Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a highly malignant tumor with few biomarkers to guide treatment options. Carbohydrate antigen 19.9 (CA19.9), the most frequently used biomarker for PDAC, is not sensitive and specific enough for the detection of the disease. This study aimed to evaluate serum periostin (POSTN) and CA242 as potential diagnostic biomarkers complementing CA19.9 in detecting pancreatic cancer. Blood samples were from 362 participants, including 213 patients with different stages of PDAC, 75 patients with benign pancreatic disease, and 74 healthy individuals. All samples were randomly divided into a training set and a validation set. Carbohydrate antigen 19.9, CA242, POSTN, as well as carcinoembryonic antigen, were measured by ELISA or automated immunoassay. The receiver operating characteristic curve analysis revealed that the performance of CA19.9 in the validation group were improved by the marker panel composed of CA19.9, POSTN, and CA242, to discriminate early stage PDAC not only from healthy controls (area under the curve [AUC]CA19.9 = 0.94 vs AUCCA19.9 + POSTN + CA242 = 0.98, P < .05) but also from benign conditions (AUCCA19.9 = 0.87 vs AUCCA19.9 + POSTN + CA242 = 0.90, P < .05). In addition, POSTN retained significant diagnostic capabilities to distinguish PDAC CA19.9‐negative from healthy controls (AUCPOSTN = 0.87) as well as from benign conditions (AUCPOSTN = 0.84) in the whole set. This study suggested that POSTN and CA242 are potential diagnostic serum biomarkers complementing CA19.9 in detecting early pancreatic cancer.
Keywords: biomarker panel, CA19.9, CA242, pancreatic ductal adenocarcinoma, periostin
Abbreviations
- AJCC
American Joint Committee on Cancer
- AUC
area under curve
- CA
carbohydrate antigen
- CEA
carcinoembryonic antigen
- IHC
immunohistochemistry
- PDAC
pancreatic ductal adenocarcinoma
- POSTN
periostin
- ROC
receiver operating characteristic
1. INTRODUCTION
Pancreatic ductal adenocarcinoma accounts for 2.2% of all cancers and is the fourth most common cause of cancer‐related death.1 Due to the lack of early symptoms, aggressive growth, and early dissemination, most patients with PDAC are diagnosed with advanced and metastatic disease. As a result, palliative chemotherapy is always the only therapeutic option for these cases.2 Although a minority of 10%‐20% of cases diagnosed with resectable disease, the recurrence rates of the disease approach close to 80% owing to the development of local recurrence or distant metastases.3, 4 Moreover, the incidence of PDAC is rising globally and is expected to be the third leading cause of cancer‐related death, due to diagnostic delays and slow progress of treatment options, by 2030.5
Visualization technology, such as computerized tomography, MRI, endoscopic ultrasound, and laparoscopy, are used to identify the presence of pancreatic cancer lesions.6 However, due to the cost, time, invasiveness, and often unpleasant nature of these screening tools, none has proven to be effective for patients in both general and high‐risk populations. On the contrary, non‐invasive blood tests are widely accepted and routinely used for the determination of biomarkers.
Attempts have been made at establishing a method of detecting pancreatic cancer earlier through the use of biomarkers that can signal the presence of malignancy. Carbohydrate antigen 19.9 is the most frequently used biomarker for patients with PDAC, but its sensitivity and specificity are unsatisfactory, especially for the diagnosis of early stage PDAC and for discrimination of PDAC from benign pancreatic disease. Therefore, CA19.9 should not be used for screening purposes.7, 8 In addition, although relatively few PDAC patients will be CA19.9‐negative, taking into account such a high rate of pancreatic cancer mortality, any false negatives using the marker are unacceptable. For these reasons, research interests have shifted to the development of biomarker panels.9, 10 Taken together, it is crucial to explore novel and reliable non‐invasive biomarkers that complement CA19.9 to improve the diagnostic sensitivity and specificity of diagnosing early PDACs.
Through bioinformatics analysis of publicly available expression data in the Oncomine database (http://www.oncomine.com), we found that POSTN was a candidate with great potential to serve as a new circulating biomarker for PDAC. Carbohydrate antigen 242 was identified relatively late as a biomarker for the diagnosis, prognosis, and surveillance of gastrointestinal malignancies,11 and a high correlation between serum levels of CA242 and CA19.9 were observed.12 However, its complementary role for CA19.9 in the early diagnosis of PDAC has not been fully elucidated. Carcinoembryonic antigen, the most widely used biomarker for gastrointestinal malignancies, was also evaluated.
In this work, we investigated serum POSTN, CA242, and CEA levels in PDAC patients and evaluated their complementary role, with CA19.9, in the diagnosis of PDAC. We also developed a biomarker panel consisting of CA19.9, CA242, and POSTN, and validated its improved performance compared with CA19.9 alone. The discriminating ability of POSTN and CA242 for CA19.9‐negative PDAC was also fully evaluated.
2. MATERIALS AND METHODS
2.1. Patients and specimens
This study analyzed 362 serum specimens that were obtained from 213 patients with PDAC at various stages, 75 patients with benign pancreatic disease, and 74 sex‐ and age‐matched healthy controls without any gastrointestinal or malignant diseases at Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) between June 2015 and February 2018. All procedures carried out in studies involving human participants were approved by the Research Ethics Committee of Tianjin Medical University Cancer Institute and Hospital and in accordance with the 1964 Helsinki Declaration ethical standards. Written informed consent was obtained from all patients, and the study was approved by the local Ethical Board.
Serum samples were harvested before surgery or treatment and were collected, aliquoted, and snap frozen at −80°C until use. Data on clinical parameters, including gender, age, and clinical stage were collected. Blood samples and tissue specimens were obtained postoperatively from 32 patients with PDAC, including 23 patients without metastasis and 9 patients with metastasis at surgery. The disease was staged according to the AJCC TNM classification.13 According to AJCC, IIA and IIB are the cut‐off points of resectable and non‐resectable PDAC, respectively. In our study, AJCC stages IA, IB, and IIA were classified as early stage PDAC and AJCC stages IIB, III, and IV as late stage PDAC. All of the samples were randomly divided into the training set and the validation set. Clinical characteristics of PDAC patients and control subjects are summarized in Table 1.
