Antibacterial activity screening of a collection of Xenorhabdus strains led to the discovery of the odilorhabdins, a new antibiotic class with broad-spectrum activity against Gram-positive and Gram-negative pathogens. Odilorhabdins inhibit bacterial translation by a new mechanism of action on ribosomes.
KEYWORDS: inhibitor, bacterial translation, carbapenem-resistant Enterobacteriaceae, preclinical candidate
ABSTRACT
Antibacterial activity screening of a collection of Xenorhabdus strains led to the discovery of the odilorhabdins, a new antibiotic class with broad-spectrum activity against Gram-positive and Gram-negative pathogens. Odilorhabdins inhibit bacterial translation by a new mechanism of action on ribosomes. A lead optimization program identified NOSO-502 as a promising candidate. NOSO-502 has MIC values ranging from 0.5 to 4 μg/ml against standard Enterobacteriaceae strains and carbapenem-resistant Enterobacteriaceae (CRE) isolates that produce KPC, AmpC, or OXA enzymes and metallo-β-lactamases. In addition, this compound overcomes multiple chromosome-encoded or plasmid-mediated resistance mechanisms of acquired resistance to colistin. It is effective in mouse systemic infection models against Escherichia coli EN122 (extended-spectrum β-lactamase [ESBL]) or E. coli ATCC BAA-2469 (NDM-1), achieving a 50% effective dose (ED50) of 3.5 mg/kg of body weight and 1-, 2-, and 3-log reductions in blood burden at 2.6, 3.8, and 5.9 mg/kg, respectively, in the first model and 100% survival in the second, starting with a dose as low as 4 mg/kg. In a urinary tract infection (UTI) model with E. coli UTI89, urine, bladder, and kidney burdens were reduced by 2.39, 1.96, and 1.36 log10 CFU/ml, respectively, after injection of 24 mg/kg. There was no cytotoxicity against HepG2, HK-2, or human renal proximal tubular epithelial cells (HRPTEpiC), no inhibition of hERG-CHO or Nav 1.5-HEK current, and no increase of micronuclei at 512 μM. NOSO-502, a compound with a new mechanism of action, is active against Enterobacteriaceae, including all classes of CRE, has a low potential for resistance development, shows efficacy in several mouse models, and has a favorable in vitro safety profile.
INTRODUCTION
Antibiotic-resistant infections are spreading around the world. The urgent need to discover new families of antibacterial agents to counter the threat of drug-resistant infection is widely recognized. The U.S. Centers for Disease Control and Prevention (CDC) recently published a report outlining the top 18 drug-resistant threats. Three were classified as “urgent” in terms of threat level: carbapenem-resistant Enterobacteriaceae (CRE), Clostridium difficile, and Neisseria gonorrhoeae (1). Carbapenems are broad-spectrum β-lactam antibiotics saved for the treatment of the most serious infections. CRE have become resistant to all or nearly all antibiotics available and cause many types of serious infections, such as infections of the respiratory tract, urinary tract and abdomen and bacteremia (2). The CDC estimates that 9,300 health care-associated infections are caused each year in the United States by the two most common types of CRE, carbapenem-resistant Klebsiella species and Escherichia coli species, which are responsible for approximately 600 deaths (1). In China, among the 664 CRE cases reported in 2015 in 25 hospitals, most were caused by Klebsiella pneumoniae (73.3%), E. coli (16.6%), or Enterobacter cloacae (7.1%), and the overall mortality rate was 33.5% (2).
Antibacterial activity screening of a collection of Xenorhabdus strains led to the discovery of the odilorhabdins (ODLs), a new antibiotic class with broad-spectrum activity against Gram-positive and Gram-negative pathogens (3). Odilorhabdins inhibit bacterial translation by a new mechanism of action on ribosomes (3). Their chemical tractability made them suitable for a lead optimization program by medicinal chemistry that led to the preclinical candidate NOSO-502 (Fig. 1).
FIG 1.
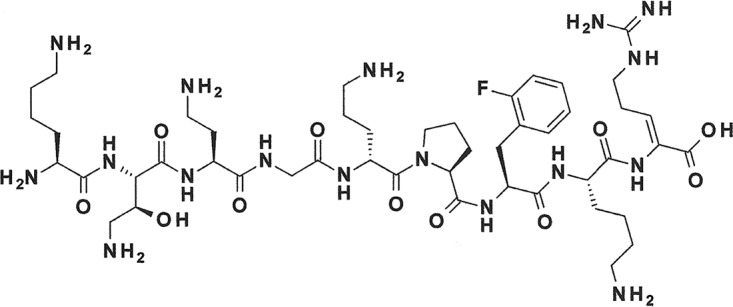
Chemical structure of NOSO-502.
We report here the in vitro and in vivo characterization of NOSO-502. The data demonstrate that NOSO-502 is active against a panel of Gram-positive and Gram-negative bacteria, including carbapenem-resistant and polymyxin-resistant strains, and exhibits promising in vivo activity in various murine infection models, a favorable in vitro safety profile, and a low potential for resistance development.
RESULTS
NOSO-502 exhibits potent antibacterial activity.
The antibacterial activity spectrum of NOSO-502 was assessed by testing a panel of Gram-positive and Gram-negative wild-type strains. The compound was active against Gram-negative pathogens of the Enterobacteriaceae family, such as E. coli or K. pneumoniae, with MIC values between 0.5 and 4 μg/ml, as well as Stenotrophomonas maltophilia. In comparison, the MIC values of NOSO-502 against Pseudomonas aeruginosa and Acinetobacter baumannii were >64 μg/ml. For Gram-positive species, NOSO-502 was more active against staphylococci than Enterococcus or Streptococcus strains (Table 1).
TABLE 1.
Bacterial susceptibility profile of NOSO-502 against reference bacterial strainsa
| Strain | MIC (μg/ml) of antibiotic |
|||||
|---|---|---|---|---|---|---|
| NOS | CIP | GEN | IPM | TGC | PMB | |
| Citrobacter freundii DSM 30039 | 2 | ≤0.125 | 0.5 | 1 | 1 | 0.5 |
| Citrobacter kozeri DSM 4595 | 2 | ≤0.125 | 0.25 | 4 | 1 | 0.25 |
| Enterobacter aerogenes DSM 30053 | 2 | ≤0.125 | 0.25 | 2 | 1 | 0.5 |
| Enterobacter cloacae DSM 14563 | 2 | ≤0.125 | 0.5 | 1 | 4 | 1 |
| Escherichia coli ATCC 25922 | 4 | ≤0.125 | 1 | 0.25 | 0.25 | 0.5 |
| Klebsiella pneumoniae ATCC 43816 | 1 | ≤0.125 | 0.25 | 1 | 2 | 1 |
| Serratia marcescens DSM 17174 | 4 | ≤0.125 | 0.5 | 2 | 4 | >32 |
| Acinetobacter baumannii ATCC 19606 | >64 | 2 | 16 | 0.5 | 1 | 0.5 |
| Pseudomonas aeruginosa DSM 1117 | >64 | 1 | 1 | 2 | >8 | 1 |
| Stenotrophomonas maltophilia ATCC 13637 | 16 | 1 | 4 | >64 | 0.5 | 1 |
| Enterococcus faecalis DSM 2570 | >64 | 2 | 16 | 1 | 0.25 | >32 |
| Enterococcus faecium DSM 20477 | 64 | 16 | 8 | 4 | 0.125 | >32 |
| Staphylococcus aureus ATCC 29213 | 1 | 0.25 | 0.5 | ≤0.125 | 0.5 | 16 |
| Staphylococcus epidermidis ATCC 12228 | 0.25 | 0.25 | ≤0.125 | ≤0.125 | 0.5 | 16 |
| Streptococcus pneumoniae DSM 2134 | 64 | 1 | 8 | ≤0.125 | 0.125 | >32 |
NOS, NOSO-502; CIP, ciprofloxacin; GEN, gentamicin; PMB, polymyxin B; IPM, imipenem; TGC, tigecycline.
