Abstract
Accumulating evidence indicates the importance of natural killer (NK) cells in controlling tumor growth and metastasis. NK cell subsets display diversities in their function and tissue distribution and Mac‐1hi CD27lo NK cells are the predominant population of lung‐resident NK cells. Although the lung is a major organ where primary tumor develops and cancer cells metastasize, there is no clear evidence whether circulating NK cells and/or tissue‐resident NK cells control tumor growth in the lung. In the present study, we examined an antitumor function of lung‐resident NK cells to control pulmonary tumor growth. In an orthotopic lung tumor model, NK cells controlled pulmonary tumor growth, and mature circulating NK cell subsets were increased in tumor‐bearing lungs through a C‐X‐C motif chemokine receptor 3 (CXCR3)‐dependent mechanism. Although such increase in migratory NK cell subsets can be blocked by anti‐CXCR3 treatment, there was no difference in pulmonary tumor growth in anti‐CXCR3‐treated mice compared with control mice. In addition to pulmonary tumor growth, lung‐resident NK cells, but not migratory NK cells, play a dominant role in controlling metastatic growth of cancer cells in lung. These results strongly indicate an importance of lung‐resident NK cells for controlling pulmonary tumor growth.
Keywords: CXCR3, IFN‐γ, lung cancer, metastasis, NK cell
1. INTRODUCTION
Natural killer (NK) cells can be dissected into functionally distinct subsets by the expression of Mac‐1 and CD27.1, 2, 3, 4 Along with the phenotypically immature Mac‐1lo CD27hi NK cell subset (Mac‐1lo), we showed that CD27 expression further dissects the mature Mac‐1hi NK cell population into CD27hi and CD27lo subsets.1, 2 Such NK cell subset shows distinct tissue distribution as well as functional diversity. Interestingly, the CD27lo subset is comparatively excluded from bone marrow (BM) and lymph nodes (LN) and is dominant in blood circulation or in peripheral tissues.1, 2 In addition, NK cells are rapidly recruited to tissues at the site of immune responses including tumor, and the chemokine receptor C‐X‐C motif chemokine receptor 3 (CXCR3) has been known to play an important role in NK cell recruitment to the tumor microenvironment.5, 6, 7, 8 Among NK cell subpopulations, Mac‐1lo and CD27hi NK cell subsets constitutively express CXCR3; therefore, those NK cell subsets are dominantly recruited into tumor.1, 6, 7
Pulmonary NK cells play an important role in the pathology of respiratory diseases, including infectious diseases, allergy, and cancer.9, 10, 11 Accumulating evidence suggests that lung tissue‐specific NK cells can be functionally and phenotypically distinct from other NK cells.1, 12 Under steady‐state conditions, NK cells account for approximately 10% of the total lymphocyte population in the lung, and those lung‐resident NK cells are mostly the CD27lo subset. The CD27lo NK cell subset expresses a highly mature phenotype bearing self‐recognizing inhibitory receptors that are tightly regulated in their responsiveness.1, 13 Although the importance of NK cells in tumor surveillance has been widely appreciated, a contribution of such tissue‐resident NK cells is not well understood. Although lung is a major organ where primary tumors develop and cancer cells metastasize, the role of lung tissue‐resident NK cells for tumor control is not clear. In the present study, we examined the role of lung‐resident NK cells in controlling primary lung tumor growth using an orthotopic lung tumor model. We showed that lung‐resident NK cells, but not migratory NK cells, play a dominant role in controlling pulmonary primary and metastatic tumor growth.
2. MATERIALS AND METHODS
2.1. Mice
Wild‐type C57BL/6 (B6) mice were purchased from CLEA Japan, Inc. (Tokyo, Japan). Interferon‐γ−/− (IFN‐γ−/−) mice on B6 background were kindly provided by Dr Y. Iwakura (Tokyo University of Science, Chiba, Japan) and maintained at the Laboratory Animal Research Center, Institute of Medical Science, The University of Tokyo. In some experiments, groups of mice were treated with either antiasialo‐GM1 antibody (anti‐asGM1, 150 μg/mouse; Wako Chemicals, Osaka, Japan) on days −3 and −1, anti‐CXCR3 mAb (500 μg/mouse, clone CXCR3‐173; Bio X Cell, West Lebanon, NH, USA) or control Ig (250 μg/mouse, InVivoMAb polyclonal rat IgG; Bio X Cell) on days −1, 0, 2, 4 and 6, or FTY720 (1 mg/kg; Sigma, St Louis, MO, USA) or vehicle (100 μL saline) daily from days 0 to 9 (where day 0 was the day of primary tumor inoculation). All experiments were approved and carried out according to the Care and Use of Laboratory Animals of University of Toyama and the Animal Care and Use Committee of Institute of Medical Science of The University of Tokyo.
