accordance with Japanese regulatory requirements called Good Post‐Marketing Study Practice. In addition, all personal information related to the surveillance was managed to be anonymous in accordance with privacy protection laws. The Ethics Committee of Keio University School of Medicine (Tokyo, Japan) approved the retrospective pharmacodynamic analysis using anonymous data collected by the postmarketing surveillance of eribulin.
2.2. Postmarketing surveillance data
Postmarketing surveillance data for eribulin treatment included gender, age, ECOG performance status (PS), history of treatment with cytotoxic agents, complete blood counts (including absolute neutrophil counts at baseline [BNEU]), serum chemistries (serum albumin [ALB], total bilirubin [BILI], and alkaline phosphatase [ALP]), injection date, and dose of eribulin mesilate. Collection of all laboratory parameters, including neutrophil counts, was arbitrary with respect to time and frequency because examination and treatment schedules varied with the patient's clinical situation. The observation period for neutrophil counts and the timing of eribulin dose reduction were also different for each patient.
2.3. Establishment of a population pharmacokinetic/pharmacodynamic model for eribulin
The population pharmacokinetic (PK)/pharmacodynamic (PD) model for eribulin is shown in Figure 1. Plasma eribulin concentrations were simulated based on a population PK model developed by Majid et al,11 who reported that eribulin PK could be described by a three‐compartment model with linear elimination from the central compartment and overall steady‐state exposure (area under the curve) that increased proportionally with the total eribulin dose. The following PK parameters were calculated from the Majid et al population PK model using individual patient demographic data to simulate the PK profile: clearance (CL [L/h]), volume of compartments (V1, V2 and V3 [L]), and intercompartmental clearance (Q2 and Q3 [L/h]). CL, Q2, and Q3 were normalized by body weight (WGT). CL was dependent on the values of ALB, ALP, and BILI.
Figure 1.
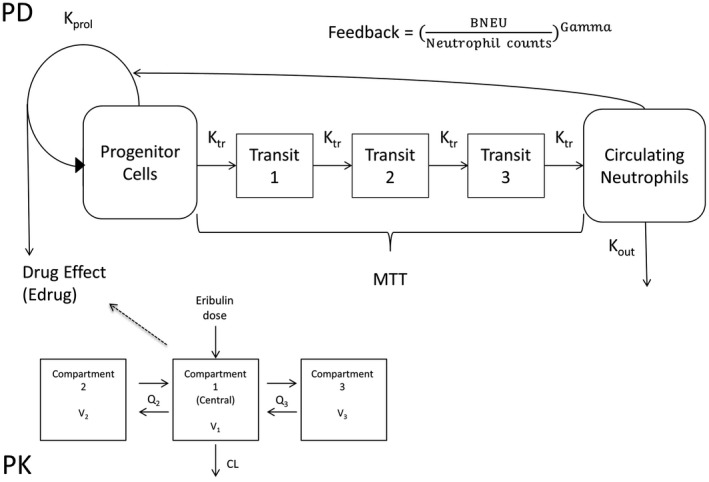
A, Mechanistic pharmacokinetic/pharmacodynamic model to describe neutrophil count profiles. BNEU, absolute neutrophil count at baseline; CL, clearance; Gamma, feedback constant; Kout, neutrophil elimination rate constant; Kprol, neutrophil proliferation rate constant; Ktr, neutrophil transition rate constant; MTT, mean transit time; Q, intercompartmental clearance; V, volume of compartment
The given dose of eribulin was converted to the free base equivalent, which was used in the calculations. An eribulin mesilate i.v. infusion dose of 1.4 mg/m2 was equivalent to an eribulin‐free base dose of 1.23 mg/m2.
