Single amino acid substitutions in the Ω loop of KPC β-lactamases are known to lead to resistance to the ceftazidime-avibactam combination. Here, we investigate this mechanism of resistance in CTX-M enzymes, which are the most widely spread extended-spectrum β-lactamases worldwide.
KEYWORDS: β-lactamase inhibitor, Ω loop, avibactam, CTX-M, ceftazidime
ABSTRACT
Single amino acid substitutions in the Ω loop of KPC β-lactamases are known to lead to resistance to the ceftazidime-avibactam combination. Here, we investigate this mechanism of resistance in CTX-M enzymes, which are the most widely spread extended-spectrum β-lactamases worldwide. Nine single amino acid polymorphisms were identified in the Ω loop of the 172 CTX-M sequences present in the Lahey database of β-lactamases. The corresponding modifications were introduced in CTX-M-15 by site-directed mutagenesis. None of the nine substitutions was associated with ceftazidime-avibactam resistance in Escherichia coli TOP10. However, two substitutions led to 4-fold (P167S) and 16-fold (L169Q) increases in the MIC of ceftazidime. We determined whether these substitutions favor the in vitro selection of mutants resistant to ceftazidime-avibactam. The selection provided mutants for the L169Q substitution but not for the P167S substitution or for the parental enzyme CTX-M-15. Resistance to the drug combination (MIC of ceftazidime, 16 μg/ml in the presence of 4 μg/ml of avibactam) resulted from the acquisition of the S130G substitution by CTX-M-15 L169Q. Purified CTX-M-15 with the two substitutions, L169Q and S130G, was only partially inhibited by avibactam at concentrations as high as 50,000 μM but retained ceftazidime hydrolysis activity with partially compensatory decreases in kcat and Km. These results indicate that emergence of resistance to the ceftazidime-avibactam combination requires more than one mutation in most CTX-M-encoding genes. Acquisition of resistance could be restricted to rare variants harboring predisposing polymorphisms such as Q at position 169 detected in a single naturally occurring CTX-M enzyme (CTX-M-93).
INTRODUCTION
The CTX-M β-lactamases confer resistance to most β-lactam antibiotics except carbapenems and cephamycins (1, 2). These enzymes are the most widespread extended-spectrum β-lactamases (ESBLs) worldwide. CTX-M-producing Enterobacteriaceae account for up to 90% of ESBL producers in many countries (3), with blaCTX-M-14 and blaCTX-M-15 being the two predominant genes (4). Coresistance to other major classes of antibiotics, such as the fluoroquinolones and the aminoglycosides, has led to an increased use of carbapenems and to the subsequent emergence of carbapenem-resistant Enterobacteriaceae (5). The association of CTX-M ESBLs with various β-lactam resistance mechanisms, such as the production of OXA-48 or of closely related carbapenemases, and impaired transport of β-lactams through porins may lead to resistance to all β-lactams (6, 7). In this context, carbapenem- and cephalosporin-resistant Enterobacteriaceae have been classified as a top priority for the development of new antibiotics by the WHO (8) since there is often no therapeutic alternative for these strains.
Avibactam is a new non-β-lactam β-lactamase inhibitor belonging to the diazabicyclooctane family (9). It displays a broad spectrum of inhibition, which includes class A, class C, and some class D β-lactamases. Avibactam, which has been approved in combination with ceftazidime, offers an alternative to second-line antibiotics such as colistin, fosfomycin, and tigecycline.
The combination of ceftazidime and avibactam is almost uniformly active against CTX-M-producing Enterobacteriaceae (10), including most carbapenem-nonsusceptible isolates with porin defects (11). A recently reported exception involves resistance to the ceftazidime-avibactam combination in Klebsiella pneumoniae. In this case, resistance required production of a CTX-M-14 derivative with a P167S substitution and additional uncharacterized β-lactam resistance mechanisms (12).
Similar to CTX-M ESBLs, carbapenemases of the KPC family are almost universally inhibited by avibactam (13). Exceptions involve the production of KPC-3 derivatives with D179Y, alone or in combination with additional amino acid substitutions, which were associated with treatment failure and microbiological resistance to the ceftazidime-avibactam combination (14). Detailed analysis of the in vitro activity of KPC-2 D179Y revealed that resistance was due to impaired inhibition by avibactam combined with significant residual activity for ceftazidime hydrolysis (15).
The substitutions D179Y in KPC and P167S in CTX-M-14, which have emerged under treatment, are located in the Ω loop of the β-lactamases (Ambler positions 164 to 179). The critical role of residues of the Ω loop in the substrate specificity and in the inhibition profile of class A β-lactamases has also been documented by several studies based on site-directed mutagenesis (16–18). In KPC enzymes, Arg164 or Asp179 is in hydrogen interaction. Amino acid substitutions individually affecting Arg164 or Asp179 result in resistance to the ceftazidime-avibactam combination, indicating the key role of this interaction. Substitutions at other positions of the Ω loop did not result in resistance to the combination. Site-directed mutagenesis of the penicillinase SHV-1 did not identify Ω loop substitutions conferring resistance to ceftazidime-avibactam (19). The possibility for emergence of resistance by acquisition of single amino acid changes appears, therefore, limited to a restricted number of enzymes, such as KPC β-lactamases, and a very small number of positions, such as Ambler positions 164 and 179.