The compound was also tested against a recent panel of Enterobacteriaceae clinical isolates. MIC90 values were between 2 and 8 μg/ml against E. coli, K. pneumoniae, Enterobacter cloacae, and Citrobacter freundii. The MIC range of NOSO-502 was narrow against C. freundii and E. cloacae (1 to 4 μg/ml) but wider against E. coli (1 to 32 μg/ml) and K. pneumoniae (0.5 to 16 μg/ml). Nevertheless, only 3 isolates of E. coli and K. pneumoniae out of 101 and 57 tested, respectively, exhibited MIC values slightly higher than the MIC90 (E. coli, 2 isolates at 16 μg/ml and 1 at 32 μg/ml; K. pneumoniae, 1 isolate at 4 μg/ml, 1 at 8 μg/ml, and 1 at 16 μg/ml). The antibacterial activity of NOSO-502 was conserved against fluoroquinolone-, aminoglycoside-, and polymyxin B-resistant strains in the panel (Table 2).
TABLE 2.
MICs of NOSO-502 and comparators against a panel of recent clinical bacterial strainsa
| Organism (no. of isolates) | Antibiotic | MIC (μg/ml) |
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Citrobacter freundii (16) | NOS | 1–4 | 2 | 2 |
| CIP | 0.008–>1 | 0.03 | >1 | |
| GEN | 0.5–>32 | 1 | >32 | |
| PMB | 0.25–1 | 0.5 | 1 | |
| Enterobacter cloacae (13) | NOS | 1–4 | 1 | 2 |
| CIP | 0.016–>1 | >1 | >1 | |
| GEN | 1–>32 | 32 | >32 | |
| PMB | 0.5–16 | 0.5 | 8 | |
| Escherichia coli (101) | NOS | 2–32 | 4 | 8 |
| CIP | 0.008–>1 | 0.03 | >1 | |
| GEN | 0.5–>32 | 1 | 2 | |
| PMB | 0.25–32 | 0.5 | 1 | |
| Ciprofloxacin-resistant E. coli (19) | NOS | 2–32 | 4 | 8 |
| CIP | >1 | >1 | >1 | |
| GEN | 0.13–>32 | 0.5 | >32 | |
| PMB | 0.5–32 | 0.5 | 1 | |
| Gentamicin-resistant E. coli (6) | NOS | 4 | ||
| CIP | 0.25–1 | |||
| GEN | 32–>32 | |||
| PMB | 0.25–32 | |||
| Polymyxin B-resistant E. coli (2) | NOS | 4–8 | ||
| CIP | 1–>1 | |||
| GEN | 1–>32 | |||
| PMB | 4–32 | |||
| Klebsiella pneumoniae (56) | NOS | 0.5–16 | 1 | 2 |
| CIP | 0.008–>1 | 0.5 | >1 | |
| GEN | 0.5–>32 | 1 | >32 | |
| PMB | 0.25–32 | 0.5 | 4 | |
| Ciprofloxacin-resistant K. pneumoniae (27) | NOS | 0.5–16 | 1 | 2 |
| CIP | >1 | >1 | >1 | |
| GEN | 0.13–>32 | 32 | >32 | |
| PMB | 0.5–>32 | 0.5 | 1 | |
| Gentamicin-resistant K. pneumoniae (16) | NOS | 0.5–2 | 1 | 2 |
| CIP | 0.5–>1 | >1 | >1 | |
| GEN | 32–>32 | >32 | >32 | |
| PMB | 0.5–1 | 0.5 | 1 | |
MIC50 and MIC90 were calculated for populations with >10 isolates.
MIC values of NOSO-502 were determined against selected CRE and colistin-resistant isolates. The CRE strains tested produce KPC enzymes (Ambler class A carbapenemase-producing strains), metallo-β-lactamases, such as NDM, VIM, or IMP (Ambler class B carbapenemase-producing strains), AmpC (Ambler class C carbapenem-resistant strains), and OXA-48 enzymes (Ambler class D carbapenemase-producing strains). NOSO-502 exhibited potent activity against all carbapenemase-producing Enterobacteriaceae strains (Table 3) and overcame multiple mechanisms of colistin acquired resistance (chromosome-encoded mutations or deletions of pmrA, pmrB, mgrB, or phoQ or expression of mcr-1, mcr-2, or mcr-3), except mechanisms involving mutations of the crrB gene (Table 4). The crrB gene belongs to a two-component system named crrAB. Mutations in this gene are responsible for the acquisition of colistin resistance via a lipopolysaccharide (LPS) modification and an upregulation of an RND-type efflux pump (4). The crrAB locus is variably present in K. pneumoniae genomes and absent in E. coli (5).
TABLE 3.
Activities of NOSO-502 and comparators against carbapenem-resistant Enterobacteriaceae strains
| Type of enzyme or organism and strain | β-Lactamase content | MIC (μg/ml) of antibiotic |
|||||
|---|---|---|---|---|---|---|---|
| NOS | CIP | GEN | IPM | TGC | PMB | ||
| Ambler class A carbapenemase | |||||||
| Escherichia coli PSP | KPC-2 + TEM-1 + OXA-1 | 2 | 16 | >64 | 8 | 0.5 | 0.5 |
| Escherichia coli COL | KPC-2 + TEM-1 + CTX-M9 | 2 | >64 | >64 | 8 | 0.5 | 0.5 |
| Escherichia coli MIN | KPC-3 + OXA-9 | 4 | <0.25 | 0.5 | 8 | 0.25 | 0.5 |
| Klebsiella pneumoniae ATCC BAA-1905 | KPC-2 | 1 | >64 | 32 | >64 | 4 | 0.5 |
| Klebsiella pneumoniae A33504 | KPC-2+ SHV-11 + TEM-1 + CTX-M-2 + OXA-9 | 1 | 32 | >64 | 16 | 2 | 0.5 |
| Klebsiella pneumoniae ATCC BAA-1904 | KPC-3 | 2 | 0.25 | 16 | 32 | 2 | 0.5 |
| Enterobacter cloacae KBM15 | KPC-2 + TEM-1 + OXA-9 | 1 | 32 | 8 | 32 | 8 | 0.5 |
| Ambler class B carbapenemase | |||||||
| Escherichia coli BAA-2469 | NDM-1 | 2 | 16 | >64 | 32 | 1 | 0.25 |
| Escherichia coli BAA-2471 | NDM-1 | 4 | >64 | >64 | >64 | 1 | 0.25 |
| Escherichia coli MON | NDM-5 + CTX-M15 + TEM-1 | 4 | >64 | 0.5 | 32 | 0.5 | 0.5 |
| Escherichia coli GAL | NDM-6 + OXA-1 + CTX-M15 | 2 | >64 | 2 | 32 | 0.5 | 0.25 |
| Escherichia coli EGB957 | VIM-1 + OXA-48 + TEM-1 + CMY-4 + OXA-1 | 4 | >64 | >64 | >64 | 1 | 0.5 |
| Klebsiella pneumoniae ATCC BAA-2146 | NDM-1 + CTX-M15 + TEM-1 + CMY-6 + OXA-1 + SHV-1 | 0.5 | >64 | >64 | >64 | 32 | 1 |
| Klebsiella pneumoniae NCTC 13443 | NDM-1 | 1 | >64 | >64 | >64 | 4 | 0.5 |
| Klebsiella pneumoniae LAM | NDM-4 + SHV-11 + CTX-M15 | 1 | >64 | >64 | 32 | 2 | 0.5 |
| Klebsiella pneumoniae NCTC 13439 | VIM-1 | 1 | 16 | 1 | 32 | 2 | 0.5 |
| Enterobacter cloacae 3047 | NDM-1 + CTX-M15 + TEM-1 + OXA-1 | 1 | 2 | 32 | 16 | 4 | 0.5 |
| Ambler class C carbapenem resistant | |||||||
| Enterobacter cloacae 10.72 | AmpC overexpressed + TEM-1 + OXA-1 | 1 | >64 | >64 | 4 | 8 | 0.5 |
| Citrobacter freundii MAU | AmpC overexpressed + TEM-3 | 1 | >64 | 2 | 4 | 8 | 0.5 |
| Ambler class D carbapenemase | |||||||
| Escherichia coli DOV | OXA-48 + TEM-1 + CTX-M15 + OXA-1 | 2 | >64 | 32 | 4 | 2 | 0.5 |
| Klebsiella pneumoniae NCTC 13442 | OXA-48 | 1 | 4 | 0.25 | 16 | 2 | 0.5 |
| Klebsiella pneumoniae DUB | OXA-48 + CTX-M15 + TEM-1 + SHV-1 + OXA-1 + CMY-2 | 0.5 | >64 | >64 | 16 | 4 | 0.5 |
| Enterobacter cloacae YAM | OXA-48 + CTX-M15 + TEM-1 + OXA-1 + DHA-1 | 2 | >64 | >64 | 4 | 4 | 1 |
| Enterobacter cloacae BEU | OXA-48 + CTX-M15 + SHV-12 + TEM-1 + OXA-1 + DHA-1 | 1 | 32 | >64 | 16 | 16 | 16 |
TABLE 4.
