Although the stability of β-lactam antibiotics is a known issue, none of the previously reported bioanalytical methods had an adequate evaluation of the stability of these drugs. In the current study, the stability of cefepime, meropenem, piperacillin, and tazobactam under various conditions was comprehensively evaluated.
KEYWORDS: LC-MS/MS, β-lactams, human plasma, stability, validation
ABSTRACT
Although the stability of β-lactam antibiotics is a known issue, none of the previously reported bioanalytical methods had an adequate evaluation of the stability of these drugs. In the current study, the stability of cefepime, meropenem, piperacillin, and tazobactam under various conditions was comprehensively evaluated. The evaluated parameters included stock solution stability, short-term stability, long-term stability, freeze-thaw stability, processed sample stability, and whole-blood stability. When stored at −20°C, the stock solution of meropenem in methanol was stable for up to 3 weeks, and the stock solutions of cefepime, piperacillin, and tazobactam were stable for up to 6 weeks. All four antibiotics were stable in human plasma for up to 3 months when stored at −80°C and were stable in whole blood for up to 4 h at room temperature. Short-term stability results indicated that all four β-lactams were stable at room temperature for 2 h, but substantial degradation was observed when the plasma samples were stored at room temperature for 24 h, with the degradation rates for cefepime, meropenem, piperacillin, and tazobactam being 30.1%, 75.6%, 49.0%, and 37.7%, respectively. Because the stability information is method independent, our stability results can be used as a reference by other research groups that work with these antibiotics.
INTRODUCTION
β-Lactam antibiotics are the mainstay for the treatment of bacterial infections in critically ill patients. It has been reported that the pharmacokinetics (PK) of β-lactam antibiotics is highly variable and unpredictable, which increases the potential for suboptimal antibiotic concentrations and provides a significant challenge to clinicians in ensuring appropriate antibiotic doses (1). Emerging evidence suggests that poor antibiotic exposure represents a potential cause of inadequate clinical outcomes. The time above the MIC (T>MIC) is one of the most important parameters that is associated with the efficacy of β-lactam antibiotics. Knowledge of the antibiotic plasma concentrations and the bacterial susceptibility, evaluated in terms of MIC, may enable improvement in treatment efficacy. Therefore, it is very important to monitor plasma concentrations of β-lactam antibiotics in critically ill patients. Although therapeutic drug monitoring (TDM) has not been set as a standard practice for β-lactam antibiotics, several studies have shown the clinical utility of this methodology in critically ill patients (2, 3).
Accompanying the growing interest in β-lactam TDM, there has been an increasing effort to develop a reliable and rapid method for the simultaneous measurement of multiple β-lactam antibiotics to ensure quick decisions on dose adjustments in critically ill patients, who often receive several antibiotics concomitantly (4–10). In line with this concept, we developed a rapid and reproducible liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the simultaneous measurement of cefepime, meropenem, piperacillin, and tazobactam in human plasma. It has been reported that the most frequently used β-lactam classes in intensive care units (ICUs) are penicillins, cephalosporins, and carbapenems (11). The β-lactam antibiotics included in our method (cefepime, meropenem, and piperacillin) cover these three β-lactam classes and represent the most frequently used antibiotics of their respective antibiotic classes. Compared to other high-performance liquid chromatography (HPLC) and LC-MS/MS methods available in the literature, our method is more robust because of a combination of the following features: (i) a simple sample extraction procedure, (ii) a short sample run time, (iii) a wide linearity range, and (iv) the small plasma sample volume needed. Another valuable part of this work is the comprehensive assay validation that we performed, including a thorough evaluation of stability. Although the stability of β-lactam antibiotics is a known issue, surprisingly, none of the reported HPLC and LC-MS/MS methods have been evaluated adequately for drug stability. Extremely limited information is available regarding the stability of these antibiotics in whole blood (whole-blood stability), their short-term and long-term stabilities, and their stock solution stability (4, 6, 8, 9, 12–16). Since this stability information is critical for ensuring the reliability and accuracy of β-lactam concentration data, it is highly important to acquire details about these stability-related validation parameters.
Among the 17 validation parameters that we evaluated, 6 are related to stability, including stock solution stability, short-term stability, long-term stability, freeze-thaw stability, processed sample stability, and whole-blood stability. As most of the stability data are method independent, our results can be used as a reference by other research groups, even if a different method is employed. Because the stability data for β-lactam antibiotics are highly useful, instead of burying these data in a general assay development and validation manuscript, we chose to highlight the stability results in the present, stand-alone article (part 2 of our work). The LC-MS/MS method development and non-stability-related assay validation work is presented in a separate article (17).
RESULTS
Stock solution stability.