In this study, we evaluate whether naturally occurring polymorphisms in the Ω loop of CTX-M enzymes affect the ability of avibactam to restore the activity of ceftazidime. We also evaluate whether these polymorphisms could affect single-step acquisition of mutations leading to increased resistance to ceftazidime in the presence of avibactam.
RESULTS AND DISCUSSION
Impact of polymorphisms in the Ω loop of CTX-M enzymes on the activity of ceftazidime-avibactam.
Seventeen of the 172 CTX-M isoforms present in the Lahey database were found to harbor variations in the Ω loop (20) (https://www.lahey.org/Studies/). Each of these 17 isoforms differed from the Ω loop consensus sequence by one amino acid (Table 1). There were a total of nine variations in the 17 isoforms since three substitutions were found to be present in more than one isoform (P167T, P167S, and T171S). We introduced each of these nine substitutions in CTX-M-15 by site-directed mutagenesis of a derivative of plasmid pTRC-99k harboring the gene blaCTX-M-15 under the control of the promoter Ptrc, which is inducible by isopropyl-β-d-1-thiogalactopyranoside (IPTG) (21). MIC determinations for Escherichia coli TOP10 expressing these genes are reported in Table 2. Compared to the wild-type enzyme, 7 out of the 9 variants conveyed similar or decreased ceftazidime MICs (range, 4 to 16 μg/ml in the variants versus 16 μg/ml for the parental enzyme). Increased MICs of ceftazidime were observed for the remaining variants, P167S (64 μg/ml) and L169Q (256 μg/ml). None of the nine substitutions conferred resistance to ceftazidime-avibactam. In the presence of avibactam (4 μg/ml), a minor increase in the MIC of ceftazidime (from 0.5 to 2 μg/ml) was observed for a single variant (P167S). Thus, all variants remained in the susceptible range (≤8 μg/ml) according to EUCAST 2017 interpretive criteria (version 7.1) (22).
TABLE 1.
Variations in the Ω loop of CTX-M β-lactamases from Enterobacteriaceae
| Variation in the Ω loop | CTX-M ESBLa |
||
|---|---|---|---|
| Group 1 | Group 2 | Group 9 | |
| R164G | M-72 | ND | ND |
| P167T | M-23 M-42 M-58 | M-74 | ND |
| P167S | M-52 M-62 | ND | M-19 M-99 |
| P167Q | M-54 | ND | ND |
| P167L | ND | ND | M-87 |
| P167A | ND | ND | M-126 |
| L169Q | ND | ND | M-93 |
| T171S | ND | M-4 M-6 M-7 | ND |
| P174Q | M-117 | ND | ND |
CTX-M ESBLs are assigned to five groups (groups 1, 2, 8, 9, and 25) based on identity of ≥95% for sequences belonging to the same group (20). No Ω loop variants were described for CTX-M β-lactamases belonging to groups 8 and 25. Sequences were obtained from the Lahey website (https://www.lahey.org/Studies/). ND, the variation was not detected in any variant of the group.
TABLE 2.
Resistance phenotypes conferred by CTX-M-15 Ω loop variants in E. coli TOP10
| CTX-M-15 variant | MIC (μg/ml)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CAZ | CAZ-AVI | AMX | AMC | CF | FOX | CTX | ATM | FEP | ETP | IPM | |
| Noneb | 0.25 | 0.25 | 2 | 2 | 16 | 4 | 0.06 | 0.25 | 0.023 | 0.003 | 0.25 |
| M-15 | 16 | 0.5 | >256 | 4 | >256 | 8 | >32 | 16 | 1.5 | 0.006 | 0.38 |
| M-15 R164G | 4 | 0.5 | >256 | 6 | 256 | 8 | 1 | 0.75 | 0.25 | 0.003 | 0.19 |
| M-15 P167L | 4 | 0.5 | >256 | 4 | >256 | 8 | 0.38 | 0.75 | 0.19 | 0.012 | 0.25 |
| M-15 P174Q | 4 | 0.5 | >256 | 4 | 256 | 12 | 0.75 | 0.75 | 0.75 | 0.008 | 0.25 |
| M-15 P167Q | 8 | 0.5 | >256 | 4 | >256 | 8 | 0.75 | 0.75 | 0.25 | 0.008 | 0.38 |
| M-15 P167A | 8 | 0.5 | >256 | 4 | >256 | 12 | 2 | 1.5 | 0.38 | 0.012 | 0.25 |
| M-15 P167T | 16 | 0.5 | >256 | 6 | >256 | 8 | 1 | 2 | 0.38 | 0.008 | 0.25 |
| M-15 T171S | 16 | 0.5 | >256 | 4 | >256 | 8 | >32 | 12 | 2 | 0.006 | 0.38 |
| M-15 P167S | 64 | 2 | >256 | 6 | >256 | 12 | 8 | 3 | 2 | 0.032 | 0.25 |
| M-15 L169Q | 256 | 0.5 | 16 | 4 | 32 | 8 | 32 | 6 | 3 | 0.023 | 0.25 |
| M-15 L169Q G240D | 64 | 1 | 32 | 4 | 64 | 8 | 6 | >32 | 4 | 0.032 | 0.19 |
| M-15 L169Q S130G | 32 | 16 | 24 | 12 | 32 | 8 | 0.25 | 0.25 | 0.75 | 0.004 | 0.25 |
| M-15 L169Q S130G G240D | 8 | 4 | 32 | 24 | 8 | 8 | 0.38 | 0.38 | 0.38 | 0.004 | 0.19 |
| M-15 S130G | 1 | 0.5 | >256 | 24 | 32 | 3 | 0.023 | 0.047 | 0.016 | 0.003 | 0.094 |
CAZ, ceftazidime; AVI, avibactam (4 μg/ml); AMX, amoxicillin; AMC, amoxicillin combined with clavulanate (2 μg/ml); CF, cephalothin; FOX, cefoxitin; CTX, cefotaxime; ATM, aztreonam; FEP, cefepime; ETP, ertapenem; IPM, imipenem.
