The acute phase of Chagas disease (CD) is characterized by high parasitic proliferation and intense inflammation, exacerbating the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). These reactive molecules are also increased by the metabolism of the nitroheterocyclic compounds benznidazole (BZ) and nifurtimox, the only drugs available for the treatment of CD.
KEYWORDS: Chagas disease, ascorbic acid, benznidazole, oxidative stress
ABSTRACT
The acute phase of Chagas disease (CD) is characterized by high parasitic proliferation and intense inflammation, exacerbating the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). These reactive molecules are also increased by the metabolism of the nitroheterocyclic compounds benznidazole (BZ) and nifurtimox, the only drugs available for the treatment of CD. This oxidative environment, associated with the intracellular multiplication of Trypanosoma cruzi, leads to tissue destruction, triggering the pathogenic process. Both drugs have limited efficacy and serious side effects, which demonstrates the need to seek alternative therapies. Due to the difficulty in developing new drugs, reviewing therapeutic regimens appears advantageous, and the use of BZ in low doses associated with antioxidants, such as ascorbic acid (AA), would be a valid alternative to attenuate oxidative stress. In our in vivo studies, mice receiving the combination of 7.14 mg/kg of body weight/day AA and 10 mg/kg/day BZ10 (AA+BZ10) showed a reduction in parasitemia that was more effective than that with those receiving BZ or AA alone. The combined treatment was effective in decreasing intracellular ROS and lipid peroxidation in cardiac tissue. Histological and PCR analyzes showed that AA also reduced the cardiac parasitism. However, the greatest benefit was seen in AA+BZ10 group, since cardiac inflammation was significantly reduced. In addition, the combined therapy prevented the hepatic damage induced by the infection. Our findings suggest that AA combined with a low dose of BZ may improve the trypanocidal activity and attenuate the toxic effects of BZ. The decrease in oxidative damage and inflammation observed in mice treated with AA+BZ10 could result in increased cardioprotection.
INTRODUCTION
The high social mobilization by migratory phenomena provides favorable conditions for the spread of diseases (1). Naturally, Chagas' disease is caused by the flagellate protozoan Trypanosoma cruzi, and it is transmitted from a cycle dependent on a vector, the blood-sucking bug triatomine, and a mammalian host (2). However, immigration of Latin people, associated with the vulnerability of prevention, especially in blood banks in areas lacking vectorial transmission (3), has triggered the dissemination of this disease.
Benznidazole (BZ) and nifurtimox are the only antitrypanosomal drugs available to treat Trypanosoma cruzi infection. Unsurprisingly, because it is a neglected disease, there is little interest for the pharmaceutical industries in the development of new chemical entities (NCE), although there are about 7 million cases of the disease registered worldwide (4, 5). Despite the in-depth understanding of parasite biochemistry and biology, the complexity and heterogeneity of disease reveal many gaps in what still needs to be discovered for the development of more effective drugs (6).
Some studies, such as BENEFIT, have demonstrated the effectiveness of BZ in decreasing antibody titers. However, it is perceived that the progression of the disease remains unchanged in those patients with already-established cardiac disease compared to that in patients who do not receive treatment during the chronic phase (7, 8), and several studies have shown that BZ has a number of side effects causing patients to withdraw from treatment (9, 10).
BZ is a nitroheterocyclic compound that undergoes bioactivation by NADPH-dependent nitroreductases (type I or II), which are responsible for the reduction of the nitro group (NO2) bound to the aromatic ring (11). NADPH-dependent type II nitroreductases lead to the formation of reactive intermediates, such as nitro anion radical (R-NO2−), and their reoxidation results in the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Alternatively, the reduction of BZ by NADPH-dependent type I nitroreductases does not appear to trigger the oxidative function but generates reactive intermediates, such as hydroxylamine, which has high affinity for macromolecules of the parasite (12). Currently, researchers believe that these mechanisms confer the trypanocidal action of the drug but could be associated with the manifestation of adverse effects during the treatment of patients (13, 14).
In addition, the intense inflammatory response, especially in the acute phase, leads to increased generation of ROS and nitric oxide (NO), due to elevated levels of some cytokines and chemokines, in an attempt to contain the infection. In particular, interferon gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), and interleukin-1β (IL-1β) increase the release of mitochondrial ROS by cardiomyocytes, resulting in a large amount of these molecules in the myocardium of individuals infected with Trypanosoma cruzi (15). Despite having innumerable biological functions, when ROS exceed the antioxidant capacity of the cell, they may oxidize biomolecules and consequently aggravate the process of tissue damage (16).
In this context, the use of BZ in combination with antioxidants seems to be an effective measure to attenuate the deleterious effects of reactive species in the host. Antioxidants are increasingly recognized as potential health promoters, combating aging, working on degenerative diseases (17), and reducing the risk of cardiovascular disease (18, 19).
It is known that ascorbic acid (AA) is an effective antioxidant, which acts directly by reacting with aqueous peroxyl radicals and indirectly by restoring the antioxidant properties of vitamin E. The benefits of AA for heart health have also been demonstrated, evidencing that the substance can prevent atherosclerosis and the formation of atheromas (20, 21), as well as the deleterious effects induced by acute myocardial infarction (22, 23).