Bacterial susceptibility profile of NOSO-502 against colistin-resistant strains
| Type of strain and gene mutation or gene conferring resistance (no. of isolates) | MIC range (μg/ml) |
|
|---|---|---|
| NOS | CSTa | |
| Escherichia coli, colistin-resistant (25) | ||
| mcr-1 (21) | 1–4 | 4–16 |
| mcr-2 (1) | 1 | 8 |
| mcr-3 (1) | 1 | 16 |
| Unknown (2) | 1–2 | 16 |
| Klebsiella pneumoniae, colistin resistant (46) | ||
| pmrA G53S substitution (2) | 1–2 | 32–128 |
| pmrB T157P substitution (6) | 0.25–16 | 8–32 |
| pmrB L17Q substitution (1) | 1 | 32 |
| phoQ R16C substitution (1) | 2 | >128 |
| mgrB full deletion (5) | 0.5–1 | 16–>128 |
| mgrB premature termination (6) | 0.5–1 | 32–128 |
| mgrB IS5 between +74 and +75 (4) | 0.5–2 | 16–128 |
| mgrB W20R substitution (1) | 0.5 | 32 |
| mgrB W47R substitution (1) | 1 | 8 |
| mgrB IS1R into promoter between −45 and −46 (3) | 0.5–1 | 32–128 |
| mgrB M27K substitution (1) | 2 | 32 |
| mgrB N42Y and K43I substitutions (1) | 2 | 32 |
| mgrB ISKpn14 into promoter between −28 and −29 (2) | 0.5–2 | 64 |
| mgrB IS903B between +69 and +70 (2) | 0.25–1 | 64 |
| mgrB IS1R between +44 and +45 (2) | 1 | 128 |
| mgrB ISKpn26-like between +74 and +75 (2) | 1 | 128–>128 |
| crrB P151L substitution (1) | 128 | >128 |
| crrB G183V substitution (1) | 64 | >128 |
| crrB F84S substitution (1) | 8 | 128 |
| crrB N141Y substitution (1) | 16 | >128 |
| mcr-1 (1) | 0.5 | 32 |
| Unknown (1) | 0.5 | 32 |
CST, colistin.
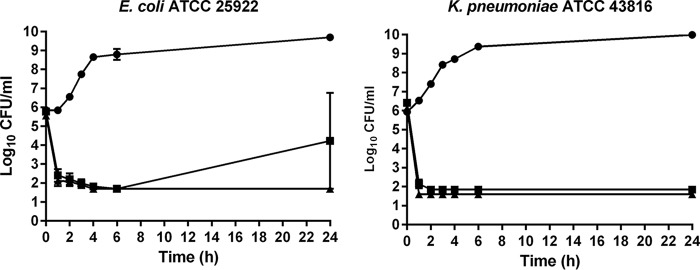
NOSO-502 had rapid bactericidal activity against E. coli ATCC 25922 and K. pneumoniae ATCC 43816, causing a 3-log decrease in CFU per milliliter at 1 h (4× and 8× MIC) (Fig. 2). We observed regrowth of E. coli at 4× MIC. Such regrowth at 24 h is not uncommon and has previously been reported for bactericidal antimicrobials, such as ciprofloxacin against E. coli (6).
FIG 2.
Bactericidal activity of NOSO-502 at 4× and 8× MIC against E. coli ATCC 25922 and K. pneumoniae ATCC 43816. Closed circles, drug-free control; closed squares, NOSO-502 at 4× MIC; closed triangles, NOSO-502 at 8× MIC. Experiments were performed in triplicate. Each symbol represents the mean, and error bars indicate the SEMs.
The propensity of bacteria to develop resistance to NOSO-502 was assessed by determining the spontaneous frequency of resistance (FoR) to the compound with E. coli ATCC 25922 and K. pneumoniae ATCC 43816. Mutants of E. coli resistant to 4× MIC (16 μg/ml) or 8× MIC (32 μg/ml) of NOSO-502 were isolated at frequencies of 3.0 × 10−9 and <5.0 × 10−10, respectively. The frequencies of resistance of K. pneumoniae were 2.4 × 10−9 at 4× MIC (4 μg/ml) and <7 × 10−10 at 8× MIC (8 μg/ml).
NOSO-502 has a good in vitro safety profile.
The potential nephrotoxicity of NOSO-502 was assessed in cells derived directly from human kidney tissue, human renal proximal tubular epithelial cells (HRPTEpiC) and HK-2 cells. A multiplexed assay with HRPTEpiC was used to assess cellular stress induced in vitro by NOSO-502. Three parameters were measured: a decrease in cell viability, the expression of heat shock protein 27 (HSP27), and the level of kidney injury molecule 1 (KIM-1). A decrease in cell viability is a very sensitive marker to detect general toxicity but is not sufficient to predict nephrotoxicity, whereas increases in the level of biomarkers, such as KIM-1 or HSP27, are well correlated with dose levels of known nephrotoxic compounds (7, 8). HSP27 is expressed in response to cellular stress to block the apoptotic pathway. KIM-1 is a well-accepted marker of renal proximal tubule injury. NOSO-502 showed no cytotoxicity to HRPTEpiC and the molecule did not significantly increase (5-fold) KIM-1 or HSP27 levels at concentrations up to 100 μM (0.1-fold and 1.5-fold increases at 100 μM in KIM-1 and HSP27 signals, respectively). Polymyxin B and gentamicin, used as comparators in this study, showed different toxicity profiles. Polymyxin B was cytotoxic at low concentrations (50% inhibitory concentration [IC50] = 11.8 μM) and induced a significant increase of KIM-1 and HSP27 levels at 12.1 and 9.7 μM, respectively. Gentamicin was not cytotoxic and did not increase KIM-1 levels at concentrations up to 100 μM but induced a 5-fold increase of HSP27 levels at 22.4 μM. NOSO-502 did not show any effect on HK-2 cell viability at concentrations up to 512 μM (0% inhibition from 16 to 256 μM and 9.4% inhibition at 512 μM).
The cardiotoxic effect of NOSO-502 was evaluated using the automated patch clamp human ether-a-go-go related gene (hERG) potassium channel assay. This test is now accepted as an early predictor of potential cardiotoxicity and is used routinely at an early stage in the drug discovery process. NOSO-502 did not significantly inhibit hERG currents at concentrations up to 512 μM (2.6% inhibition at 256 μM and 1.9% inhibition at 512 μM). We also measured the effect of the compound on the voltage-gated cardiac sodium ion channel Nav 1.5. This channel is a key component for the initiation and transmission of the electrical signal throughout the heart. The IC50 of NOSO-502 in the patch clamp Nav 1.5 sodium channel assay was higher than 512 μM.
The genotoxic potential of NOSO-502 was investigated using the micronucleus (MN) assay. This test detects both aneugenic (whole chromosome) and clastogenic (chromosome breakage) damage in interphase cells (9). There was no significant increase of micronuclei in cells treated with 512 μM NOSO-502 versus an S9 medium negative control (0.61% cells with micronuclei for NOSO-502 versus 0.7% for S9 medium).
NOSO-502 had no cytotoxic effect against mammalian HepG2 (human hepatocellular carcinoma) cells at concentrations up to 512 μM (0% inhibition from 16 to 256 μM and 4.2% inhibition at 512 μM) and did not show any hemolytic activity at 100 μM. The compound (10 μM) had no significant activity against any of the 55 cell surface receptors or enzymes tested in a broad-based screen.
NOSO-502 is resistant to biotransformation by hepatocytes and microsomes.