2.2. Cells and reagents
Mouse Lewis Lung carcinoma cell line (3LL) was kindly provided by Dr Kazuyoshi Takeda (Juntendo University), and the luciferase‐expressing 3LL cell line (3LL‐Luc2) was prepared as previously described.14 pGL4.50 [luc2/CMV/Hygro] vector, and d‐luciferin were obtained from Promega (Madison, WI, USA). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA, USA). Hygromycin B was obtained from Nacalai Tesque (Kyoto, Japan).
2.3. Tumor cell inoculation and bioluminescent imaging
Intrapulmonary implantation procedure was described previously.14 Briefly, B6 mice were anesthetized with isoflurane and a small skin incision in the left chest wall was made. 3LL‐Luc2 cells were suspended (5 × 105 cells/mL) in PBS with 500 μg/mL Matrigel (BD Bioscience, San Jose, CA, USA). On observing the motion of the left lung, 20 μL cell suspension was directly injected into the lung with a 29‐gauge needle attached to a 0.3 mL syringe (Becton Dickinson, Franklin Lakes, NJ, USA). The skin incision was closed with a surgical skin clip. For the lung metastasis model, 3LL‐Luc2 cells (104) were inoculated i.v. into B6 mice from the tail vein. To obtain bioluminescent images, mice were injected with d‐luciferin (150 mg/kg ip; Promega) and luminescence was measured with an in vivo imaging system (IVIS Lumina II; Perkin Elmer, Waltham, MA, USA) 10 minutes after the d‐luciferin injection.
2.4. Flow cytometry
3LL‐Luc2 cells were (104) were inoculated intrapulmonarily. Ten days after the inoculation, lung tissues were dissected, minced and digested with 2 mg/mL collagenase (Roche Diagnostics GmbH, Mannheim, Germany) and 0.1 mg/mL DNase I (Roche Diagnostics GmbH) in serum‐free RPMI 1640 for 1 hour at 37°C. Samples were further homogenized through wire mesh. For flow cytometry analysis, cells were first preincubated with anti‐CD16/32 (2.4G2) mAb to avoid nonspecific binding of antibodies to FcγR. The cells were then incubated with a saturating amount of fluorophore‐conjugated mAb. Antibodies against CD3ε (2C11), NK1.1 (PK136), CD11b (M1/70), and CD27 (LG.3A10) were purchased from Biolegend (San Diego, CA, USA), eBioscience (San Diego, CA, USA) or Tombo Bioscience (San Diego, CA, USA). Flow cytometry analysis was carried out with a FACS Canto (BD Bioscience) and the data were analyzed with FlowJo software (FlowJo, LLC, Ashland, OR, USA).
2.5. Cytotoxicity and cytokine production assay
Cytotoxic activity was assessed against YAC‐1 target cells by bioluminescent imaging.15 The YAC‐1 cells stably expressing firefly luciferase with an EF‐promoter using the lentiviral expression system (kindly provided by Dr Hiroyuki Miyoshi, Keio University) were established as previously described.16 Briefly, for the production of lentiviral vectors, 293FT cells (Invitrogen) were transfected with an expression vector construct (CSII‐EF‐Luc2, kindly provided by Dr Yoko Katsuno, Graduate School of Medicine, The University of Tokyo), a VSV‐G‐ and Rev‐expressing construct (pCMV‐VSV‐G‐RSV‐Rev) and a packaging vector construct (pCAG‐HIVgp). The cultured supernatants with lentiviral particles were collected, concentrated with Lenti‐X Concentrator (Clontech, Mountain View, CA, USA) and used for lentiviral vector infections. Stable transfectants were cloned by limiting dilution. NK cells were isolated from lung and peripheral blood using magnetic sorting (>80% purity, MojoSort Mouse NK cell Isolation Kit; Biolegend). Target cells (104/well) were incubated in a total volume of 200 μL effector cells and d‐luciferin (150 μg/mL; Promega) in 96‐well black plates. The plates were centrifuged before incubation, and bioluminescence measured after 18 hours in an in vivo imaging system (IVIS Lumina II; Perkin Elmer). For measuring IFN‐γ production, lung (105/well) or peripheral blood (5 × 104/well) NK cells were stimulated with PMA (50 ng/mL; Sigma) and ionomycin (500 ng/mL; Sigma) in RPMI 1640 medium. After 24‐hour incubation, cell‐free supernatants were harvested and subjected to ELISA. Amounts of IFN‐γ were quantitated by specific sandwich ELISA (Biolegend).