Moreover, due to the difficulty in developing new therapeutic alternatives for CD, which belongs to the group of neglected tropical diseases (NTDs), researchers have been interested in studies that use the so-called “drug-sparing” regimens, where current therapies are used in low doses or shorter treatment periods with the intention of bringing improvements to the usual therapy (24, 25).
Thus, we evaluated the role of AA in Trypanosoma cruzi infection during the acute phase. We hypothesized that the antioxidant could enhance the effectiveness of BZ against cardiac damage due its cardioprotective properties and as a ROS scavenger. Motivated by dose reduction regimens, AA was combined with a low dose of BZ (10 mg/kg of body weight/day) based on previous experiments from our group (data not shown) in which BZ, at this dose, decreased the parasite load but did not lead to parasite clearance, which allows an evaluation of possible synergistic effects. The effects of this combination on the cardiac damage induced by Trypanosoma cruzi were evaluated.
RESULTS
Parasitemia.
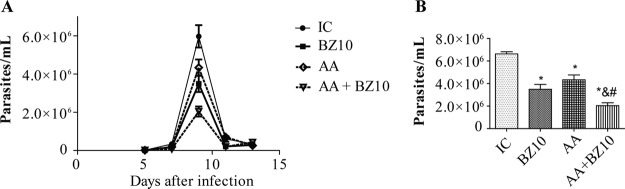
The parasitemic peak was observed on the 9th day after infection (Fig. 1A). All proposed treatments promoted parasite load reduction compared to the infected control (IC) group (Fig. 1B). The AA and BZ10 groups showed statistically significant reductions of 27.5% and 41%, respectively. However, a greater decrease was observed when the drugs were combined (7.14 mg/kg of body weight/day AA and 10 mg/kg/day BZ10 [AA+BZ10]), where the reduction in parasitemia reached 66% and was statistically different from the IC, AA, and BZ10 groups.
FIG 1.
Parasitemia rates of mice infected with Trypanosoma cruzi Y strain. (A) Parasitemic curve. Parasitemia was monitored on days 5, 7, 9, 11, and 13 after infection. (B) Statistical analysis was performed, and significant differences were observed during the parasitemic peak (on the 9th day). The results are shown as the means ± SEM of n = 5 animals. *, compared to IC group; &, compared to BZ10 group; #, compared to AA group (P < 0.05, two-way ANOVA followed by a Tukey test).
Intracellular ROS.
Trypanosoma cruzi infection did not change intracellular ROS levels in macrophages (Fig. 2A). On the other hand, an increase in the amount of ROS was detected in macrophages isolated from mice treated with BZ. The increase in ROS induced by BZ was not observed when animals were treated with the combination of AA and BZ. Thus, the combination seems to control the levels of ROS at basal levels, since there was no statistical difference for this group from the control (CT) and IC groups.
FIG 2.
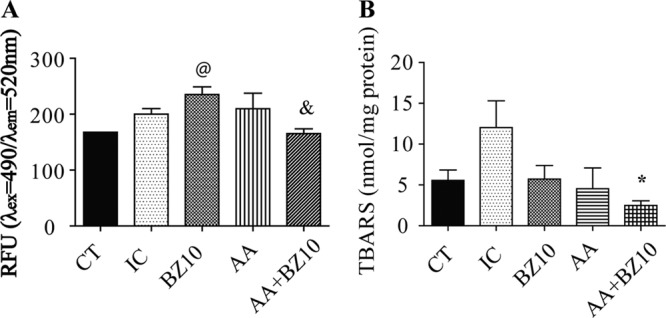
Effect of Trypanosoma cruzi infection on ROS generation in macrophages and cardiac lipoperoxidation. (A) Levels of intracellular ROS in macrophages were determined fluorometrically. (B) The concentration of TBARS in the heart was determined colorimetrically as an estimative of lipoperoxidation. Results are shown as the mean ± SEM of n = 5 animals. @, compared to CT group; *, compared to IC group; &, compared to BZ10 group (P < 0.05, one-way ANOVA followed by a Tukey test).
TBARS levels in cardiac tissue.
A significant decrease in the thiobarbituric acid reactive substance (TBARS) levels was observed in mice treated with the AA+BZ10 combination compared to the IC group (Fig. 2B). Moreover, although there is no statistical difference between the CT and IC groups, it was observed that Trypanosoma cruzi infection increased the mean concentration of TBARS in cardiac tissue (Fig. 2B).
Quantification of parasitic DNA in the heart.
The evaluation performed by quantitative real-time PCR (qPCR) showed that BZ10 and AA+BZ10 decreased tissue parasitism compared to the IC group (Fig. 3). These reductions reach 67.7% for BZ10 and 69.7% for the AA+BZ10 group. However, a greater reduction in numbers of T. cruzi copies in cardiac tissue was detected in mice treated with AA, which is equivalent to 76% in relation to the IC group.
FIG 3.