A mechanistic PD model for neutropenia during eribulin treatment reported by Friberg et al12 was used to describe neutrophil count vs time profiles with simulated plasma concentrations (C) of eribulin in individual patients. The model consisted of four system‐dependent and drug‐dependent parts (Figure 1): (i) proliferation of the progenitor cell compartment; (ii) maturation, represented in the model by three transit compartments (Tr1, Tr2, and Tr3); (iii) elimination of circulating neutrophils; and (iv) homeostatic feedback regulation. Steps (i) – (iv) can be described by the following PD parameters: mean transit time through the neutrophil maturation delay chain (MTT [h]), neutrophil proliferation rate constant (Kprol [h−1]), neutrophil elimination rate constant (Kout [h−1]), feedback constant (Gamma), and linear coefficient of drug effect (Slope [mL/ng]). Edrug means drug effect, and Ktr is the transit rate (Tr) constant from one compartment to the next. MTT was converted as 4/Ktr in the following formulae.
Computation was carried out using Phoenix NLME software version 7.0 (Certara, Princeton, NJ, USA) with a first‐order method on a HP Z640 workstation (Intel Xeon E5 processor, 2.60 GHz, 28 cores).
2.4. Determination of clinical factors that affect safety
We undertook the multivariate analysis using a stepwise method to search for clinical factors that could influence neutropenia. The potential factors analyzed included age, ECOG PS, laboratory data (BNEU and ALB), and the number of previous chemotherapy regimens. Final covariate selection was carried out using the likelihood ratio test based on differences in the objective function value. P < .05 was considered significant. Based on the final model, a Monte Carlo simulation was carried out to estimate the predictability of neutropenia of grade 3 (<1000/μL) and grade 4 (<500/μL) according to the Common Terminology Criteria for Adverse Events (version 4.0). The simulations were conducted according to three treatment scenarios: (i) i.v. infusion on day 1 and day 8 every 21 days (standard scenario); (ii) i.v. infusion on day 1 and day 15 every 28 days (biweekly scenario); and (iii) i.v. infusion on day 1 every 21 days (triweekly scenario).
3. RESULTS
3.1. Study population and patient characteristics
Patients with RBC/MBC who were receiving eribulin treatment for the first time and had not received granulocyte colony‐stimulating factor (G‐CSF) were enrolled in this study. A flowchart showing selection of the final study population is presented in Figure 2. Of the 608 patients whose data were collected, a total of 207 were excluded for the following reasons: 182 patients lacked data for ALB, and/or ALP, and/or BILI; and 25 patients lacked data for BNEU. Finally, 401 patients with a total of 5199 neutrophil count measurements were eligible for the PD analysis.
Figure 2.
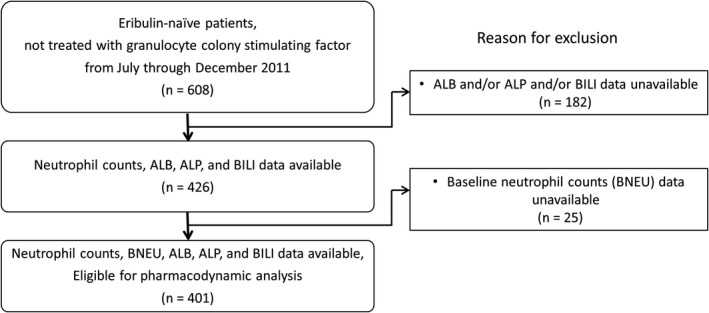
Overview of the study population of eribulin‐treated recurrent or metastatic breast cancer patients. Of the 608 starting patient population who had not been treated with granulocyte colony‐stimulating factor, a total of 401 patients were eligible for pharmacodynamic analysis. ALB, serum albumin level; ALP, alkaline phosphatase level; BILI, total bilirubin level; BNEU, absolute neutrophil count at baseline
Characteristics of the study population are shown in Table 1. The median age was 58 years (range, 26‐84 years), and the median number of previous chemotherapy regimens, including taxanes, was 4 (range, 0‐13). The planned eribulin treatment regimen was i.v. administration of 1.4 mg/m2 on days 1 and 8 every 3 weeks. Depending on the individual patient's condition (eg, disorder of liver function such as elevated aspartate aminotransferase or alanine aminotransferase), the eribulin dose was reduced to 0.7 mg/m2, the treatment schedule was modified, doses were skipped, or treatment was discontinued based on the physicians’ decision.