NOSO-502 was resistant to biotransformation when incubated in mouse, rat, dog, monkey, and human liver microsomes and hepatocytes during the in vitro study conducted to evaluate metabolic stability. After 45 min, 70.5 to 78.6% of NOSO-502 remained after incubation with microsomes of the different species. The half-lives of NOSO-502 were 116, 129, 101, 147, and 145 min in mouse, rat, dog, monkey, and human liver microsomes, respectively. After 60 min, 79.5 to 91.9% of NOSO-502 remained after incubation with hepatocytes of the different species. The half-lives of NOSO-502 in hepatocytes were 192, 194, 483, 698, and 329 min for mouse, rat, dog, monkey, and human microsomes, respectively.
NOSO-502 shows variable stability in plasma of different species.
NOSO-502 showed variable stability to biotransformation when incubated in mouse, rat, dog, monkey, and human plasma; 10.1 to 61.2% of NOSO-502 remained after incubation with plasma of the different species over the 120-min test period. The half-lives of NOSO-502 were 54, 36, 158, 96, and 79 min in mouse, rat, dog, monkey, and human plasma, respectively.
Pharmacokinetics.
The pharmacokinetics of NOSO-502 were evaluated in normal female CD-1 mice or normal female Sprague-Dawley rats. NOSO-502 was administered intravenously at 30 mg/kg of body weight to mice and 15 mg/kg to rats. The concentration-versus-time curves and the results of the pharmacokinetic analysis are summarized in Fig. 3. In mice, NOSO-502 displayed moderate clearance (1.13 liters/h/kg), a moderate volume of distribution (0.66 liters/kg), and a half-life of 25 min. The pharmacokinetics of NOSO-502 in rats showed a longer half-life (90 min) but were consistent with the results in mice, with a plasma clearance of 1.92 liters/h/kg and a volume of distribution of 0.94 liter/kg. NOSO-502 showed moderate plasma protein binding, with 19.8, 20.5, 17.6, and 18.7% unbound in mouse, rat, dog, and human plasma, respectively.
FIG 3.
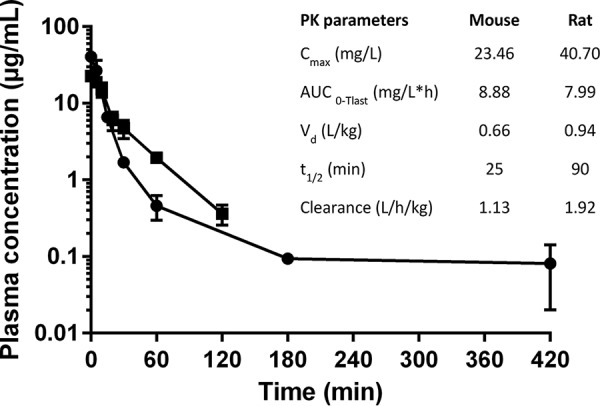
Pharmacokinetic studies with CD-1 mice (closed squares) and Sprague-Dawley rats (closed circles) following intravenous dosing at 30 and 15 mg/kg, respectively. Each symbol represents the mean, and error bars indicate the SEMs.
NOSO-502 shows efficacy in several murine infection models.
The efficacy of NOSO-502 was evaluated in murine infection models to determine whether NOSO-502 has potential as a clinical therapy. In vivo efficacy studies were conducted by administering NOSO-502 subcutaneously (s.c.). The efficacy of NOSO-502 was first assessed in a neutropenic murine sepsis infection model. This model, with E. coli EN122 (extended-spectrum β-lactamase [ESBL], clinical isolate; MIC of NOSO-502 = 4 μg/ml), was established in female NMRI mice. NOSO-502 was administered subcutaneously 1 h postinoculation at set concentrations of 1.3, 2.5, 5, 10, 20, and 40 mg/kg, whereas colistin was administered by the same route at 5 mg/kg. Five hours postchallenge, blood samples were collected, and the mice were euthanized. Blood was serially plated and colonies enumerated to determine the CFU per milliliter of blood. NOSO-502 was highly effective, achieving a 50% effective dose (ED50) of 3.5 mg/kg and 1-, 2-, and 3-log reductions in blood burden at 2.6, 3.8, and 5.9 mg/kg, respectively (Fig. 4).
FIG 4.
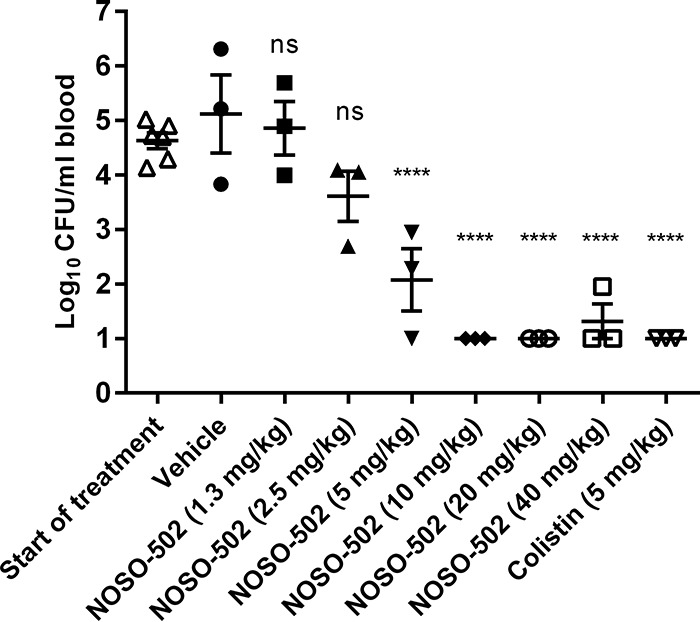
Efficacy of NOSO-502 and colistin in a neutropenic murine sepsis infection model against E. coli EN122. Each symbol represents an individual mouse, and the horizontal line indicates the mean. Error bars indicate the SEMs. Statistically significant reduction versus vehicle control (one-way analysis of variance [ANOVA], Dunnett's comparison) is indicated as follows: ns, not significant; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
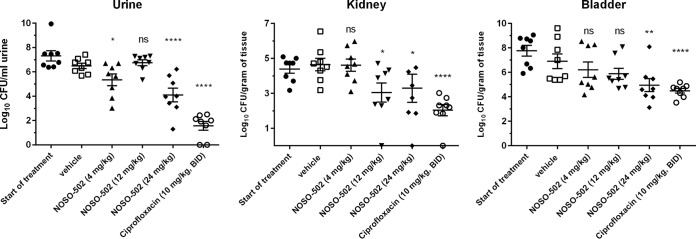
A mouse E. coli UTI89 (MIC of NOSO-502 = 4 μg/ml) upper urinary tract infection model was established in female C3H/HeN mice. Administration of 24 mg/kg of NOSO-502 once daily resulted in a statistically significant reduction in urine, bladder, and kidney burdens relative to vehicle control animals. At 4 days postinfection, NOSO-502 reduced the urine burden by 2.39 log10 CFU/ml (P ≤ 0.0001), the bladder burden by 1.96 log10 CFU/ml (P = 0.0012), and the kidney burden by 1.36 log10 CFU/ml (P = 0.0123) relative to vehicle (Fig. 5).
FIG 5.