2.6. Statistical analysis
All data were obtained from a group of 6‐9 mice and are representative of at least 2 independent experiments. Data were analyzed for statistical significance using Student's t test. P‐values < .05 were considered significant.
3. RESULTS
3.1. Characterization of NK cells in pulmonary tumor
To understand the role of NK cells in controlling lung cancer, we first analyzed NK cells in primary lung tumors by orthotopically implanting murine 3LL‐Luc2 cells. Either tumor‐bearing or control lung lobes were harvested 10 days after tumor inoculation and the NK cell population was analyzed by flow cytometry. Although there was no significant difference in the total NK cell population, subsets of NK cells showed a significant difference between control and tumor‐bearing lungs (Figure 1). Considering the number of mononuclear cells (MNC) were increased in tumor‐bearing lung (Figure 1A), the absolute number of NK cells was also increased in tumor‐bearing lung compared to control lung. The subsets of mouse NK cells can be determined by the expression of two markers Mac‐1 (CD11b) and CD27, and the functional and phenotypic differences in those NK cell subsets are well characterized.1 In tumor‐bearing lungs, the population of migratory Mac‐1lo and CD27hi NK cell subsets were significantly increased, whereas no obvious change was observed in tissue‐resident CD27lo NK cell subset (Figure 1). There was no difference in CD3+ T cell population between control and tumor‐bearing lungs (data not shown).
Figure 1.
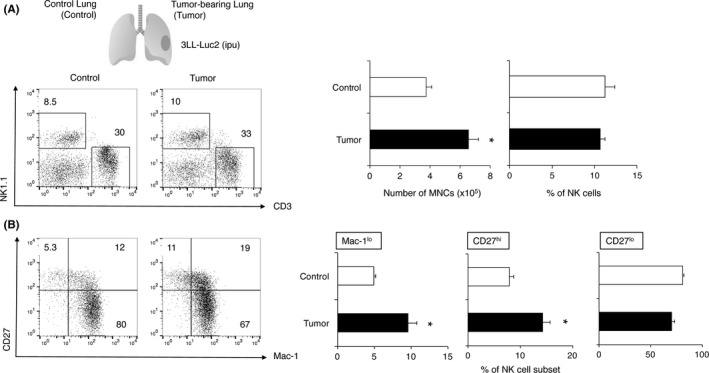
Characterization of (NK) cells in pulmonary 3LL‐Luc2 tumor. B6 mice were inoculated intrapulmonarily with 3LL‐Luc2 (104) and lungs were harvested 10 d after the inoculation. Mononuclear cells were isolated from control lung (Control) or tumor‐bearing lung (Tumor) and then subjected to cell count and flow cytometry analysis. Representative FACS plots from the indicated groups of mice are shown and numbers represent percentage of cells in the different gates. A, Number of mononuclear cells (MNC) and the proportion of NK cells (NK1.1+ CD3− cells) or B, NK cell subsets (Mac‐1lo : Mac‐1lo CD27hi, CD27hi : Mac‐1hi CD27hi, CD27lo : Mac‐1hi CD27lo, electronically gated on NK1.1+ CD3− cells) from the indicated lung samples are presented. Data represent mean ± SEM and representative of 2 experiments. *P < .05 compared with control group
Next, we examined whether NK cells can be recruited from circulation into the tumor‐bearing lung by a CXCR3‐ or sphingosine 1‐phosphate (S1P)‐dependent mechanism, which are known to be important for in vivo NK cell trafficking.1, 5, 6, 17, 18 As shown in Figure 2A, the population of migratory Mac‐1lo and CD27hi NK cell subsets in the tumor‐bearing lungs were significantly decreased in mice treated with anti‐CXCR3 (aCXCR3). In contrast, there was no such difference in NK cell subsets of tumor‐bearing lungs in FTY720‐treated mice (Figure 2B). Considering a significant reduction in CD3+ T cells in the FTY720 treated tumor‐bearing lungs was observed (data not shown), the trafficking of Mac‐1lo and CD27hi NK cells to primary lung tumor should be dependent on CXCR3, but not on S1P, similar to that of subcutaneous tumor.6, 7
Figure 2.