Table 1.
Characteristics of samples from pancreatic ductal adenocarcinoma (PDAC) patients and control subjects
| Sample characteristic | Training set | Validation set | Total |
|---|---|---|---|
| Healthy controls | 37 | 37 | 74 |
| Benign disease | 32 | 43 | 75 |
| Chronic pancreatitis | 21 | 28 | 49 |
| Other benign diseasesa | 11 | 15 | 26 |
| PDAC early stage | 30 | 38 | 68 |
| PDAC IA | 2 | 3 | 5 |
| PDAC IB | 3 | 5 | 8 |
| PDAC IIA | 25 | 30 | 55 |
| PDAC late stage | 68 | 77 | 145 |
| PDAC IIB | 38 | 36 | 74 |
| PDAC III | 8 | 15 | 23 |
| PDAC IV | 22 | 26 | 48 |
| PDAC CA19.9‐negative | 27 | 29 | 56 |
| Total | 167 | 195 | 362 |
| Gender, female/male | 80/87 | 92/103 | 172/190 |
| Median (mean) age | 64.7 | 65.4 | 64.6 |
Other benign diseases including benign intraductal papillary mucinous neoplasm and solid pseudopapillary neoplasm.
CA, carbohydrate antigen. Total number of patients in groups with subcategories were shown in bold.
2.2. Serum assays for POSTN using ELISA
Serum levels of POSTN in patients and control subjects were measured by the ELISA method using a commercial kit (R&D Systems, Minneapolis, MN, USA), according to the protocol from the manufacturer. Briefly, 96‐well ELISA microplates were coated overnight with 100 μL POSTN antibody at a final concentration of 2 μg/mL in PBS. After washing with PBS/0.05% (w/v) Tween‐20 (PBST, pH 7.4), the wells were blocked with 300 μL blocking buffer at room temperature for 1 hour. Serum samples (100 μL) were then added and incubated at room temperature for 2 hours. Similarly, 100 μL PBS‐lacking Ab was used as a negative control. Following three washes with PBST, 100 μL detection antibody diluted to a concentration of 0.5 μg/mL was added. After incubation at room temperature for 2 hours, 100 μL avidin‐HRP‐conjugated secondary Ab (at 1:200 dilution) was added, and the plates were incubated at room temperature for 20 minutes. The excess conjugate was removed by washing the plates three times with PBST. The amount of bound conjugate was determined by adding the TMB substrate solution (Biolegend, San Diego, CA, USA) to each well, and the plates were incubated at room temperature for color development. Stop Solution (50 μL, Biolegend) was added to each well. The absorbance was measured at 450 nm using a Model 680 microplate reader (Bio‐Rad, Hercules, CA, USA). All samples were tested in duplicate.
2.3. Immunohistochemical staining for POSTN
Tissue sections were incubated with anti‐POSTN antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:100 dilution overnight at 4°C. The scoring method, which combined intensity and percentage of positivity, was previously described,14 and extent and intensity measures for each core were combined as weak (score 1), moderate (score 2), and strong (score 3).
2.4. Serum assays for CA19.9, CA242, and CEA
Serum CA19.9, CA242, and CEA were measured using electrochemiluminescence immunoassays on the Roche Cobas E601 immunoassay analyzer (Roche Diagnostics, Mannheim, Germany) equipped with dedicated reagents, according to the instructions from the manufacturer. All of the assays were carried out at the department of Laboratory Medicine, Tianjin Medical University Cancer Institute and Hospital.
2.5. Statistical analysis
Comparisons of levels of markers between groups were carried out using the Mann‐Whitney test. Non‐parametric Spearman's correlation coefficients method was used to evaluate the association among serum POSTN, CA19.9, and CA242 levels. Because of the wide distribution of CA19.9, CA242, and CEA levels, values were plotted on a logarithmic scale. Receiver operating characteristic curves were generated to assess diagnostic efficiency. The 95% confidence intervals for AUC and P‐values for comparison of related ROC curves were obtained by the method described by DeLong and coworkers.15 Values of P < 0.05 were considered statistically significant. All of these statistical analyses were undertaken with spss 23.0 software (SPSS, Chicago, IL, USA).
3. RESULTS
3.1. Elevated serum levels of POSTN and CA242 in patients with PDAC
A meta‐analysis of expression studies from the Oncomine database was undertaken to explore new biomarker candidates for PDAC. All of the eight pancreatic cancer datasets in Oncomine containing a differential analysis of PDAC and normal tissues were included in this study. The following approaches were combined: (i) comparing analyses across the eight datasets identified the 20 most highly ranked genes that were overexpressed in tumor tissues (Figure S1); (ii) signal peptide analysis showed that fibronectin 1, laminin, gamma 2, POSTN, thrombospondin 2, and versican were predicted secreted proteins; and (iii) analysis of published works revealed that POSTN was involved in many aspects of PDAC tumorigenesis.16, 17, 18, 19, 20 Therefore, we focused our attention on POSTN in the subsequent study.
Serum levels of CA19.9, POSTN, CA242, and CEA were evaluated in healthy controls, patients with benign pancreatic disease, and patients with PDAC (divided into two groups: PDAC early stage and PDAC late stage). As shown in Figure 1, serum levels of POSTN and CA242, with analogous expression patterns of CA19.9, were significantly increased in PDAC patients compared with those with benign disease and healthy controls in both the training and validation cohorts. In addition, the serum levels of the two markers in the PDAC early stage group were also significantly elevated compared to the control groups. Both POSTN and CA242 showed promising potential as PDAC biomarkers. In contrast, CEA showed relatively poor performances. Although CEA levels in PDAC patients were higher than those in benign controls, no statistically significant differences were observed between the PDAC early stage group and the healthy or benign disease groups (Figure S2). Carcinoembryonic antigen might not be a valuable biomarker used for early diagnosis of PDAC and was left out of subsequent analyses.
Figure 1.