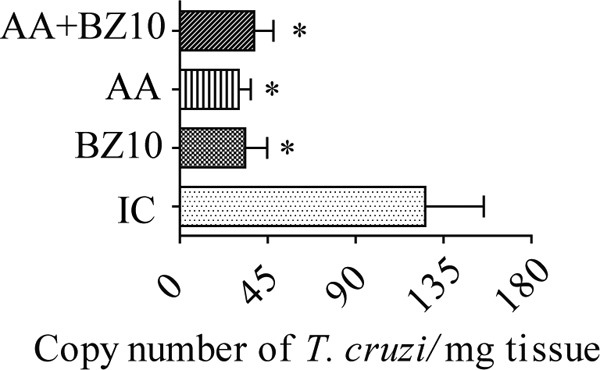
Measurement of Trypanosoma cruzi DNA in cardiac tissue. Evaluation by qPCR at 17 days after infection. Results are shown as the mean ± SEM of n = 5 animals. *, compared to IC group (P < 0.05, one-way ANOVA followed by a Tukey test).
Histological analysis of the heart.
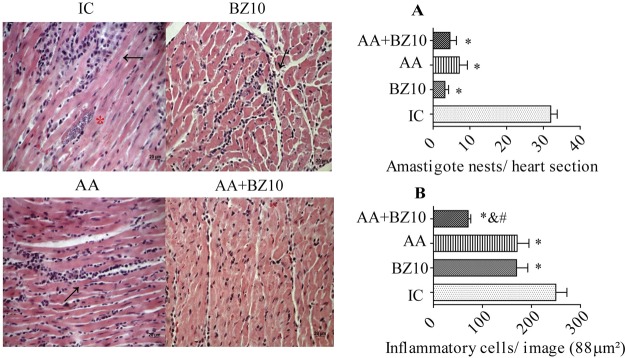
All treatments similarly reduced the amastigote nests compared to the IC group (Fig. 4A). The evaluation of the inflammatory infiltrate showed significant reductions of 31.6% and 32% for the AA and BZ10 groups, respectively. In the AA+BZ10 group, there was a greater decrease in the number of inflammatory cells, reaching 71.6%. (Fig. 4B). The statistical analysis showed that the inflammatory infiltrates the cardiac tissue of mice treated with AA+BZ10 were smaller than those observed in mice from the IC, AA, and BZ10 groups.
FIG 4.
Histological evaluation of the heart. Cardiac photomicrographs illustrate the histopathological changes induced by Trypanosoma cruzi infection. Arrows indicate the inflammatory infiltrate, and asterisks indicate amastigote nests. (A) Amastigote nests in the cardiac tissue expressed as amastigote nests/tissue section. (B) Quantification of inflammatory cells in the heart in 88 μm2 of tissue. Results are shown as the mean ± SEM of n = 5 animals. *, compared to IC group; &, compared to BZ10 group; #, compared to AA group (P < 0.05, one-way ANOVA followed by a Tukey test).
Functional markers of hepatic and renal damage.
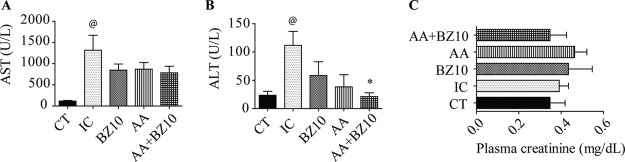
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in the plasma of the experimental animals were higher in the IC group than in the CT group (Fig. 5A and B, respectively). The combination of AA and BZ10 reduced the levels of ALT compared to those in IC group (Fig. 5B).
FIG 5.
Plasma levels of AST, ALT, and creatinine. The plasma levels of AST (A), ALT (B), and creatinine (C) were determined colorimetrically. Results are shown as the mean ± SEM of n = 5 animals. @, compared to CT group; *, compared to IC group (P < 0.05, one-way ANOVA followed by a Tukey test).
The evaluation of the creatinine levels demonstrated that neither the infection nor the therapies employed altered the functionality of the kidneys (Fig. 5C).
DISCUSSION
In a clinical trial, Barbosa et al. (26) showed that AA associated with BZ at a clinical dosage has a therapeutic impact on the reduction of ventricular arrhythmias in chronic chagasic patients with an advanced degree of cardiac damage. Nevertheless, studies combining low doses of BZ and AA are still poorly explored.
In this context, drug-sparing regimes would be an inevitable path to neglected diseases, since the discovery of new drugs is costly and time-consuming (27, 28). Reducing treatment time or dose without changing the benefits of therapy appears to be a valid alternative when the search for new trypanocidal agents fails. For this reason, the number of studies using BZ at doses lower than the usual clinical dose, associated or not with other compounds, has increased (29, 30).
During the BZ metabolism, the reactive intermediates produced not only cause strong trypanocidal activity but also numerous toxic effects to the host. Some of the major side effects of BZ include cutaneous manifestations, lymphadenopathy, and, more rarely, bone marrow suppression (31). A recent clinical study indicated that 91.5% of chagasic patients had at least one side effect during treatment with BZ (10). The same study showed that the dose administered is intrinsically related to the severity of the adverse effects and to the abandonment of treatment.
In parallel, Santos et al. (32) demonstrated that noninfected Swiss mice treated with BZ (100 mg/kg) presented increased levels of nitric oxide, malondialdehyde, and carbonyl protein. The authors also showed that the activity of antioxidant enzymes was increased, indicating an adaptive upregulation in response to oxidative damage. These data indicate that the metabolization of the drug, independently of infection, may result in the establishment of oxidative damage.
A subtherapeutic dose of BZ (25 mg/kg) was previously assessed by Cevey et al. (30) and showed the ability to reduce the parasitemia significantly, as well as to promote the survival of infected animals with a highly virulent strain of T. cruzi. In the present study, parasitemia was also reduced with BZ at a low dose (10 mg/kg), but when AA was added, it could be more effectively controlled, which represented a potentiation of the trypanocidal effect by 25%.