None, plasmid control without blaCTX-M-15.
Comparison of the MICs of amoxicillin in the absence or presence of clavulanate (2 μg/ml) suggested that none of the substitutions impaired inhibition of CTX-M-15 by clavulanate. The L169Q substitution led to a reduction in the MIC of amoxicillin, as expected from the previous characterization of CTX-M-93, which contains a Q residue at position 169 (23). All substitutions, except L169Q and T171S, abolished resistance to cefotaxime and aztreonam. As expected, the parental enzyme and the variants did not confer resistance to imipenem.
The association of the S130G and L169Q substitutions confers resistance to ceftazidime-avibactam.
Our next objective was to investigate in vitro acquisition of resistance to ceftazidime-avibactam. Selection was performed on agar plates containing a fixed concentration of avibactam (4 μg/ml) and 2-fold increasing concentrations of ceftazidime (0.12 to 16 μg/ml). This procedure was applied to E. coli TOP10 cells producing CTX-M-15 and the two CTX-M-15 variants harboring the substitutions that led to increases in the MICs of ceftazidime (P167S and L169Q) (see above). No mutant was recovered for strains producing CTX-M-15 and CTX-M-15 P167S (frequency, <2 × 10−10). For CTX-M-15 L169Q, mutants were obtained at a frequency of 2 × 10−8 on agar plates containing ceftazidime (2 μg/ml) and avibactam (4 μg/ml). Sequencing of the blaCTX-M-15 gene from five of these putative mutants revealed the same nucleotide change (A397G) leading to a serine-to-glycine substitution at Ambler position 130, which corresponds to the first position of the conserved SDN motif. All five mutants retained the initial L169Q substitution. The plasmid encoding CTX-M-15 with S130G and L169Q was introduced into E. coli TOP10 and produced resistance to the ceftazidime-avibactam combination (MIC of 16 μg/ml in the presence of 4 μg/ml of avibactam) (Table 2). The MIC of ceftazidime in the absence of avibactam (32 μg/ml) was only 2-fold higher, suggesting that inhibition of CTX-M-15 S130G L169Q is severely impaired. In the L169Q background, S130G led to 8-, 128-, and 24-fold decreases in the MICs of ceftazidime, cefotaxime, and aztreonam, respectively. Thus, both the efficacy of inhibition of the β-lactamase by avibactam and the efficacy of hydrolysis of β-lactams appear to be reduced following acquisition of S130G by CTX-M-15 L169Q. Similarly, the efficacy of inhibition of the β-lactamase by clavulanate appears to be reduced by the S130G substitution. This result was expected from the phenotypic analysis of E. coli producing the CTX-M-15 S130G variant (Table 2), which showed a decrease in the level of resistance to all cephalosporins and an increase in the level of resistance to amoxicillin in the presence of clavulanate. Similar results were previously reported for the impact of the S130G substitution in CTX-M β-lactamases and other class A enzymes (19, 24–28). For example, introduction of S130G in SHV-9, TEM-3, and OXY-2, generating SHV-10, TEM-89, and IRKO-1, respectively, impaired the inhibitory activity of clavulanate and the efficacy of hydrolysis of cephalosporins but not that of penicillins (29–31).
Quantitative assessment of the impact of amino acid substitutions on the catalytic constants of CTX-M-15.
The parental enzyme CTX-M-15 and the two variants harboring the substitution P167S or the combination of S130G and L169Q were purified by metal affinity and size exclusion chromatography (see Table S2 in the supplemental material for the mass of the enzymes). As described above (Table 2), the P167S substitution was associated with 4-fold increases of the MICs of ceftazidime and ceftazidime-avibactam. However, these differences were not associated with any significant difference in the kinetic parameters for hydrolysis of ceftazidime or for carbamoylation of the enzyme by avibactam (Fig. 1 and Table 3). The basis for this discrepancy remains unknown. Similar results were previously obtained for the analysis of the impact of the P167S substitution on the hydrolysis of ceftazidime in the CTX-M-14 background (32). The authors of this study proposed that an increase in ceftazidime resistance in the absence of any modification of the kinetic parameters could result from the instability of the β-lactamase harboring P167S.