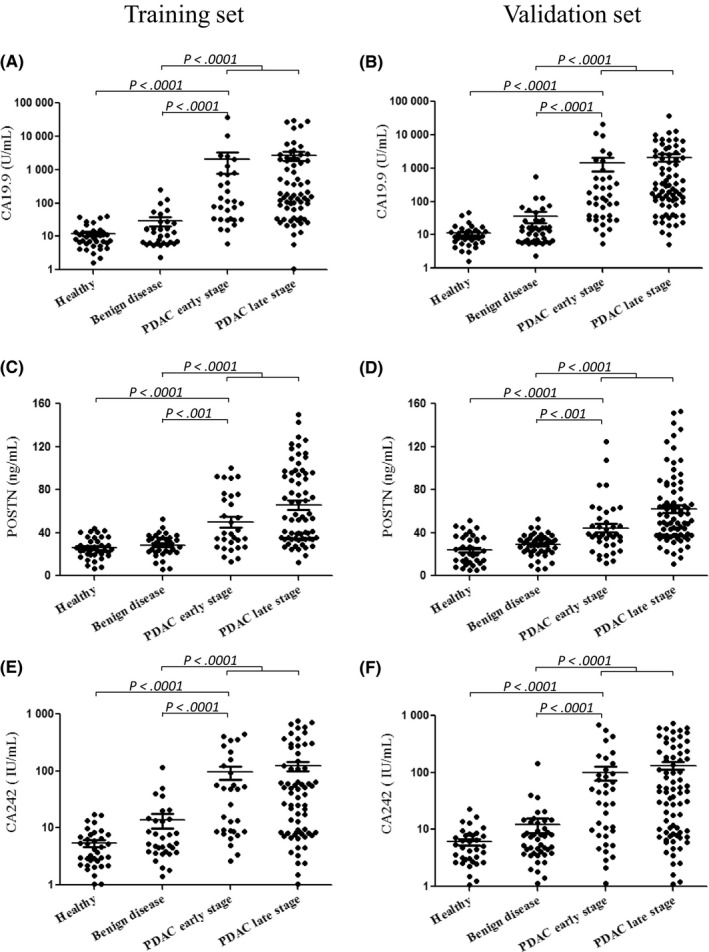
Scatter plots of carbohydrate antigen 19.9 (CA19.9), periostin (POSTN), and CA242 in pancreatic ductal adenocarcinoma (PDAC) patients and control subjects. Scatter plots are shown for CA19.9 (A, B), POSTN (C, D), and CA242 (E, F) in the training and validation cohorts, respectively. For each marker in the dataset, there are four groups: healthy controls (Healthy), patients with benign pancreatic disease (Benign disease), PDAC early stage, and PDAC late stage
The association between serum POSTN levels and IHC were also evaluated. Representative figures for immunostaining score are shown (Figure S3A); POSTN protein was mainly detected in the ECM components adjacent to tumor cells, but the tumor cells themselves showed no immunoreactivity except for occasional weak intracytoplasmic staining. As shown in Figure S3B, there was a significant correlation between serum POSTN levels and IHC scores.
Serum POSTN levels were compared in samples collected prior to and 2 weeks after surgery. As shown in the Figure S4, the postoperative levels of POSTN in PDAC patients without metastasis were decreased significantly (P = 0.006). However, no significant changes were observed in PDAC patients with metastasis (P = 0.24).
3.2. Performances of POSTN and CA242 as individual biomarkers for the diagnosis of PDAC
Performances of POSTN and CA242 as individual biomarkers were evaluated by the ROC curves, and compared with CA19.9. As shown in the Table S1 and Figure S5, all three markers showed remarkable performance in distinguishing PDAC early stage from healthy controls in the training set (AUCCA19.9 = 0.94, AUCPOSTN = 0.85, AUCCA242 = 0.88) and the validation set (AUCCA19.9 = 0.94, AUCPOSTN = 0.85, AUCCA242 = 0.85). As shown in Table 2 and Figure 2, AUCs for CA242 to discriminate PDAC early stage from healthy controls were comparable with CA19.9 in the training set (AUCCA19.9 = 0.94, AUCCA242 = 0.89) and in the validation set (AUCCA19.9 = 0.94, AUCCA242 = 0.83). To discriminate PDAC from benign disease, both markers had similar performances with CA19.9 in both the training set (AUCCA19.9 = 0.88, AUCPOSTN = 0.82, AUCCA242 = 0.79) and the validation set (AUCCA19.9 = 0.88, AUCPOSTN = 0.82, AUCCA242 = 0.79). Similarly, to distinguish PDAC early stage from benign disease, the performances of the three markers were comparable in both the training set (AUCCA19.9 = 0.88, AUCPOSTN = 0.81, AUCCA242 = 0.78) and the validation set (AUCCA19.9 = 0.88, AUCPOSTN = 0.82, AUCCA242 = 0.79).
Table 2.