Table 1.
Characteristics of study population of eribulin‐treated recurrent or metastatic breast cancer patients
| Treatment schedule | Total (n = 401) | Standard (n = 275) | Biweekly (n = 64) | Triweekly (n = 50) |
|---|---|---|---|---|
| Dose (mg/m2) | ||||
| Median | 1.4 | |||
| Range | 0.7‐1.4 | |||
| Age | ||||
| Median | 58 | 58 | 58.5 | 59 |
| Range | 26‐84 | 26‐81 | 33‐84 | 40‐74 |
| ECOG performance status (n) | ||||
| 0‐1 | 192 | 138 | 24 | 22 |
| 2 | 172 | 121 | 27 | 20 |
| ≥3 | 37 | 16 | 13 | 8 |
| Number of previous CTx regimens (n) | ||||
| 0 | 11 | 9 | 1 | 1 |
| 1 | 31 | 17 | 6 | 5 |
| 2‐4 | 194 | 127 | 33 | 28 |
| ≥5 | 165 | 122 | 24 | 16 |
| Serum albumin (g/dL) | ||||
| Median | 3.9 | 3.9 | 3.7 | 3.7 |
| Range | 1.3‐5.1 | 1.4‐5 | 1.7‐4.8 | 1.4‐4.8 |
| Baseline neutrophil count (/μL) | ||||
| Median | 3200 | 3432 | 3204 | 2972 |
| Range | 943‐15 000 | 943‐15 002 | 1090‐9430 | 1040‐6712 |
CTx, chemotherapy.
3.2. Pharmacokinetic/pharmacodynamic model and clinical factors affecting toxicity
We developed a population PK/PD model describing eribulin‐induced neutropenia using the postmarketing surveillance data collected from 401 patients. The following parameters were estimated: MTT (h), Kprol (h−1), Kout (h−1), Gamma, and Slope (mL/ng). Clinical factors that could influence the severity of neutropenia were searched using a shotgun algorithm. We found that age (<65 or ≥65 years), ECOG PS (<2 or ≥2), and the number of previous chemotherapy regimens (<5 or ≥5) were not related to neutropenia. However, BNEU and ALB were suggested to influence neutropenic toxicity, in that lower BNEU was associated with higher Kprol and lower ALB was associated with higher Kprol, lower MTT, and lower Kout. In contrast, Slope was not influenced by any factors.
The final PK/PD model incorporating the clinical factors that influenced neutropenia is shown in Table 2. The key estimated mean parameters were as follows: MTT = 104.5 h, Kprol = 0.0377 h−1, Kout = 0.0295 h−1, Gamma = 0.203, and Slope = 0.0413 mL/ng. We confirmed the validity and robustness of the obtained PD parameters by a bootstrap method. A total of 182 of the 200 bootstrap runs reached successful convergence, and the bootstrap mean/final estimate ratio was within a reasonable range (97.5%‐142.8%). Final estimates of the model parameters were similar to those previously reported using premarketing clinical trial data.13
Table 2.