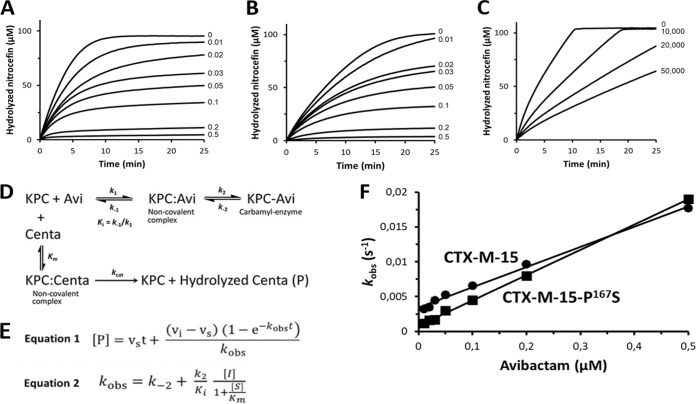
FIG 1.
Kinetics of CTX-M-15 (A), CTX-M-15 P167S (B), and CTX-M-15 S130G L169Q (C) inhibition by avibactam. The β-lactamases CTX-M-15 (0.002 μM), CTX-M-15 P167S (0.002 μM), and CTX-M-15 S130G L169Q (1 μM) were incubated with nitrocefin (100 μM) and avibactam at the indicated concentrations at 20°C in 100 mM 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 6.4). (D) The efficacy of the carbamoylation reaction was evaluated based on inhibition of nitrocefin hydrolysis (Δε486 nm = 14,600 M−1 cm−1), as previously described (9, 15). Avi, avibactam. (E) Equation 1 was fitted to data to determine the value of the observed rate constant (kobs) for the various concentrations of avibactam. The carbamoylation efficacy (k2/Ki) was determined by plotting the values of kobs as a function of the inhibitor concentration (F) and fitting equation 2 to the data. Abbreviations: [P], concentration of hydrolyzed nitrocefin; vi, uninhibited enzyme velocity; vs, fully inhibited enzyme velocity; [S], initial concentration of nitrocefin; [I], avibactam concentration; Km, Michaelis constant for hydrolysis of nitrocefin by CTX-M β-lactamases.
TABLE 3.
Impact of substitutions in CTX-M-15 on the hydrolysis of β-lactams and inhibition by avibactam
| Compound and parameter | Value of the parameter for: |
||
|---|---|---|---|
| CTX-M-15 | CTX-M-15 P167S | CTX-M-15 S130G L169Q | |
| Amoxicillin | |||
| kcat (s−1) | 47 ± 4 | 6.5 ± 0.4 | (9.0 ± 0.5) x 10−4 |
| Km (μM) | 42 ± 14 | 47 ± 9 | <50 |
| kcat/Km (M−1 s−1) | (1.1 ± 0.4) × 106 | (1.4 ± 0.3) × 105 | >20 |
| Cephalothin | |||
| kcat (s−1) | (2.2 ± 0.2) × 103 | 170 ± 2 | (6.8 ± 0.5) × 10−4 |
| Km (μM) | 77 ± 13 | 22 ± 1 | <30 |
| kcat/Km (M−1 s−1) | (2.9 ± 0.7) × 107 | (7.7 ± 0.4) × 106 | >20 |
| Ceftazidime | |||
| kcat (s−1) | >1.8 | >0.7 | (8.0 ± 1.2) × 10−3 |
| Km (μM) | >200 | >200 | <22a |
| kcat/Km (M−1 s−1) | (8.2 ± 0.4) × 103 | (3.5 ± 0.2) × 103 | >3.6 × 102 |
| Cefotaxime | |||
| kcat (s−1) | 110 ± 12 | 44 ± 3 | (1.0 ± 0.5) × 10−4 |
| Km (μM) | 24 ± 10 | 74 ± 12 | <30 |
| kcat/Km (M−1 s−1) | (4.6 ± 2.0) × 106 | (6.0 ± 1.1) × 105 | >3 |
| Clavulanate | |||
| kcat (s−1) | NAb | NAb | NAb |
| Km (μM) | NAb | NAb | NAb |
| kcat/Km (M−1 s−1) | NAb | NAb | NAb |
| Avibactam | |||
| k2/Ki (M−1 s−1) | (1.8 ± 0.1) × 105 | (2.0 ± 0.0) × 105 | NAc |
| k−2 (s−1) | (3.4 ± 0.2) × 10−3 | (8.6 ± 1.0) × 10−4 | NAc |
The Km for ceftazidime hydrolysis by CTX-M-15 S130G L169Q was determined using nitrocefin (100 μM) as a reporter substrate.
NA, not applicable as hydrolysis of clavulanate (100 μM) was not detected at the highest β-lactamase concentration tested (10 μM).
NA, not applicable as hydrolysis of nitrocefin (100 μM) by CTX-M-15 S130G L169Q (1 μM) was only partially inhibited at the highest avibactam concentration that could be tested (50,000 μM).