Stock solution stability was tested after 3 and 6 weeks for each of the four antibiotics. Stock solutions of cefepime, piperacillin, and tazobactam were found to be stable for up to 6 weeks. On comparing the mean area ratio for 50-μg/ml replicates prepared using the 6-week-old stock solution and the mean area ratio for replicates prepared using fresh stock solution, the difference for cefepime was 3.9%, and the coefficient of variation percentage (CV %) for both stocks was less than 15%. For piperacillin and tazobactam, the differences were −8.1% and −2.1%, respectively. The stock solution for meropenem was found to be stable for only up to 3 weeks, with the differences for a 3-week stock and a 6-week stock versus a freshly prepared stock solution being −8.3% and −18.4%, respectively.
Short-term stability.
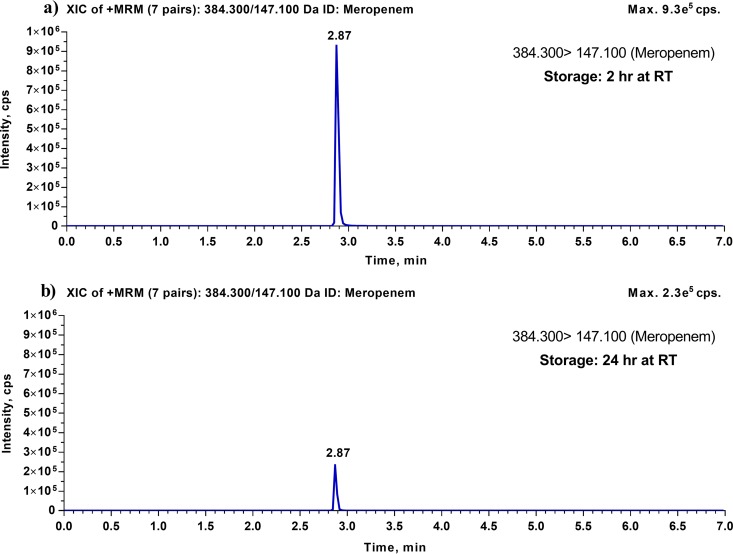
For each analyte, we evaluated short-term stability in human plasma under the following conditions: at room temperature (RT) for 2 h, on ice for 2 h, at RT for 24 h, and in the refrigerator (∼4°C) for 24 h. Table 1 summarizes the results of the short-term stability studies of cefepime, meropenem, piperacillin, and tazobactam. These results indicate that cefepime, meropenem, piperacillin, and tazobactam are all stable in human plasma for 2 h, both at RT and on ice. When these four compounds were evaluated for 24 h stability at 4°C, cefepime, piperacillin, and tazobactam were stable, with accuracy and precision limits meeting the acceptance criteria of ±15.0%. However, meropenem was unstable after 24 h at 4°C, with an average degradation of 25.9%. On the other hand, after 24 h at RT, all four antibiotics were found to be unstable, with average degradation ranging from 30.1% for cefepime to 75.6% for meropenem. Figure 1 shows representative chromatograms for the meropenem short-term stability test after 2 h at RT and 24 h at RT.
TABLE 1.
Short-term stability of cefepime, meropenem, piperacillin, and tazobactam in human plasma for 2 h and 24 ha
| Antibiotic | QC level (concn [μg/ml]) | 2 h |
24 h |
||||||
|---|---|---|---|---|---|---|---|---|---|
| RT |
On ice |
RT |
4°C |
||||||
| Bias (%) | CV (%) | Bias (%) | CV (%) | Bias (%) | CV (%) | Bias (%) | CV (%) | ||
| Cefepime | Low (1.50) | 12.1 | 5.0 | 14.4 | 5.5 | −26.8 | 13.9 | −7.8 | 13.9 |
| High (120) | −5.3 | 2.5 | 7.5 | 3.7 | −33.4 | 10.1 | −8.9 | 9.1 | |
| Meropenem | Low (0.300) | 1.8 | 4.1 | 9.3 | 7.2 | −75.4 | 10.5 | −26.4 | 8.3 |
| High (120) | −3.8 | 3.7 | −5.0 | 3.6 | −75.8 | 7.4 | −25.4 | 3.9 | |
| Piperacillin | Low (0.300) | −7.8 | 7.3 | −2.6 | 6.3 | −45.7 | 7.8 | 4.4 | 5.6 |
| High (120) | −3.7 | 10.9 | −3.7 | 5.1 | −52.3 | 4.5 | −6.3 | 5.1 | |
| Tazobactam | Low (0.750) | −4.2 | 3.0 | −0.4 | 7.0 | −36.6 | 4.2 | 6.8 | 9.0 |
| High (120) | 9.3 | 4.0 | 11.9 | 4.6 | −38.7 | 6.8 | −5.6 | 6.3 | |
n = 6 for each QC level.
FIG 1.