Final parameter estimates and bootstrap validation of the population pharmacodynamic model for eribulin
| Parametersa | Final estimates of the model parameters | Results of 182 bootstrap simulations | Bootstrap mean/final estimate ratio (%) | ||
|---|---|---|---|---|---|
| Mean | 95% CI | ||||
| tvKprol (h−1) | 0.0377 | 0.0388 | 0.0303 to 0.0472 | 102.9 | |
| tvMTT (h) | 104.5 | 103.1 | 82.1 to 124.1 | 98.7 | |
| tvKout (h−1) | 0.0295 | 0.0315 | 0.0142 to 0.0489 | 106.8 | |
| tvGamma | 0.203 | 0.198 | 0.157 to 0.239 | 97.5 | |
| tvSlope (mL/ng) | 0.0413 | 0.0408 | 0.0320 to 0.0496 | 98.8 | |
| θALBKprol | −0.759 | −0.768 | −1.110 to −0.427 | 101.2 | |
| θALBMTT | 0.605 | 0.626 | 0.278 to 0.973 | 103.5 | |
| θALBKout | 0.357 | 0.403 | −0.144 to 0.950 | 112.9 | |
| θBNEUKprol | 0.0704 | 0.0693 | 0.0432 to 0.0953 | 98.4 | |
|
|
0.00417 | 0.00409 | 0.00212 to 0.00606 | 98.1 | |
|
|
0.374 | 0.534 | −0.270 to 1.340 | 142.8 | |
|
|
0.163 | 0.168 | 0.106 to 0.229 | 103.1 | |
| σ (/nL) | 1.15 | 1.13 | 1.03 to 1.23 | 98.3 | |
, , , variances of interindividual variability; σ, standard deviation of residual variability; θALBMTT, effect of ALB on MTT; θALBKout, effect of ALB on Kout; θALBKprol, effect of ALB on Kprol; θBNEUKprol, effect of BNEU on Kprol; ALB, albumin; BNEU, absolute neutrophil count at baseline; CI, confidence interval; Gamma, feedback constant; Kout, neutrophil elimination rate constant; Kprol, neutrophil proliferation rate constant; MTT, mean transit time; Slope, linear coefficient of drug effect; tv, typical value.
Population PD model parameter estimates for eribulin:
If baseline neutrophil counts <3000, then BNEU3 = 1; if ≥3000, then BNEU3 = 0.
3.3. Model‐based simulation according to the treatment scenario
We next investigated the absolute neutrophil counts during the 21 days of cycle 1 of eribulin treatment (Figure 3). Neutropenic toxicity was influenced by both ALB levels (Figure 3A) and BNEU (Figure 3B), although the impact varied with the treatment schedule. In patients with low albumin levels, the toxicity, in terms of nadir counts and delayed recovery, was most severe in group 1 (standard treatment scenario, n = 275) compared with group 2 (biweekly scenario, n = 64) and group 3 (triweekly scenario, n = 50). Twelve patients with other treatment scenarios were excluded from this analysis.
Figure 3.
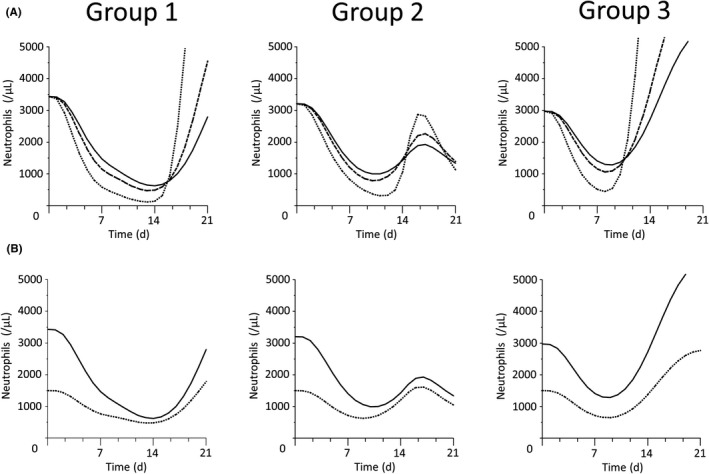
Effects of serum albumin level and baseline neutrophil counts on neutropenia in eribulin‐treated recurrent or metastatic breast cancer patients (n = 401). A, Effect of albumin levels. Black, dashed, and dotted lines indicate normal (3.9 g/dL), low (3.0 g/dL), and severely reduced (1.5 g/dL) serum albumin levels, respectively. Earlier and deeper nadirs were observed in patients with low albumin levels in each group. B, Effect of baseline neutrophil counts. Black and dotted lines indicate normal (3200/μL) and severely reduced (1500/μL) neutrophil counts, respectively. The difference between normal and severely reduced absolute neutrophil counts was greater in group 3 than in groups 1 or 2. For A and B, group 1, standard treatment scenario (n = 275), i.v. infusion on day 1 and day 8 every 21 days; group 2, biweekly scenario (n = 64), i.v. infusion on day 1 and day 15 every 28 days; group 3, triweekly scenario (n = 50), i.v. infusion on day 1 every 21 days
3.4. Risk prediction based on the PD simulation
Based on the simulated absolute neutrophil counts in cycle 1 obtained with the PK/PD model, we ran simulated analyses of 401 patients in the standard, biweekly, and triweekly treatment scenarios to predict the severity of neutropenia. From this, the probability of grade ≥3 and ≥4 neutropenia was estimated to be 69% and 23% on the standard scenario, 27% and 3% on the biweekly scenario, and 27% and 3% on the triweekly scenario, respectively (Figure 4).