Introduction of both S130G and L169Q in CTX-M-15 was associated with a >225-fold decrease in the value of kcat for hydrolysis of ceftazidime (Table 3). However, the value of Km was also reduced (at least 9-fold), leading to substantial residual catalytic efficacy (kcat/Km values of >360 M−1 s−1 and 8,200 M−1 s−1 for CTX-M-15 S130G L169Q and CTX-M-15, respectively). Inhibition of the enzyme by avibactam was only partial at the highest concentration that was tested (50,000 μM) (Fig. 1). The low efficacy of CTX-M-15 S130G L169Q inhibition is in agreement with the minor difference observed between the MICs of ceftazidime in the presence or absence of avibactam (16 μg/ml or 32 μg/ml, respectively) (Table 2).
The introduction of the G240D substitution in the CTX-M-15 L169Q background prevents the emergence of resistance to ceftazidime-avibactam.
The D240G substitution present in CTX-M-15 but not in most other CTX-M enzymes is known to be essential for efficacious hydrolysis of ceftazidime by CTX-M-15. To further explore the role of the L169Q substitution in the emergence of resistance to ceftazidime-avibactam, we introduced the G240D substitution into the CTX-M-15 L169Q background. As expected, production of CTX-M-15 L169Q G240D conferred a lower level of resistance to ceftazidime to E. coli TOP10 than that conferred by the parental enzyme CTX-M-15 L169Q (MICs of 64 μg/ml versus 256 μg/ml, respectively). The in vitro acquisition of resistance to ceftazidime-avibactam by E. coli TOP10 producing CTX-M-15 L169Q G240D was also explored. No mutant resistant to the combination was obtained in three independent experiments (frequency of <2 × 10−10). Thus, the D240G substitution was essential for the in vitro acquisition of resistance to ceftazidime-avibactam. Since attempts to obtain resistant mutants with E. coli TOP10 producing CTX-M-15 L169Q G240D were negative, we constructed the triple mutant CTX-M-15 L169Q S130G G240D. In comparison to CTX-M-15 L169Q S130G, introduction of G240D reduced the MIC of ceftazidime both in the absence of avibactam (from 32 μg/ml to 8 μg/ml) and in the presence of the β-lactamase inhibitor (from 16 μg/ml to 4 μg/ml). These observations indicate both that the L169Q and G240D substitutions contribute to an increase in the efficacy of ceftazidime hydrolysis and that efficacious hydrolysis of this drug is a prerequisite for acquisition of ceftazidime-avibactam resistance mediated by S130G. This is accounted for by the fact that the S130G substitution impairs both ceftazidime hydrolysis and inhibition by avibactam.
Conclusions.
Ceftazidime combined with avibactam was previously found to be uniformly active on large collections of Enterobacteriaceae producing class A β-lactamases (10, 33), indicating that resistance did not exist prior to the clinical use of the combination. However, emergence of resistance to ceftazidime-avibactam has been reported in 3 of 37 patients treated with this combination (14). Microbiological failure was associated with acquisition of a mutation leading to the D179Y substitution in the Ω loop of the β-lactamase KPC-3. The D179Y substitution is sufficient for ceftazidime-avibactam resistance, and this correlates with the expected single-step in vitro acquisition of resistance due to the same substitution (15). In contrast, we show here that derivatives of CTX-M-15 conferring ceftazidime-avibactam resistance cannot be obtained using the same selection procedure (frequency of <2 × 10−10). We also show that previously reported polymorphisms in the Ω loop of CTX-M enzymes (Table 1) are not associated with resistance to ceftazidime-avibactam following their introduction into the CTX-M-15 background (Table 2). However, two substitutions (P167S and L169Q) led to significant increases in the MIC of ceftazidime (4- and 16-fold). Application of the in vitro selection procedure to these two variants led to acquisition of ceftazidime-avibactam resistance only for the L169Q substitution. The resulting double mutant produced a CTX-M-15 β-lactamase with the S130G and L169Q substitutions that was only partially inhibited by avibactam at concentrations as high as 50,000 μM (Fig. 1). The β-lactamase retained substantial activity for hydrolysis of ceftazidime since the large decrease in the value of kcat was partially compensated by a decrease in Km (Table 3). These results account for the fact that derivatives of CTX-M-15 conferring ceftazidime-avibactam resistance cannot be obtained in a single step since resistance requires at least two amino acid substitutions. These results also suggest that emergence of resistance to the combination might be restricted to CTX-M variants with Q at position 169. This polymorphism has been detected in a single group 9 CTX-M enzyme (CTX-M-93), which has only been reported once in 2009 in an E. coli clinical isolate from France (23). However, CTX-M-93 does not harbor the D240G substitution present in CTX-M-15, which was shown to be important for acquisition of resistance to the ceftazidime-avibactam combination. Thus, two mutations are predicted to be required for acquisition of resistance to the combination for the CTX-M-93 variant, as experimentally shown for CTX-M-15 in our study. In conclusion, acquisition of ceftazidime-avibactam resistance in a single step appears unlikely for all CTX-M variants identified in clinical isolates.
MATERIALS AND METHODS
Construction of Ω loop variants of CTX-M-15 by site-directed mutagenesis.