Representative LC-MS/MS chromatograms for meropenem from its short-term stability test. (a) Typical chromatogram for human plasma with lithium heparin, spiked with meropenem (QC high), after storage at RT for 2 h. (b) Typical chromatogram for human plasma with lithium heparin, spiked with meropenem (QC high), after storage at RT for 24 h.
Long-term stability.
The long-term stability of cefepime, meropenem, piperacillin, and tazobactam in human plasma was evaluated after 1 and 3 months of storage at −80°C. The results are presented in Table 2 and show that all four analytes were stable in human plasma for up to 3 months of storage at −80°C.
TABLE 2.
Long-term stability of cefepime, meropenem, piperacillin, and tazobactam in human plasma for 1 and 3 monthsa
| Antibiotic | QC level (concn [μg/ml]) | 1 mo |
3 mo |
||
|---|---|---|---|---|---|
| Bias (%) | CV (%) | Bias (%) | CV (%) | ||
| Cefepime | Low (1.50) | −7.1 | 6.9 | 4.4 | 4.4 |
| High (120) | −3.5 | 4.2 | 8.3 | 9.2 | |
| Meropenem | Low (0.300) | −14.1 | 10.0 | 13.3 | 6.3 |
| High (120) | −5.7 | 5.6 | −0.3 | 2.2 | |
| Piperacillin | Low (0.300) | 11.3 | 7.2 | −1.3 | 9.2 |
| High (120) | −14.4 | 7.9 | 5.7 | 6.7 | |
| Tazobactam | Low (0.750) | 8.3 | 2.9 | −3.6 | 8.7 |
| High (120) | −3.9 | 5.9 | 9.3 | 4.3 | |
n = 6 for each QC level.
Freeze-thaw stability.
The influence of the physical processes of freezing and thawing on analyte stability in human plasma was evaluated by analyzing quality control (QC) plasma samples with low and high concentrations of each antibiotic (QC low and QC high) that were subjected to three freeze-thaw cycles prior to analysis, with six replicates at each QC level.
Table 3 summarizes the freeze-thaw stability results and confirms that cefepime, meropenem, piperacillin, and tazobactam were stable in human plasma after three freeze-thaw cycles. Precision and accuracy for all four analytes were within the acceptance limits of ±15.0%.
TABLE 3.
Freeze-thaw stability, reinjection reproducibility, and autosampler stability of cefepime, meropenem, piperacillin, and tazobactam in human plasmaa
| Antibiotic | QC level (concn [μg/ml]) | Freeze-thaw stability |
Reinjection reproducibility |
Autosampler stability |
|||
|---|---|---|---|---|---|---|---|
| Bias (%) | CV (%) | Bias (%) | CV (%) | Bias (%) | CV (%) | ||
| Cefepime | Low (1.50) | −2.6 | 7.2 | −1.7 | 6.8 | 7.0c | 6.8c |
| High (120) | −5.3 | 6.7 | −5.8 | 2.7 | 4.2c | 5.0c | |
| Meropenem | Low (0.300) | 10.5b | 9.4b | 2.4 | 10.1 | 10.6 | 11.1 |
| High (120) | −8.8 | 2.2 | −14.1 | 3.0 | 0.6 | 3.3 | |
| Piperacillin | Low (0.300) | −4.9 | 7.7 | 5.8b | 5.9b | 5.3b | 6.4b |
| High (120) | 1.7 | 3.0 | −4.0 | 3.9 | 6.5 | 4.1 | |
| Tazobactam | Low (0.750) | 9.1 | 14.0 | 14.6 | 3.6 | −7.0 | 9.8 |
| High (120) | 14.3 | 11.4 | −0.1 | 4.9 | −12.9 | 6.5 | |
n = 6 for each QC level, unless noted otherwise.
n = 5 (an outlier was excluded).
Cefepime autosampler stability after 12 h.
Reinjection reproducibility.
An analyte batch consisting of calibration standards, QC low, and QC high samples was subjected to storage at 4°C for 24 h and then reinjected. Precision and accuracy were within the limits of acceptance for cefepime, meropenem, piperacillin, and tazobactam and demonstrated the reinjectability of samples within 24 h. These data are presented in Table 3.
Autosampler stability.
Autosampler stability, also known as processed sample stability or extract stability, was evaluated by storing QC samples at two levels, QC low and QC high, in the autosampler at 4°C for 24 h and then analyzing them with freshly prepared calibration standards. The results are summarized in Table 3 and show that meropenem, piperacillin, and tazobactam in processed samples were stable for up to 24 h in the autosampler. However, processed cefepime samples did not meet the acceptance criteria if they were kept at 4°C for 24 h, and the average percent accuracy was 72.5% above nominal concentration values. On further investigation, it was found that the internal standard, 2H6-meropenem, degraded on storage for 24 h, resulting in a decreased peak area for the IS, and hence in higher IS-normalized peak area ratios for cefepime. As a result, the calculated cefepime concentrations for these samples were much higher than those for fresh QC samples. Therefore, in order to determine the autosampler stability for cefepime, processed QC samples were analyzed after 3, 6, 9, and 12 h of storage in the autosampler. Cefepime was found to be stable in processed samples for up to 12 h in the autosampler at 4°C, and these data are presented in Table 3.