Our results demonstrated that treatment with BZ increases intracellular ROS levels, but this response was not observed in mice treated with AA+BZ10, which confirms the action of AA in the neutralization of ROS. In addition, we did not note a statistical difference between the CT and IC groups, indicating that the increase in the ROS concentration observed is directly related to the treatment and not to the infection by itself. This result corroborates with previous findings by Novaes et al. (14) showing that treatment with BZ, independently of infection by Trypanosoma cruzi, causes oxidative damage to the lipids and proteins in the liver of male C57BL/6 mice.
Lipid peroxidation is the main biochemical consequence of oxidative attack on cell membranes, generating a wide range of reactive aldehydes (e.g., malondialdehyde and 4-hydroxy-2-nonenal [4-HNE]). These products are relatively stable and able to diffuse and attack targets that are near or distant from their site of origin (33). The chagasic heart suffers substantial oxidative damage due to increased oxidation of biomolecules, such as lipids, during the infection and development of cardiomyopathy (34).
The role of AA in the inflammation and lipid peroxidation during the acute phase of Chagas disease has been studied. In this sense, Marim et al. (35), using the Trypanosoma cruzi QM1 strain, showed that AA at the same concentration used in the present work was able to reduce inflammation in cardiac tissue more effectively than a lower dose (0.85 mg/kg). However, this study also showed that AA at 7.14 mg/kg increased the lipid peroxidation levels in the plasma and myocardium of infected mice, evidenced by TBARS levels. This result is not in line with our findings and could be explained by the employed experimental design, which included a longer treatment time, and that the strain used by them, besides presenting low virulence, seems to exhibit a tropism by the skeletal muscle, reaching the heart less intensely (36). In our study, where a strain that is highly virulent and known to cause cardiac damage was used, AA+BZ10 reduced cardiac TBARS levels compared to those in mice from the IC group.
Given the close relationship between oxidative stress and inflammation (15, 37), this result corroborates findings from the histological analysis, where there was an important reduction in the inflammatory infiltrate in the cardiac tissue of mice from the AA+BZ10 group. This finding strengths the idea of the benefits of a combined treatment, since myocardial inflammation associated with local mononuclear infiltrate is commonly observed during the acute phase and is pointed out as an important pathogenic mechanism; therefore, its reduction could lead to a lower impairment in cardiac function (38). The histological data also showed that all treatments promoted similar reductions in the number of amastigote nests compared to the IC group. Moreover, the treatment with AA reduced the number of inflammatory cells in the heart similar to what was found with BZ10. AA still promoted the greatest reduction of parasitic DNA in the heart, showing that this antioxidant alone may decrease tissue parasitism.
The levels of ALT and AST were determined to evaluate hepatic function, since the liver regulates the metabolic homeostasis of lipids, carbohydrates, and proteins, which are essential functions to host health (39). The T. cruzi infection may cause liver injury, since there may be parasitism in this organ, and alterations, such as tissue inflammation and increased collagen deposition and AST/ALT ratio, have been demonstrated (40). Our results revealed that the IC group showed increased levels of AST and ALT, but in the treated groups, where the infection was partially controlled, this increase was not observed, evidencing that this finding could be directly related to the parasitism, as previously demonstrated by Penas et al. (40). Additionally, for ALT dosage, a decrease was observed in AA+BZ10 compared with IC, which may suggest the relation of this result with the reduction of parasitism and inflammation in the group treated with the antioxidant combined with the drug, indicating systemic benefit.
Besides that, BZ is metabolized by liver enzymes (41), and studies have shown that its administration may elevate the levels of transaminases (28, 39). For Novaes et al. (14), BZ therapy at a clinical dose (100 mg/kg) elevates ALT and AST levels in C57BL/6J mice treated for 15 days, independently of Trypanosoma cruzi infection. In our study, animals treated with the subclinical dose for the same period did not show an increase in transaminase levels. These data suggest that reduced doses of BZ may be beneficial when associated with other trypanocidal compounds.
It is important to mention that during its metabolism, AA is converted to oxalate. Therefore, excessive intake of AA has been implicated as a risk factor for the formation of calcium oxalate stones and changes in renal function (42). For this reason, we also evaluated plasma creatinine levels as an estimative of renal function. Our findings show that neither Trypanosoma cruzi infection nor treatment with AA or BZ affected the renal function. Thus, we can conclude that the use of AA and low doses of BZ does not cause renal dysfunction.
Finally, Trypanosoma cruzi has an advanced antioxidant machinery as well as mechanisms to repair the damage caused by oxidative stress (43). This condition seems to be crucial in the process of the establishment of tissue damage in Chagas disease (15, 16). For this reason, it is believed that therapeutic alternatives that attenuate this process can be beneficial.
Our results show that AA combined with a low dose of BZ may improve the trypanocidal activity and attenuate the toxic effects of BZ. The decrease in oxidative damage and inflammation observed in mice treated with AA+BZ10 could result in increased cardioprotection. Our findings encourage further studies using low doses of BZ as an alternative to usual therapy.
MATERIALS AND METHODS
Parasites, animals, and ethical aspects.