Performances of biomarkers for the diagnosis of pancreatic ductal adenocarcinoma (PDAC)
| Training set | Validation set | |||||
|---|---|---|---|---|---|---|
| AUC (95% CI) | Sensitivity (%) | Specificity (%) | AUC (95% CI) | Sensitivity (%) | Specificity (%) | |
| Healthy vs PDAC early stage | ||||||
| CA19.9 | 0.94 (0.86‐0.99) | 96.7 | 83.8 | 0.94 (0.86‐0.98) | 86.8 | 94.6 |
| POSTN | 0.78 (0.66‐0.87) | 70.0 | 75.7 | 0.78 (0.66‐0.86) | 63.2 | 78.4 |
| CA242 | 0.89 (0.79‐0.95) | 83.3 | 81.1 | 0.83 (0.73‐0.91) | 57.9 | 100 |
| CA19.9 + POSTN | 0.97 (0.90‐1.00) | 93.3 | 94.6 | 0.95 (0.88‐0.99) | 86.8 | 97.3 |
| CA19.9 + CA242 | 0.96 (0.88‐0.99) | 90.0 | 94.6 | 0.97 (0.90‐1.00) | 86.8 | 97.3 |
| POSTN + CA242 | 0.90 (0.80‐0.96) | 83.3 | 86.5 | 0.92 (0.84‐0.97) | 79.0 | 94.6 |
| CA19.9 + POSTN + CA242 | 0.98 (0.90‐1.00)* | 96.7 | 94.6 | 0.98 (0.92‐1.00)* | 92.1 | 97.3 |
| Benign disease vs all PDAC | ||||||
| CA19.9 | 0.88 (0.82‐0.93) | 85.7 | 81.3 | 0.88 (0.82‐0.93) | 84.4 | 81.4 |
| POSTN | 0.81 (0.74‐0.88) | 64.3 | 87.5 | 0.82 (0.76‐0.88) | 77.4 | 79.1 |
| CA242 | 0.78 (0.70‐0.85) | 58.1 | 87.5 | 0.79 (0.72‐0.85) | 60.0 | 93.0 |
| CA19.9 + POSTN | 0.93 (0.88‐0.97)* | 84.7 | 90.6 | 0.92 (0.87‐0.96) | 83.5 | 93.0 |
| CA19.9 + CA242 | 0.89 (0.83‐0.94) | 75.5 | 90.6 | 0.90 (0.84‐0.94) | 77.4 | 88.4 |
| POSTN + CA242 | 0.87 (0.80‐0.92) | 67.4 | 96.9 | 0.89 (0.83‐0.93) | 84.4 | 83.7 |
| CA19.9 + POSTN + CA242 | 0.94 (0.88‐0.97)** | 84.7 | 90.6 | 0.93 (0.88‐0.96)* | 80.0 | 97.7 |
| Benign disease vs PDAC early stage | ||||||
| CA19.9 | 0.88 (0.77‐0.95) | 86.7 | 81.3 | 0.87 (0.78‐0.93) | 86.8 | 79.1 |
| POSTN | 0.74 (0.61‐0.84) | 53.3 | 87.5 | 0.72 (0.61‐0.82) | 65.8 | 79.1 |
| CA242 | 0.78 (0.66‐0.88) | 83.3 | 62.5 | 0.77 (0.66‐0.85) | 57.9 | 93.0 |
| CA19.9 + POSTN | 0.90 (0.80‐0.96) | 96.7 | 75.0 | 0.84 (0.75‐0.92) | 65.8 | 93.0 |
| CA19.9 + CA242 | 0.90 (0.79‐0.96) | 90.0 | 78.1 | 0.92 (0.83‐0.97)* | 76.3 | 93.0 |
| POSTN + CA242 | 0.80 (0.68‐0.89) | 56.7 | 96.9 | 0.84 (0.74‐0.91) | 76.3 | 86.1 |
| CA19.9 + POSTN + CA242 | 0.92 (0.82‐0.97)* | 96.7 | 75.0 | 0.90 (0.81‐0.96)* | 83.6 | 88.4 |
*P < 0.05 in comparison with carbohydrate antigen 19.9 (CA19.9); **P < 0.01 in comparison with CA19.9.
AUC, area under the curve; CI, confidence interval; POSTN, periostin.
Figure 2.
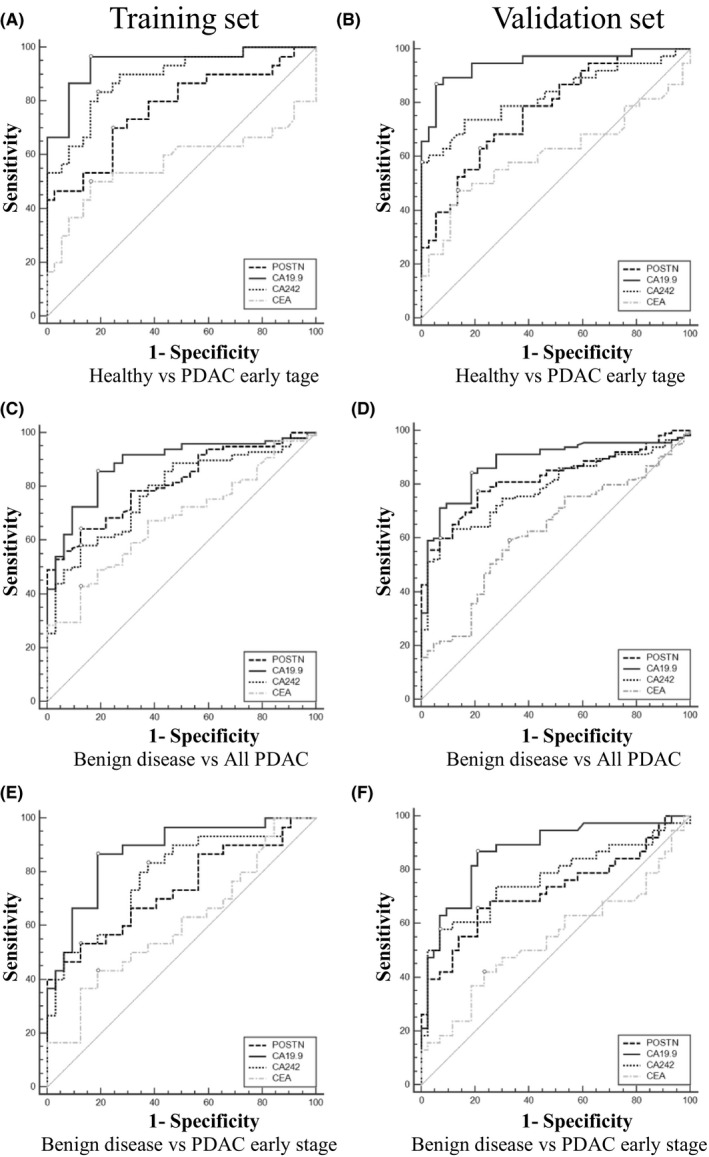
Performance of carbohydrate antigen 19.9 (CA19.9), periostin (POSTN), CA242, and carcinoembryonic antigen (CEA) in the diagnosis of pancreatic ductal adenocarcinoma (PDAC) as individual biomarkers. Receiver operating characteristic curves to distinguish PDAC early stage from healthy controls (A, B), all PDAC from benign disease (C, D), and PDAC early stage from benign disease (E, F), in the training and validation cohorts, respectively
As shown in Figures 2 and S5 and Tables 2 and S1, CA19.9 was observed with the best discriminatory performance in both the training and validation sets. However, if determined by its clinically used cut‐off (37 U/mL), as many as 27 (27.6%) patients in the training set and 29 (25.2%) patients in the validation set would be missed by this marker. The optimal cut‐off values for each marker in all of the analyses were obtained by optimizing Youden's index21 and are shown in Table S1.