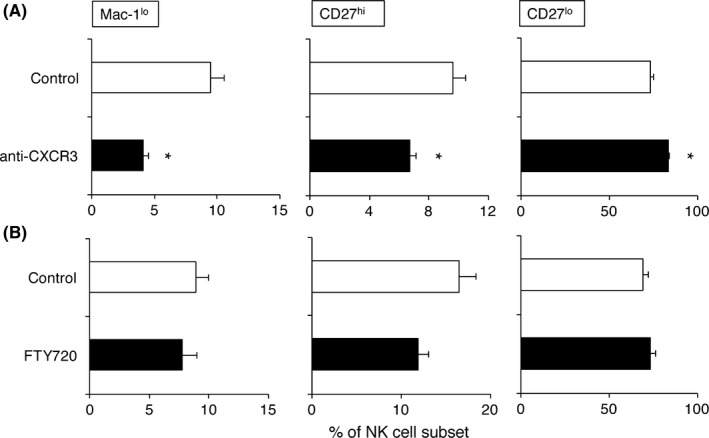
C‐X‐C motif chemokine receptor 3 (CXCR3) controls migratory natural killer (NK) cell accumulation in pulmonary 3LL‐Luc2 tumor. 3LL‐Luc2 (104) were inoculated intrapulmonarily into B6 mice. A, To block CXCR3, mice were treated with anti‐CXCR3 (500 μg/mouse, ip) on days −1, 0, 2, 4 and 6. B, FTY720 (1 mg/kg, ip) were treated daily from days 0 to 9 (day 0 = tumor inoculation). Mononuclear cells were isolated from tumor‐bearing lung and then subjected to flow cytometry analysis. Proportion of NK cell subsets (Mac‐1lo : Mac‐1lo CD27hi, CD27hi : Mac‐1hi CD27hi, CD27lo : Mac‐1hi CD27lo, electronically gated on NK1.1+ CD3− cells) from the indicated lung samples are presented. Data represent mean ± SEM and representative of 2 experiments. *P < .05 compared with control group
3.2. Lung‐resident NK cells control pulmonary tumor growth and metastasis
To determine the importance of NK cells for controlling primary lung tumor, we examined the growth of 3LL‐Luc2 tumors in lung of NK cell‐depleted mice (NK dep) treated with antiasialo‐GM1 antibody. In NK cell‐depleted mice, lung tumor growth was significantly enhanced compared with control B6 mice (Figure 3A,B), indicating NK cells significantly contribute to antitumor immunity in controlling pulmonary tumor growth. Such NK cell‐dependent antitumor immune response against primary lung tumor required IFN‐γ because there was no difference in the presence or absence of NK cells for controlling primary lung tumor growth in IFN‐γ‐deficient mice (Figure 3C). These results clearly indicate that NK cells control lung primary tumor growth in an IFN‐γ‐dependent mechanism.
Figure 3.
