Abstract
In the human genome, miR‐451a, miR‐144‐5p (passenger strand), and miR‐144‐3p (guide strand) reside in clustered microRNA (miRNA) sequences located within the 17q11.2 region. Low expression of these miRNAs is significantly associated with poor prognosis of patients with renal cell carcinoma (RCC) (miR‐451a: P = .00305; miR‐144‐5p: P = .00128; miR‐144‐3p: P = 9.45 × 10−5). We previously reported that miR‐451a acted as an antitumor miRNA in RCC cells. Involvement of the passenger strand of the miR‐144 duplex in the pathogenesis of RCC is not well understood. Functional assays showed that miR‐144‐5p and miR‐144‐3p significantly reduced cancer cell migration and invasive abilities, suggesting these miRNAs acted as antitumor miRNAs in RCC cells. Analyses of miR‐144‐5p targets identified a total of 65 putative oncogenic targets in RCC cells. Among them, high expression levels of 9 genes (FAM64A, F2,TRIP13,ANKRD36,CENPF,NCAPG,CLEC2D,SDC3, and SEMA4B) were significantly associated with poor prognosis (P < .001). Among these targets, expression of SDC3 was directly controlled by miR‐144‐5p, and its expression enhanced cancer cell aggressiveness. We identified genes downstream by SDC3 regulation. Data showed that expression of 10 of the downstream genes (IL18RAP,SDC3,SH2D1A,GZMH,KIF21B,TMC8,GAB3,HLA‐DPB2,PLEK, and C1QB) significantly predicted poor prognosis of the patients (P = .0064). These data indicated that the antitumor miR‐144‐5p/oncogenic SDC3 axis was deeply involved in RCC pathogenesis. Clustered miRNAs (miR‐451a, miR‐144‐5p, and miR‐144‐3p) acted as antitumor miRNAs, and their targets were intimately involved in RCC pathogenesis.
Keywords: antitumor, microRNA, miR‐144‐5p, renal cell carcinoma, SDC3
1. INTRODUCTION
Renal cell carcinoma (RCC) is the most common form of adult kidney cancer. It accounts for approximately 3.8% of all newly diagnosed malignancies, and more than 140 000 people die worldwide every year.1 Approximately 80% of RCC patients are classified with clear cell RCC.2 Approximately 20%‐30% of patients are found with advanced RCC at diagnosis, and the frequency of 5‐year survival is only 12.1%. The treatment strategy of metastatic RCC remains confused.3 Recently developed molecularly targeted therapeutics and immunotherapies have improved the prognosis of patients with advanced RCC, but recurrence, progression of distant metastasis, and side‐effects remain important issues associated with these treatments.4 Searching for new therapeutic targets and developing useful prognostic markers are important issues to overcome in new treatments for RCC.
MicroRNAs (miRNAs), which are short, single‐strand RNAs (19‐22 nucleotides) belong to a group of noncoding RNA molecules that act as pivotal agents responsible for fine‐tuning RNA expression in a sequence‐dependent manner.5 A vast number of studies have reported that miRNAs are closely involved in the physiological and pathological processes of disease.6 In cancer cells, abnormal expression of miRNAs can disrupt regulatory networks and lead to cancer cell development, progression, metastasis, and drug resistance.5, 7, 8 We have identified antitumor miRNAs (miR‐10a‐5p, miR‐29s, miR‐101, miR‐149, and miR‐451a) and their targets that are involved in the pathogenesis of RCC.9, 10, 11, 12, 13 This strategy is a novel approach to identify new molecular targets and prognostic markers for RCC.
Previous miRNA biogenesis posits that the passenger strand of miRNA is degraded and does not regulate gene expression. Contrary to this concept, our miRNA expression signature of RCC showed that some miRNA passenger strands are aberrantly expressed in cancer tissues, for example, miR‐139‐3p, miR‐144‐5p, miR‐145‐3p, and miR‐150‐3p.14, 15, 16, 17 In fact, we found that some passenger strands actually act as antitumor miRNAs (miR‐144‐5p, miR‐145‐3p, miR‐149‐3p, miR‐150‐3p, and miR‐199a‐3p) through their targeting of oncogenes in several cancers.12, 15, 16, 17, 18, 19 These studies suggested the importance of analyzing passenger strands of miRNA duplex in cancer cells.
Our recent study showed that miR‐451a was significantly downregulated in RCC tissues and acted as an antitumor miRNA in RCC cells.13 Interestingly, miR‐451a‐regulated oncogenic targets were significantly associated with RCC pathogenesis.13 In the human genome, miR‐451a, miR‐144‐5p (the passenger strand), and miR‐144‐3p (the guide strand) are clustered together in chromosomal region 17q11.2. The Cancer Genome Atlas (TCGA) database analyses showed that low expression of miR‐144‐5p and miR‐144‐3p was significantly associated with poor prognosis of RCC patients (P = .00128 and P = 9.45 × 10−5 , respectively).
In this study, we focused on miR‐144‐5p because the functional significance of miRNA passenger strands in RCC pathogenesis is obscure. Here, we studied the antitumor roles of miR‐144‐5p and identified the oncogenic targets involved in the pathogenesis of RCC. We suggest that identification of novel functions of miRNA passenger strands and the RNA networks they regulate might enhance our understanding of the molecular pathogenesis of RCC.
2. MATERIALS AND METHODS
2.1. Clinical RCC specimens and cell lines
We obtained a total of 18 clinical tissue specimens from RCC patients who underwent total nephrectomy at Chiba University Hospital (Chiba, Japan) between 2008 and 2015 (Table 1). All patients in our study provided signed informed consent, and the study protocol was approved by the Institutional Review Board of Chiba University (approval no. 484). We used 2 cell lines, 786‐O and A498, obtained from ATCC (Manassas, VA, USA).
Table 1.
Clinical features of 18 patients with clear cell renal cell carcinoma
| No. | Age, years | Gender | Grade | pT | INF | v | ly | eg or ig | fc | im | rc | rp | s | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | F | G2 | T1a | a | 0 | 0 | eg | 1 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 2 | 74 | M | G1 > G2 | T1a | a | 0 | 0 | eg | 1 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 3 | 59 | M | G3 > G2 | T1b | a | 0 | 0 | eg | 1 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 4 | 52 | M | G2 > G3 > G1 | T1a | a | 0 | 0 | eg | 1 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 5 | 64 | M | G2 > G3 | T1b | a | 0 | 0 | eg | 1 | 1 | 0 | 0 | 0 | qRT‐PCR |
| 6 | 67 | M | G2 > G3 > G1 | T3a | b | 1 | 0 | ig | 0 | 1 | 1 | 0 | 0 | qRT‐PCR |
| 7 | 67 | M | G2 > G3 > G1 | T3a | b | 1 | 0 | ig | 1 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 8 | 59 | M | G3 > G2 | T3a | b | 1 | 0 | ig | 0 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 9 | 73 | M | G1 > G3 | T2a | a | 0 | 1 | eg | 1 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 10 | 77 | M | G1 > G2 | T1b | a | 0 | 0 | eg | 1 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 11 | 77 | M | G2 > G1 | T3a | a | 1 | 0 | eg | 1 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 12 | 51 | M | G2 > G1 | T1b | a | 0 | 0 | eg | 0 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 13 | 78 | M | G2 > G1 > G3 | T1b | b | 0 | 0 | eg | 1 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 14 | 57 | M | G1 > G2 | T1a | a | 0 | 0 | eg | 1 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 15 | 54 | M | G2 > G1 | T3a | a | 0 | 0 | eg | 0 | 0 | 1 | 0 | 0 | qRT‐PCR |
| 16 | 54 | M | G1 > >G3 | T2b | a | 0 | 0 | eg | 1 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 17 | 74 | F | G1 > G2 | T2a | b | 0 | 0 | ig | 1 | 0 | 0 | 0 | 0 | qRT‐PCR |
| 18 | 65 | M | G1 > G2 | T1b | b | 0 | 0 | ig | 1 | 0 | 0 | 0 | 0 | IHC |
a, clearly bounded with noncancer surrounding tissue; b, intermediate type; eg, expansive growth; F, female; fc, capsular formation; ig, infiltrative growth; IHC, immunohistochemistry; im, intrarenal metastasis; INF, infiltration; ly, lymph node; M, male; qRT‐PCR, quantitative RT‐PCR; rc, renal capsule invasion; rp, pelvis invasion; s, sinus invasion; v, vein.
2.2. Transfection of mature miRNA and siRNA into RCC cells
The following RNA species were used in this study: mature miRNAs, pre‐miR miRNA precursors (hsa‐miR‐144‐5p, assay ID: PM12631; hsa‐miR‐144‐3p, assay ID: PM11051; Applied Biosystems, Foster City, CA, USA), negative control miRNA (assay ID: AM 17111; Applied Biosystems), and siRNA (Stealth Select RNAi siRNA; si‐SDC3, P/N: HSS145253 and HSS145254; Invitrogen, Carlsbad, CA, USA). The transfection methods were described previously.11, 20
2.3. Quantitative RT‐PCR
The procedures for PCR quantification were described previously.11, 20 TaqMan probes and primers for SDC3 (P/N:Hs01568665_m1; Applied Biosystems) were assay‐on‐demand gene expression products. Quantitative RT‐PCRs (qRT‐PCRs) for miR‐144‐5p (P/N:002148; Applied Biosystems) and miR‐144‐3p (P/N:002676) were used to identify the expression levels of miRNAs according to the manufacturer's protocol. To normalize the data for quantification of mRNA and miRNAs, we used human GAPDH (P/N: Hs02786624_g1; Applied Biosystems), GUSB (P/N: Hs99999908_m1; Applied Biosystems), and RNU48 (assay ID: 001006; Applied Biosystems).
2.4. Cell proliferation, migration, and invasion assays
Cell proliferation abilities were determined by XTT assays using Cell Proliferation Kit II (Sigma‐Aldrich, St. Louis, MO, USA). Cell migration was characterized with wound healing assays. Cell invasion abilities were determined with modified Boyden chambers containing Transwell‐precoated Matrigel membrane filter inserts.11, 20
2.5. Incorporation of miR‐144‐5p or miR‐144‐3p into the RNA‐induced silencing complex by Ago2 immunoprecipitation
786‐O cells were transfected with 10 nmol/L miRNAs by reverse transfection. After 48 hours, immunoprecipitation was carried out using a human AGO2 miRNA isolation kit (Wako, Osaka, Japan).16 Expression levels of miR‐144‐5p or miR‐144‐3p were evaluated by qRT‐PCR. MicroRNA data were normalized to the expression of miR‐26a (P/N:000405; Applied Biosystems), which was not influenced by miR‐144‐5p or miR‐144‐3p transfection.