To assess how the serum biomarkers behaved in each group of subjects, we constructed scatter plots between each marker. As illustrated in Figure 3, no correlation was found between serum CA19.9 and POSTN (R = 0.219, P < .001) or CA242 and POSTN (R = 0.295, P < .001). However, there was a significant positive correlation between serum levels of CA19.9 and CA242 (R = 0.617, P < .001).
Figure 3.
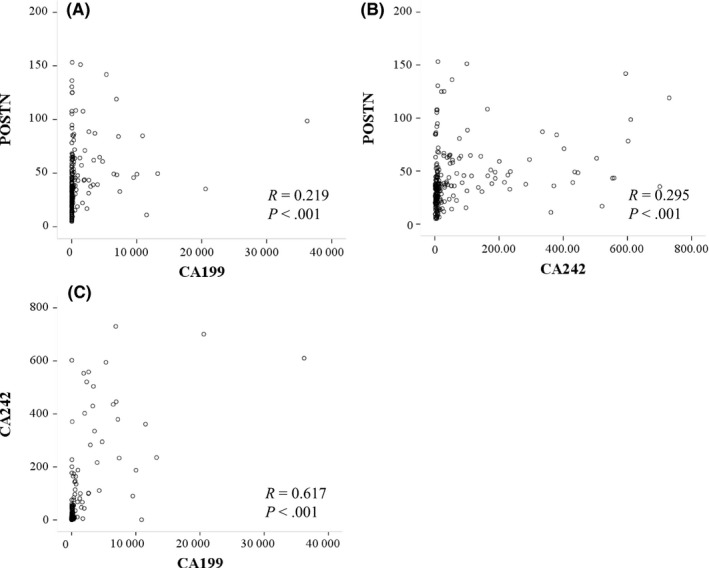
Correlations of serum carbohydrate antigen 19.9 (CA19.9) and periostin (POSTN) (A), POSTN and CA242 (B), and CA19.9 and CA242 (C) levels in the diagnosis of pancreatic ductal adenocarcinoma, analyzed using the non‐parametric Spearman's correlation coefficients method
3.3. Complementary performances of POSTN and CA242 for CA19.9 in the diagnosis of PDAC
To estimate whether the discrimination ability of CA19.9 could be improved by POSTN and CA242, marker panel models were constructed. As shown in Figures 4 and S6 and Tables 2 and S1, the three‐marker panels composed of CA19.9, POSTN, and CA242 were observed with significantly improved AUCs than CA19.9 alone in all of the analysis groups (healthy vs all PDAC, healthy vs PDAC early‐stage, benign disease vs all PDAC, and benign disease vs PDAC early stage). To discriminate PDAC early stage from healthy controls, significant improvements were shown in both the training set (AUCCA19.9 = 0.94 vs AUCCA19.9 + POSTN + CA242 = 0.98, P < .05) and the validation set (AUCCA19.9 = 0.94 vs AUCCA19.9 + POSTN + CA242 = 0.98, P < .05). As a single marker, CA19.9 showed particularly poor performances in discriminating benign disease from all PDAC or from PDAC early stage (Figure 2, Table 2). The AUCs were improved to 0.94 (training set, AUC19.9 = 0.88, P < .01) as well as 0.93 (validation set, AUC19.9 = 0.88, P < .05) to distinguish benign disease from all PDAC, and 0.92 (training set, AUC19.9 = 0.88, P < .05) as well as 0.90 (training set, AUC19.9 = 0.87, P < .05) to distinguish benign disease from PDAC early stage by the three‐marker panel, respectively.
Figure 4.
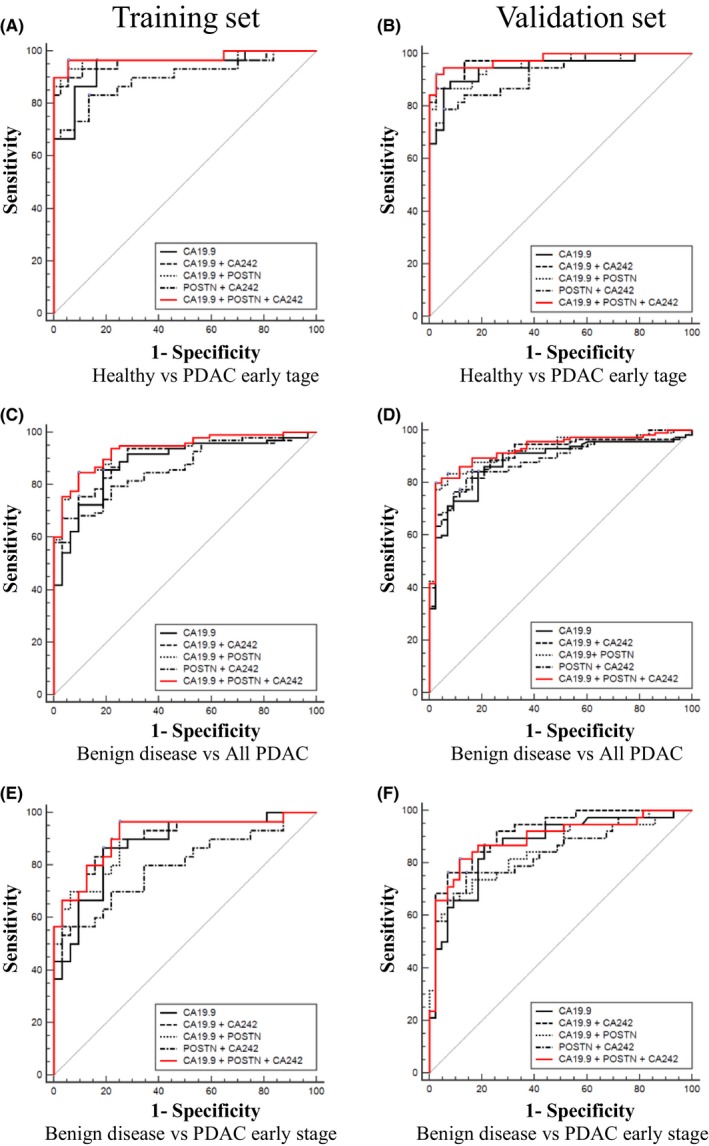
Complementarity of periostin (POSTN) and carbohydrate antigen 242 (CA242) for CA19.9 in the diagnosis of pancreatic ductal adenocarcinoma (PDAC). Receiver operating characteristic curves of CA19.9, CA19.9 + POSTN, CA19.9 + CA242, POSTN + CA242, and CA19.9 + POSTN + CA242 in the training and validation cohorts. To distinguish PDAC early stage from healthy controls (A, B), all PDAC from benign disease (C, D), and PDAC early stage from benign disease (E, F), in the training and validation cohorts, respectively
3.4. Performance of POSTN and CA242 in the diagnosis of CA19.9‐negative PDAC patients