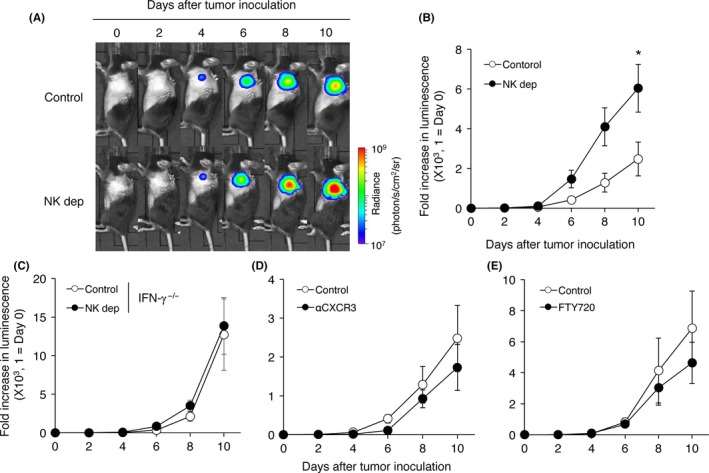
Lung‐resident natural killer (NK) cells control pulmonary 3LL‐Luc2 tumor growth. 3LL‐Luc2 (104) were inoculated intrapulmonarily to B6 WT mice or interferon (IFN)γ−/− mice. To deplete NK cells (NK dep), mice were treated with antiasialo‐GM1 (anti‐asGM1) antibody (150 μg/mouse, ip) on days −3 and −1 (day 0 = tumor inoculation). To block C‐X‐C motif chemokine receptor 3 (CXCR3), mice were treated with anti‐CXCR3 (500 μg/mouse, ip) on days −1, 0, 2, 4 and 6. FTY720 (1 mg/kg, ip) were treated daily from days 0 to 9 (day 0 = tumor inoculation). Representative bioluminescent images of mice bearing orthotopic 3LL‐Luc2 tumor are shown (A). Bioluminescence of 3LL‐Luc2 tumor was monitored in WT mice (B, D, E) or in IFNγ−/− mice (C). Luminescence was normalized by that of the individual mouse on day 0. Data were obtained from a group of 6‐9 mice and presented as the mean ± SEM. *P < .05 compared with control group
We next examined the contribution of migratory Mac‐1lo and CD27hi NK cells and/or tissue‐resident CD27lo NK cells in controlling primary lung tumor. Trafficking of Mac‐1lo and CD27hi NK cells to primary lung tumor was blocked by treating mice with anti‐CXCR3 (Figure 2A). As shown in Figure 3D, there were no differences in the growth of lung tumor between control and anti‐CXCR3‐treated mice. There was also no difference in the growth of lung tumor with or without FTY720 treatment (Figure 3E). Collectively, these results indicate the importance of lung‐resident NK cells, rather than NK cells recruited from circulation, in controlling pulmonary tumor growth.
The importance of NK cells for protecting lung metastasis has been widely recognized, and we previously reported that IFN‐γ production by lung NK cells is critical for such metastasis protection.11 As shown in Figure 4, NK cell depletion significantly enhanced the metastatic growth of 3LL‐Luc2 cells in lung, whereas anti‐CXCR3 treatment did not show any difference to control mice in lung metastasis of 3LL‐Luc2 cells. Along with our presented finding in controlling pulmonary tumor growth, lung‐resident NK cells, but not NK cells recruited by CXCR3, play an important role in controlling metastatic growth of 3LL‐Luc2 cells. Furthermore, we tested the functional competence of lung‐resident NK cells by comparison with circulating blood NK cells (Figure 5). The freshly isolated lung NK cells did not show any significant direct cytotoxicity against 3LL cells (data not shown), similar to our previous observation in splenic NK cells even though they expressed NKG2D ligand.19 Instead, NK cells from both lung and blood were capable of killing Yac‐1 target cells, and produce IFN‐γ upon stimulation with PMA and ionomycin (Figure 5); therefore, both lung‐resident and circulating NK cells showed comparable effector function as mature NK cells. Collectively, these results further emphasize the general importance of lung‐resident NK cells for pulmonary immune‐surveillance of cancer.
Figure 4.
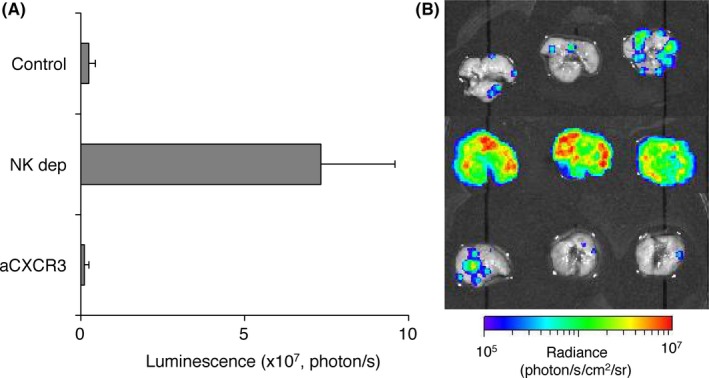
Lung‐resident natural killer (NK) cells control pulmonary 3LL‐Luc2 tumor metastases. 3LL‐Luc2 (104) were inoculated i.v. to B6 mice. To deplete NK cells (NK dep), mice were treated with antiasialo‐GM1 (anti‐asGM1) antibody (200 μg/mouse, ip) on days −3 and −1 (day 0 = tumor inoculation). To block C‐X‐C motif chemokine receptor 3 (CXCR3), mice were treated with anti‐CXCR3 (500 μg/mouse, ip) on days −1, 0 and 2. Bioluminescence of lungs was measured at 4 d after tumor inoculation. Summary of lung metastases (A) and representative bioluminescent images of inoculated lung (B) are shown. Data are presented as the mean ± SEM.