Efficacy of NOSO-502 and ciprofloxacin in a murine UTI model against E. coli UTI89. Each symbol represents an individual mouse, and the horizontal line indicates the mean. Error bars indicate the SEMs. Statistically significant reduction versus vehicle control (Kruskal-Wallis statistical test, multiple comparison) is indicated as follows: ns, not significant; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
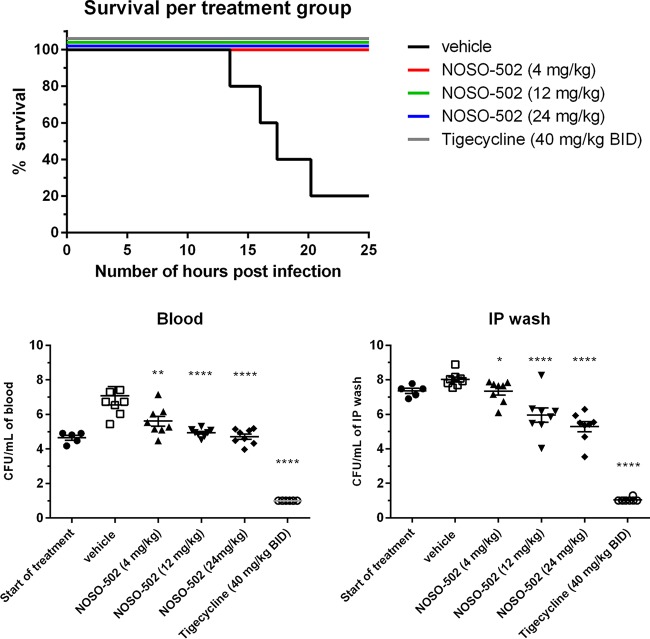
A neutropenic mouse E. coli ATCC BAA-2469 (NDM-1; MIC of NOSO-502 = 2 μg/ml) intraperitoneal (i.p.) sepsis infection model was established in male CD-1/ICR mice. Ninety percent of the vehicle-treated mice succumbed to infection prior to the end of the study. All NOSO-502-treated mice (4, 12, and 24 mg/kg) survived up to the end of the study at 24 h (P = 0.0009 relative to vehicle). The vehicle group had mean and median survival times of 19.8 h and 20.2 h, respectively. One subcutaneous administration of NOSO-502 resulted in statistically significant dose-dependent reductions in blood and i.p. wash burdens relative to vehicle control animals at all doses. Treatment with 4 mg/kg of NOSO-502 reduced the blood and i.p. wash burdens by 1.48 log10 CFU/ml (P = 0.0081) and 0.68 log10 CFU/ml (P = 0.0145), respectively. Treatment with 12 mg/kg reduced the blood burden by 2.14 log10 CFU/ml (P < 0.0001) and the i.p. wash burden by 2.07 log10 CFU/ml (P ≤ 0.0001), and treatment with 24 mg/kg reduced the blood burden by 2.37 log10 CFU/ml (P ≤ 0.0001) and the i.p. wash burden by 2.74 log10 CFU/ml (P ≤ 0.0001) (Fig. 6).
FIG 6.
Efficacy of NOSO-502 and tigecycline in a survival neutropenic sepsis infection model against E. coli ATCC BAA-2469 (NDM-1). Each symbol represents an individual mouse, and the horizontal line indicates the mean. Error bars indicate the SEMs. Statistically significant reduction versus vehicle control (Kruskal-Wallis statistical test, multiple comparison) is indicated as follows: ns, not significant; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
A neutropenic mouse K. pneumoniae NCTC 13442 (OXA-48; MIC of NOSO-502 = 1 μg/ml) lung infection model was established in male CD-1/ICR mice. NOSO-502 was administered subcutaneously 2 h, 8 h, 14 h, and 20 h postinoculation at set concentrations of 2, 6, and 20 mg/kg (equivalent to 8, 24, and 80 mg/kg/day), whereas tigecycline was administered by the same route at 40 mg/kg (equivalent to 160 mg/kg/day). NOSO-502 was also administered once 2 h postinoculation at 80 mg/kg. Twenty-six hours postchallenge, mice were euthanized, and the lungs were collected. Administration of NOSO-502 resulted in statistically significant reductions in lung burdens relative to vehicle control animals at all doses. Treatment with 8, 24, and 80 mg/kg/day of NOSO-502 reduced the lung burden by 2.69, 3.99, and 4.07 log10 CFU/gram of lung tissue, respectively (P ≤ 0.0001). Treatment with 80 mg/kg once reduced the lung burden by 3.98 log10 CFU/gram of lung tissue (P ≤ 0.0001), and treatment with 160 mg/kg/day of tigecycline reduced the lung burden by 3.14 log10 CFU/gram of lung tissue (P ≤ 0.0001) (Fig. 7).
FIG 7.
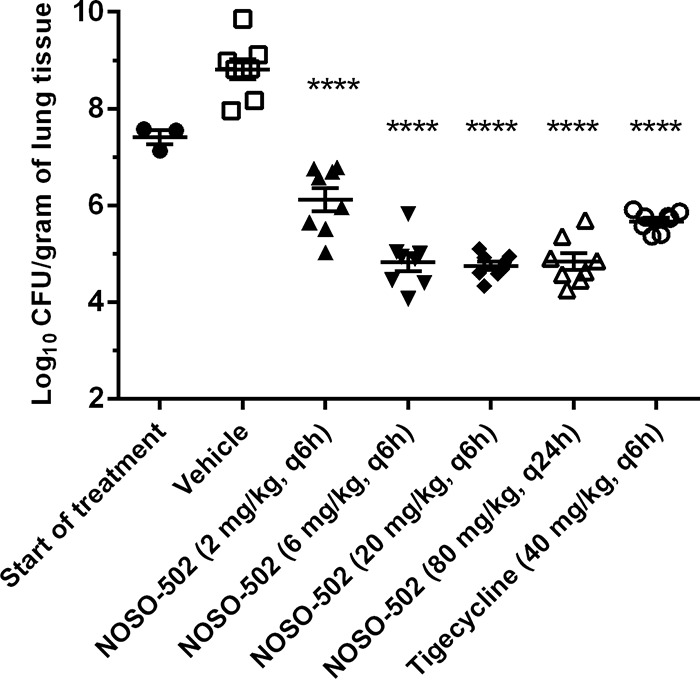
Efficacy of NOSO-502 and tigecycline in a murine lung infection model against K. pneumoniae NCTC 13442 (OXA-48). Each symbol represents an individual mouse, and the horizontal line indicates the mean. Error bars indicate the SEMs. Statistically significant reduction versus vehicle control (one-way ANOVA, Dunnett's comparison) is indicated as follows: ns, not significant; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
DISCUSSION
The urgent need to discover new antibiotics active against Gram-negative bacteria with a novel mechanism of action to counter the threat of drug-resistant infection is widely recognized. NOSO-502 is the first preclinical candidate of a novel antibiotic class, the odilorhabdins (ODLs). ODLs are cationic peptides that inhibit bacterial translation by a novel mechanism of action. ODLs bind to the small subunit of bacterial ribosomes at a site not exploited by any known ribosome-targeting antibiotic. When bound to the ribosome, ODLs make contacts with both the rRNA and tRNA and kill bacteria by interfering with the decoding of genetic information and inhibiting ribosome progression along the mRNA in a context-specific manner (3).
NOSO-502 is active against Enterobacteriaceae, including CRE belonging to all classes of the Ambler classification and resistant to gentamicin, polymyxin B, or tigecycline. This is crucial, because these antibiotics, classically used for the treatment of such infections, are associated with high levels of resistance, ranging from 9.7 to 51.3% (mean, 22.6%) for colistin, 5.6 to 85.4% (mean, 43.5%) for gentamicin, and 0 to 33% (mean, 15.2%) for tigecycline (10, 11, 12, 13, 14, 15, 16, 17, 18, 19). Current options to address these resistance issues are not entirely satisfactory, because none of the recently approved antibiotics or those under development are effective against all CRE. The combination ceftazidime-avibactam displays in vitro activity against CRE isolates that produce KPC, AmpC, and OXA enzymes. However, this drug is not active against metallo-β-lactamases, such as NDM, IMP, or VIM (20). This combination was approved by the U.S. Food and Drug Administration (FDA) in 2015 and by the European Medicines Agency in 2016 for treating complicated urinary tract (cUTI) and intra-abdominal infections. Another drug approved by the FDA in 2017 for treatment of adult patients with cUTI (including acute pyelonephritis) is the combination meropenem-vaborbactam. But this drug is active only against KPC-producing strains within the CRE group (21). Except eravacycline (a new tetracycline), none of the investigational antimicrobials (plazomicin, a new aminoglycoside, or omadacycline, a new tetracycline) or novel combinations, such as aztreonam and avibactam or imipenem and relebactam-cilastatin, are effective against all classes of carbapenemases like NOSO-502 (22, 23). Recently, CRE have caused numerous outbreaks of severe nosocomial infections and have become endemic in several countries (24, 25, 26, 27, 28). These infections have been associated with mortality rates exceeding 50% in some reports (29, 30, 31, 32). NOSO-502 can overcome multiple mechanisms of colistin-resistant strains. Furthermore, the compound demonstrated rapid bactericidal activity and a low potential for the development of resistance.