2.6. Western blot analysis
Immunoblotting was carried out with monoclonal anti‐SDC3 antibodies (1:400 dilution; SAB4301620; Sigma‐Aldrich). We used anti‐GAPDH antibodies (1:10 000 dilution; ab8245; Abcam, Cambridge, UK) as an internal control.11, 20
2.7. Identification of candidate genes regulated by miR‐144‐5p and miR‐144‐3p in RCC cells
Candidate genes regulated by miR‐144‐5p and miR‐144‐3p were identified by a combination of in silico and genomewide gene expression analyses. Genes possessing sequences regulated by miR‐144‐5p and miR‐144‐3p were obtained from the TargetScan database (http://www.targetscan.org/vert_71/). Upregulated genes in RCC were identified from publicly available datasets in the Gene Expression Omnibus (GEO; accession no. GSE36895) and we narrowed down the candidate genes as explained below. Oligo microarrays (Human GE 60K; Agilent Technologies, Santa Clara, CA, USA) were used for gene expression analyses. The microarray data were deposited into GEO (http://www.ncbi.nlm.nih.gov/geo/), with accession number GSE106791. The Genomics Analysis and Visualization Platform was used for visualization of gene expression heat maps and clustering.21 The normalized mRNA expression values in the RNA sequencing data were processed and provided as Z scores. In the present study, patients were divided into two groups: Z‐score ≥ 0 and Z‐score < 0.
2.8. Plasmid construction and dual‐luciferase reporter assay
The partial wild‐type sequence of the SDC3 3′‐UTR was inserted between the SgfI‐PmeI restriction sites in the 3′‐UTR of the hRluc gene in the psiCHECK‐2 vector (C8021; Promega, Madison, WI, USA). We used sequences that were missing the miR‐144‐5p target sites (position 2166‐2172). The synthesized DNA was cloned into the psiCHECK‐2 vector.11, 20
2.9. Immunohistochemistry
Tissue sections were incubated overnight at 4°C with anti‐SDC3 antibodies diluted 1:50 (SAB4301620; Sigma‐Aldrich).11, 20
2.10. Regulation of targets downstream of SDC3 in RCC
We further investigated pathways regulated by SDC3 in RCC cells. We analyzed gene expression using si‐SDC3‐transfected 786‐O cells. Microarray data were used for expression profiling of si‐SDC3 transfectants. The microarray data were deposited into GEO (accession no. GSE113066).
2.11. Clinical data analysis based on TCGA datasets
To investigate the clinical significance of miRNAs and genes in RCC, we used the RNA sequence database in TCGA (https://tcga-data.nci.nih.gov/tcga/). The gene expression and clinical data were obtained from cBioPortal (http://www.cbioportal.org/, the provisional data downloaded on 1 December 2017).22, 23, 24
2.12. Statistical analysis
Relationships between 2 or 3 variables and numerical values were analyzed with Mann‐Whitney U tests or Bonferroni‐adjusted Mann‐Whitney U‐tests. Spearman's rank tests were used to analyze the correlations of the expressions. Expert StatView software (version 5.0; SAS Institute, Cary, NC, USA) was used for these analyses. Univariate and multivariate Cox proportional hazard regression models were used to determine prognostic factors with JMP Pro 13 (SAS Institute Inc., Cary, NC, USA).
3. RESULTS
3.1. Expression levels of miR‐144‐5p and miR‐144‐3p in RCC clinical specimens
As shown in Figure 1, the expression levels of miR‐144‐5p and miR‐144‐3p were significantly lower in cancer tissues compared with those in adjacent noncancerous tissues (P = .0325 and P = .0329, respectively; Figure 1A,B). Furthermore, Spearman's rank test showed a positive correlation between the expression levels of miR‐144‐5p and miR‐144‐3p in clinical specimens (R = 0.891, P < .0001; Figure 1C).
Figure 1.
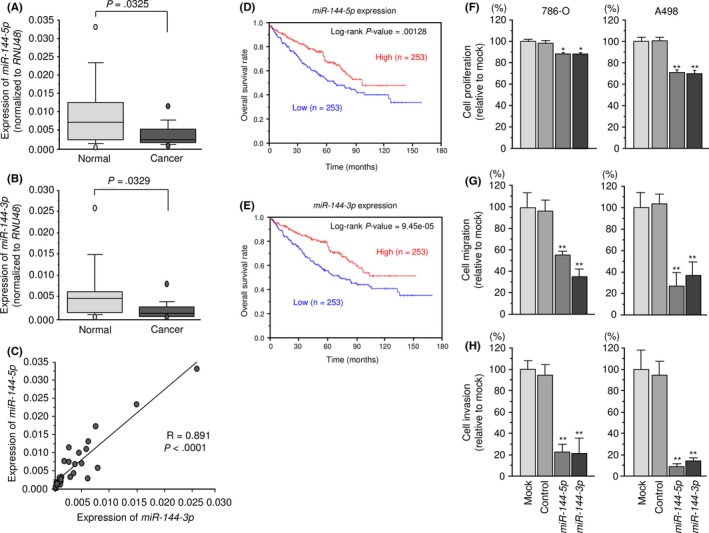
Expression levels, clinical significance, and functional roles of miR‐144‐5p and miR‐144‐3p in renal cell carcinoma (RCC). A‐C, Expression levels of miR‐144‐5p and miR‐144‐3p in RCC clinical specimens. RNU48 was used as an internal control. Spearman's rank test showed a positive correlation between the expression levels of miR‐144‐5p and miR‐144‐3p. D,E, From The Cancer Genome Atlas database, patients with low expression levels of either miR‐144‐5p or miR‐144‐3p had significantly reduced overall survival. F‐H, Cell proliferation was determined by XTT assays. Cell migration activity was determined using migration assays. Cell invasion activity was determined using Matrigel invasion assays. *P < .005; **P < .0001
3.2. Clinical significance and functional roles of miR‐144‐5p and miR‐144‐3p in RCC
From TCGA database, patients with low expression levels of both miR‐144‐5p and miR‐144‐3p were significantly associated with poor prognosis (P = .00128 and P = 9.45 × 10−5, respectively; Figure 1D,E).
We undertook gain‐of‐function assays using miRNA transfection into two RCC cell lines. Ectopic expression of miR‐144‐5p and miR‐144‐3p showed that both miR‐144‐5p and miR‐144‐3p reduced cancer cell proliferation, migration, and invasive abilities in comparison with mock and miR‐control transfectants (Figure 1F‐H).
3.3. Incorporation of miR‐144‐5p into the RNA‐induced silencing complex in RCC cells
We carried out immunoprecipitation with antibodies targeting Ago2, which plays a pivotal role in the RNA‐induced silencing complex (RISC). After transfection with miR‐144‐5p and immunoprecipitation by anti‐Ago2 antibodies, miR‐144‐5p levels were significantly higher than those of mock‐ or miR‐control‐transfected cells or those of miR‐144‐3p‐transfected 786‐O cells (P < .0001; Figure S1A). Similarly, after miR‐144‐3p transfection, miR‐144‐3p was detected by Ago2 immunoprecipitation (P < .0001; Figure S1B).
3.4. Identification of candidate targets of miR‐144‐5p and miR‐144‐3p regulation in RCC cells
We searched for candidate targets using a combination of genomewide gene expression and in silico database analyses. The strategy for identification of miR‐144‐5p and miR‐144‐3p target genes is shown in Figure S2. First, we identified 2078 and 1043 genes that had putative target sites for miR‐144‐5p and miR‐144‐3p, respectively in their 3′‐UTRs based upon the TargetScanHuman 7.1 database. Next, we narrowed down those presumptive targets to 227 and 268 genes, respectively based on expression levels that were upregulated (fold change >1.5) in RCC tissues using the GEO database. Next, we identified 65 and 34 genes that were downregulated after miR‐144‐5p and miR‐144‐3p transfection, respectively into RCC cells (Log2 ratio < −0.5; Tables 2, 3). In this study, we focused on miR‐144‐5p, the passenger strand of the miR‐144 duplex. As shown in Figure 2, 65 candidate target genes of miR‐144‐5p were analyzed, allowing us to construct a heat map. Among those genes, 9 were significantly associated with poor prognosis in RCC patients (P < .001; Figure 3). Heat map visualization of those genes is shown in Figure 4A. Patients with high gene signature expression (Z‐score ≥ 0) had poorer outcomes (disease‐free survival and overall survival) than those with low gene signature expression (Z‐score < 0) (P < .0001; Figure 4B,C). In the present study, we focused on syndecan‐3 (SDC3), reportedly related to carcinogenesis in several types of cancers.