The Y strain of Trypanosoma cruzi was used in this study. The parasites were maintained in the Laboratory of Parasitology of the School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo (USP), through weekly passages in mice.
Six-week-old male Swiss mice (25 to 30 g) were purchased from the animal facilities of the USP, Ribeirão Preto, Brazil. All procedures were carried out with mice were approved by the Ethics Committee on the Use of Animals (no. 16.1.91.60.7) and are in accordance with guidelines of the National Council for the Control of Animal Experimentation (CONCEA, Brazil). The animals were maintained on a 12-h light/12-h dark cycle in ventilated racks, with free access to laboratory rodent chow and filtered water.
Experimental infection, groups, and treatments.
The animals were intraperitoneally infected with 1 × 104 trypomastigote forms collected from the blood of infected mice. The mice were randomly distributed into 5 groups: control animals without infection or treatment (CT; n = 5), infected control (IC; n = 5), infected and treated with BZ at 10 mg/kg/day (BZ10; n = 5), infected and treated with AA at 7.14 mg/kg/day (AA; n = 5), infected and treated with the combination of the two compounds in the same dose (AA+BZ10; at 7.14:10 mg/kg/day) (n = 5).
The compounds used in the treatment were purchased from Sigma-Aldrich (St. Louis, MO, USA) and were diluted in water with 5% dimethyl sulfoxide (DMSO). The IC group received the vehicle used for the dilution.
The treatment was administered in an oral (p.o.) gavage in a daily dose during 15 days. The treatment started 2 days after infection. Therefore, all experimental protocols were carried out at 17 days postinfection.
Parasitemia.
Parasitemia was evaluated on alternate days from days 5 to 13 postinfection. With this purpose, samples (5 μl) of fresh blood were collected from the tail of each animal and examined by optical microscopy (×40). Fifty fields were counted and quantified using Brenner's method (44).
Concentration of intracellular ROS.
To evaluate the amount of intracellular ROS, intraperitoneal macrophages were obtained by injection of 3 ml of 0.9% saline solution, followed by abdominal massage and aspiration. Briefly, the samples were treated with red cell lysis buffer and counted in a hemocytometer to adjust the cell concentration (5 × 105 cells/well). The levels of ROS were determined fluorometrically using a commercially available kit (catalog no. MAK143; Sigma-Aldrich, St. Louis, MO, USA). ROS present in the samples react with a fluorogenic sensor, resulting in a fluorometric product (λex = 490 nm/λem = 520 nm).
Menadione (200 mM) was used as internal control for the reaction by inducing oxidative stress (45). The results are expressed in relative fluorescence units (RFU) (λex = 490 nm/λem = 520 nm).
TBARS measurement in cardiac tissue.
A heart sample was collected and stored at −80°C. The cardiac tissue was transferred to tubes containing 200 ml of cell lysis solution and homogenized using a glass tissue homogenizer. The concentration of TBARS was determined colorimetrically according to the instructions of a commercially available kit (catalog no. 10009055; Cayman Chemical, Ann Arbor, MI, USA), as previously described by do Valle et al. (46). The concentration of protein in each sample was determined by the Lowry protein assay (Bio-Rad Laboratories, Hercules, CA, USA). The results are expressed as nanomoles per milligram of protein.
Quantitative real-time PCR.
Quantitative PCR was used to determine the amount of parasitic DNA in the heart. Briefly, the DNA was purified from 20 mg of heart tissue using ReliaPrep genomic DNA (gDNA) tissue miniprep system (Promega, Madison, WI, USA), according to the manufacturer's instructions. Subsequently, the samples were evaluated for their integrity by electrophoretic separation on a 1% agarose gel in Tris-acetate-EDTA (TAE) buffer (pH 8.0). The DNA concentration was then adjusted to 10 ng/μl. Two primers were used for Trypanosoma cruzi, the sequences Cruzi 1 (5′-ASTCGGCTGATCGTTTTCGA-3′) and Cruzi 2 (5′-ATTCCTCCAAGCAGCGGATA-3′) (47), both at a concentration of 1 μM. The reaction was performed using the SYBR master mix kit (Kapa Biosystems, Wilmington, MA, USA) (final reaction volume, 19 μl). The reaction was carried out in an Illumina Eco real-time PCR equipment, and the analyses were made using the Eco Study software. The final result was expressed in number of copies of T. cruzi/milligram of tissue.
Histological analysis.
Portions of the heart were fixed in paraffin, sectioned at 5-μm thickness, and stained with hematoxylin-eosin (H&E). For each animal, serial sections were made. The slices were visualized using an Axioscope 40 microscope (Zeiss, Thornwood, NY, USA) with a charge-coupled camera.
The amastigote nests of the 3 sections were counted and the average number obtained for each animal. These results were demonstrated in the number of amastigote nests/tissue section. To evaluate the inflammatory infiltration, 10 random images of each sample (×400 magnification) were obtained, and the number of inflammatory cells was determined using the ImageJ program (National Institutes of Health, USA), as previously described (48). The results are expressed as inflammatory cells/image (88 μm2 of cardiac tissue).
Evaluation of hepatic and renal function.