Site-directed mutagenesis was performed on plasmid pTRC-99kΩblaCTX-M-15 with the oligonucleotides depicted in Table S1 in the supplemental material, as previously described (21). The sequence of the blaCTX-M-15 variant was verified by double-strand sequencing. In the resulting plasmids, the blaCTX-M-15 variants were expressed under the control of the IPTG-inducible Ptrc promoter of the vector pTRC-99k (21). The plasmids encoding blaCTX-M-15 variants were introduced by transformation into E. coli TOP10, with selection for resistance to kanamycin (50 μg/ml) conveyed by the vector pTRC-99k.
MIC determinations.
E. coli TOP10 was used as the host for expression of the blaCTX-M-15 variants cloned into the vector pTRC-99k. The precultures were grown in Mueller-Hinton (MH) broth (Difco) containing IPTG (0.5 mM) for the induction of the Ptrc promoter and kanamycin (50 μg/ml) for plasmid maintenance. MICs of ceftazidime (PANPharma) were determined by the microdilution method in MH broth containing IPTG (0.5 mM), according to the Clinical and Laboratory Standards Institute (CLSI) recommendations (34). Avibactam (PANPharma) was used at a fixed concentration (4 μg/ml). The reported MIC values are the medians of five determinations.
MICs of amoxicillin, amoxicillin-clavulanate, cephalothin, cefoxitin, cefotaxime, cefepime, ertapenem, imipenem, and aztreonam were determined in duplicate in MH agar supplemented with IPTG (0.5 mM) by the Etest method, according to the manufacturer's instructions (bioMérieux, Marcy-l'Étoile, France). The two determinations led to the same MIC values.
Production and purification of CTX-M β-lactamases.
Derivatives of plasmid pET-tobacco etch virus (TEV) were used for the production and purification of CTX-M β-lactamases, as previously described for class A β-lactamases from mycobacteria (35). Briefly, a fragment of the blaCTX-M-15 gene encoding residues 31 to 288 of the β-lactamase was cloned into the pET-TEV vector, generating a translational fusion with a sequence from the vector encoding an N-terminal 6×His tag, followed by a cleavage site for the TEV protease. The same approach was used to clone the genes encoding the CTX-M-15 variants with the P167S or the S130G and L169Q substitutions (see above). The β-lactamases were produced in E. coli BL21(DE3) and purified from clarified lysates by affinity chromatography (Ni-nitrilotriacetic acid [NTA] agarose; Sigma-Aldrich) and size exclusion chromatography in 25 mM Tris-HCl (pH 7.5) containing 300 mM NaCl (Superdex 200 HL26/60; Amersham Pharmacia Biotech), as previously described (35). The β-lactamases were stored at −20°C in the same buffer. For determination of the mass of the β-lactamases (see Table S2 in the supplemental material), samples were filtered (0.45-μm pore size), desalted (HiTrap desalting column, 5 ml; General Electrics), and diluted in water (1:5), and 0.5 μl was injected at a flow rate of 50 μl/min (acetonitrile 20%, formic acid 0.1%). Mass spectra were acquired in the positive mode (maXis II ETD electrospray ionization-quadrupole time of flight [ESI-QTOF] mass spectrometer; Bruker). Protein concentration was determined by a Bio-Rad protein assay using bovine serum albumin as a standard.
Kinetic analyses.
All kinetic experiments were performed at 20°C in 2-(N-morpholino)ethanesulfonic acid ([MES] 100 mM, pH 6.4). Steady-state kinetic parameters for hydrolysis of ceftazidime (kcat, Km, and kcat/Km) were determined by spectrophotometry (Δε256 nm = −9,800 M−1 cm−1) with a Cary 300 spectrophotometer (Agilent) (15), except for the Km for ceftazidime hydrolysis by CTX-M-15 S130G L169Q, which was determined using nitrocefin (100 μM) as a reporter substrate (21). Inhibition of nitrocefin (100 μM) hydrolysis by avibactam was evaluated by spectrophotometry (Δε486 nm = 14,600 M−1 cm−1), as previously reported (9, 15). The Km values for hydrolysis of nitrocefin by CTX-M-15, CTX-M-15 P167S, and CTX-M-15 S130G L169Q were 19 μM, 22 μM, and <1 μM, respectively.
In vitro selection of mutants resistant to the ceftazidime-avibactam combination.
The selection procedure was applied to E. coli TOP10 harboring derivatives of plasmid pTRC-99k encoding β-lactamases CTX-M-15, CTX-M-15 P167S, CTX-M-15 L169Q, and CTX-M-15 L169Q G240D. Briefly, overnight precultures were grown in brain heart infusion (BHI) broth (Difco) containing IPTG (0.5 mM) and kanamycin (50 μg/ml). Approximately 6 × 109 CFU were plated on MH agar (Difco) containing IPTG (0.5 mM), a fixed concentration of avibactam (4 μg/ml), and increasing (2-fold) concentrations of ceftazidime (0.12 to 16 μg/ml). Agar plates were inspected once a day for 7 days for the presence of CFU. For each selection experiment, 10 colonies were randomly chosen from the plate containing the highest ceftazidime concentration enabling growth. Plasmids harboring putative blaCTX-M-15 variants were extracted using a QIAprep Spin Miniprep kit (Qiagen, Courtaboeuf, France) and introduced into E. coli TOP10 by transformation. Transformants were selected for resistance to kanamycin (50 μg/ml) conveyed by the pTRC-99k vector. The MICs of ceftazidime in the absence or presence (4 μg/ml) of avibactam were determined. The blaCTX-M-15 gene of transformants exhibiting increased resistance was sequenced (Eurofins Genomics).