Figure 5.
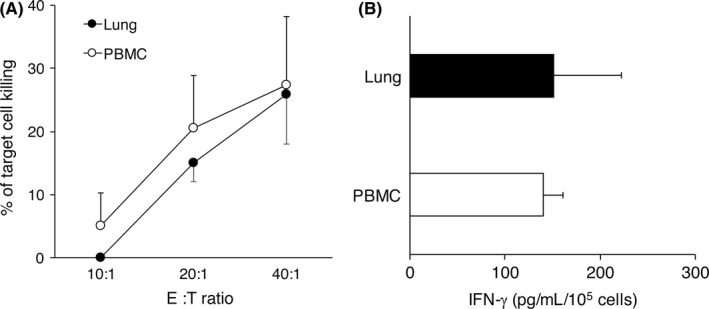
Functional competence of lung‐resident natural killer (NK) cells. NK cells were isolated from B6 mice lung and peripheral blood. A, Cytotoxicity was determined against Yac‐1 target cells using bioluminescent assay. B, NK cells were stimulated with PMA (50 ng/mL) and ionomycin (500 ng/mL) for 24 h. Cell‐free supernatants were harvested and the amounts of interferon (IFN)‐γ were quantitated by specific sandwich ELISA. Data are presented as the mean ± SEM
4. DISCUSSION
The importance of NK cells in controlling tumor growth and metastasis has been widely recognized.20, 21, 22 Although NK cells have been known to play a critical role in resistance to lung metastases,11 it is not clear whether circulating NK cells and/or tissue‐resident NK cells control tumor growth in lung. Although mature CD27hi and CD27lo NK cells reside in both lymphoid and nonlymphoid organs, the CD27lo subset is a predominant NK cell population within lung.1, 2 In the present study, we examined the role of lung‐resident NK cells in controlling primary lung tumor growth. In an orthotopic lung tumor model, NK cells controlled pulmonary tumor growth, and mature circulating NK cell subsets, both Mac‐1lo and CD27hi subsets, were increased in tumor‐bearing lungs through a CXCR3‐dependent mechanism. Although such an increase in migratory NK cell subsets can be blocked by anti‐CXCR3 treatment, there was no difference in pulmonary tumor growth in anti‐CXCR3‐treated mice compared with control mice. In addition to pulmonary tumor growth, lung‐resident NK cells, but not migratory NK cells, play a dominant role in controlling lung metastatic growth. These results strongly indicate the importance of lung‐resident NK cells for controlling pulmonary tumor growth.
Natural killer cells account for about 10% of circulating lymphocytes and can also be found in peripheral tissues including spleen, liver, gut, lung lymph node and uterus.1, 9, 23 The presence of NK cells in those peripheral organs has been believed to be a result of chemokine‐ and S1P‐dependent distribution from the circulation.17, 18, 24, 25, 26 In lung tissue, NK cells play an important role in several pulmonary diseases other than cancer, including influenza infection, asthma, tuberculosis and others.27 It has been suggested that pulmonary NK cells are also recruited from the periphery;12, 28 however, the importance of lung‐resident NK cells in those pathologies is not yet clear. We previously reported that the importance of liver‐resident NK cells in controlling metastasis of cancer through a tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL)‐dependent mechanism, and such NK cell surveillance in liver metastases was dependent on a unique NK cell subset residing in liver.29, 30 In addition, we also reported that IFN‐γ production by lung NK cells is critical for metastasis protection of murine melanoma cells.11 Further study is required for understanding whether lung‐resident NK cells display any functional distinction to NK cells in other organs.