Blood samples were collected with heparin and centrifuged at 9,000 × g for 5 min. Plasma was collected from the supernatant for liver and kidney biochemical function assays. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were quantified using commercial enzyme kits (no. 108 and 109; Labtest, Lagoa Santa, Minas Gerais, Brazil) to analyze hepatic function. Creatinine was used as a biomarker of renal function, and its dosage was performed using a kit purchased from Labtest (no. 96). The results are expressed as units per liter (for AST and ALT) and milligrams per deciliter (for creatinine).
Statistical analysis.
The data are expressed as the means ± standard error of the mean (SEM). The results were analyzed by one- or two-way analysis of variance (ANOVA), followed by a Tukey test. The results of statistical tests with a P value of <0.05 were considered significant. The analyses were performed using GraphPad Prism 5.01 (GraphPad Software, Inc., San Diego, CA, USA).
Data availability.
All data presented are publicly available on the “Issues” tab at https://github.com/MVProvidello/Role-of-ascorbic-acid-as-supporting-in-the-therapeutic-of-Chagas-disease-benefits-in-the-associa.
ACKNOWLEDGMENTS
This work was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP, Brazil, grant 2016/03632-6).
The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
We thank Carla Duque Lopes for the technical support provided for this study.
REFERENCES
- 1.Molyneux DH, Savioli L, Engels D. 2017. Neglected tropical diseases: progress towards addressing the chronic pandemic. Lancet 389:312–325. doi: 10.1016/S0140-6736(16)30171-4. [DOI] [PubMed] [Google Scholar]
- 2.Rassi A Jr, Rassi A, Marcondes de Rezende J. 2012. American trypanosomiasis (Chagas disease). Infect Dis Clin North Am 26:275–291. doi: 10.1016/j.idc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Bonney KM. 2014. Chagas disease in the 21st century: a public health success or an emerging threat? Parasite 21:11. doi: 10.1051/parasite/2014012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dias JCP, Coura JR, Yasuda MAS. 2014. The present situation, challenges, and perspectives regarding the production and utilization of effective drugs against human Chagas disease. Rev Soc Bras Med Trop 47:123–125. doi: 10.1590/0037-8682-0248-2013. [DOI] [PubMed] [Google Scholar]
- 5.WHO. 2018. Chagas disease (American trypanosomiasis). World Health Organization, Geneva, Switzerland: http://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis). [Google Scholar]
- 6.Andrade LO, Andrews NW. 2005. Opinion: the Trypanosoma cruzi–host-cell interplay: location, invasion, retention. Nat Rev Microbiol 3:819–823. doi: 10.1038/nrmicro1249. [DOI] [PubMed] [Google Scholar]
- 7.Zingales B, Miles MA, Moraes CB, Luquetti A, Guhl F, Schijman AG, Ribeiro I. 2014. Drug discovery for Chagas disease should consider Trypanosoma cruzi strain diversity. Mem Inst Oswaldo Cruz 109:828–833. doi: 10.1590/0074-0276140156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, Guhl F, Velazquez E, Bonilla L, Meeks B, Rao-Melacini P, Pogue J, Mattos A, Lazdins J, Rassi A, Connolly SJ, Yusuf S, BENEFIT Investigators . 2015. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med 373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 9.Priegue M, Almuedo A, Rodríguez I, Rovira O, Soler N, Pardo C, Pola N, Mas P, Modamio P, Mariño EL. 2017. Pharmacist intervention in patients receiving treatment for Chagas disease: an emerging challenge for non-endemic countries. Infect Dis Health 22:219–226. doi: 10.1016/j.idh.2017.07.004. [DOI] [Google Scholar]
- 10.Olivera MJ, Cucunubá ZM, Valencia-Hernández CA, Herazo R, Agreda-Rudenko D, Flórez C, Duque S, Nicholls RS. 2017. Risk factors for treatment interruption and severe adverse effects to benznidazole in adult patients with Chagas disease. PLoS One 12:e0185033. doi: 10.1371/journal.pone.0185033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitmore GF, Varghese AJ. 1986. The biological properties of reduced nitroheterocyclics and possible underlying biochemical mechanisms. Biochem Pharmacol 35:97–103. doi: 10.1016/0006-2952(86)90565-4. [DOI] [PubMed] [Google Scholar]
- 12.Hall BS, Wilkinson SR. 2012. Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob Agents Chemother 56:115–123. doi: 10.1128/AAC.05135-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedrosa RC, de Bem AF, Locatelli C, Pedrosa RC, Geremias R, Wilhelm Filho D. 2001. Time-dependent oxidative stress caused by benznidazole. Redox Rep 6:265–270. doi: 10.1179/135100001101536328. [DOI] [PubMed] [Google Scholar]
- 14.Novaes RD, Santos EC, Cupertino MC, Bastos DSS, Oliveira JM, Carvalho TV, Neves MM, Oliveira LL, Talvani A. 2015. Trypanosoma cruzi infection and benznidazole therapy independently stimulate oxidative status and structural pathological remodeling of the liver tissue in mice. Parasitol Res 114:2873–2881. doi: 10.1007/s00436-015-4488-x. [DOI] [PubMed] [Google Scholar]