In order to further investigate the complementarity of POSTN and CA242 for CA19.9 in the diagnosis of PDAC, we assessed the performance of the two markers for patients with PDAC that were missed by CA19.9, based on the clinically used threshold 37 U/mL. Considering the relatively small number of cases, the data for the whole set was also assessed. As shown in Figure 5 and Table 3, POSTN retained significant ability to discriminate PDAC CA19.9‐negative from healthy controls in the training set (AUCPOSTN = 0.89), the validation set (AUCPOSTN = 0.85), and the whole set (AUCPOSTN = 0.87). Moreover, the performance for POSTN to distinguish PDAC CA19.9‐negative from benign disease was still noticeable (AUCPOSTN = 0.87, training set; AUCPOSTN = 0.82, validation set; and AUCPOSTN = 0.84, whole set).
Figure 5.
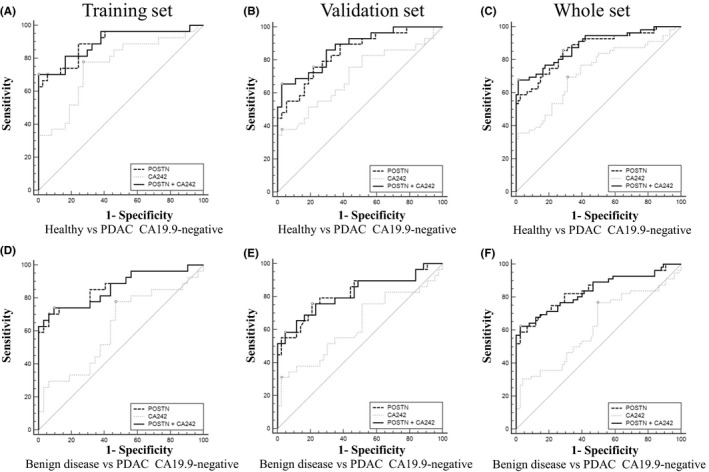
Performance of periostin (POSTN) and carbohydrate antigen 242 (CA242) in diagnosis of CA19.9‐negative pancreatic ductal adenocarcinoma (PDAC) patients. Receiver operating characteristic curves of POSTN, CA242, and POSTN + CA242 in the training, validation, and whole cohorts. To distinguish PDAC CA19.9‐negative from healthy controls (A‐C), and benign disease (D‐F) in the training, validation, and whole cohorts, respectively
Table 3.
Performance of periostin (POSTN) and carbohydrate antigen 242 (CA242) in diagnosis of CA19.9‐negative pancreatic ductal adenocarcinoma (PDAC) patients
| AUC (95% CI) | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|
| Training set | |||
| Healthy vs PDAC CA19.9‐negative | |||
| POSTN | 0.89 (0.79‐0.96) | 70.4 | 94.6 |
| CA242 | 0.76 (0.63‐0.85) | 77.8 | 73.0 |
| POSTN + CA242 | 0.90 (0.80‐0.96) | 70.4 | 100 |
| Benign disease vs PDAC CA19.9‐negative | |||
| POSTN | 0.87 (0.75‐0.94) | 70.4 | 93.8 |
| CA242 | 0.62 (0.49‐0.74) | 77.8 | 53.1 |
| POSTN + CA242 | 0.86 (0.75‐0.94) | 74.1 | 90.6 |
| Validation set | |||
| Healthy vs PDAC CA19.9‐negative | |||
| POSTN | 0.85 (0.74‐0.92) | 75.9 | 78.4 |
| CA242 | 0.70 (0.58‐0.81) | 38.0 | 97.3 |
| POSTN + CA242 | 0.88 (0.77‐0.94) | 65.5 | 97.3 |
| Benign disease vs PDAC CA19.9‐negative | |||
| POSTN | 0.82 (0.71‐0.90) | 75.9 | 79.1 |
| CA242 | 0.63 (0.51‐0.74) | 31.0 | 97.7 |
| POSTN + CA242 | 0.82 (0.71‐0.90) | 58.6 | 95.4 |
| Whole set | |||
| Healthy vs PDAC CA19.9‐negative | |||
| POSTN | 0.87 (0.80‐0.92) | 85.7 | 71.6 |
| CA242 | 0.73 (0.64‐0.80) | 69.6 | 68.9 |
| POSTN + CA242 | 0.89 (0.82‐0.94) | 67.9 | 98.7 |
| Benign disease vs PDAC CA19.9‐negative | |||
| POSTN | 0.84 (0.77‐0.90) | 58.9 | 97.3 |
| CA242 | 0.62 (0.53‐0.71) | 76.8 | 50.7 |
| POSTN + CA242 | 0.84 (0.76‐0.90) | 62.5 | 97.3 |
AUC, area under the curve; CI, confidence interval.