Table 2.
miR‐144‐5p candidate target genes in renal cell carcinoma
| Entrez gene ID | Gene symbol | Gene name | Site count | GEO expression data fold change (tumor/normal) | A498 miR‐144‐5p transfection (Log2 ratio) | 786‐O miR‐144‐5p transfection (Log2 ratio) | Average A498/786‐O miR‐144‐5p transfection (Log2 ratio) | Cytoband | TCGA data for OS (high vs low expression: P‐value) |
|---|---|---|---|---|---|---|---|---|---|
| 54478 | FAM64A | Family with sequence similarity 64, member A | 1 | 2.400 | −1.290 | −0.933 | −1.111 | hs|17p13.2 | 1.79E‐07 |
| 2147 | F2 | Coagulation factor II (thrombin) | 1 | 2.673 | −0.234 | −0.925 | −0.579 | hs|11p11.2 | 3.68E‐07 |
| 9319 | TRIP13 | Thyroid hormone receptor interactor 13 | 1 | 2.551 | −1.164 | −0.652 | −0.908 | hs|5p15.33 | 9.70E‐07 |
| 375248 | ANKRD36 | Ankyrin repeat domain 36 | 1 | 1.775 | −0.874 | −0.841 | −0.857 | hs|2q11.2 | 4.23E‐05 |
| 1063 | CENPF | Centromere protein F, 350/400 kDa | 1 | 2.699 | −0.717 | −0.360 | −0.539 | hs|1q41 | 7.01E‐05 |
| 64151 | NCAPG | Non‐SMC condensin I complex, subunit G | 1 | 2.746 | −1.624 | −0.840 | −1.232 | hs|4p15.31 | 7.27E‐05 |
| 29121 | CLEC2D | C‐type lectin domain family 2, member D | 1 | 2.558 | −1.014 | −1.455 | −1.235 | hs|12p13.31 | 9.14E‐05 |
| 9672 | SDC3 | Syndecan 3 | 1 | 2.432 | −0.894 | −0.977 | −0.936 | hs|1p35.2 | 0.000271 |
| 10509 | SEMA4B | Sema domain, immunoglobulin domain (Ig), transmembrane domain (TM) and short cytoplasmic domain, (semaphorin) 4B | 1 | 2.298 | −0.692 | −0.934 | −0.813 | hs|15q26.1 | 0.000821 |
| 81552 | VOPP1 | Vesicular, overexpressed in cancer, prosurvival protein 1 | 1 | 1.842 | −0.406 | −1.035 | −0.720 | hs|7p11.2 | 0.004190 |
| 727936 | GXYLT2 | Glucoside xylosyltransferase 2 | 3 | 2.640 | −0.590 | −0.814 | −0.702 | hs|3p13 | 0.004620 |
| 3832 | KIF11 | Kinesin family member 11 | 1 | 2.461 | −1.241 | −1.236 | −1.238 | hs|10q23.33 | 0.004640 |
| 29028 | ATAD2 | ATPase family, AAA domain containing 2 | 1 | 2.606 | −0.844 | −0.507 | −0.676 | hs|8q24.13 | 0.006000 |
| 3090 | HIC1 | Hypermethylated in cancer 1 | 1 | 2.709 | −0.994 | −0.022 | −0.508 | hs|17p13.3 | 0.009480 |
| 51060 | TXNDC12 | Thioredoxin domain containing 12 (endoplasmic reticulum) | 1 | 1.579 | −0.564 | −0.765 | −0.665 | hs|1p32.3 | 0.009760 |
| 710 | SERPING1 | Serpin peptidase inhibitor, clade G (C1 inhibitor), member 1 | 1 | 2.015 | −0.558 | −0.536 | −0.547 | hs|11q12.1 | 0.016900 |
| 59345 | GNB4 | Guanine nucleotide‐binding protein (G protein), beta polypeptide 4 | 1 | 1.862 | −0.881 | −1.421 | −1.151 | hs|3q26.33 | 0.053200 |
| 1356 | CP | Ceruloplasmin (ferroxidase) | 1 | 15.420 | −1.753 | −1.380 | −1.566 | hs|3q24 | 0.070000 |
| 5272 | SERPINB9 | Serpin peptidase inhibitor, clade B (ovalbumin), member 9 | 1 | 1.797 | −0.462 | −1.730 | −1.096 | hs|6p25.2 | 0.078200 |
| 5046 | PCSK6 | Proprotein convertase subtilisin/kexin type 6 | 1 | 7.374 | −0.930 | −1.827 | −1.379 | hs|15q26.3 | 0.080000 |
| 586 | BCAT1 | Branched chain amino acid transaminase 1, cytosolic | 2 | 3.076 | −0.850 | −1.324 | −1.087 | hs|12p12.1 | 0.100000 |
| 54437 | SEMA5B | Sema domain, seven thrombospondin repeats (type 1 and type 1‐like), transmembrane domain (TM) and short cytoplasmic domain, (semaphorin) 5B | 1 | 7.089 | −0.706 | −2.687 | −1.696 | hs|3q21.1 | 0.109000 |
| 317 | APAF1 | Apoptotic peptidase activating factor 1 | 1 | 1.839 | −0.973 | −1.014 | −0.994 | hs|12q23.1 | 0.121000 |
| 10718 | NRG3 | Neuregulin 3 | 1 | 1.977 | −1.645 | −0.389 | −1.017 | hs|10q23.1 | 0.213000 |
| 51316 | PLAC8 | Placenta‐specific 8 | 2 | 2.750 | −0.630 | −1.962 | −1.296 | hs|4q21.22 | 0.249000 |
| 7436 | VLDLR | Very low density lipoprotein receptor | 1 | 2.186 | −0.455 | −0.817 | −0.636 | hs|9p24.2 | 0.254000 |
| 1050 | CEBPA | CCAAT/enhancer binding protein (C/EBP), alpha | 1 | 1.531 | −0.877 | −0.648 | −0.763 | hs|19q13.11 | 0.320000 |
| 64919 | BCL11B | B‐cell CLL/lymphoma 11B (zinc finger protein) | 1 | 2.484 | −0.178 | −1.121 | −0.649 | hs|14q32.2 | 0.340000 |
| 56950 | SMYD2 | SET and MYND domain containing 2 | 1 | 1.657 | −0.501 | −0.762 | −0.631 | hs|1q41 | 0.343000 |
| 11096 | ADAMTS5 | ADAM metallopeptidase with thrombospondin type 1 motif, 5 | 2 | 1.523 | −0.188 | −0.946 | −0.567 | hs|21q21.3 | 0.394000 |
| 1009 | CDH11 | Cadherin 11, type 2, OB‐cadherin (osteoblast) | 1 | 1.848 | −0.792 | −1.789 | −1.290 | hs|16q21 | 0.426000 |
| 149628 | PYHIN1 | Pyrin and HIN domain family, member 1 | 1 | 1.968 | −1.154 | −1.032 | −1.093 | hs|1q23.1 | 0.474000 |
| 27010 | TPK1 | Thiamin pyrophosphokinase 1 | 1 | 1.578 | −0.810 | −0.591 | −0.701 | hs|7q35 | 0.487000 |
| 8357 | HIST1H3H | Histone cluster 1, H3 h | 1 | 3.446 | −0.690 | −1.521 | −1.105 | hs|6p22.1 | 0.516000 |
| 4082 | MARCKS | Myristoylated alanine‐rich protein kinase C substrate | 2 | 2.769 | −1.310 | −2.252 | −1.781 | hs|6q21 | 0.528000 |
| 23468 | CBX5 | Chromobo × homolog 5 | 2 | 1.659 | −1.157 | −1.216 | −1.187 | hs|12q13.13 | 0.549000 |
| 79627 | OGFRL1 | Opioid growth factor receptor‐like 1 | 2 | 2.107 | −0.940 | −0.167 | −0.553 | hs|6q13 | 0.587000 |
| 571 | BACH1 | BTB and CNC homology 1, basic leucine zipper transcription factor 1 | 1 | 1.649 | −0.197 | −1.127 | −0.662 | hs|21q21.3 | 0.622000 |
| 23102 | TBC1D2B | TBC1 domain family, member 2B | 1 | 1.654 | −0.974 | −0.531 | −0.752 | hs|15q24.3 | 0.693000 |
| 4481 | MSR1 | Macrophage scavenger receptor 1 | 1 | 2.887 | −1.581 | −1.135 | −1.358 | hs|8p22 | 0.705000 |
| 493 | ATP2B4 | ATPase, Ca++ transporting, plasma membrane 4 | 1 | 2.282 | −1.285 | −0.928 | −1.106 | hs|1q32.1 | 0.723000 |
| 56124 | PCDHB12 | Protocadherin beta 12 | 1 | 2.095 | −0.179 | −0.844 | −0.512 | hs|5q31.3 | 0.765000 |
| 3556 | IL1RAP | Interleukin 1 receptor accessory protein | 1 | 1.775 | −0.170 | −1.024 | −0.597 | hs|3q28 | 0.774000 |
| 9201 | DCLK1 | Doublecortin‐like kinase 1 | 1 | 3.633 | −1.282 | −0.906 | −1.094 | hs|13q13.3 | 0.804000 |
| 488 | ATP2A2 | ATPase, Ca++ transporting, cardiac muscle, slow twitch 2 | 1 | 1.522 | −1.297 | −0.891 | −1.094 | hs|12q24.11 | 0.816000 |
| 9545 | RAB3D | RAB3D, member RAS oncogene family | 1 | 1.956 | −1.106 | −0.074 | −0.590 | hs|19p13.2 | 0.846000 |
| 4330 | MN1 | Meningioma (disrupted in balanced translocation) 1 | 1 | 1.682 | −0.170 | −0.855 | −0.512 | hs|22q12.1 | 0.846000 |
| 23036 | ZNF292 | Zinc finger protein 292 | 2 | 2.177 | −0.792 | −0.573 | −0.683 | hs|6q14.3 | 0.900000 |
| 9770 | RASSF2 | Ras association (RalGDS/AF‐6) domain family member 2 | 1 | 6.147 | −1.030 | −0.058 | −0.544 | hs|20p13 | 0.911000 |
| 11120 | BTN2A1 | Butyrophilin, subfamily 2, member A1 | 1 | 1.520 | −1.328 | −0.533 | −0.930 | hs|6p22.2 | 0.912000 |
| 11237 | RNF24 | Ring finger protein 24 | 1 | 1.606 | −0.831 | −0.578 | −0.704 | hs|20p13 | 0.918000 |
| 23023 | TMCC1 | Transmembrane and coiled‐coil domain family 1 | 1 | 4.679 | −0.791 | −0.349 | −0.570 | hs|3q22.1 | 0.945000 |
| 636 | BICD1 | Bicaudal D homolog 1 (Drosophila) | 1 | 2.423 | −0.557 | −0.590 | −0.574 | hs|12p11.21 | 0.955000 |
| 6424 | SFRP4 | Secreted frizzled‐related protein 4 | 1 | 1.786 | −1.625 | −1.025 | −1.325 | hs|7p14.1 | 0.980000 |
| 54769 | DIRAS2 | DIRAS family, GTP‐binding RAS‐like 2 | 3 | 6.202 | −0.204 | −2.678 | −1.441 | hs|9q22.2 | 0.001190a |
| 196 | AHR | Aryl hydrocarbon receptor | 1 | 1.745 | −0.816 | −1.261 | −1.039 | hs|7p21.1 | 0.011700a |
| 283 | ANG | Angiogenin, ribonuclease, RNase A family, 5 | 2 | 1.617 | −0.554 | −0.906 | −0.730 | hs|14q11.2 | 0.015300a |
| 8490 | RGS5 | Regulator of G protein signaling 5 | 1 | 4.721 | −0.938 | −0.705 | −0.821 | hs|1q23.3 | 0.031700a |
| 54941 | RNF125 | Ring finger protein 125, E3 ubiquitin protein ligase | 1 | 1.527 | −0.789 | −0.321 | −0.555 | hs|18q12.1 | 0.038200a |
| 80854 | SETD7 | SET domain containing (lysine methyltransferase) 7 | 1 | 2.225 | −0.662 | −0.854 | −0.758 | hs|4q31.1 | 0.045900a |
| 81575 | APOLD1 | Apolipoprotein L domain containing 1 | 1 | 3.953 | −1.531 | −1.101 | −1.316 | hs|12p13.1 | 2.21E‐06a |
| 143872 | ARHGAP42 | Rho GTPase activating protein 42 | 1 | 2.075 | −1.289 | −1.614 | −1.452 | hs|11q22.1 | 4.85E‐05a |
| 642273 | FAM110C | Family with sequence similarity 110, member C | 1 | 2.149 | −1.376 | −0.041 | −0.708 | hs|2p25.3 | 5.9E‐06a |
| 375287 | RBM43 | RNA binding motif protein 43 | 1 | 1.630 | −0.377 | −0.994 | −0.685 | hs|2q23.3 | 6.29E‐05a |
| 4601 | MXI1 | MAX interactor 1, dimerization protein | 1 | 1.987 | −1.649 | −0.513 | −1.081 | hs|10q25.2 | 9.79E‐05a |
Poor prognosis with low gene expression.