Whole-blood stability.
Whole-blood stability was tested at 15 min, 1 h, 2 h, and 4 h for cefepime, meropenem, piperacillin, and tazobactam. The 4 h whole-blood stability samples met the acceptance criteria for all four drugs, with mean stability values within ±15.0% of the IS-normalized peak area ratio for the 15-min (T0) sample. The results in Table 4 show that cefepime, meropenem, piperacillin, and tazobactam are stable in whole blood for up to 4 h. Red blood cell (RBC)-to-plasma distribution ratios for cefepime, meropenem, piperacillin, and tazobactam were determined to be 0.954, 0.507, 0.306, and 0.347, respectively.
TABLE 4.
Whole-blood stability and RBC-to-plasma distribution ratios for cefepime, meropenem, piperacillin, and tazobactama
| Antibiotic | 15 min (T0) |
1 h (T1) |
2 h (T2) |
4 h (T3) |
RBC-to-plasma ratio | |||
|---|---|---|---|---|---|---|---|---|
| CV (%) | Difference from T0 (%) | CV (%) | Difference from T0 (%) | CV (%) | Difference from T0 (%) | CV (%) | ||
| Cefepime | 7.1 | 2.1 | 4.6 | −3.7 | 6.6 | −1.2 | 11.3 | 0.954 |
| Meropenem | 5.7 | 0.5 | 2.2 | −6.7 | 5.8 | −10.5 | 4.0 | 0.507 |
| Piperacillin | 3.9 | −0.1 | 3.8 | −0.2 | 5.2 | −1.2 | 3.5 | 0.306 |
| Tazobactam | 3.6 | 1.2 | 2.0 | 0.7 | 2.6 | 1.3 | 2.8 | 0.347 |
A concentration of 5 μg/ml was used for each antibiotic, and n = 6 for each antibiotic.
DISCUSSION
In the current study, we performed a comprehensive evaluation of the stability of cefepime, meropenem, piperacillin, and tazobactam under various conditions, using different matrices (methanol, human plasma, and human whole blood), different temperatures (RT, 4°C, −20°C, and −80°C), and different times. As noted earlier, this stability information is method independent and therefore can be used as a reference by any other research group that works with these antibiotics. We observed that when it was stored at −20°C, a stock solution of meropenem in methanol was stable for up to 3 weeks, and stock solutions of cefepime, piperacillin, and tazobactam were stable for up to 6 weeks. Our long-term stability results showed that all four antibiotics were stable for up to 3 months if their plasma samples were stored at −80°C. In addition, cefepime, meropenem, piperacillin, and tazobactam were found to be stable in human plasma after three freeze-thaw cycles. These results are consistent with those of previously reported stability studies. Three consecutive freeze-thaw cycles resulted in no degradation of cefepime, meropenem, piperacillin, and tazobactam (9, 16).
Regarding the processed sample stability data, the results for cefepime and meropenem should be interpreted carefully; this is particularly true for bioanalytical laboratories that use different internal standards. Based on our results, although the cefepime 24 h autosampler stability at 4°C did not pass the acceptance criteria, cefepime by itself in processed samples did not have a stability issue. The degradation of the internal standard, 2H6-meropenem, in the processed samples led to the failed cefepime 24 h autosampler stability test. On the other hand, meropenem's 24 h autosampler stability at 4°C passed the acceptance criteria because 2H6-meropenem, the internal standard, degraded in the processed sample in the same way and therefore masked the unstable behavior of the unlabeled meropenem. If a different and more stable internal standard was used for meropenem, meropenem in processed samples very likely would not be stable for up to 24 h. This is consistent with previous studies in which meropenem was found to be stable in the autosampler for at least 12 h at 4°C (12, 15). However, it contrasts with a recent report which concluded that meropenem in processed samples in the autosampler at 20°C was stable for up to 48 h (14). From our stability studies, meropenem was found to be the most unstable among the tested compounds, and hence, we have reason to believe that this reported result should be used with caution.