Although NK cell trafficking under homeostatic conditions is not yet clearly defined, the rapid accumulation of NK cells in the tumor microenvironment has been known to be controlled by CXCR3.5, 6, 8 In mice, CD27hi NK cells (either Mac‐1hi or Mac‐1lo), but not CD27lo NK cells, are known to express CXCR3 and migrate to its ligands.1 In contrast to the findings of subcutaneous tumors,6, 7, 31 CXCR3 did not show any significant role in controlling tumor growth in lung. Moreover, blocking of the S1P pathway by treatment with FTY720 did not affect NK cell‐dependent tumor control in lung, although S1P5‐dependent NK cell trafficking was shown to be resistant to FTY720,17 suggesting NK cell recruitment through either a CXCR3 or a S1P pathway is not involved in pulmonary tumor control. Alternatively, another chemokine receptor, such as CXCR4 and CX3CR1, may contribute to NK cell‐dependent pulmonary tumor control considering its expression in mature NK cell subsets and their involvement in NK cell recruitment.1, 32, 33 It has been reported that the maintenance of lung NK cells is dependent on interleukin (IL)‐15.34, 35 In mice, lung‐resident NK cells are mostly the CD27lo NK cell subset which shows hypo‐responsiveness to those homeostatic cytokines for NK cells including IL‐15.1, 3 The mature CD27lo NK cell subset expresses higher levels of inhibitory Ly‐49 and CD94/NKG2 complexes;13 therefore, lung‐resident NK cells are assumed to be tightly regulated for their responsiveness. Nevertheless, further study will be required to fully understand NK cell behavior in the lung tissue microenvironment for maximizing their antitumor function against lung cancer and developing new NK cell‐targeted immunotherapy.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
ACKNOWLEDGMENTS
We are grateful to Hiroyuki Miyoshi and Yoko Katsuno for providing reagents, and Asuka Asami and Setsuko Nakayama for their technical assistance. This work was partly supported by a Grant‐in‐Aid for Scientific Research on Innovative Areas (17H06398), The Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (Y.H.).
Yamamoto Y, Miyazato K, Takahashi K, Yoshimura N, Tahara H, Hayakawa Y. Lung‐resident natural killer cells control pulmonary tumor growth in mice. Cancer Sci. 2018;109:2670–2676. 10.1111/cas.13703
REFERENCES
- 1. Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176(3):1517‐1524. [DOI] [PubMed] [Google Scholar]
- 2. Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47‐55. [DOI] [PubMed] [Google Scholar]
- 3. Huntington ND, Tabarias H, Fairfax K, et al. NK cell maturation and peripheral homeostasis is associated with KLRG1 up‐regulation. J Immunol. 2007;178(8):4764‐4770. [DOI] [PubMed] [Google Scholar]
- 4. Silva A, Andrews DM, Brooks AG, Smyth MJ, Hayakawa Y. Application of CD27 as a marker for distinguishing human NK cell subsets. Int Immunol. 2008;20(4):625‐630. [DOI] [PubMed] [Google Scholar]
- 5. Martin‐Fontecha A, Thomsen LL, Brett S, et al. Induced recruitment of NK cells to lymph nodes provides IFN‐gamma for T(H)1 priming. Nat Immunol. 2004;5(12):1260‐1265. [DOI] [PubMed] [Google Scholar]
- 6. Wendel M, Galani IE, Suri‐Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN‐gamma and CXCR3 ligands. Cancer Res. 2008;68(20):8437‐8445. [DOI] [PubMed] [Google Scholar]
- 7. Hayakawa Y, Sato‐Matsushita M, Takeda K, Iwakura Y, Tahara H, Irimura T. Early activation and interferon‐gamma production of tumor‐infiltrating mature CD27 high natural killer cells. Cancer Sci. 2011;102(11):1967‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watt SV, Andrews DM, Takeda K, Smyth MJ, Hayakawa Y. IFN‐gamma‐dependent recruitment of mature CD27(high) NK cells to lymph nodes primed by dendritic cells. J Immunol. 2008;181(8):5323‐5330. [DOI] [PubMed] [Google Scholar]
- 9. Lysakova‐Devine T, O'Farrelly C. Tissue‐specific NK cell populations and their origin. J Leukoc Biol. 2014;96(6):981‐990. [DOI] [PubMed] [Google Scholar]
- 10. Small CL, McCormick S, Gill N, et al. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J Immunol. 2008;180(8):5558‐5568. [DOI] [PubMed] [Google Scholar]
- 11. Takeda K, Nakayama M, Sakaki M, et al. IFN‐gamma production by lung NK cells is critical for the natural resistance to pulmonary metastasis of B16 melanoma in mice. J Leukoc Biol. 2011;90(4):777‐785. [DOI] [PubMed] [Google Scholar]