GEO, Gene Expression Omnibus; OS, overall survival; TCGA, The Cancer Genome Atlas.
Table 3.
miR‐144‐3p candidate target genes in renal cell carcinoma
| Entrez gene ID | Gene symbol | Gene name | Conserved site count | Poorly conserved site count | GEO expression data fold change (tumor/normal) | A498 miR‐144‐3p transfection (Log2 ratio) | 786‐O miR‐144‐3p transfection (Log2 ratio) | Average A498/786‐O miR‐144‐3p transfection (Log2 ratio) | Cytoband | TCGA data for OS (high vs low expression: P‐value) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5373 | PMM2 | Phosphomannomutase 2 | 1 | 0 | 1.580 | −1.617 | −1.020 | −1.319 | hs|16p13.2 | 2.18E‐07 |
| 55165 | CEP55 | Centrosomal protein 55 kDa | 1 | 1 | 4.202 | −1.743 | −1.130 | −1.437 | hs|10q23.33 | 6.94E‐07 |
| 79733 | E2F8 | E2F transcription factor 8 | 1 | 0 | 4.133 | −0.537 | −0.722 | −0.630 | hs|11p15.1 | 0.00145 |
| 9134 | CCNE2 | Cyclin E2 | 1 | 0 | 2.430 | −0.591 | −1.823 | −1.207 | hs|8q22.1 | 0.00664 |
| 23657 | SLC7A11 | Solute carrier family 7 (anionic amino acid transporter light chain, xc‐ system), member 11 | 1 | 5 | 2.678 | −0.418 | −1.195 | −0.806 | hs|4q28.3 | 0.02340 |
| 1462 | VCAN | Versican | 1 | 1 | 5.753 | −0.695 | −0.883 | −0.789 | hs|5q14.3 | 0.04670 |
| 2335 | FN1 | Fibronectin 1 | 1 | 1 | 5.453 | −1.470 | −0.105 | −0.787 | hs|2q35 | 0.07790 |
| 5738 | PTGFRN | Prostaglandin F2 receptor inhibitor | 1 | 0 | 2.242 | −0.565 | −0.981 | −0.773 | hs|1p13.1 | 0.08260 |
| 57561 | ARRDC3 | Arrestin domain containing 3 | 1 | 2 | 1.705 | −0.381 | −0.940 | −0.660 | hs|5q14.3 | 0.11100 |
| 11116 | FGFR1OP | FGFR1 oncogene partner | 1 | 1 | 1.551 | −0.499 | −0.881 | −0.690 | hs|6q27 | 0.17000 |
| 7436 | VLDLR | Very low density lipoprotein receptor | 1 | 2 | 2.186 | −0.455 | −0.817 | −0.636 | hs|9p24.2 | 0.25400 |
| 1050 | CEBPA | CCAAT/enhancer binding protein (C/EBP), alpha | 1 | 0 | 1.531 | −0.877 | −0.648 | −0.763 | hs|19q13.11 | 0.32000 |
| 4154 | MBNL1 | Muscleblind‐like splicing regulator 1 | 3 | 0 | 1.743 | −0.610 | −0.947 | −0.779 | hs|3q25.2 | 0.32100 |
| 64919 | BCL11B | B‐cell CLL/lymphoma 11B (zinc finger protein) | 1 | 0 | 2.484 | −0.178 | −1.121 | −0.649 | hs|14q32.2 | 0.34000 |
| 11096 | ADAMTS5 | ADAM metallopeptidase with thrombospondin type 1 motif, 5 | 1 | 2 | 1.523 | −0.188 | −0.946 | −0.567 | hs|21q21.3 | 0.39400 |
| 1009 | CDH11 | Cadherin 11, type 2, OB‐cadherin (osteoblast) | 1 | 0 | 1.848 | −0.792 | −1.789 | −1.290 | hs|16q21 | 0.42600 |
| 3796 | KIF2A | Kinesin heavy chain member 2A | 1 | 2 | 2.008 | −0.922 | −1.005 | −0.963 | hs|5q12.1 | 0.44500 |
| 55205 | ZNF532 | Zinc finger protein 532 | 1 | 0 | 1.899 | −0.790 | −1.560 | −1.175 | hs|18q21.32 | 0.50400 |
| 4082 | MARCKS | Myristoylated alanine‐rich protein kinase C substrate | 1 | 1 | 2.769 | −1.310 | −2.252 | −1.781 | hs|6q21 | 0.52800 |
| 79627 | OGFRL1 | Opioid growth factor receptor‐like 1 | 1 | 2 | 2.107 | −0.940 | −0.167 | −0.553 | hs|6q13 | 0.58700 |
| 22795 | NID2 | Nidogen 2 (osteonidogen) | 1 | 0 | 1.527 | −0.935 | −0.208 | −0.571 | hs|14q22.1 | 0.62800 |
| 2200 | FBN1 | Fibrillin 1 | 2 | 0 | 2.173 | −0.605 | −1.049 | −0.827 | hs|15q21.1 | 0.63000 |
| 10957 | PNRC1 | Proline‐rich nuclear receptor coactivator 1 | 1 | 0 | 1.724 | −0.640 | −0.875 | −0.757 | hs|6q15 | 0.72000 |
| 79365 | BHLHE41 | Basic helix‐loop‐helix family, member e41 | 1 | 2 | 9.461 | −0.947 | −0.568 | −0.758 | hs|12p12.1 | 0.89500 |
| 23036 | ZNF292 | Zinc finger protein 292 | 1 | 1 | 2.177 | −0.792 | −0.573 | −0.683 | hs|6q14.3 | 0.90000 |
| 23023 | TMCC1 | Transmembrane and coiled‐coil domain family 1 | 1 | 0 | 4.679 | −0.791 | −0.349 | −0.570 | hs|3q22.1 | 0.94500 |
| 4131 | MAP1B | Microtubule‐associated protein 1B | 1 | 0 | 2.795 | −0.448 | −0.880 | −0.664 | hs|5q13.2 | 0.99300 |
| 8445 | DYRK2 | Dual‐specificity tyrosine‐(Y)‐phosphorylation regulated kinase 2 | 2 | 0 | 1.729 | −0.494 | −0.518 | −0.506 | hs|12q15 | 0.000356a |
| 1003 | CDH5 | Cadherin 5, type 2 (vascular endothelium) | 1 | 0 | 2.616 | −0.439 | −0.643 | −0.541 | hs|16q21 | 0.00935a |
| 23097 | CDK19 | Cyclin‐dependent kinase 19 | 1 | 2 | 2.174 | −0.145 | −0.891 | −0.518 | hs|6q21 | 0.01660a |
| 2908 | NR3C1 | Nuclear receptor subfamily 3, group C, member 1 (glucocorticoid receptor) | 1 | 0 | 2.111 | −0.667 | −1.105 | −0.886 | hs|5q31.3 | 0.01780a |
| 54492 | NEURL1B | Neuralized E3 ubiquitin protein ligase 1B | 1 | 0 | 2.907 | −0.637 | −0.810 | −0.723 | hs|5q35.1 | 0.03430a |
| 54941 | RNF125 | Ring finger protein 125, E3 ubiquitin protein ligase | 1 | 1 | 1.527 | −0.789 | −0.321 | −0.555 | hs|18q12.1 | 0.03820a |
| 114800 | CCDC85A | Coiled‐coil domain containing 85A | 1 | 0 | 2.334 | −1.409 | −0.189 | −0.799 | hs|2p16.1 | 3.69E‐05a |
Poor prognosis with low gene expression.
GEO, Gene Expression Omnibus; OS, overall survival; TCGA, The Cancer Genome Atlas.
Figure 2.
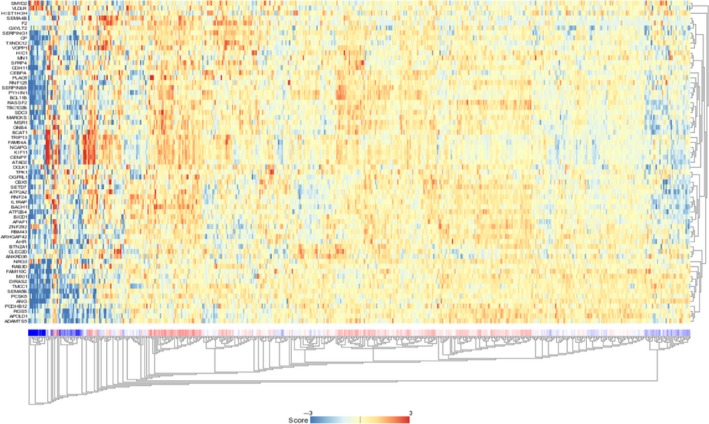
Heat map showing the expression of 65 genes targeted by miR‐144‐5p
Figure 3.