The stability of β-lactam antibiotics is a known issue (9, 14), and this is clearly reflected by our short-term stability data. Substantial degradation was observed for all four antibiotics when their plasma samples were stored at RT for 24 h. During a 24 h period at RT, cefepime, meropenem, piperacillin, and tazobactam degraded by 30.1%, 75.6%, 49.0%, and 37.7%, respectively. We also evaluated the 24 h stability of these four antibiotics in human plasma at 4°C and found that, except for meropenem, which degraded by more than 25%, all other antibiotics were stable under these storage conditions. These short-term stability data are very beneficial and may be used as important references when questions (e.g., “Can leftover blood samples of β-lactam antibiotics from clinical laboratory tests be used for TDM?”) are raised. For critically ill patients who undergo multiple phlebotomies for clinical laboratory testing, using salvaged blood samples for β-lactam antibiotic TDM could avoid additional blood draws and therefore has been advocated as a potential PK sample collection strategy. However, based on our short-term stability data, using leftover blood samples potentially may lead to inaccurate drug concentration data. As an example, at the University of Iowa Hospitals & Clinics, clinical laboratories usually store blood samples in the refrigerator for 3 days (in case the samples need to be reanalyzed) before discarding them. This means that the β-lactam antibiotics in these salvaged blood samples, once obtained, may have already undergone significant degradation. Therefore, extreme caution is advised for using salvaged blood samples to measure the concentrations of β-lactam antibiotics.
Although whole-blood stability is not a required assay validation parameter according to the FDA guidance, the information may be very vital, since it provides a time window regarding the separation of plasma from whole blood. In our study, we evaluated the stability of cefepime, meropenem, piperacillin, and tazobactam in whole blood at various time points, and our results showed that all four antibiotics are stable at RT for up to 4 h. In general, for a PK sample, once the blood is drawn, the samples are usually processed within 30 min to separate plasma. However, this may not be practical in situations where blood samples require some transit time or when the research team is not available immediately. These whole-blood stability data may be very useful and can also be used as an important reference by other research teams.
Conclusions.
In conclusion, we obtained stability data for cefepime, meropenem, piperacillin, and tazobactam under various conditions. All four antibiotics were stable in human plasma for up to 3 months at −80°C, in whole blood for up to 4 h at RT, and for at least 2 h in human plasma at RT and on ice. Among the four antibiotics evaluated, meropenem was the least stable one. None of the four antibiotics were stable at RT for 24 h, with meropenem being degraded the most. A similar trend was also observed for stock solution stability, as meropenem was stable for only up to 3 weeks, while the other three antibiotics were stable for up to 6 weeks. The short-term stability data indicated that using salvaged blood samples for measurement of β-lactam antibiotics from clinical laboratory tests for TDM may not be appropriate.
MATERIALS AND METHODS
Chemicals and reagents.
For the evaluation of stability, cefepime, meropenem, piperacillin sodium salt, and tazobactam were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). The deuterated internal standards, 2H5-piperacillin sodium salt and 2H6-meropenem, were purchased from Alsachim Lab (Strasbourg, France). Formic acid (>99.5%), water, acetonitrile, and methanol (each LC-MS grade) were bought from Fisher Scientific (Fairlawn, NJ, USA). Human plasma with lithium heparin was purchased from BioreclamationIVT (Westbury, NY, USA). Whole blood with lithium heparin was obtained from a healthy blood donor at the University of Iowa Hospitals & Clinics. All chemicals were of the highest purity available from commercial providers.
Chromatographic and mass spectrometric conditions.
Chromatographic analysis was carried out using a Shimadzu UFLC-20AD system (Shimadzu, Japan), which includes an LC-20AD binary pump, a SIL-20AC UFLC autosampler, a CTO-20A column oven, and a DGU-20A3 degasser. Mass spectrometric detection was carried out using a triple-quadrupole API4000 mass spectrometer (AB Sciex LLC, Redwood City, CA, USA) operated in positive-ion mode with a TurboIonSpray probe. The instrument control and data processing software Analyst 1.6.2 (AB Sciex LLC, Redwood City, CA, USA) was used for data acquisition and processing.
Analytical separation of cefepime, meropenem, piperacillin, tazobactam, 2H6-meropenem, and 2H5-piperacillin was carried out using a Phenomenex Kinetex C18 100 Å LC column (50 mm × 2.1 mm × 2.6 μm) coupled with a Phenomenex SecurityGuard Ultra UPLC Evo C18 cartridge (both from Phenomenex, Torrance, CA, USA) under gradient elution conditions with a total flow rate of 0.25 ml/min. The mobile phase used for analysis was a mixture of solvent A (water with 0.1% formic acid) and solvent B (acetonitrile with 0.1% formic acid). The total run time was 7.0 min, and gradient elution was performed as follows: 2% solvent B (0 to 0.3 min), 40% solvent B (0.3 to 2.8 min), and a hold at 2% solvent B until the end of the run.