NOSO-502 is effective in mouse models of serious hospital-acquired infections. It provided significant protection against the Gram-negative pathogens E. coli and K. pneumoniae, the highest-incidence pathogens in complicated intra-abdominal and urinary tract infections, in septicemia following peritoneal challenge, and in acute pyelonephritis. NOSO-502 was active in mouse infection models against E. coli strains expressing the metallo-β-lactamase NDM-1 and resistant to other major antibiotic classes, including fluoroquinolones, macrolides, aminoglycosides, β-lactams, cephalosporins, and carbapenems. These results are encouraging and show the strong potential for in vivo efficacy of NOSO-502. Effective doses will be optimized after the best dosing schedule is defined during a pharmacokinetic-pharmacodynamic (PKPD) study.
NOSO-502 showed a good safety profile, with no in vitro nephrotoxicity, cardiotoxicity, genotoxicity, or cytotoxicity at concentrations up to 512 μM. Nephrotoxicity is a serious side effect of many drugs, including cationic antibiotics aminoglycosides and polymyxins (33, 34, 35). Polymyxins accumulate extensively within proximal tubular cells (PTCs) of the kidneys, where they induce damage, which may lead to acute kidney injury (AKI) in patients (36). AKI is the major dose-limiting adverse effect of this class of antibiotics and affects 50 to 60% of patients receiving them (35, 37). Aminoglycosides are filtered across the glomerulus and then excreted, with 5 to 10% of a parenteral dose being taken up and sequestered by the PTCs, in which the aminoglycoside can achieve high concentrations (38). AKI due to acute tubular necrosis is a relatively common complication of aminoglycoside therapy and affects 10 to 20% of patients (33, 34). The results of NOSO-502 on HRPTEpiC and HK-2 cells are promising but must be confirmed by histopathological examination of kidney cells following in vivo administration to animals, the standard assay for studying nephrotoxicity effects.
Cardiotoxicity issues are associated with many antibiotics, including macrolides, ketolides, and fluoroquinolones. These classes have been associated with prolongation of cardiac repolarization. All these agents produce a blockage of the hERG channel-dependent potassium current in myocyte membranes, resulting in a prolonged QTc interval, which may give rise to ventricular fibrillation or tachycardia (39). Nav 1.5 is another channel involved in cardiotoxicity issues. Its activation induces depolarization of the cell membrane. Failure of the Nav 1.5 sodium channel to adequately conduct the electrical current across the cell membrane can result in a potentially fatal disorder. NOSO-502 did not show any effects on hERG or Nav 1.5 channels at high concentrations.
In this study, we confirmed that NOSO-502, like many other therapeutic peptides, is safe and highly selective. NOSO-502 interacts strongly with a specific site on the 30S subunit of bacterial ribosomes but has no significant activity against any of the 55 cell surface receptors, transporters, or ion channels tested. There is increasing interest in peptides in pharmaceutical research and development (R&D), and approximately 140 are currently being evaluated in clinical trials and more than 500 are in preclinical development (40, 41). The main limitation of peptides is their predisposition to enzymatic degradation. Thus, most do not circulate in blood for more than a few minutes, preventing their usefulness as therapeutic agents. However, NOSO-502 showed good stability in plasma, microsomes, and hepatocytes, probably due to the presence in its structure of three nonstandard amino acid residues: α,γ-diamino-β-hydroxybutyric acid [Dab(βOH)] at position 2 (N terminus), α,β-dehydroarginine (Dha) at position 9 (C terminus), and d-ornithine at position 5. This translates into relatively long half-lives in mice and rats.
NOSO-502 represents a new class of very promising bacterial ribosomal inhibitors to combat bacterial multidrug resistance.
MATERIALS AND METHODS
Bacterial strains and antimicrobial agents.
Reference strains are from the German Collection of Microorganisms and Cell Cultures (DSMZ), the American Type Culture Collection (ATCC), the National Collection of Type Cultures (NCTC), and the Medical and Molecular Microbiology, Faculty of Science and Medicine, University of Fribourg, Switzerland. Clinical strains used to determine the MIC90 of NOSO-502 came from hospitals in Warsaw, Poland, Copenhagen, Denmark, Cardiff, UK, and Madrid, Spain. NOSO-502 was synthesized at Nosopharm, Nîmes, France. Ciprofloxacin (Sigma-Aldrich; no. 1134335), gentamicin (Sigma-Aldrich; no. G1397), imipenem (Sigma-Aldrich; no. IO160), polymyxin B (Sigma-Aldrich; no. 92283), and tigecycline (Sigma-Aldrich; no. PZ0021) were provided by the manufacturer as standard powders except for gentamicin and polymyxin B, in solution at 50 and 20 mg/ml, respectively.
MIC.
MIC values were determined using Clinical and Laboratory Standards Institute (CLSI) broth microdilution methodology, colony direct suspension, as described in CLSI document M07-A10 (42).
Time-dependent killing.
Time-kill assays were performed by the broth macrodilution method, according to the CLSI guideline M26-A (43).
Determination of mutation frequency.
Bacterial strains were grown in antibiotic-free Luria-Bertani broth at 35°C for 18 h. Approximately 109 CFU of each strain was plated in duplicate onto Mueller-Hinton agar (MHA) plates containing NOSO-502 concentrations at 4× and 8× the MIC values. The plates were read after 24 and 48 h of incubation at 35°C. The frequency of selected resistant mutants was calculated as the ratio of the number of bacteria growing divided by the number of bacteria in the original inoculum, which was calculated by plating several dilutions of the original inoculum.
Multiplexed HRPTEpiC cytotoxicity assay.
The multiplexed cytotoxicity assay on human renal proximal tubule epithelial cells (HRPTEpiC) was conducted by Eurofins Panlabs (Eurofins Panlabs, Inc., St. Charles, MO) by using an image-based high content analysis (HCA) technique where cells were fixed and stained with nuclear dye to visualize nuclei and fluorescently labeled antibodies to detect drug-induced cellular injury and cellular stress arising from oxidative and chemical stress. Cells were seeded into 384-well plates and grown in RPMI 1640, 10% fetal bovine serum (FBS), 2 mM l-alanyl-l-glutamine, and 1 mM sodium pyruvate in a humidified atmosphere of 5% CO2 at 37°C. NOSO-502, gentamicin, and polymyxin B were added 24 h after cell seeding. Compounds were serially diluted 3.16-fold and assayed over 10 concentrations in a final assay concentration of 0.5% dimethyl sulfoxide (DMSO) from 100 μM to 3.7 nM. At the same time, a time zero untreated cell plate was generated. After a 48-h incubation period, cells were fixed and stained with fluorescently labeled antibodies and nuclear dyed to allow visualization of nuclei, injured cells, and stressed cells. Injured cells were detected using a KIM-1 (kidney injury molecule 1) antibody. Stressed cells were detected using an anti-HSP27 (heat shock protein 27) antibody. Cell proliferation was measured by the signal intensity of the incorporated nuclear dye. The cell proliferation assay output was referred to as the relative cell count. To determine the cell proliferation endpoint, the cell proliferation data output was transformed to percentage of control (POC) using the following formula: POC = relative cell count in compound wells/relative cell count in vehicle wells × 100. The signal intensity of the incorporated cellular stress and injury measurements were normalized with the relative cell count from each well. Automated fluorescence microscopy was carried out using a Molecular Devices ImageXpress micro-imager, and images were collected with a 4× objective.
Cytotoxicity testing.
HK-2 and HepG2 cell cytotoxicity assays were run by Eurofins-Cerep (Cerep cytotoxicity profile; Eurofins-Cerep SA, Poitiers, France) as described in reference 44. Cell viability was measured using a luciferase-coupled ATP-quantitation assay (CellTiter-Glo; Promega, Madison, WI). In this assay, the luminescent signal is proportional to the amount of ATP and thus to the number of metabolically competent cells; cell injury and death result in a marked decrease in intracellular ATP levels. HK-2 and HepG2 cells were dispensed at 6,000 to 3,000 cells/5 μl/well in white tissue-culture treated 96-well solid-bottom assay plates and incubated at 37°C for 16 h to allow cell attachment, followed by the addition of NOSO-502 at 16, 32, 64, 128, 256, and 512 μM. After compound addition, plates were incubated for 48 h at 37°C. At the end of the incubation period, 5 μl of CellTiter-Glo reagent was added, the plates were incubated at room temperature for 30 min, and the luminescence intensity of each well was determined. Each experiment was carried out in duplicate.
hERG tail current inhibition.