4. DISCUSSION
Pancreatic ductal adenocarcinoma is a common gastrointestinal malignancy characterized by aggressive growth and early dissemination, and many patients with PDAC are diagnosed with advanced stages.1, 2 Carbohydrate antigen 19.9 is the most commonly used biomarker for patients with PDAC, but with unsatisfactory sensitivity and specificity, especially for early stage disease.7, 8 As our study showed, although observed with the best performances in all analysis groups, CA19.9 performed relatively poorly in distinguishing benign disease from all PDAC or early stages of PDAC. Moreover, if used the clinically used cut‐off (37 U/mL), as many as 27 (27.6%) patients in the training set and 29 (25.2%) patients in the validation set would be considered negative. Therefore, it is essential to explore novel and reliable non‐invasive biomarkers to complement CA19.9 to improve the diagnosis of PDAC. As an important strategy to improve the diagnosis of PDAC, marker panel studies have gained increased attention in recent years.9, 10 In the present study, we identified that POSTN and CA242 could be potential diagnostic serum biomarkers complementing CA19.9 in detecting early pancreatic cancer.
Periostin is a secreted matrix N‐glycoprotein that plays vital roles in numerous cancers, especially in gastrointestinal malignancy.16, 20, 22, 23, 24, 25, 26 For pancreatic cancer, it is reported that POSTN has a biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells,17 participates in the process of tumor angiogenesis,16 and could promote invasiveness and resistance of pancreatic cancer cells to induce cell death.17, 27 However, few studies have focused on the clinical significance of serum POSTN in patients with PDAC. The diagnostic value of POSTN as a non‐invasive serological biomarker for PDAC was assessed in this study. The serum POSTN levels were found to be significantly elevated in patients with PDAC, which were consistent with previous studies reporting increased levels of POSTN mRNA in PDAC tissues.17, 27 Moreover, the result that postoperative levels of POSTN in PDAC patients without metastasis were decreased significantly, as well as the result that there was a significant correlation between serum POSTN levels and IHC scores, all revealed one fact: pancreatic cancer tissues were probably the main source of POSTN in the circulation. No obvious changes were observed for those patients with metastasis. The explanation for this might lay in the fact that metastatic tumors could also be the source of serum POSTN, therefore, levels will not dramatically drop after primary tumor resection. Altogether, these data suggested that serum POSTN was upregulated in patients with PDAC.
The expression of POSTN in the serum of patients with PDAC was correlated with tumor stage, and patients with advanced PDAC tended have higher levels of POSTN than those with early stages of PDAC. As most advanced cases of PDAC are metastasis tumors, these results are consistent with the previous report that POSTN was involved in the process of PDAC metastasis.17, 18, 19 The performance of POSTN in diagnosing PDAC as an individual biomarker was significant, particularly in distinguishing resectable or early stage PDAC patients from control subjects. Additionally, as a component of the marker panel, POSTN could improve the performance of CA19.9, which illustrated that POSTN might provide an essential complementary effect for CA19.9. In addition, this study found that POSTN retained moderate diagnosis capabilities for patients with CA19.9‐negative PDAC. Taken together, these data indicated that POSTN was an exceedingly promising diagnosis biomarker for PDAC. Periostin, as a recently discovered serum tumor biomarker, not only plays promising roles in the diagnosis of malignant diseases, but also has important prognostic value for multiple tumors, such as hepatocellular carcinoma,28 esophageal squamous cell carcinoma,29 non‐small‐cell lung cancer,30 and breast cancer.31 However, whether high levels of serum POSTN are associated with poor prognosis of patients with PDAC is not yet known. In this study, it has been shown that serum levels of POSTN in late‐stage PDAC were higher than in PDAC early stage. This is probably due to the infiltrative growth or distant metastasis of tumor cells, which are also important signs of poor prognosis. Long‐term follow‐up of PDAC patients is necessary to reveal the potential prognostic implications of serum POSTN levels.
Carbohydrate antigen 242 was identified relatively late as a diagnostic, prognostic, and surveillance biomarker of gastrointestinal malignancy.11 Preliminary results on the serum expression of CA242 in pancreatic cancer were described in 1991 by Kuusela et al.12 They reported a high correlation between the serum levels of CA242 and CA19.9, which is consistent with the results of our study.12 In spite of the similarity of the markers, there are significant differences in the expression pattern of the two antigens in many patients and the reason remains unknown.32 The complementary role of CA242 for CA19.9 in the early diagnosis of PDAC has also not been fully elucidated. Although there is a high correlation between serum CA19.9 and CA242, our study indicated that the discriminating capabilities of CA19.9 for PDAC could be significantly improved by CA242, especially to make up for the shortcomings of CA19.9 in the diagnosis of early stage PDAC. All of these data indicated that CA242 was of great clinical application value.
However, compared with POSTN, CA242 retained relatively poor performance to distinguish between CA19.9‐negative PDAC and control subjects. One possible reason is that there is no correlation between the expression of POSTN and CA19.9, but CA242 and CA19.9 do have a correlation of expression.12 Patients with PDAC missed by CA19.9 tend to be those with lower CA242 levels. However, the diagnostic ability of POSTN for pancreatic cancer is completely independent of CA19.9. Therefore, POSTN, rather than CA242, might be more suitable to serve as a rescuer for patients with PDAC missed by CA19.9. The two markers play different complementary roles for CA19.9 in the diagnosis of PDAC.
In conclusion, this is the first study to fully illustrate the potential value of serum POSTN as a diagnostic biomarker for PDAC, as well as the complementary role of CA242 for CA19.9 in the early diagnosis of PDAC. Periostin and CA242 could improve the performance of CA19.9 in the early diagnosis of PDAC. The performance of marker panels were fully investigated in this study. A marker panel composed of CA19.9, POSTN, and CA242 showed significantly improved performance compared with CA19.9 alone, especially to distinguish patients with early stage PDAC from control subjects. The data presented in our study could provide valuable information for the early diagnosis of PDAC using CA19.9, POSTN, and CA242 as a marker panel in clinical work. In conclusion, the present study suggested that POSTN and CA242 were potential diagnostic serum biomarkers complementing CA19.9 in detecting pancreatic cancer.
CONFLICT OF INTEREST
Authors declare no conflict of interests for this article.
Supporting information
ACKNOWLEDGMENTS
This study was supported by research grants from the National Natural Science Foundation of China (nos. 81502519, 81402174, and 81201653), and the Natural Science Foundation of Tianjin (16JCYBJC26000).