The samples were analyzed in multiple-reaction-monitoring (MRM) mode. The following mass transitions were used to monitor cefepime, meropenem, piperacillin, and tazobactam: m/z 481.400 > 396.100, 384.300 > 147.100, 540.300 > 398.100, and 301.400 > 168.100, respectively. For the internal standards, the following mass transitions were used to monitor 2H6-meropenem and 2H5-piperacillin: m/z 390.33 > 147.100 and 545.300 > 398.100, respectively.
Detailed MS parameters that gave the best sensitivities for detecting the analytes of interest are specified in part 1 of our work, entitled “Quantification of Cefepime, Meropenem, Piperacillin, and Tazobactam in Human Plasma by Using a Sensitive and Robust Liquid Chromatography-Tandem Mass Spectrometry Method, Part 1: Assay Development and Validation” (17).
Preparation of calibration standards and quality control samples.
Stock solutions of antibiotics and internal standards (2H5-meropenem and 2H6-piperacillin) were prepared in methanol to final concentrations of 2 mg/ml and 1 mg/ml, respectively, and were stored at −20°C. Working calibrator and quality control (QC) solutions for cefepime, meropenem, piperacillin, and tazobactam were prepared by appropriate serial dilution in methanol. Linearity concentrations ranged from 0.5 μg/ml to 150 μg/ml for cefepime, from 0.1 μg/ml to 150 μg/ml for meropenem and piperacillin, and from 0.25 μg/ml to 150 μg/ml for tazobactam. Calibration standards and QC samples at two levels, QC low and QC high, for all four antibiotics were prepared by spiking blank plasma containing lithium heparin with the appropriate working solution. The QC low concentration was 1.5 μg/ml for cefepime, 0.3 μg/ml for meropenem and piperacillin, and 0.75 μg/ml for tazobactam. The QC high concentration was 120 μg/ml for all four analytes. The appropriate IS was then used to spike the calibration standards and QC samples, and the final concentration of IS in each sample was 2.5 μg/ml.
Sample preparation.
Plasma samples were prepared by spiking 90 μl of blank human plasma containing lithium heparin with 10 μl of the appropriate antibiotic working solution and 10 μl of the IS working solution. Double-blank samples were prepared by spiking 90 μl of blank human plasma with 20 μl of methanol, and blank samples were prepared by spiking blank human plasma with 10 μl of methanol and 10 μl of IS working solution. After vortex mixing, 400 μl of acetonitrile was added to each sample for protein precipitation. The samples were further vortexed for 30 s and then centrifuged at 17,000 × g for 15 min at 4°C. Following this, 10 μl of supernatant from each tube was diluted 10-fold using 90 μl of water with 0.1% formic acid (mobile phase A). Subsequently, the contents were transferred to LC-MS vials for analysis.
Method validation. (i) Stock solution stability.
Stock solutions (2 mg/ml) of cefepime, meropenem, piperacillin, and tazobactam were made in methanol and stored at −20°C. The stock solution stability of each analyte was assessed individually after 3 and 6 weeks of storage at −20°C. This was done by comparing the signal response for six replicates of 50 μg/ml made using the freshly prepared stock solution (new) with the signal response for six replicates of 50 μg/ml made using the stock solution prepared 3 or 6 weeks earlier (old). The old stock solution was considered stable if the percent difference of the mean IS-normalized peak areas between the two stock solution samples was no more than ±15.0% and the CV % was less than 15.0%. The formula for calculating the difference is as follows: % difference = [(mean IS-normalized peak area for old stock − mean IS-normalized peak area for new stock)/mean IS-normalized peak area for new stock] × 100.
(ii) Short-term stability.
To assess the short-term stability of cefepime, meropenem, piperacillin, and tazobactam in human plasma, QC samples at two concentration levels, QC low and QC high, were maintained unextracted at room temperature (RT) and on ice for 2 h and 24 h and then evaluated in six replicates against freshly prepared calibration standards. The analyte was considered stable if the mean of the obtained concentrations at each level was within ±15.0% of the nominal concentration and the % CV was no more than 15.0%.
(iii) Long-term stability.
The long-term storage stability of all four antibiotics in lithium heparin-human plasma was assessed by preparing QC low and QC high samples and maintaining them at a nominal freezer temperature of −80°C for 1 and 3 months. The QC samples were assayed in replicates of six against freshly prepared calibration standards. The antibiotics were considered stable if the accuracy values were within ±15.0% and the % CV was within 15.0%.
(iv) Freeze-thaw stability.
The stability of the four analytes in human plasma through three freeze-thaw cycles was assessed at two concentrations, QC low and QC high, with six replicates at each concentration level. QC samples stored at −80°C were subjected to three freeze-thaw cycles, and the concentrations were measured against freshly prepared calibration standards. A freeze-thaw cycle consisted of keeping the QC samples frozen for at least 12 h at −80°C and then completely thawing them at room temperature, with the first freezing period lasting at least 24 h. The analytes were considered stable if the accuracy of the mean value was within ±15.0% of the nominal concentration and the % CV was less than 15.0%.