Inhibition of the human ether-a-go-go-related gene (hERG) cardiac potassium ion channel was determined by Eurofins Panlabs (Eurofins Panlabs, Inc., St. Charles, MO) in CHO-K1 (Chinese hamster ovary) cells stably transfected with human hERG cDNA using QPatch automated whole-cell patch clamp electrophysiology as described in reference 45. NOSO-502 was tested at 64, 256, and 512 μM. In this method, the extracellular solution (control) is applied first and the cell is stabilized in the solution for 5 min. Then the test compound is applied from low to high concentrations sequentially on the same cell, with 5 min for each test concentration, at room temperature.
Nav 1.5 peak current inhibition.
Inhibition of the Nav 1.5 human sodium ion channel was determined by Eurofins Panlabs in HEK-293 cells stably transfected with human Nav1.5 cDNA (type V voltage-gated sodium channel alpha subunit, GenBank accession number NM_000335) using IonWorks Quattro automated whole-cell patch clamp electrophysiology. NOSO-502 was tested at 4, 8, 16, 32, 64, 128, 256, and 512 μM. In this method, the voltage protocol is applied prior to compound addition (Pre), the compounds are added and incubated for 600 s at room temperature, and then the voltage protocol is applied a final time (Post) on the IonWorks Quattro.
In vitro micronucleus assay.
The test was conducted by Eurofins Panlabs. CHO-K1 cells were preloaded with a cell dye that stains the cytoplasm, after which the cells were treated with NOSO-502 at 32, 64, 128, 256, and 512 μM for 24 h. At the end of the incubation period the cells were fixed, and their DNA was stained with Hoechst. The visualization and scoring of the cells was done using an automated fluorescence microscope coupled with proprietary automated image analysis software (46). The percent micronucleated cells was calculated. A marginally positive result (“−/+”) is defined as a value significantly higher than controls (t test, P < 0.05) and at least 2-fold higher than controls. A positive result (“+”) is defined as a value significantly higher than controls (t test, P < 0.05) and at least 3-fold higher than controls.
Hemolytic activity.
Mouse red blood cells were washed with phosphate-buffered saline (PBS) and resuspended in PBS to 10% (vol/vol). NOSO-502 was tested at a final concentration of 100 μM. PBS and deionized water were used as 0 and 100% hemolytic controls, respectively. Assays were incubated at 35°C for 45 min. The release of hemoglobin in the supernatant was monitored by absorbance at 540 nm. Experiments were performed in triplicate.
Hepatocyte stability.
The hepatocyte metabolic stability assays were performed by Cyprotex Discovery Ltd. (Macclesfield, UK). This assay utilizes cryopreserved pooled hepatocytes from different species (mouse, rat, dog, monkey, and human), stored in liquid nitrogen prior to use. Williams E media supplemented with 2 mM l-glutamine, 25 mM HEPES, and NOSO-502 (NOSO-502 final substrate concentration of 1 μM, test compound prepared in water; control compound final substrate concentration of 3 μM, final DMSO concentration of 0.25%) were preincubated at 37°C prior to the addition of a suspension of cryopreserved hepatocytes (final cell density of 0.5 × 106 viable cells/ml in Williams E media supplemented with 2 mM l-glutamine and 25 mM HEPES) to initiate the reaction. The final incubation volume was 500 μl. Two control compounds were included with each species alongside an appropriate vehicle control. The reactions were stopped by transferring an aliquot of the mixture to 40% trichloroacetic acid (TCA) in water, containing an internal standard for the test compounds (NOSO-95216; 1 μM final concentration) or methanol for the control compounds, at various time points (0, 5, 10, 20, 40, and 60 min). The termination plates were centrifuged at 2,500 rpm at 4°C for 30 min to precipitate the protein. Following protein precipitation, the test compound sample supernatants were diluted with analytical-grade water, whereas the control compounds were diluted with an internal standard (metoprolol) in water. The test compound samples were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Microsome stability.
The microsome metabolic stability assays were performed by Cyprotex Discovery Ltd. (Macclesfield, UK). Pooled microsomes from different species (mouse, rat, dog, monkey, and human) were stored at −80°C prior to use. Microsomes (final protein concentration of 0.5 mg/ml), 0.1 M phosphate buffer (pH 7.4), and NOSO-502 (test compound final substrate concentration of 1 μM, test compound prepared in water; control compound final substrate concentration of 3 μM, final DMSO concentration of 0.25%) were preincubated at 37°C prior to the addition of NADPH (final concentration of 1 mM) to initiate the reaction. The final incubation volume was 500 μl. A minus cofactor control incubation was included for each compound tested, in which 0.1 M phosphate buffer (pH 7.4) was added instead of NADPH (minus NADPH). Two control compounds were included with each species. Each compound was incubated for 0, 5, 15, 30, and 45 min. The control (minus NADPH) was incubated for 45 min only. The reactions were stopped by transferring an aliquot of the mixture to 40% TCA in water containing an internal standard (NOSO-95216, 1 μM final concentration) for the test compounds, or methanol for the control compounds, at the desired time points. The termination plates were centrifuged at 2,500 rpm for 20 min at 4°C to precipitate the protein. Following protein precipitation, the test compound sample supernatants were diluted with analytical-grade water, whereas the control compounds were diluted with an internal standard (metoprolol) in water. The test compound samples were analyzed by LC-MS/MS.
Plasma stability.
The plasma stability assays were performed by Cyprotex Discovery Ltd. Species-specific plasma (heparin anticoagulant) was adjusted to pH 7.4 at 37°C, and NOSO-502 or control compound (test compound final substrate concentration of 10 μM, test compound prepared in water; control compound final substrate concentration of 1 μM, final DMSO concentration of 2.5%) was added to initiate the reaction. The final incubation volume was 200 μl. All incubations were performed singularly for each compound at each time point. A vehicle control incubation was included using either water or DMSO, along with a control compound known to be metabolized specifically by each species. Each compound was incubated for 0, 15, 30, 60, and 120 min at 37°C. The reactions were stopped by the addition of 40% TCA in water containing an internal standard (NOSO-95216, 1 μM final concentration) for the test compounds, or methanol for the control compounds, at the appropriate time points. The vehicle control incubation was incubated for 120 min only. The termination plates were centrifuged at 3,000 rpm for 45 min at 4°C to precipitate the protein. Following protein precipitation, the test compound sample supernatants were diluted with analytical-grade water, whereas the control compounds were diluted with an internal standard (metoprolol) in water. The test compound samples were analyzed by LC-MS/MS.
Selectivity profile.
The affinity of NOSO-502 tested at 10 μM was assessed using radioligand binding assays for 55 cell surface receptors, transporters, and ion channels by Eurofins-Cerep (Cerep diversity profile; Eurofins-Cerep SA, Poitiers, France). Receptors tested included those to adenosine (A1, A2A, and A3), adrenergic agonists (alpha1, alpha2, beta1, and beta2), angiotensin II (AT1), bradykinin (B2), cannabinoid (CB1), chemokines (CCCR1 and CXCR2) cholecystokinin (CCK1), dopamine (D1 and D2S), endothelin (ETa), γ-aminobutyric acid (GABA) nonselective, galanine, (GAL2), histamine (H1 and H2), melanocortin (MC4), muscarinic agonists (M1, M2, and M3), neurokinin (NK2 and NK3), neuropeptide Y (Y1 and Y2), neurotensin (NTS1), opioid and opioid-like substances (delta2, kappa, mu, NOP), prostanoid (EP4), serotonin (5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT5A, 5-HT6, and 5-HT7), somatostatin (sst), vasoactive intestinal peptide (VPAC1), and vasopressin (V1a). Transporters tested included the dopamine transporter (DAT), norepinephrine transporter (NET), and serotonin transporter (5-HT). Ion channels tested included those for potassium (Kv and SKca channels) calcium (Ca2+ channel, L-type, verapamil site), sodium (Na+ channel site 2), GABA (BZD and Cl− channel GABA gated), and serotonin (5-HT3). Receptor, transporter, or ion channel binding by a specific ligand was defined as the difference between total and nonspecific binding, determined in the presence of an excess of unlabeled ligand. Results are expressed as the percent inhibition of control-specific binding or the percent variation of control values obtained in the presence of NOSO-502.