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Dubost and A. Marie for technical assistance in the collection of mass spectra and Z. Edoo for helpful discussion.
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00357-18.
REFERENCES
- 1.Woerther P-L, Burdet C, Chachaty E, Andremont A. 2013. Trends in human fecal carriage of extended-spectrum β-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev 26:744–758. doi: 10.1128/CMR.00023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calbo E, Garau J. 2015. The changing epidemiology of hospital outbreaks due to ESBL-producing Klebsiella pneumoniae: the CTX-M-15 type consolidation. Future Microbiol 10:1063–1075. doi: 10.2217/fmb.15.22. [DOI] [PubMed] [Google Scholar]
- 3.Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, Arlet G, Ayala J, Coque TM, Kern-Zdanowicz I, Luzzaro F, Poirel L, Woodford N. 2007. CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother 59:165–174. doi: 10.1093/jac/dkl483. [DOI] [PubMed] [Google Scholar]
- 4.Bevan ER, Jones AM, Hawkey PM. 2017. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother 72:2145–2155. doi: 10.1093/jac/dkx146. [DOI] [PubMed] [Google Scholar]
- 5.Hawkey PM. 2015. Multidrug-resistant Gram-negative bacteria: a product of globalization. J Hosp Infect 89:241–247. doi: 10.1016/j.jhin.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Fernández J, Montero I, Martínez Ó, Fleites A, Poirel L, Nordmann P, Rodicio MR. 2015. Dissemination of multiresistant Enterobacter cloacae isolates producing OXA-48 and CTX-M-15 in a Spanish hospital. Int J Antimicrob Agents 46:469–474. doi: 10.1016/j.ijantimicag.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Martínez L. 2008. Extended-spectrum beta-lactamases and the permeability barrier. Clin Microbiol Infect 14(Suppl 1):S82–S89. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 9.Ehmann DE, Jahić H, Ross PL, Gu R-F, Hu J, Kern G, Walkup GK, Fisher SL. 2012. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc Natl Acad Sci U S A 109:11663–11668. doi: 10.1073/pnas.1205073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castanheira M, Mills JC, Costello SE, Jones RN, Sader HS. 2015. Ceftazidime-avibactam activity tested against Enterobacteriaceae isolates from U.S. hospitals (2011 to 2013) and characterization of β-lactamase-producing strains. Antimicrob Agents Chemother 59:3509–3517. doi: 10.1128/AAC.00163-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupont H, Gaillot O, Goetgheluck A-S, Plassart C, Emond J-P, Lecuru M, Gaillard N, Derdouri S, Lemaire B, Girard de Courtilles M, Cattoir V, Mammeri H. 2016. Molecular characterization of carbapenem-nonsusceptible enterobacterial isolates collected during a prospective interregional survey in France and susceptibility to the novel ceftazidime-avibactam and aztreonam-avibactam combinations. Antimicrob Agents Chemother 60:215–221. doi: 10.1128/AAC.01559-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Both A, Büttner H, Huang J, Perbandt M, Belmar Campos C, Christner M, Maurer FP, Kluge S, König C, Aepfelbacher M, Wichmann D, Rohde H. 2017. Emergence of ceftazidime/avibactam non-susceptibility in an MDR Klebsiella pneumoniae isolate. J Antimicrob Chemother 72:2483–2488. doi: 10.1093/jac/dkx179. [DOI] [PubMed] [Google Scholar]
- 13.Falcone M, Paterson D. 2016. Spotlight on ceftazidime/avibactam: a new option for MDR Gram-negative infections. J Antimicrob Chemother 71:2713–2722. doi: 10.1093/jac/dkw239. [DOI] [PubMed] [Google Scholar]
- 14.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Compain F, Arthur M. 2017. Impaired inhibition by avibactam and resistance to the ceftazidime-avibactam combination due to the D179Y substitution in the KPC-2 β-lactamase. Antimicrob Agents Chemother 61:e00451-17. doi: 10.1128/AAC.00451-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palzkill T, Le QQ, Venkatachalam KV, LaRocco M, Ocera H. 1994. Evolution of antibiotic resistance: several different amino acid substitutions in an active site loop alter the substrate profile of beta-lactamase. Mol Microbiol 12:217–229. doi: 10.1111/j.1365-2958.1994.