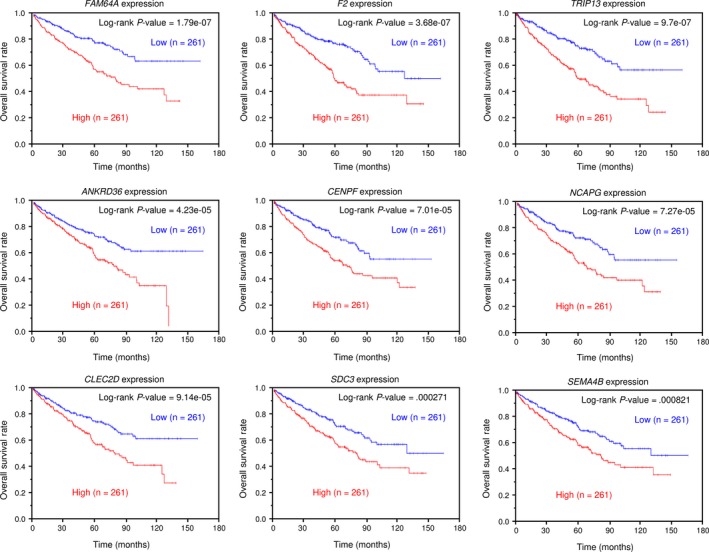
The Cancer Genome Atlas database analysis of putative targets of miR‐144‐5p in renal cell carcinoma. Kaplan‐Meier plots of overall survival with log‐rank tests for 9 genes regulated by miR‐144‐5p with high and low gene expression from The Cancer Genome Atlas database
Figure 4.
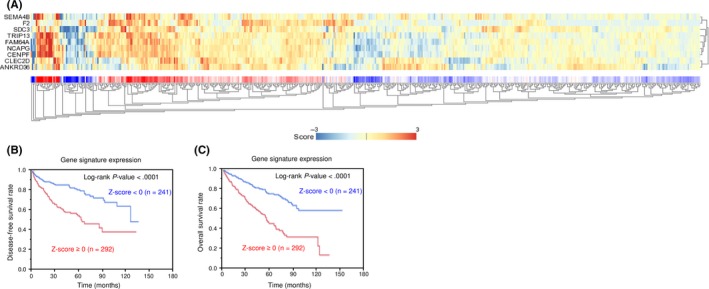
Heat map showing gene expression and Kaplan‐Meier analysis of 9 candidate genes in renal cell carcinoma. A, Heat map visualization of 9 candidate genes. B, Kaplan‐Meier analysis of disease‐free survival of patients with high gene signature expression and those with a low gene signature expression. C, Kaplan‐Meier analysis of overall survival of patients with high gene signature expression and those with a low gene signature expression
3.5. Direct regulation of SDC3 by miR‐144‐5p in RCC cells
We asked whether the expression of the SDC3 gene and SDC3 protein decreased in miR‐144‐5p‐transfected RCC cells. As shown in Figure 5A,B, both mRNA and protein levels were significantly decreased by miR‐144‐5p transfection compared with the mock, miR‐control, or miR‐144‐3p transfectants.
Figure 5.
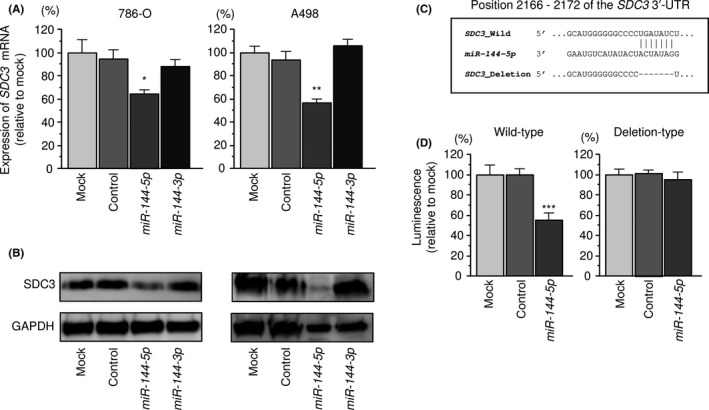
Regulation of SDC3 expression by miR‐144‐5p in renal cell carcinoma cells. A, Expression levels of SDC3 mRNA 48 hours after transfection with 10 nmol/L miR‐144‐5p or miR‐144‐3p into cell lines. GAPDH was used as an internal control. *P < .0001. B, Protein expression of syndecan‐3 (SDC3) 72 hours after transfection with miR‐144‐5p or miR‐144‐3p. GAPDH was used as a loading control. C, miR‐144‐5p binding sites in the 3′‐UTR of SDC3 mRNA. D, Dual‐luciferase reporter assays using vectors encoding putative miR‐144‐5p target sites (positions 2166‐2172) in the SDC3 3′‐UTR for both wild‐type and deletion‐type. Normalized data were calculated as the ratio of Renilla/firefly luciferase activities. *P < .005; **P < .001; ***P < .05
Next, luciferase reporter assays with a vector that included the 3′‐UTR of SDC3 were undertaken to confirm that miR‐144‐5p directly regulated SDC3 in a sequence‐dependent manner. The TargetScanHuman database predicted that there was a binding site for miR‐144‐5p in the 3′‐UTR of SDC3 (position 2166‐2172; Figure 5C). Cotransfection with miR‐144‐5p and vectors significantly decreased luciferase activity in comparison with those in mock and miR‐control transfectants (Figure 5D).
3.6. Effects of silencing SDC3 on cell proliferation, migration, and invasion in RCC cells
We confirmed that the expression levels of SDC3 mRNA and SDC3 protein were decreased by si‐SDC3 in RCC cells (Figure 6A,B). Furthermore, we investigated the effects of silencing SDC3 on cell proliferation, migration, and invasion in RCC cells. Cancer aggressiveness was significantly inhibited in si‐SDC3 transfectants in comparison with that in mock‐ or miR‐control‐transfected cell lines (Figure 6C‐E).
Figure 6.
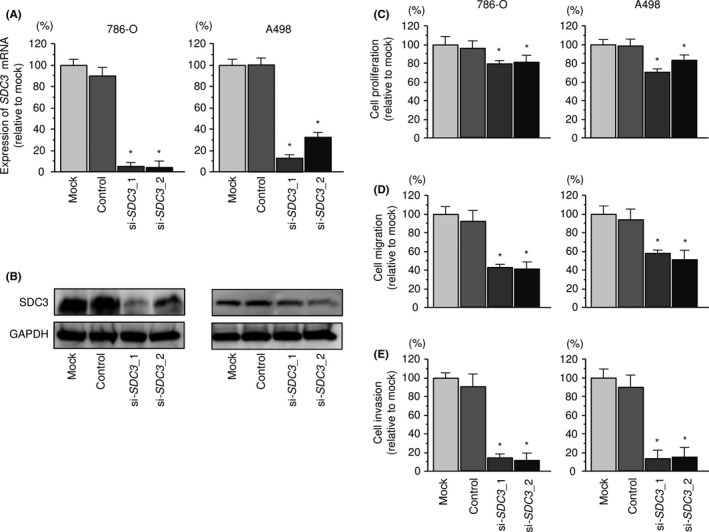
Effects of silencing SDC3 in renal cell carcinoma cell lines. A, SDC3 mRNA expression 72 hours after transfection with 10 nmol/L si‐SDC3_1 or si‐SDC3_2 into renal cell carcinoma cell lines. GAPDH was used as an internal control. B, Syndecan‐3 (SDC3) protein expression 72 hours after transfection with si‐SDC3_1 or si‐SDC3_2. GAPDH was used as a loading control. C, Cell proliferation was determined with XTT assays 72 hours after transfection with 10 nmol/L si‐SDC3_1 or si‐SDC3_2. D, Cell migration activity was determined by migration assays. E, Cell invasion activity was determined using Matrigel invasion assays. *P < .0001
3.7. Expression of SDC3 in RCC clinical specimens
We examined the mRNA expression levels of SDC3 in 17 RCC clinical specimens using qRT‐PCR. The mRNA expression levels of SDC3 were significantly upregulated in cancer tissues compared with those in adjacent noncancerous tissues (Figure 7A). Spearman's rank test revealed a negative correlation between the expression of SDC3 and miR‐144‐5p (P = .0409, R = −0.356, Figure 7B). Next, we investigated the expression levels of SDC3 in RCC clinical specimens by immunostaining. It was found that SDC3 was strongly overexpressed in several cancer lesions compared with that in adjacent noncancerous lesions with the same staining intensity (Figure 7C).
Figure 7.
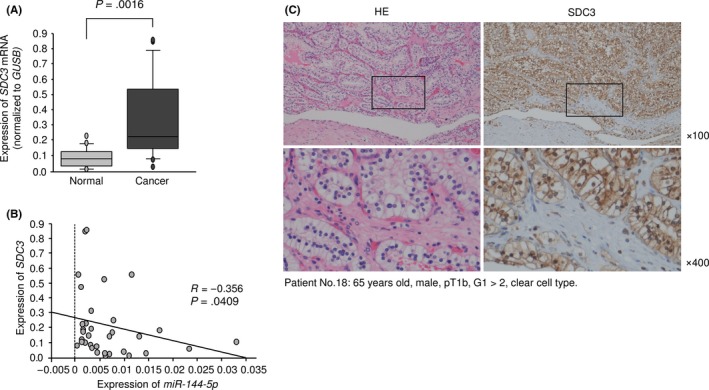
Expression of SDC3 in clinical specimens of renal cell carcinoma. A, Expression levels of SDC3 in RCC clinical specimens. GUSB was used as an internal control. B, Spearman's rank test showed the negative correlation between SDC3 expression and miR‐144‐5p. C, Immunostaining showed that SDC3 was strongly expressed in cancer lesions (100× and 400× magnification field)
3.8. Downstream genes affected by silencing of SDC3 in RCC cells
Finally, we undertook a genomewide gene expression analysis using si‐SDC3‐treated 786‐O cells to investigate which genes were modulated by SDC3. A SurePrint G3 Human GE 60K v3 microarray (Agilent Technologies) was used for genomewide expression analysis. We focused on genes that were significantly downregulated by transfection of both si‐SDC3_1 and si‐SDC3_2 (log2 [average‐si‐SDC3/mock] < −1.0). SDC3 was the most significantly downregulated gene, indicating that the array data were worthy of evaluation. We identified 26 candidate genes (Table 4), from which a gene expression heat map was constructed (Figure 8A). In the heat map, we focused on a gene cluster including SDC3 (IL18RAP, SDC3, SH2D1A, GZMH, KIF21B, TMC8, GAB3, HLA‐DPB2, PLEK, and C1Qb) (Figure 8B). Furthermore, patients with high gene signature expression (Figure 8B, red square) were significantly associated with a lower overall survival rate than those with low gene signature expression (Figure 8B, blue square) (P = 0.0064, Figure 8C). Furthermore, high expression of 7 genes (SDC3, PLXDC1, IL18RAP, GZMH, ATP8B3, TBX15, and TMC8) was significantly associated with poor prognosis of RCC patients by TCGA datasets (Figure S3).