(v) Reinjection reproducibility.
To assess the reinjection reproducibility for cefepime, meropenem, piperacillin, and tazobactam, an analyte batch containing standard curve, QC low, and QC high samples was reinjected after being kept at 4°C for 24 h after the initial injection. The analyte batch met the validation acceptance criteria if it had accuracy and precision values within 15.0% of the nominal values, except for the lower limit of quantification (LLOQ), for which the acceptance criterion was 20.0%.
(vi) Autosampler stability.
To evaluate autosampler stability, processed QC samples at two concentration levels for each antibiotic, QC low and QC high, were kept at the autosampler temperature (4°C) for 24 h, reinjected after the initial injection, and assessed against freshly prepared calibration standards. The analyte was considered stable if the mean of the obtained concentrations at each level was within ±15.0% of the nominal concentration and the % CV was no more than 15.0%.
(vii) Whole-blood stability.
The stability of cefepime, meropenem, piperacillin, and tazobactam in human whole blood was evaluated at room temperature at four different time points, 15 min and 1, 2, and 4 h, after spiking of whole blood collected in lithium heparin with the analyte of interest. Samples were prepared in human whole blood obtained from a healthy blood donor at the University of Iowa Hospitals & Clinics, with a final concentration of 5 μg/ml for each analyte. A working solution was prepared in 0.9% saline solution. To allow for adequate equilibrium and red blood cell partitioning, the tube containing spiked whole blood was gently inverted for 5 min and kept on the bench for an additional 10 min. At each predetermined time point, plasma was separated by centrifugation at approximately 1,300 × g for 5 min. The plasma samples were then assayed according to the regular protocol (n = 6 replicates at each time point). Passing criteria were defined as the mean IS-normalized peak areas for each analyte at 1, 2, and 4 h at RT being in agreement with the mean area ratio at 15 min, which means that the difference was less than 15%.
Pharmacokinetic parameters are usually determined by the analysis of drug concentrations in plasma rather than whole blood and may be misleading if drug concentrations differ between plasma and red blood cells as a consequence of differential binding to specific blood components. The red blood cell (RBC)-to-plasma ratio is an important parameter for predicting whole-body pharmacokinetics and therefore represents a drug distribution parameter of interest. It is defined as the ratio of the total drug concentration in blood cells to its total concentration in plasma at equilibrium. In order to calculate it, 150 μl of whole donor blood was transferred to a 0.5-ml Eppendorf tube, and a hematological analysis was performed using a Sysmex XE-2100 instrument (Sysmex, Kobe, Japan) to measure hematocrit content. For LC-MS/MS analysis, calibration curves were prepared for each of the four analytes. The IS-normalized peak area for the respective analyte in the reference plasma was then back calculated for an analyte concentration of 5 μg/ml. The analyte IS-normalized peak area obtained from the LC-MS/MS analysis for the donor plasma at 15 min was then used to calculate the RBC-to-plasma ratio, as follows (18): RBC-to-plasma ratio = (1/H) × (IREF.Pl/IPl − 1) + 1, where H is the hematocrit (percentage of total blood cells in donor whole-blood sample [vol/vol]), which was 40.6% for the donor blood used for this test, IREF.Pl is the IS-normalized peak area for the analyte in the reference plasma, and IPl is the IS-normalized peak area for the analyte in donor plasma.
ACKNOWLEDGMENT
This work was supported by the Division of Microbiology and Infectious Disease, National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant HHSN272200800008C).
Footnotes
For a companion article on this topic, see https://doi.org/10.1128/AAC.00859-18.