Figure 4.
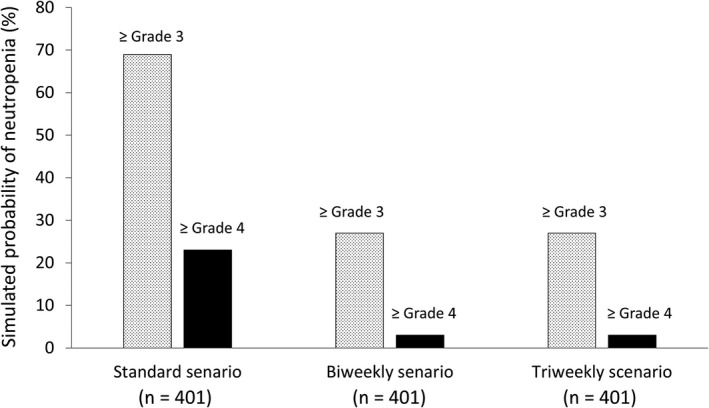
Simulated probability of neutropenia in eribulin‐treated recurrent or metastatic breast cancer patients (n = 401). Shaded and black bars indicate the probability of grade ≥3 or ≥4 neutropenia, respectively. Pharmacodynamic simulation showed that a biweekly treatment scenario (i.v. infusion on day 1 and day 15 every 28 days) reduced the probability of neutropenia compared with the standard scenario (i.v. infusion on day 1 and day 8 every 21 days). Triweekly scenario, i.v. infusion on day 1 every 21 days
4. DISCUSSION
This is the first large‐scale PD study of eribulin therapy in RBC/MBC patients using postmarketing surveillance safety data. Population PK/PD analyses of eribulin‐associated neutropenia published to date have been based on data obtained in premarketing clinical trials that had strict eligibility criteria and treatment schedules.13 However, our study here shows that postmarketing surveillance data can also be used for a model‐based safety analysis. The use of postmarketing data to investigate drug safety profiles is advantageous because it is derived from patients with broader backgrounds in more realistic clinical settings. Furthermore, the variability in treatment schedules based on each patient's physical condition provides additional information about treatment schedules that differ from the standard regimen.
Data on plasma concentrations of drug were not available in this postmarketing surveillance; therefore, plasma eribulin concentrations were simulated using the population PK model reported by Majid et al. In their analysis, efficacy and safety data from seven phase I studies, one phase II study, and one phase III study were combined to characterize the PK and exposure‐efficacy relationship of eribulin. The results of that study suggested that their PK model was also applicable for analyzing the safety of eribulin treatment, especially dose‐limiting toxicities.
In the present study, the PD simulations revealed that low ALB and low BNEU were both associated with severe neutropenia. A PK/PD model of docetaxel, for which neutropenia is also a dose‐limiting toxicity, showed that ALB influenced the CL and EC50 of docetaxel; indeed, both factors had a strong impact on the development of neutropenia.14 Although it seems reasonable that a lower BNEU would lead to more severe neutropenia after eribulin treatment, the mechanism by which ALB influences the nadir neutrophil count remains unclear. Malnutrition‐related low ALB levels have been suggested to influence drug PD.15, 16 Binding of eribulin to human plasma proteins ranges from 49% to 65% at concentrations from 100 to 1000 ng/mL.17 Therefore, it is unlikely that the severity of neutropenic toxicity is caused by elevation of plasma‐free eribulin concentrations resulting from low ALB levels. Further studies will be needed to clarify the mechanism.