Table 4.
Candidate downstream genes of SDC3 in renal cell carcinoma cells
| Gene symbol | Gene name | Log2 (si‐SDC3_1/mock) | Log2 (si‐SDC3_2/mock) | Average Log2 (si‐SDC3/mock) | GEO expression data fold change (tumor/normal) | Cytoband | TCGA data OS (P‐value) |
|---|---|---|---|---|---|---|---|
| SDC3 | Syndecan 3 | −2.319 | −2.821 | −2.570 | 2.432 | hs|1p35.2 | 0.000271 |
| GAB3 | GRB2‐associated binding protein 3 | −1.599 | −1.879 | −1.739 | 2.467 | hs|Xq28 | 0.200000 |
| PLXDC1 | Plexin domain containing 1 | −0.481 | −2.365 | −1.423 | 3.144 | hs|17q12 | 0.001860 |
| SH2D1A | SH2 domain containing 1A | −1.092 | −1.692 | −1.392 | 2.214 | hs|Xq25 | 0.133000 |
| SFMBT2 | Scm‐like with four mbt domains 2 | −1.240 | −1.434 | −1.337 | 2.189 | hs|10p14 | 0.009770a |
| NFATC2 | Nuclear factor of activated T cells, cytoplasmic, calcineurin‐dependent 2 | −1.036 | −1.624 | −1.330 | 2.259 | hs|20q13.2 | 0.002260a |
| KIF21B | Kinesin family member 21B | −1.385 | −1.231 | −1.308 | 2.701 | hs|1q32.1 | 0.148000 |
| NLGN1 | Neuroligin 1 | −0.971 | −1.518 | −1.244 | 2.423 | hs|3q26.31 | 0.039100a |
| PREX2 | Phosphatidylinositol‐3,4,5‐trisphosphate‐dependent Rac exchange factor 2 | −1.088 | −1.390 | −1.239 | 2.213 | hs|8q13.2 | 0.069000 |
| CALHM2 | Calcium homeostasis modulator 2 | −1.858 | −0.617 | −1.237 | 2.940 | hs|10q24.33 | 0.135000 |
| IL18RAP | Interleukin 18 receptor accessory protein | −0.431 | −1.976 | −1.203 | 3.967 | hs|2q12.1 | 0.001070 |
| PLEK | Pleckstrin | −1.275 | −1.123 | −1.199 | 3.395 | hs|2p13.3 | 0.121000 |
| PECAM1 | Platelet/endothelial cell adhesion molecule 1 | −0.465 | −1.931 | −1.198 | 2.831 | hs|17q23.3 | 0.036500a |
| ZNF660 | Zinc finger protein 660 | −0.452 | −1.913 | −1.183 | 2.274 | hs|3p21.31 | 0.155000 |
| ELTD1 | EGF, latrophilin, and seven transmembrane domain containing 1 | −0.634 | −1.612 | −1.123 | 2.297 | hs|1p31.1 | No data |
| KCNJ8 | Potassium channel, inwardly rectifying subfamily J, member 8 | −0.465 | −1.720 | −1.093 | 2.002 | hs|12p12.1 | 0.495000 |
| ITGA4 | Integrin, alpha 4 (antigen CD49D, alpha 4 subunit of VLA‐4 receptor) | −0.369 | −1.788 | −1.079 | 2.336 | hs|2q31.3 | 0.573000 |
| GZMH | Granzyme H (cathepsin G‐like 2, protein h‐CCPX) | −0.273 | −1.882 | −1.077 | 5.323 | hs|14q12 | 0.012900 |
| ATP8B3 | ATPase, aminophospholipid transporter, class I, type 8B, member 3 | −0.470 | −1.647 | −1.059 | 2.941 | hs|19p13.3 | 7.35E‐07 |
| ZG16B | Zymogen granule protein 16B | −1.156 | −0.955 | −1.056 | 2.080 | hs|16p13.3 | 0.596000 |
| HLA‐DPB2 | Major histocompatibility complex, class II, DP beta 2 (pseudogene) | −0.988 | −1.111 | −1.050 | 3.123 | hs|6p21.32 | 0.968000 |
| TBX15 | T‐box 15 | −0.442 | −1.631 | −1.036 | 4.119 | hs|1p12 | 0.001930 |
| C1QB | Complement component 1, q subcomponent, B chain | −1.363 | −0.661 | −1.012 | 6.547 | hs|1p36.12 | 0.070700 |
| TMC8 | Transmembrane channel‐like 8 | −0.651 | −1.370 | −1.011 | 2.786 | hs|17q25.3 | 0.001460 |
| SLITRK5 | SLIT and NTRK‐like family, member 5 | −1.372 | −0.636 | −1.004 | 5.478 | hs|13q31.2 | 0.016200a |
| HECW2 | HECT, C2, and WW domain containing E3 ubiquitin protein ligase 2 | −0.984 | −1.017 | −1.000 | 2.663 | hs|2q32.3 | 0.000152a |
Poor prognosis with low expression.
GEO, Gene Expression Omnibus; OS, overall survival; TCGA, The Cancer Genome Atlas.
Figure 8.
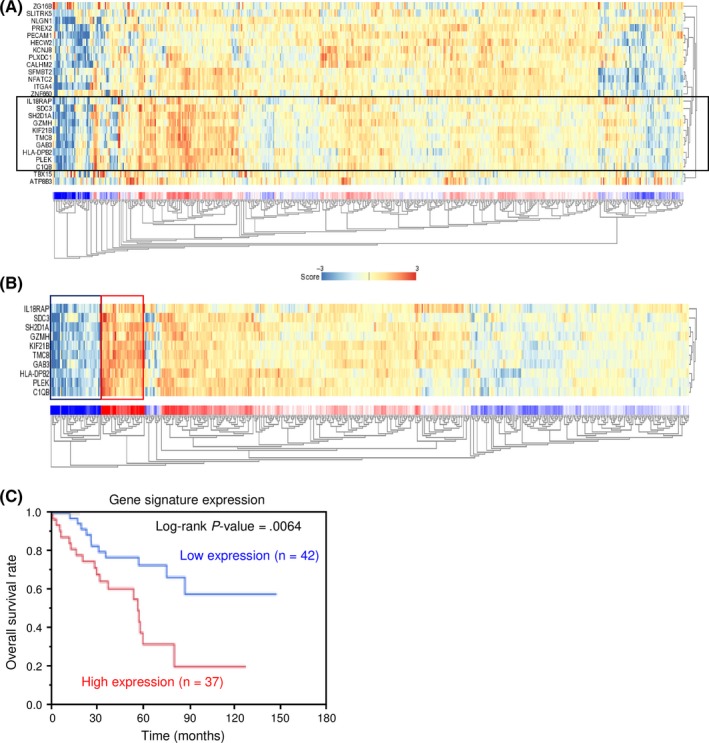
Heat map showing gene expression and Kaplan‐Meier analysis in renal cell carcinoma cells. A, Heat map visualization of candidate genes downstream from SDC3. B, Heat map visualization of a gene signature including SDC3 (black square). C, Kaplan‐Meier analysis of overall survival of patients with high gene signature expression (red square) and those with a low gene signature expression (blue square)
3.9. Analysis of pre‐miR‐144 and the SDC family in RCC pathogenesis and clinical outcome from TCGA database
Figure 9A shows that patients with high expression of SDC3 had shorter disease‐free survival. Furthermore, high expression of SDC3 was significantly associated with advanced tumor stage and high pathological grade (Figure 9B‐F).
Figure 9.
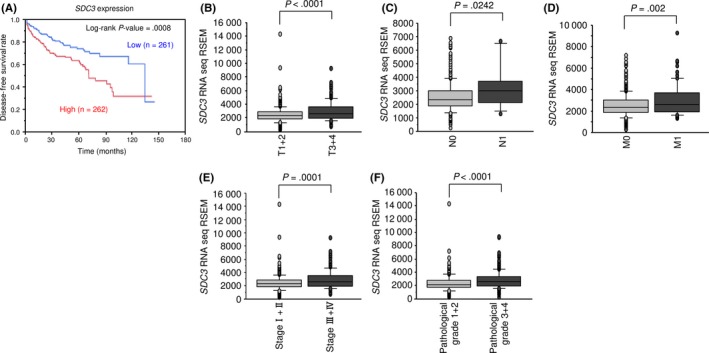
The Cancer Genome Atlas database analysis of SDC3 in renal cell carcinoma. A, Patients with high SDC3 expression had shorter disease‐free survival than those with low expression. B‐F, High SDC3 expression was significantly associated with advanced tumor stage and pathological grade
Conversely, low expression levels of miR‐144‐5p and miR‐144‐3p were significantly associated with shorter disease‐free survival and advanced tumor stage (Figure S4).
The univariate and multivariate Cox proportional hazards model showed that high expression of SDC3 was an independent predictive factor for survival (hazard ratio, 1.77; 95% confidence interval, 1.07‐2.97; P = 0.0249), as were well‐known clinical prognostic factors such as T stage, M stage, and hemoglobin level (Table 5).
Table 5.