Dong D, Jia L, Zhang L, et al. Periostin and CA242 as potential diagnostic serum biomarkers complementing CA19.9 in detecting pancreatic cancer. Cancer Sci. 2018;109:2841–2851. 10.1111/cas.13712
Funding information
National Natural Science Foundation of China (nos. 81502519, 81402174, and 81201653); Natural Science Foundation of Tianjin (16JCYBJC26000).
REFERENCES
- 1. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:2140‐2141. [DOI] [PubMed] [Google Scholar]
- 3. Arvold ND, Ryan DP, Niemierko A, et al. Long‐term outcomes of neoadjuvant chemotherapy before chemoradiation for locally advanced pancreatic cancer. Cancer. 2012;118:3026‐3035. [DOI] [PubMed] [Google Scholar]
- 4. Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut. 2007;56:1134‐1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Can Res. 2014;74:2913‐2921. [DOI] [PubMed] [Google Scholar]
- 6. Bhutani MS, Koduru P, Joshi V, et al. The role of endoscopic ultrasound in pancreatic cancer screening. Endosc Ultrasound. 2016;5:8‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duffy MJ, Sturgeon C, Lamerz R, et al. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010;21:441‐447. [DOI] [PubMed] [Google Scholar]
- 8. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19‐9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266‐270. [DOI] [PubMed] [Google Scholar]
- 9. Chan A, Diamandis EP, Blasutig IM. Strategies for discovering novel pancreatic cancer biomarkers. J Proteomics. 2013;81:126‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brand RE, Nolen BM, Zeh HJ, et al. Serum biomarker panels for the detection of pancreatic cancer. Clin Cancer Res. 2011;17:805‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haglund C, Lindgren J, Roberts PJ, Kuusela P, Nordling S. Tissue expression of the tumour associated antigen CA242 in benign and malignant pancreatic lesions. A comparison with CA 50 and CA 19‐9. Br J Cancer. 1989;60:845‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuusela P, Haglund C, Roberts PJ. Comparison of a new tumour marker CA 242 with CA 19‐9, CA 50 and carcinoembryonic antigen (CEA) in digestive tract diseases. Br J Cancer. 1991;63:636‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471‐1474. [DOI] [PubMed] [Google Scholar]
- 14. Goode EL, Chenevix‐Trench G, Hartmann LC, et al. Assessment of hepatocyte growth factor in ovarian cancer mortality. Cancer Epidemiol Biomarkers Prev. 2011;20:1638‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837‐845. [PubMed] [Google Scholar]
- 16. Liu Y, Li F, Gao F, et al. Periostin promotes tumor angiogenesis in pancreatic cancer via Erk/VEGF signaling. Oncotarget. 2016;7:40148‐40159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanno A, Satoh K, Masamune A, et al. Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int J Cancer. 2008;122:2707‐2718. [DOI] [PubMed] [Google Scholar]
- 18. Grupp K, Melling N, Bogoevska V, et al. Expression of ICAM‐1, E‐cadherin, periostin and midkine in metastases of pancreatic ductal adenocarcinomas. Exp Mol Pathol. 2018;104:109‐113. [DOI] [PubMed] [Google Scholar]
- 19. Baril P, Gangeswaran R, Mahon PC, et al. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia‐induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene. 2007;26:2082‐2094. [DOI] [PubMed] [Google Scholar]
- 20. Fukushima N, Kikuchi Y, Nishiyama T, Kudo A, Fukayama M. Periostin deposition in the stroma of invasive and intraductal neoplasms of the pancreas. Mod Pathol. 2008;21:1044‐1053. [DOI] [PubMed] [Google Scholar]
- 21. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32‐35. [DOI] [PubMed] [Google Scholar]
- 22. Hu WW, Chen PC, Chen JM, et al. Periostin promotes epithelial‐mesenchymal transition via the MAPK/miR‐381 axis in lung cancer. Oncotarget. 2017;8:62248‐62260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Z, Xiong S, Mao Y, et al. Periostin promotes immunosuppressive premetastatic niche formation to facilitate breast tumour metastasis. J Pathol. 2016;239:484‐495. [DOI] [PubMed] [Google Scholar]
- 24. Sung PL, Jan YH, Lin SC, et al. Periostin in tumor microenvironment is associated with poor prognosis and platinum resistance in epithelial ovarian carcinoma. Oncotarget. 2016;7:4036‐4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sriram R, Lo V, Pryce B, et al. Loss of periostin/OSF‐2 in ErbB2/Neu‐driven tumors results in androgen receptor‐positive molecular apocrine‐like tumors with reduced Notch1 activity. Breast Cancer Res. 2015;17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park SY, Piao Y, Jeong KJ, Dong J, de Groot JF. Periostin (POSTN) regulates tumor resistance to antiangiogenic therapy in glioma models. Mol Cancer Ther. 2016;15:2187‐2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Erkan M, Reiser‐Erkan C, Michalski CW, et al. Cancer‐stellate cell interactions perpetuate the hypoxia‐fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia. 2009;11:497‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lv Y, Wang W, Jia WD, et al. High preoparative levels of serum periostin are associated with poor prognosis in patients with hepatocellular carcinoma after hepatectomy. Eur J Surg Oncol. 2013;39:1129‐1135. [DOI] [PubMed] [Google Scholar]
- 29. Wang W, Sun QK, He YF, et al. Overexpression of periostin is significantly correlated to the tumor angiogenesis and poor prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:593‐601. [PMC free article] [PubMed] [Google Scholar]
- 30. Xu CH, Wang W, Lin Y, et al. Diagnostic and prognostic value of serum periostin in patients with non‐small cell lung cancer. Oncotarget. 2017;8:18746‐18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nuzzo PV, Rubagotti A, Argellati F, et al. Prognostic value of preoperative serum levels of periostin (PN) in early breast cancer (BCa). Int J Mol Sci. 2015;16:17181‐17192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haglund C, Lundin J, Kuusela P, Roberts PJ. CA 242, a new tumour marker for pancreatic cancer: a comparison with CA 19‐9, CA 50 and CEA. Br J Cancer. 1994;70:487‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