Our simulation showed that the beginning, duration, and depth of the nadir in on‐treatment neutrophil counts varied with the eribulin treatment schedules. These predictions cannot be led from simple summarization of the observed data because actual intervention can carry a selection bias; only patients with particular characteristics were allocated to the alternative schedule. Modeling and simulation can be a useful tool for investigating and evaluating an optimal treatment strategy in a variety of virtual treatment options.
Physicians were permitted any treatment modification based on the patient's clinical situation, and the surveillance data indicated that treatment schedules were modified to avoid severe toxicity. Of note, the surveillance data revealed that approximately 30% of patients required a reduction in dosing frequency due to neutropenic toxicity during the first cycle of eribulin treatment. Several prospective studies exploring alternative treatment schedules of eribulin have been carried out worldwide.18, 19 The multicenter phase II study undertaken in Japan (JUST‐STUDY) investigated a new dosing regimen aimed at controlling eribulin toxicity, mainly febrile neutropenia. The study found that biweekly eribulin administration showed comparable efficacy and helped to control eribulin toxicity for women with previously treated MBC who were unable to continue on the standard schedule of eribulin.18 In the present study, our simulation also suggests that the probability of severe neutropenia (grade ≥3) would be reduced from 69% on the standard scenario to 27% on the biweekly scenario. Although the triweekly scenario was also shown to be less toxic, its efficacy might be compromised by the lower total dose. Further phase III studies of biweekly eribulin are needed to verify comparable efficacy and improved safety.
This study is the first large‐scale PD analysis of eribulin toxicity, although it is limited by its retrospective nature. Various patient factors and comorbidities could influence the eribulin toxicity profiles, because data collected by postmarketing surveillance are more heterogeneous than data from prospectively controlled studies. Therefore, our results are expected to reflect a real‐world setting in which follow‐up intervals and dosing schedules are tailored to each patient. However, in the present study, 35% of the total patients were excluded from the original surveillance data to avoid the influence of G‐CSF. This means that the recovery process from neutropenia by treatment with G‐CSF was not analyzed in the present study.
In conclusion, neutrophil count data collected by a postmarketing surveillance method were successfully applied to a model‐based safety analysis for eribulin in patients with RBC/MBC. Our study showed that ALB levels and BNEU affected the severity of neutropenia. Using our PK/PD model, we simulated the severity of neutropenic toxicity under different treatment scenarios and found that a biweekly scenario could lower the probability of severe neutropenia. This analysis of real‐world safety data reflecting authentic clinical settings will provide useful information on the safety and potential risk factors of eribulin.
CONFLICT OF INTEREST
Yukinori Sakata, Toshiyuki Matsuoka, and Mika Ishii are employees of Eisai Co. Ltd. Hidefumi Kasai is an employee of Certara G. K. The other authors declare no conflict of interest. No financial support was provided to the present study.
ACKNOWLEDGMENTS
We acknowledge the Japanese Society for the Promotion of Science (JSPS), the Swedish Foundation for International Cooperation in Research and Higher Education (STINT), the Swedish Foundation for Strategic Research (SSF), and Martin Adiels, PhD. We also thank Anne M. O'Rourke, PhD, from Edanz Group for editing a draft of this manuscript (http://www.edanzediting.com/ac).