Univariable and multivariable Cox hazard regression models for overall survival in renal cell carcinoma
| Variable | Group | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | ||
| SDC3 expression | High/low | 1.73 | 1.28‐2.36 | 0.0003 | 1.77 | 1.07‐2.97 | 0.0249 |
| Age, years | ≥60/<60 | 1.84 | 1.35‐2.54 | 0.0001 | 1.51 | 0.91‐2.57 | 0.1131 |
| Gender | Male/female | 0.97 | 0.72‐1.34 | 0.8684 | – | – | – |
| T stage | 3 + 4/1 + 2 | 3.05 | 2.26‐4.14 | <0.0001 | 2.94 | 1.05‐10.44 | 0.0381 |
| N stage | Positive/negative | 3.07 | 1.49‐5.65 | 0.0038 | 0.66 | 0.19‐1.95 | 0.4708 |
| M stage | Positive/negative | 4.27 | 3.11‐5.82 | <0.0001 | 5.11 | 2.57‐10.07 | <0.0001 |
| Stage | III + IV/I + II | 3.72 | 2.72‐5.13 | <0.0001 | 0.55 | 0.14‐1.82 | 0.3423 |
| Histological grade | G3 + 4/G1 + 2 | 2.59 | 1.86‐3.68 | <0.0001 | 1.06 | 0.62‐1.86 | 0.8232 |
| Serum Ca level | High/normal | 4.38 | 2.06‐8.18 | 0.0005 | 0.74 | 0.19‐2.33 | 0.6173 |
| Serum Hb level | Low/normal | 2.13 | 1.52‐3.05 | <0.0001 | 1.67 | 1.00‐2.89 | 0.0488 |
–, not included in analysis. Ca, calcium; CI, confidence interval; Hb, hemoglobin; HR, hazard ratio.
In further analyses, we investigated the relationships between other genes in the syndecan family (SDC1, SDC2, and SDC4) and RCC pathogenesis. Interestingly, no other SDC family gene had a significant relationship between its expression and patient prognosis, tumor stage, or pathological grade in RCC (Figure S5).
3.10. Effect of cotransfection of SDC3/miR‐144‐5p in 786‐O cells
In order to investigate whether the SDC3/miR‐144‐5p axis is essential for RCC pathogenesis, we applied rescue studies in 786‐O cells. Our present studies showed that cell proliferation, migration, and invasive abilities were recovered by cotransfection of SDC3 expression vector and miR‐455‐5p mature miRNA compared to miR‐144‐5p transfection alone (Figure 10). These findings suggested that overexpression of SDC3 contributed to aggressiveness of RCC cells.
Figure 10.
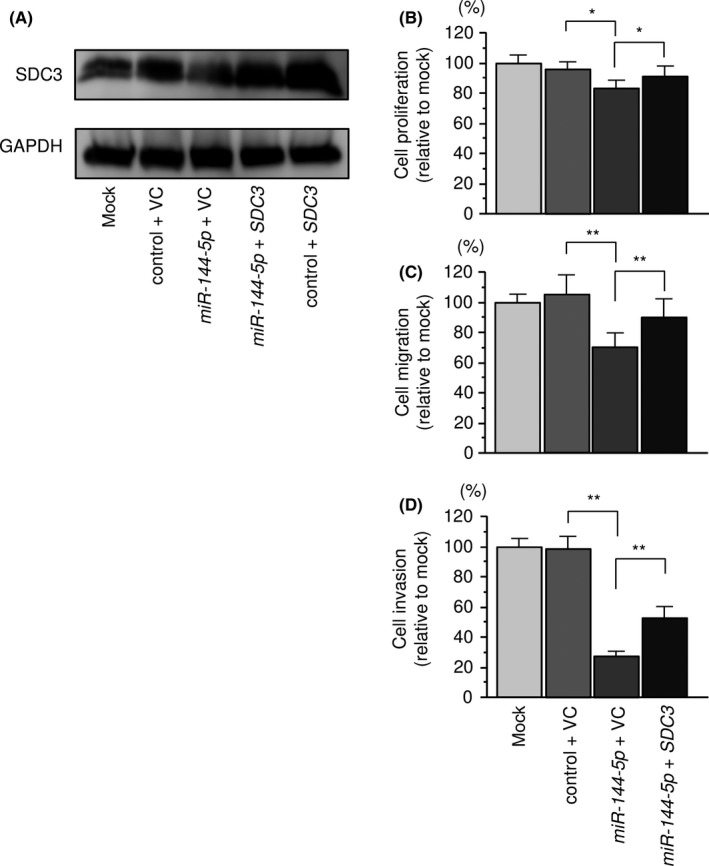
Effects of cotransfection of SDC3/miR‐144‐5p into 786‐O cells. A, Syndecan‐3 (SDC3) protein expression was evaluated by Western blot analysis of 786‐O cells. The rescue studies were evaluated 48 hours after reverse transfection with miR‐144‐5p and 24 hours after forward transfection with the SDC3 vector. GAPDH was used as a loading control. B, Cell proliferation was determined using XTT assays 72 hours after reverse transfection with miR‐144‐5p and 48 hours after forward transfection with the SDC3 vector. C, Cell migration activity was assessed by wound healing assays 48 hours after reverse transfection with miR‐144‐5p and 24 hours after forward transfection with the SDC3 vector. D, Cell invasive activity was evaluated by invasion assays 48 hours after reverse transfection with miR‐144‐5p and 24 hours after forward transfection with SDC3 vector. *P < .005, **P < .0001. VC, vector control
A schema summarizing these results of the study is shown in Figure S6.
4. DISCUSSION
The general understanding of miRNA biogenesis posits that only guide strands of miRNAs (derived from the miRNA duplex) are incorporated into the RISC and actually modulate target RNA transcripts.25 Passenger strands of miRNAs are also thought to undergo degradation, becoming nonfunctional.26 Contrary to this point of view, our miRNA signatures showed that some miRNA passenger strands were aberrantly expressed in several cancer tissues.15, 17 Our previous studies revealed that miR‐145‐3p (the passenger strand of the miR‐145 duplex) was significantly reduced in clinical specimens of prostate cancer as well as head and neck squamous cell carcinoma. Moreover, ectopic expression of miR‐145‐3p blocked cancer cell aggressiveness, suggesting that the passenger strand of the miR‐145 duplex acts as an antitumor miRNA, as does miR‐145‐5p (the guide strand).15, 16 Moreover, miR‐145‐3p was incorporated into the RISC and targeted several oncogenes (eg MELK, NCAPG, BUB1, CDK1, and MYO1B) in cancer cells.15, 16 Importantly, these miR‐145‐3p targets were deeply involved in cancer pathogenesis. For example, high expression of MELK, NCAPG, BUB1, and CDK1 significantly predicted survival in patients with prostate cancer.15
Some miRNAs are distributed in clusters on human chromosomes.27 Analyses of our miRNA signature of RCC based on RNA sequencing showed that miR‐451a was significantly downregulated in cancer tissues and it had antitumor functions.13 In the human genome, miR‐451a, miR‐451b, miR‐4732, miR‐144‐5p, and miR‐144‐3p form a miRNA cluster at 17q11.2. Among these miRNAs, low expression of miR‐451a, miR‐144‐5p, and miR‐144‐3p predicted poor prognosis of patients with RCC according to TCGA database analyses. Our data showed that both strands of miR‐144‐5p and miR‐144‐3p had antitumor functions in RCC cells. Many studies have reported that miR‐144‐3p acted as an antitumor miRNA in several types of cancers.28, 29 In contrast to recent analyses of miR‐144‐3p, few papers have examined the function of miR‐144‐5p in cancer cells. We previously showed that miR‐144‐5p had tumor‐suppressive functions through its targeting of CCNE1 and CCNE2 in bladder cancer.18 It is very interesting that members of this miRNA cluster at 17q11.2 have cancer‐suppressing effects. These results suggest that the anticancer effects of this miRNA cluster should be closely examined in many cancers.
In miRNA‐based cancer research, elucidation of target genes and RNA networks controlled by aberrantly expressed miRNAs is an important approach to better understanding the development and progression of tumors. In this study, we identified 65 putative targets of miR‐144‐5p regulation in RCC cells. Among these targets, high expression of 9 genes (FAM64A, F2, TRIP13, ANKRD36, CENPF, NCAPG, CLEC2D, SDC3, and SEMA4B) significantly predicted poor survival in patients with RCC (P < .001), suggesting they might be good prognostic markers. Among them, coagulation factor 2 (F2), which was overexpressed in advanced RCC, is related to tumor progression in several types of cancers.30 Furthermore, centromere protein F (CENPF) was previously reported to be regulated by antitumor miR‐205 and involved in prostate cancer pathogenesis.31 Non‐SMC condensin I complex, subunit G (NCAPG) was also directly regulated by miR‐145‐3p and associated with tumor development in prostate cancer.15
In the present study, we focused on SDC3 as a crucial oncogene directly regulated by miR‐144‐5p in RCC cells. The syndecan protein family consists of four transmembrane proteoglycans in mammals (SDC1‐4). In carcinogenesis, syndecans, integrins, and growth factor receptors interact and play important roles in cell signaling. They appear to be involved in both cancer initiation and progression.32 Although they are similar in molecular structure, it has been reported that their expression and biological roles in cancer cells are different. Relatively little is known about SDC3, whereas SDC1, SDC2, and SDC4 have been shown to possess oncogenic functions in several types of cancers.33, 34, 35 SDC3 is primarily expressed in nerve tissue and developed musculoskeletal tissues. Overexpression of the gene might be involved in perineural invasion and shorter survival in pancreatic cancer.32 SDC3 and perlecan were particularly strongly expressed in tumor stromal vessels, indicating that these heparan sulfate proteoglycans play pivotal roles in tumor angiogenesis.32 Furthermore, the SDC3‐mediated signaling pathway might lead to prostate cancer cell migration, invasion, and metastasis.32 These findings indicate that SDC3 expression could be associated with RCC progression.
Furthermore, we identified a gene signature of SDC3 downstream and its expressions were significantly related to cancer aggressiveness. Among 26 downstream genes, several genes have already reported roles in RCC pathogenesis. ITGA4 promoted cancer cell metastasis and the kinesin family was related to cell proliferation, invasion, and migration in RCC.36, 37 Interestingly, high expression of 7 genes (SDC3, PLXDC1, IL18RAP, GZMH, ATP8B3, TBX15, and TMC8) significantly predicted poor prognosis of RCC patients according to TCGA datasets. PLXDC1 (also known as TEM7) was initially cloned as a high expression protein from vascular endothelium of human cancer.38 Several studies showed that its expression contributed to angiogenesis.39, 40 In gastric cancer, aberrant expression of PLXDC1 enhanced cancer cell migration and invasive abilities.41 TBX15 is a member of the T‐box family of transcription factors; dysregulated expression of some TBX members is involved in human disease and carcinogenesis.42 In thyroid cancer cells, expression of TBX15 induced Bcl2 and Bcl‐XL (anti‐apoptotic proteins) expression and its overexpression played a role of anti‐apoptosis.43 These studies showed that SDC3 and its regulatory network have potential to be therapeutic targets of RCC. Further analysis of SDC3 could contribute to the development of novel therapeutic strategies for RCC.44
In conclusion, we showed that the expression of both miR‐144‐5p and miR‐144‐3p was significantly downregulated in RCC tissues and that they functioned as tumor suppressors in RCC cells. We found that SDC3 was directly regulated by miR‐144‐5p and that it is a significant gene in RCC pathogenesis. Overexpression of SDC3 was involved in the pathogenesis of RCC and acted as an oncogene. The antitumor functionality of the passenger strand of miRNA is a new concept in cancer research. Searching for RNA networks controlled by passenger strands of miRNA is a new challenge in studies of RCC pathogenesis.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
The present study was supported by KAKENHI grants 16K20125, 17K11160, 16H05462, and 15K10801.