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 17.Petrosino JF, Palzkill T. 1996. Systematic mutagenesis of the active site omega loop of TEM-1 beta-lactamase. J Bacteriol 178:1821–1828. doi: 10.1128/jb.178.7.1821-1828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levitt PS, Papp-Wallace KM, Taracila MA, Hujer AM, Winkler ML, Smith KM, Xu Y, Harris ME, Bonomo RA. 2012. Exploring the role of a conserved class A residue in the Ω-loop of KPC-2 β-lactamase: a mechanism for ceftazidime hydrolysis. J Biol Chem 287:31783–31793. doi: 10.1074/jbc.M112.348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkler ML, Papp-Wallace KM, Taracila MA, Bonomo RA. 2015. Avibactam and inhibitor-resistant SHV β-lactamases. Antimicrob Agents Chemother 59:3700–3709. doi: 10.1128/AAC.04405-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Andrea MM, Arena F, Pallecchi L, Rossolini GM. 2013. CTX-M-type β-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol 303:305–317. doi: 10.1016/j.ijmm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Ourghanlian C, Soroka D, Arthur M. 2017. Inhibition by avibactam and clavulanate of the β-lactamases KPC-2 and CTX-M-15 harboring the substitution N132G in the conserved motif SDN. Antimicrob Agents Chemother 61:e02510-16. doi: 10.1128/AAC.02510-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.EUCAST. 2017. Breakpoint tables for interpretation of MICs and zone diameters, version 7.1. EUCAST, Vaxjo, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf. [Google Scholar]
- 23.Djamdjian L, Naas T, Tandé D, Cuzon G, Hanrotel-Saliou C, Nordmann P. 2011. CTX-M-93, a CTX-M variant lacking penicillin hydrolytic activity. Antimicrob Agents Chemother 55:1861–1866. doi: 10.1128/AAC.01656-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papp-Wallace KM, Winkler ML, Taracila MA, Bonomo RA. 2015. Variants of β-lactamase KPC-2 that are resistant to inhibition by avibactam. Antimicrob Agents Chemother 59:3710–3717. doi: 10.1128/AAC.04406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ripoll A, Baquero F, Novais A, Rodríguez-Domínguez MJ, Turrientes M-C, Cantón R, Galán J-C. 2011. In vitro selection of variants resistant to beta-lactams plus beta-lactamase inhibitors in CTX-M beta-lactamases: predicting the in vivo scenario? Antimicrob Agents Chemother 55:4530–4536. doi: 10.1128/AAC.00178-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas VL, Golemi-Kotra D, Kim C, Vakulenko SB, Mobashery S, Shoichet BK. 2005. Structural consequences of the inhibitor-resistant Ser130Gly substitution in TEM β-lactamase. Biochemistry 44:9330–9338. doi: 10.1021/bi0502700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juteau JM, Billings E, Knox JR, Levesque RC. 1992. Site-saturation mutagenesis and three-dimensional modelling of ROB-1 define a substrate binding role of Ser130 in class A beta-lactamases. Protein Eng 5:693–701. doi: 10.1093/protein/5.7.693. [DOI] [PubMed] [Google Scholar]
- 28.Jacob F, Joris B, Lepage S, Dusart J, Frère JM. 1990. Role of the conserved amino acids of the “SDN” loop (Ser130, Asp131 and Asn132) in a class A beta-lactamase studied by site-directed mutagenesis. Biochem J 271:399–406. doi: 10.1042/bj2710399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neuwirth C, Madec S, Siebor E, Pechinot A, Duez JM, Pruneaux M, Fouchereau-Peron M, Kazmierczak A, Labia R. 2001. TEM-89 beta-lactamase produced by a Proteus mirabilis clinical isolate: new complex mutant (CMT 3) with mutations in both TEM-59 (IRT-17) and TEM-3. Antimicrob Agents Chemother 45:3591–3594. doi: 10.1128/AAC.45.12.3591-3594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prinarakis EE, Miriagou V, Tzelepi E, Gazouli M, Tzouvelekis LS. 1997. Emergence of an inhibitor-resistant beta-lactamase (SHV-10) derived from an SHV-5 variant. Antimicrob Agents Chemother 41:838–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirot D, Labia R, Pouedras P, Chanal-Claris C, Cerceau C, Sirot J. 1998. Inhibitor-resistant OXY-2-derived β-lactamase produced by Klebsiella oxytoca. Antimicrob Agents Chemother 42:2184–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poirel L, Naas T, Le Thomas I, Karim A, Bingen E, Nordmann P. 2001. CTX-M-type extended-spectrum beta-lactamase that hydrolyzes ceftazidime through a single amino acid substitution in the omega loop. Antimicrob Agents Chemother 45:3355–3361. doi: 10.1128/AAC.45.12.3355-3361.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livermore DM, Meunier D, Hopkins KL, Doumith M, Hill R, Pike R, Staves P, Woodford N. 2018. Activity of ceftazidime/avibactam against problem Enterobacteriaceae and Pseudomonas aeruginosa in the UK, 2015–16. J Antimicrob Chemother 73:648–657. doi: 10.1093/jac/dkx438. [DOI] [PubMed] [Google Scholar]
- 34.Clinical and Laboratory Standards Institute. 2015. Methods for dilution susceptibility tests for bacteria that grow aerobically; approved standard—10th ed CLSI document M7-A8. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 35.Soroka D, Sierra-Gallay IL de la, Dubée V, Triboulet S, Tilbeurgh H van, Compain F, Ballell L, Barros D, Mainardi J-L, Hugonnet J-E, Arthur M. 2015. Hydrolysis of clavulanate by Mycobacterium tuberculosis β-lactamase BlaC harboring a canonical SDN motif. Antimicrob Agents Chemother 59:5714–5720. doi: 10.1128/AAC.00598-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.