- 12. Wang J, Li F, Zheng M, Sun R, Wei H, Tian Z. Lung natural killer cells in mice: phenotype and response to respiratory infection. Immunology. 2012;137(1):37‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayakawa Y, Watt SV, Takeda K, Smyth MJ. Distinct receptor repertoire formation in mouse NK cell subsets regulated by MHC class I expression. J Leukoc Biol. 2008;83(1):106‐111. [DOI] [PubMed] [Google Scholar]
- 14. Fushiki H, Kanoh‐Azuma T, Katoh M, et al. Quantification of mouse pulmonary cancer models by microcomputed tomography imaging. Cancer Sci. 2009;100(8):1544‐1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karimi MA, Lee E, Bachmann MH, et al. Measuring cytotoxicity by bioluminescence imaging outperforms the standard chromium‐51 release assay. PLoS ONE. 2014;9(2):e89357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takahashi K, Ehata S, Koinuma D, et al. Pancreatic tumor microenvironment confers highly malignant properties on pancreatic cancer cells. Oncogene. 2018;37(21):2757‐2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walzer T, Chiossone L, Chaix J, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1‐phosphate receptor. Nat Immunol. 2007;8(12):1337‐1344. [DOI] [PubMed] [Google Scholar]
- 18. Jenne CN, Enders A, Rivera R, et al. T‐bet‐dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206(11):2469‐2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smyth MJ, Swann J, Kelly JM, et al. NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J Exp Med. 2004;200(10):1325‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural‐killer‐cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2(11):850‐861. [DOI] [PubMed] [Google Scholar]
- 21. Smyth MJ, Swann J, Hayakawa Y. Innate tumor immune surveillance. Adv Exp Med Biol. 2007;590:103‐111. [DOI] [PubMed] [Google Scholar]
- 22. Lopez‐Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of metastasis by NK cells. Cancer Cell. 2017;32(2):135‐154. [DOI] [PubMed] [Google Scholar]
- 23. Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ‐specific features of natural killer cells. Nat Rev Immunol. 2011;11(10):658‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gregoire C, Chasson L, Luci C, et al. The trafficking of natural killer cells. Immunol Rev. 2007;220:169‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernardini G, Sciume G, Santoni A. Differential chemotactic receptor requirements for NK cell subset trafficking into bone marrow. Front Immunol. 2013;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ponzetta A, Sciume G, Benigni G, et al. CX3CR1 regulates the maintenance of KLRG1+ NK cells into the bone marrow by promoting their entry into circulation. J Immunol. 2013;191(11):5684‐5694. [DOI] [PubMed] [Google Scholar]
- 27. Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology. 2009;128(2):151‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morrison BE, Park SJ, Mooney JM, Mehrad B. Chemokine‐mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J Clin Invest. 2003;112(12):1862‐1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takeda K, Hayakawa Y, Smyth MJ, et al. Involvement of tumor necrosis factor‐related apoptosis‐inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7(1):94‐100. [DOI] [PubMed] [Google Scholar]
- 30. Takeda K, Cretney E, Hayakawa Y, et al. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood. 2005;105(5):2082‐2089. [DOI] [PubMed] [Google Scholar]
- 31. Ogura K, Sato‐Matsushita M, Yamamoto S, et al. NK cells control tumor‐promoting function of neutrophils in mice. Cancer Immunol Res. 2018;6(3):348‐357. [DOI] [PubMed] [Google Scholar]
- 32. Yu YR, Fong AM, Combadiere C, Gao JL, Murphy PM, Patel DD. Defective antitumor responses in CX3CR1‐deficient mice. Int J Cancer. 2007;121(2):316‐322. [DOI] [PubMed] [Google Scholar]
- 33. Hertwig L, Hamann I, Romero‐Suarez S, et al. CX3CR1‐dependent recruitment of mature NK cells into the central nervous system contributes to control autoimmune neuroinflammation. Eur J Immunol. 2016;46(8):1984‐1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allavena P, Giardina G, Bianchi G, Mantovani A. IL‐15 is chemotactic for natural killer cells and stimulates their adhesion to vascular endothelium. J Leukoc Biol. 1997;61(6):729‐735. [DOI] [PubMed] [Google Scholar]
- 35. Ge N, Nishioka Y, Nakamura Y, et al. Synthesis and secretion of interleukin‐15 by freshly isolated human bronchial epithelial cells. Int Arch Allergy Immunol. 2004;135(3):235‐242. [DOI] [PubMed] [Google Scholar]