- 15.Machado FS, Tanowitz HB, Ribeiro AL. 2013. Pathogenesis of Chagas cardiomyopathy: role of inflammation and oxidative stress. J Am Heart Assoc 2:e000539. doi: 10.1161/JAHA.113.000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paiva CN, Feijó DF, Dutra FF, Carneiro VC, Freitas GB, Alves LS, Mesquita J, Fortes GB, Figueiredo RT, Souza HSP, Fantappié MR, Lannes-Vieira J, Bozza MT. 2012. Oxidative stress fuels Trypanosoma cruzi infection in mice. J Clin Invest 122:2531–2542. doi: 10.1172/JCI58525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng C, Wang X, Chen J, Jiao R, Wang L, Li YM, Zuo Y, Liu Y, Lei L, Ma KY, Huang Y, Chen Z-Y. 2014. Biology of ageing and role of dietary antioxidants. Biomed Res Int 2014:831841. doi: 10.1155/2014/831841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ames BN, Shigenaga MK, Hagen TM. 1993. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A 90:7915–7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths K, Aggarwal B, Singh R, Buttar H, Wilson D, De Meester F. 2016. Food antioxidants and their anti-inflammatory properties: a potential role in cardiovascular diseases and cancer prevention. Diseases 4:28. doi: 10.3390/diseases4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvayre R, Negre-Salvayre A, Camaré C. 2016. Oxidative theory of atherosclerosis and antioxidants. Biochimie 125:281–296. doi: 10.1016/j.biochi.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Moser MA, Chun OK. 2016. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci 17:E1328. doi: 10.3390/ijms17081328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buttros JB, Bergamaschi CT, Ribeiro DA, Fracalossi ACC, Campos RR. 2009. Cardioprotective actions of ascorbic acid during isoproterenol-induced acute myocardial infarction in rats. Pharmacology 84:29–37. doi: 10.1159/000222245. [DOI] [PubMed] [Google Scholar]
- 23.Viswanatha Swamy AH, Koti B, Ronad P, Wangikar U, Thippeswamy AH, Manjula D. 2011. Cardioprotective effect of ascorbic acid on doxorubicin-induced myocardial toxicity in rats. Indian J Pharmacol 43:507. doi: 10.4103/0253-7613.84952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viotti R, Gabriela M, Petti M, Armenti A. 2009. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev Anti Infect Ther 7:157–163. doi: 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- 25.Romanha AJ, Castro SLD, Soeiro MDNC, Lannes-Vieira J, Ribeiro I, Talvani A, Bourdin B, Blum B, Olivieri B, Zani C, Spadafora C, Chiari E, Chatelain E, Chaves G, Calzada JE, Bustamante JM, Freitas-Junior LH, Romero LI, Bahia MT, Lotrowska M, Soares M, Andrade SG, Armstrong T, Degrave W, Andrade Zde A. 2010. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem Inst Oswaldo Cruz 105:233–238. doi: 10.1590/S0074-02762010000200022. [DOI] [PubMed] [Google Scholar]
- 26.Barbosa JL, Thiers CA, de Bragança Pereira B, do Nascimento EM, Ribeiro Frazon CM, Budni P, Wilhelm Filho D, Pedrosa RC. 2016. Impact of the use of benznidazole followed by antioxidant supplementation in the prevalence of ventricular arrhythmias in patients with chronic Chagas disease: pilot study. Am J Ther 23:e1474–e1483. doi: 10.1097/MJT.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 27.Bustamante JM, Tarleton RL. 2014. Potential new clinical therapies for Chagas disease. Expert Rev Clin Pharmacol 7:317–325. doi: 10.1586/17512433.2014.909282. [DOI] [PubMed] [Google Scholar]
- 28.Álvarez MG, Hernández Y, Bertocchi G, Fernández M, Lococo B, Ramírez JC, Cura C, Albizu CL, Schijman A, Abril M, Sosa-Estani S, Viotti R. 2016. New scheme of intermittent benznidazole administration in patients chronically infected with Trypanosoma cruzi: a pilot short-term follow-up study with adult patients. Antimicrob Agents Chemother 60:833–837. doi: 10.1128/AAC.00745-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maçao LB, Filho DW, Pedrosa RC, Pereira A, Backes P, Torres MA, Fröde TS. 2007. Antioxidant therapy attenuates oxidative stress in chronic cardiopathy associated with Chagas' disease. Int J Cardiol 123:43–49. doi: 10.1016/j.ijcard.2006.11.118. [DOI] [PubMed] [Google Scholar]
- 30.Cevey ÁC, Mirkin GA, Penas FN, Goren NB. 2016. Low-dose benznidazole treatment results in parasite clearance and attenuates heart inflammatory reaction in an experimental model of infection with a highly virulent Trypanosoma cruzi strain. Int J Parasitol Drugs Drug Resist 6:12–22. doi: 10.1016/j.ijpddr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bern C. 2015. Chagas' disease. N Engl J Med 373:456–466. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 32.Santos EC, Novaes RD, Bastos DSS, Oliveira JM, Penitente AR, Gonçalves WG, Cardoso SA, Talvani A, Oliveira LL. 2015. Modulation of oxidative and inflammatory cardiac response by nonselective 1- and 2-cyclooxygenase inhibitor and benznidazole in mice: cardiac response during drug association. J Pharm Pharmacol 67:1556–1566. doi: 10.1111/jphp.12451. [DOI] [PubMed] [Google Scholar]