REFERENCES
- 1.Roberts JA, Lipman J. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 37:840–851. doi: 10.1097/CCM.0b013e3181961bff. [DOI] [PubMed] [Google Scholar]
- 2.Blondiaux N, Wallet F, Favory R, Onimus T, Nseir S, Courcol RJ, Durocher A, Roussel-Delvallez M. 2010. Daily serum piperacillin monitoring is advisable in critically ill patients. Int J Antimicrob Agents 35:500–503. doi: 10.1016/j.ijantimicag.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JA, Ulldemolins M, Roberts MS, McWhinney B, Ungerer J, Paterson DL, Lipman J. 2010. Therapeutic drug monitoring of beta-lactams in critically ill patients: proof of concept. Int J Antimicrob Agents 36:332–339. doi: 10.1016/j.ijantimicag.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Zander J, Maier B, Suhr A, Zoller M, Frey L, Teupser D, Vogeser M. 2015. Quantification of piperacillin, tazobactam, cefepime, meropenem, ciprofloxacin and linezolid in serum using an isotope dilution UHPLC-MS/MS method with semi-automated sample preparation. Clin Chem Lab Med 53:781–791. doi: 10.1515/cclm-2014-0746. [DOI] [PubMed] [Google Scholar]
- 5.Verdier MC, Tribut O, Tattevin P, Le Tulzo Y, Michelet C, Bentue-Ferrer D. 2011. Simultaneous determination of 12 beta-lactam antibiotics in human plasma by high-performance liquid chromatography with UV detection: application to therapeutic drug monitoring. Antimicrob Agents Chemother 55:4873–4879. doi: 10.1128/AAC.00533-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sime FB, Roberts MS, Roberts JA, Robertson TA. 2014. Simultaneous determination of seven beta-lactam antibiotics in human plasma for therapeutic drug monitoring and pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci 960:134–144. doi: 10.1016/j.jchromb.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Ohmori T, Suzuki A, Niwa T, Ushikoshi H, Shirai K, Yoshida S, Ogura S, Itoh Y. 2011. Simultaneous determination of eight beta-lactam antibiotics in human serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 879:1038–1042. doi: 10.1016/j.jchromb.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 8.McWhinney BC, Wallis SC, Hillister T, Roberts JA, Lipman J, Ungerer JP. 2010. Analysis of 12 beta-lactam antibiotics in human plasma by HPLC with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci 878:2039–2043. doi: 10.1016/j.jchromb.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Denooz R, Charlier C. 2008. Simultaneous determination of five beta-lactam antibiotics (cefepim, ceftazidim, cefuroxim, meropenem and piperacillin) in human plasma by high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci 864:161–167. doi: 10.1016/j.jchromb.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 10.Cazorla-Reyes R, Romero-Gonzalez R, Frenich AG, Rodriguez Maresca MA, Martinez Vidal JL. 2014. Simultaneous analysis of antibiotics in biological samples by ultra high performance liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 89:203–212. doi: 10.1016/j.jpba.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Meyer E, Gastmeier P, Deja M, Schwab F. 2013. Antibiotic consumption and resistance: data from Europe and Germany. Int J Med Microbiol 303:388–395. doi: 10.1016/j.ijmm.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Carlier M, Stove V, De Waele JJ, Verstraete AG. 2015. Ultrafast quantification of beta-lactam antibiotics in human plasma using UPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 978–979:89–94. doi: 10.1016/j.jchromb.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 13.Legrand T, Vodovar D, Tournier N, Khoudour N, Hulin A. 2016. Simultaneous determination of eight beta-lactam antibiotics, amoxicillin, cefazolin, cefepime, cefotaxime, ceftazidime, cloxacillin, oxacillin, and piperacillin, in human plasma by using ultra-high-performance liquid chromatography with ultraviolet detection. Antimicrob Agents Chemother 60:4734–4742. doi: 10.1128/AAC.00176-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinder N, Brenner T, Swoboda S, Weigand MA, Hoppe-Tichy T. 2017. Therapeutic drug monitoring of beta-lactam antibiotics—influence of sample stability on the analysis of piperacillin, meropenem, ceftazidime and flucloxacillin by HPLC-UV. J Pharm Biomed Anal 143:86–93. doi: 10.1016/j.jpba.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 15.Rigo-Bonnin R, Ribera A, Arbiol-Roca A, Cobo-Sacristan S, Padulles A, Murillo O, Shaw E, Granada R, Perez-Fernandez XL, Tubau F, Alia P. 2017. Development and validation of a measurement procedure based on ultra-high performance liquid chromatography-tandem mass spectrometry for simultaneous measurement of beta-lactam antibiotic concentration in human plasma. Clin Chim Acta 468:215–224. doi: 10.1016/j.cca.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Chen F, Hu ZY, Laizure SC, Hudson JQ. 2017. Simultaneous assay of multiple antibiotics in human plasma by LC-MS/MS: importance of optimizing formic acid concentration. Bioanalysis 9:469–483. doi: 10.4155/bio-2016-0157. [DOI] [PubMed] [Google Scholar]
- 17.D'Cunha R, Bach T, Young BA, Li P, Nalbant D, Zhang J, Winokur P, An G. 2018. Quantification of cefepime, meropenem, piperacillin, and tazobactam in human plasma using a sensitive and robust liquid chromatography-tandem mass spectrometry method, part 1: assay development and validation. Antimicrob Agents Chemother 62:e00859-18. doi: 10.1128/AAC.00859-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerep. 2013. Protein binding-blood partitioning. http://www.cerep.fr/cerep/utilisateurs/pages/downloads/Documents/Marketing/Pharmacology%20&%20ADME/Application%20notes/2013/Protein%20binding-blood%20partitioning.pdf Accessed 19 December 2017.