Kawamura T, Kasai H, Fermanelli V, et al. Pharmacodynamic analysis of eribulin safety in breast cancer patients using real‐world postmarketing surveillance data. Cancer Sci. 2018;109:2822–2829. 10.1111/cas.13708
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2. Gonzalez‐Angulo AM, Morales‐Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1‐22. [DOI] [PubMed] [Google Scholar]
- 3. Cortes J, O'Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open‐label randomised study. Lancet. 2011;377:914‐923. [DOI] [PubMed] [Google Scholar]
- 4. Kaufman PA, Awada A, Twelves C, et al. Phase III open‐label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015;33:594‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maeda S, Saimura M, Minami S, et al. Efficacy and safety of eribulin as first‐ to third‐line treatment in patients with advanced or metastatic breast cancer previously treated with anthracyclines and taxanes. Breast. 2017;32:66‐72. [DOI] [PubMed] [Google Scholar]
- 6. Inoue K, Saito T, Okubo K, et al. Phase II clinical study of eribulin monotherapy in Japanese patients with metastatic breast cancer who had well‐defined taxane resistance. Breast Cancer Res Treat. 2016;157:295‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park YH, Im Y‐H, Lee K‐S, et al. Safety of eribulin in Korean patients with metastatic breast cancer. J Clin Oncol, 2015;33(suppl 15):e12031 [Google Scholar]
- 8. Aogi K, Iwata H, Masuda N, et al. A phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancer. Ann Oncol. 2012;23:1441‐1448. [DOI] [PubMed] [Google Scholar]
- 9. Watanabe J, Ito Y, Ohsumi S, et al. Safety and effectiveness of eribulin in Japanese patients with locally advanced or metastatic breast cancer: a post‐marketing observational study. Invest New Drugs. 2017;35:791‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgan RJ, Synold TW, Longmate JA, et al. Pharmacodynamics (PD) and pharmacokinetics (PK) of E7389 (eribulin, halichondrin B analog) during a phase I trial in patients with advanced solid tumors: a California Cancer Consortium trial. Cancer Chemother Pharmacol. 2015;76:897‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Majid O, Gupta A, Reyderman L, Olivo M, Hussein Z. Population pharmacometric analyses of eribulin in patients with locally advanced or metastatic breast cancer previously treated with anthracyclines and taxanes. J Clin Pharmacol. 2014;54:1134‐1143. [DOI] [PubMed] [Google Scholar]
- 12. Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy‐induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20:4713‐4721. [DOI] [PubMed] [Google Scholar]
- 13. van Hasselt JG, Gupta A, Hussein Z, Beijnen JH, Schellens JH, Huitema AD. Population pharmacokinetic‐pharmacodynamic analysis for eribulin mesilate‐associated neutropenia. Br J Clin Pharmacol. 2013;76:412‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fukae M, Shiraishi Y, Hirota T, et al. Population pharmacokinetic‐pharmacodynamic modeling and model‐based prediction of docetaxel‐induced neutropenia in Japanese patients with non‐small cell lung cancer. Cancer Chemother Pharmacol. 2016;78:1013‐1023. [DOI] [PubMed] [Google Scholar]
- 15. Fearon KC, Hansell DT, Preston T, et al. Influence of whole body protein turnover rate on resting energy expenditure in patients with cancer. Cancer Res. 1988;48:2590‐2595. [PubMed] [Google Scholar]
- 16. Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Model‐based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6:58‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donoghue M, Lemery SJ, Yuan W, et al. Eribulin mesylate for the treatment of patients with refractory metastatic breast cancer: use of a “physician's choice” control arm in a randomized approval trial. Clin Cancer Res. 2012;18:1496‐1505. [DOI] [PubMed] [Google Scholar]
- 18. Ohtani S, Nakayama T, Yoshinami T, et al. Bi‐weekly eribulin therapy for metastatic breast cancer: a multicenter phase II prospective study (JUST‐STUDY). Breast Cancer. 2018;25:438‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith J II, Irwin A, Jensen L, et al. Phase 2 study evaluating the efficacy and safety of eribulin mesylate administered biweekly for patients with human epidermal growth factor receptor 2‐negative metastatic breast cancer. The San Antonio Breast Cancer Symposium, Cancer Res. 2018;78(Suppl 4):P6‐14‐05. [DOI] [PubMed] [Google Scholar]