Yamada Y, Arai T, Kojima S, et al. Regulation of antitumor miR‐144‐5p targets oncogenes: Direct regulation of syndecan‐3 and its clinical significance. Cancer Sci. 2018;109:2919–2936. 10.1111/cas.13722
Funding Information
Japan Society for the Promotion of Science KAKENHI grants 16K20125, 17K11160, 16H05462, and 15K10801
REFERENCES
- 1. Capitanio U, Montorsi F. Renal cancer. Lancet. 2016;387(10021):894‐906. [DOI] [PubMed] [Google Scholar]
- 2. Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60(4):615‐621. [DOI] [PubMed] [Google Scholar]
- 3. Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34(3):193‐205. [DOI] [PubMed] [Google Scholar]
- 4. Figlin R, Sternberg C, Wood CG. Novel agents and approaches for advanced renal cell carcinoma. J Urol. 2012;188(3):707‐715. [DOI] [PubMed] [Google Scholar]
- 5. Goto Y, Kurozumi A, Enokida H, Ichikawa T, Seki N. Functional significance of aberrantly expressed microRNAs in prostate cancer. Int J Urol. 2015;22(3):242‐252. [DOI] [PubMed] [Google Scholar]
- 6. Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20(8):460‐469. [DOI] [PubMed] [Google Scholar]
- 7. Kurozumi A, Goto Y, Okato A, Ichikawa T, Seki N. Aberrantly expressed microRNAs in bladder cancer and renal cell carcinoma. J Hum Genet. 2017;62(1):49‐56. [DOI] [PubMed] [Google Scholar]
- 8. Koshizuka K, Hanazawa T, Fukumoto I, Kikkawa N, Okamoto Y, Seki N. The microRNA signatures: aberrantly expressed microRNAs in head and neck squamous cell carcinoma. J Hum Genet. 2017;62(1):3‐13. [DOI] [PubMed] [Google Scholar]
- 9. Arai T, Okato A, Kojima S, et al. Regulation of spindle and kinetochore‐associated protein 1 by antitumor miR‐10a‐5p in renal cell carcinoma. Cancer Sci. 2017;108(10):2088‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nishikawa R, Goto Y, Kojima S, et al. Tumor‐suppressive microRNA‐29s inhibit cancer cell migration and invasion via targeting LAMC1 in prostate cancer. Int J Oncol. 2014;45(1):401‐410. [DOI] [PubMed] [Google Scholar]
- 11. Goto Y, Kurozumi A, Nohata N, et al. The microRNA signature of patients with sunitinib failure: regulation of UHRF1 pathways by microRNA‐101 in renal cell carcinoma. Oncotarget. 2016;7(37):59070‐59086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okato A, Arai T, Yamada Y, et al. Dual strands of Pre‐miR‐149 inhibit cancer cell migration and invasion through targeting FOXM1 in renal cell carcinoma. Int J Mol Sci. 2017;18(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamada Y, Arai T, Sugawara S, et al. Impact of novel oncogenic pathways regulated by anti‐tumor miR‐451a in renal cell carcinoma. Cancer Sci. 2018;109(4):1239‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yonemori M, Seki N, Yoshino H, et al. Dual tumor‐suppressors miR‐139‐5p and miR‐139‐3p targeting matrix metalloprotease 11 in bladder cancer. Cancer Sci. 2016;107(9):1233‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goto Y, Kurozumi A, Arai T, et al. Impact of novel miR‐145‐3p regulatory networks on survival in patients with castration‐resistant prostate cancer. Br J Cancer. 2017;117(3):409‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamada Y, Koshizuka K, Hanazawa T, et al. Passenger strand of miR‐145‐3p acts as a tumor‐suppressor by targeting MYO1B in head and neck squamous cell carcinoma. Int J Oncol. 2018;52(1):166‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koshizuka K, Nohata N, Hanazawa T, et al. Deep sequencing‐based microRNA expression signatures in head and neck squamous cell carcinoma: dual strands of pre‐miR‐150 as antitumor miRNAs. Oncotarget. 2017;8(18):30288‐30304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsushita R, Seki N, Chiyomaru T, et al. Tumour‐suppressive microRNA‐144‐5p directly targets CCNE1/2 as potential prognostic markers in bladder cancer. Br J Cancer. 2015;113(2):282‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koshizuka K, Hanazawa T, Kikkawa N, et al. Regulation of ITGA3 by the anti‐tumor miR‐199 family inhibits cancer cell migration and invasion in head and neck cancer. Cancer Sci. 2017;108(8):1681‐1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishikawa R, Chiyomaru T, Enokida H, et al. Tumour‐suppressive microRNA‐29s directly regulate LOXL2 expression and inhibit cancer cell migration and invasion in renal cell carcinoma. FEBS Lett. 2015;589(16):2136‐2145. [DOI] [PubMed] [Google Scholar]
- 21. R2: genomics analysis and visualization platform. http://r2.amc.nl. Accessed March 9, 2018.
- 22. J A. OncoLnc: linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput Sci. 2016;2:e67. [Google Scholar]
- 23. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123(4):631‐640. [DOI] [PubMed] [Google Scholar]
- 26. Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger‐strand cleavage facilitates assembly of siRNA into Ago2‐containing RNAi enzyme complexes. Cell. 2005;123(4):607‐620. [DOI] [PubMed] [Google Scholar]
- 27. Chhabra R, Dubey R, Saini N. Cooperative and individualistic functions of the microRNAs in the miR‐23a~27a~24‐2 cluster and its implication in human diseases. Mol Cancer. 2010;9:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao Z, Liu R, Liao J, et al. Possible tumor suppressive role of the miR‐144/451 cluster in esophageal carcinoma as determined by principal component regression analysis. Mol Med Rep. 2016;14(4):3805‐3813. [DOI] [PubMed] [Google Scholar]
- 29. Zhang SY, Lu ZM, Lin YF, et al. miR‐144‐3p, a tumor suppressive microRNA targeting ETS‐1 in laryngeal squamous cell carcinoma. Oncotarget. 2016;7(10):11637‐11650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. John A, Gorzelanny C, Bauer AT, Schneider SW, Bolenz C. Role of the coagulation system in genitourinary cancers: review. Clin Genitourin Cancer. 2017;16(1):e29‐e39. [DOI] [PubMed] [Google Scholar]
- 31. Nishikawa R, Goto Y, Kurozumi A, et al. MicroRNA‐205 inhibits cancer cell migration and invasion via modulation of centromere protein F regulating pathways in prostate cancer. Int J Urol. 2015;22(9):867‐877. [DOI] [PubMed] [Google Scholar]
- 32. Afratis NA, Nikitovic D, Multhaupt HA, Theocharis AD, Couchman JR, Karamanos NK. Syndecans ‐ key regulators of cell signaling and biological functions. FEBS J. 2017;284(1):27‐41. [DOI] [PubMed] [Google Scholar]
- 33. Szatmari T, Otvos R, Hjerpe A, Dobra K. Syndecan‐1 in cancer: implications for cell signaling, differentiation, and prognostication. Dis Markers. 2015;2015:796052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kousidou O, Berdiaki A, Kletsas D, et al. Estradiol‐estrogen receptor: a key interplay of the expression of syndecan‐2 and metalloproteinase‐9 in breast cancer cells. Mol Oncol. 2008;2(3):223‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saoncella S, Echtermeyer F, Denhez F, et al. Syndecan‐4 signals cooperatively with integrins in a Rho‐dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci USA. 1999;96(6):2805‐2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Markovic‐Lipkovski J, Brasanac D, Muller GA, Muller CA. Cadherins and integrins in renal cell carcinoma: an immunohistochemical study. Tumori. 2001;87(3):173‐178. [DOI] [PubMed] [Google Scholar]
- 37. Li G, Chong T, Yang J, Li H, Chen H. Kinesin motor protein KIFC1 is a target protein of miR‐338‐3p and associated with poor prognosis and progression of renal cell carcinoma. Oncol Res. 2018; 10.3727/096504018X15213115046567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. St Croix B, Rago C, Velculescu V, et al. Genes expressed in human tumor endothelium. Science. 2000;289(5482):1197‐1202. [DOI] [PubMed] [Google Scholar]
- 39. Yamaji Y, Yoshida S, Ishikawa K, et al. TEM7 (PLXDC1) in neovascular endothelial cells of fibrovascular membranes from patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49(7):3151‐3157. [DOI] [PubMed] [Google Scholar]
- 40. Bagley RG, Rouleau C, Weber W, et al. Tumor endothelial marker 7 (TEM‐7): a novel target for antiangiogenic therapy. Microvasc Res. 2011;82(3):253‐262. [DOI] [PubMed] [Google Scholar]
- 41. Zhang ZZ, Hua R, Zhang JF, et al. TEM7 (PLXDC1), a key prognostic predictor for resectable gastric cancer, promotes cancer cell migration and invasion. Am J Cancer Res. 2015;5(2):772‐781. [PMC free article] [PubMed] [Google Scholar]
- 42. Peres J, Davis E, Mowla S, et al. The highly homologous T‐Box transcription factors, TBX2 and TBX3, have distinct roles in the oncogenic process. Genes Cancer. 2010;1(3):272‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arribas J, Gimenez E, Marcos R, Velazquez A. Novel antiapoptotic effect of TBX15: overexpression of TBX15 reduces apoptosis in cancer cells. Apoptosis. 2015;20(10):1338‐1346. [DOI] [PubMed] [Google Scholar]
- 44. Rhee J, Hoff PM. Angiogenesis inhibitors in the treatment of cancer. Expert Opin Pharmacother. 2005;6(10):1701‐1711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