- 33.Wen J-J, Vyatkina G, Garg N. 2004. Oxidative damage during chagasic cardiomyopathy development: role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic Biol Med 37:1821–1833. doi: 10.1016/j.freeradbiomed.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Wen J, Dhiman M, Whorton EB, Garg NJ. 2008. Tissue-specific oxidative imbalance and mitochondrial dysfunction during Trypanosoma cruzi infection in mice. Microbes Infect 10:1201–1209. doi: 10.1016/j.micinf.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marim RG, de Gusmão AS, Castanho REP, Deminice R, Therezo ALS, Jordão Júnior AA, Martins LPA. 2012. Effects of vitamin C supplementation on acute phase Chagas disease in experimentally infected mice with Trypanosoma cruzi QM1 strain. Rev Inst Med Trop São Paulo 54:319–323. [DOI] [PubMed] [Google Scholar]
- 36.Martins LPA, Marcili A, Castanho REP, Therezo ALS, Suzuki RB, Teixeira MMG, da Rosa JA, Speranca MA. Rural Triatoma rubrovaria from southern Brazil harbors Trypanosoma cruzi of lineage IIc. 79:427–434. [PubMed] [Google Scholar]
- 37.Machado FS, Dutra WO, Esper L, Gollob KJ, Teixeira MM, Factor SM, Weiss LM, Nagajyothi F, Tanowitz HB, Garg NJ. 2012. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin Immunopathol 34:753–770. doi: 10.1007/s00281-012-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez HO, Guerrero NA, Fortes A, Santi-Rocca J, Gironès N, Fresno M. 2014. Trypanosoma cruzi strains cause different myocarditis patterns in infected mice. Acta Trop 139:57–66. doi: 10.1016/j.actatropica.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Onofrio LI, Arocena AR, Paroli AF, Cabalén ME, Andrada MC, Cano RC, Gea S. 2015. Trypanosoma cruzi infection is a potent risk factor for non-alcoholic steatohepatitis enhancing local and systemic inflammation associated with strong oxidative stress and metabolic disorders. PLoS Negl Trop Dis 9:e0003464. doi: 10.1371/journal.pntd.0003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penas FN, Cevey Á, Siffo CS, Mirkin GA, Goren NB. 2016. Hepatic injury associated with Trypanosoma cruzi infection is attenuated by treatment with 15-deoxy-Δ12,14 prostaglandin J2. Exp Parasitol 170:100–108. doi: 10.1016/j.exppara.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Masana M, de Toranzo EG, Castro JA. 1984. Reductive metabolism and activation of benznidazole. Biochem Pharmacol 33:1041–1045. doi: 10.1016/0006-2952(84)90511-2. [DOI] [PubMed] [Google Scholar]
- 42.Knight J, Madduma-Liyanage K, Mobley JA, Assimos DG, Holmes RP. 2016. Ascorbic acid intake and oxalate synthesis. Urolithiasis 44:289–297. doi: 10.1007/s00240-016-0868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machado-Silva A, Cerqueira PG, Grazielle-Silva V, Gadelha FR, Peloso Ede F, Teixeira SMR, Machado CR. 2016. How Trypanosoma cruzi deals with oxidative stress: antioxidant defence and DNA repair pathways. Mutat Res Rev Mutat Res 767:8–22. doi: 10.1016/j.mrrev.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Brener Z. 1962. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo 4:389–396. [PubMed] [Google Scholar]
- 45.Bellomo G, Mirabelli F, Vairetti M, Iosi F, Malomi W. 1990. Cytoskeleton as a target in menadione-induced oxidative stress in cultured mammalian cells. I. Biochemical and immunocytochemical features. J Cell Physiol 143:118–128. [DOI] [PubMed] [Google Scholar]
- 46.do Vale GT, Gonzaga NA, Simplicio JA, Tirapelli CR. 2017. Nebivolol prevents ethanol-induced reactive oxygen species generation and lipoperoxidation in the rat kidney by regulating NADPH oxidase activation and expression. Eur J Pharmacol 799:33–40. doi: 10.1016/j.ejphar.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 47.Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Mejia Jaramillo AM, Cura C, Auter F, Veron V, Qvarnstrom Y, Deborggraeve S, Hijar G, Zulantay I, Lucero RH, Velazquez E, Tellez T, Sanchez Leon Z, Galvão L, Nolder D, Monje Rumi M, Levi JE, Ramirez JD, Zorrilla P, Flores M, Jercic MI, Crisante G, Añez N, De Castro AM, Gonzalez CI, Acosta Viana K, Yachelini P, Torrico F, Robello C, Diosque P, Triana Chavez O, Aznar C, Russomando G, Büscher P, Assal A, Guhl F, Sosa Estani S, DaSilva A, Britto C, Luquetti A, Ladzins J. 2011. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis 5:e931. doi: 10.1371/journal.pntd.0000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sesti-Costa R, Carneiro ZA, Silva MC, Santos M, Silva GK, Milanezi C, da Silva RS, Silva JS. 2014. Ruthenium complex with benznidazole and nitric oxide as a new candidate for the treatment of Chagas disease. PLoS Negl Trop Dis 8:e3207. doi: 10.1371/journal.pntd.0003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented are publicly available on the “Issues” tab at https://github.com/MVProvidello/Role-of-ascorbic-acid-as-supporting-in-the-therapeutic-of-Chagas-disease-benefits-in-the-associa.