Pharmacokinetic analysis.
Pharmacokinetic analyses were performed by Pharmacelsus (Saarbrücken, Germany). CD-1 female mice and female Sprague-Dawley rats were injected intravenously with 30 and 15 mg/kg of NOSO-502, respectively, prepared in saline (5 ml/kg). Blood (100 μl) was collected from three animals per time point (5, 10, 20, 30, 60, and 120 min postdose for mice and 5, 15, 30, 60, 180, and 420 min postdose for rats) in tubes containing K3-EDTA as an anticoagulant. Samples were stored on ice and centrifuged at 6,000 rpm for 10 min at 4°C. A sample volume of 50 μl was mixed with 5 μl of solvent (acetonitrile-H2O-DMSO, [5/4/1, vol/vol/vol] plus 1% formic acid). A volume of 10 μl of solvent containing the internal standard and 50 μl of precipitant (10% TCA) were added to 55 μl of sample. The mixture was vortexed and centrifuged at 6,000 × g (room temperature) for 10 min. The protein-free supernatant was analyzed by LC-MS using an Ultimate 3000RS ultrahigh-performance liquid chromatograph (U-HPLC) coupled with an Orbitrap Q Exactive mass spectrometer.
Analytes were separated on a Accurore phenyl-hexyl analytical column (2.1 by 50 mm, 2.6 μm; Thermo, Germany) using a linear gradient of mobile phase A (acetonitrile-0.2% heptafluorobutyric acid)-mobile phase B (water-0.2% heptafluorobutyric acid), starting from 5% mobile phase A to 97% in 2.2 min, and a flow rate of 0.6 μl/min. Peaks were analyzed by mass spectrometry (ESI ionization in MRM mode) using Xcalibur 4.0 software. The products analyzed, [M + 2H]2+ and [M + 3H]3+, were 539.8 and 360.2 Da, respectively. PK parameters were calculated using a noncompartmental analysis model and Kinetica 5.0 software (Thermo Scientific, Waltham, MA). The mean plasma concentrations from all three mice at each time point were used in the calculation.
Plasma protein binding.
The plasma stability assays were performed by Cyprotex Discovery Ltd. (Macclesfield, UK). This study was conducted to determine the extent of binding of NOSO-502 to the proteins in human, monkey, dog, rat, and mouse plasma. Solutions of NOSO-502 or control compound (NOSO-502 final substrate concentration of 2 μM in water; control compound final substrate concentration of 2 μM, final DMSO concentration of 0.5%) were prepared in 100% species-specific plasma (collected using EDTA as the anticoagulant). The experiment was performed using equilibrium dialysis (RED device) with the two compartments separated by a semipermeable membrane. Buffer (pH 7.4) was added to one side of the membrane and the plasma solution to the other. After equilibration (4 h), samples were taken from both sides of the membrane. Calibration standards were prepared in plasma and buffer. All incubations were performed in triplicate. A control compound was included in each experiment. Incubation of the control compound samples was terminated with acetonitrile containing an internal standard (metoprolol). Incubation of the test compound samples was terminated with 40% TCA in water containing in internal standard (NOSO-95216; 1 μM final concentration). All samples were centrifuged and further diluted with water prior to analysis. The solutions for each batch of control compounds were combined into two groups (protein free and protein containing) and cassette analyzed by LC-MS/MS using two sets of calibration standards for protein-free (seven points) and protein-containing solutions (seven points).
Mouse neutropenic peritonitis/sepsis model.
NOSO-502 was tested against E. coli EN122 (MIC = 4 μg/ml, ESBL, clinical isolate 106-EC-09, Denmark) in a murine neutropenic peritonitis/sepsis model. Female NMRI mice (Taconic Biosciences A/S, Lille Skensved, Denmark) were used. Mice had ad libitum access to domestic quality drinking water and food (2016 16% protein rodent diet; Harlan, USA) and were exposed to a 12-h light/dark cycle. All animal experiments were approved by the National Committee of Animal Ethics, Denmark, and adhered to the standards of EU directive 2010/63/EU. Mice were allowed to acclimatize for 4 days, and thereafter neutropenia was induced by intraperitoneal injections of cyclophosphamide (Baxter A/S, Søborg, Denmark) 4 days (200 mg/kg) and 1 day (100 mg/kg) prior to inoculation. Overnight E. coli colonies were suspended in saline to 107 CFU/ml, and mice were inoculated intraperitoneally with 0.5 ml of the suspension. At 1 h postinoculation, mice were treated with 1.3, 2.5, 4, 10, 20, or 40 mg/kg NOSO-502, a vehicle (PBS [pH 7.4]), or 5 mg/kg of colistin (Sigma-Aldrich; no. 4461) subcutaneously as a single dose in 0.2 ml. Four hours after treatment, mice were anesthetized and blood was collected by axillary cutdown. Blood samples were serially diluted and plated on blood agar plates (SSI Diagnostica, Hillerød, Denmark), with subsequent counting of colonies after incubation overnight at 35°C in ambient air. The mice were observed during the study for clinical signs of infection, such as lack of curiosity, social withdrawal, changes in body position and patterns of movement, distress, or pain.
Mouse UTI model.
All animal studies were performed under UK Home Office license P2BC7D240 with local ethical committee clearance. All studies were performed by technicians who completed parts A, B, and C of the UK Home Office personal license course and hold current personal licenses. All experiments were performed in dedicated biohazard 2 facilities (this site holds a certificate of designation).
NOSO-502 was tested against E. coli UTI89 (MIC = 4 μg/ml) in a mouse UTI model by Evotec (Manchester, UK). Female C3H/HeN mice, 18 to 22 g (Janvier Laboratories, UK), were allowed to acclimatize for 7 days. Following acclimatization, drinking water was replaced with water containing 5% glucose from 5 days preinfection. Previously prepared frozen stocks of E. coli UTI89 were diluted to 1 × 1010 CFU/ml immediately prior to infection. Mice were infected by directly administering a 0.05-ml inoculum (5 × 108 CFU/mouse) via the urethra into the bladder under parenteral anesthesia (90 mg/kg of ketamine and 9 mg/kg of xylazine). Bladders were emptied prior to infection, and once infected, infection catheters were left in the urinary tract for 10 min to reduce the risk of the organism flowing back out. Following catheter removal, mice were allowed to fully recover in warmed humidified cages. Dose formulations of NOSO-502 were prepared in 25 mM PBS. Treatment with 4, 12, and 24 mg/kg of NOSO-502 was initiated 24 h postinfection and was administered once daily (q24h) by subcutaneous injection or intravenously (ciprofloxacin) for 3 days. Mice were euthanized 96 h postinfection (three doses administered). Ciprofloxacin (Bayer; lot BXHEFTI), administered at 10 mg/kg/dose intravenously twice a day (BID), was included as a comparator (six doses administered), and 25 mM PBS was used as a vehicle. Urine was collected 24 h postinfection from all animals and used to assess the infection level of each mouse prior to initiation of treatment; all mice were successfully infected. In addition, five mice were euthanized by pentobarbitone overdose to provide a 24-h pretreatment control group. The clinical condition and body weight of all remaining animals were assessed and urine samples were collected 96 h postinfection. Animals were then euthanized by pentobarbitone overdose and the kidneys and bladders removed and weighed. Tissue samples were homogenized using a Precellys 24 dual-bead beater in 2 ml of ice-cold sterile PBS. Homogenates and urine samples were quantitatively cultured onto MacConkey's agar plates and incubated at 37°C for 24 h before colonies were counted. The data from the culture burdens were analyzed using appropriate nonparametric statistical models (Kruskal-Wallis using Conover-Inman to make all pairwise comparisons between groups) with StatsDirect software v. 2.7.8 and compared to pretreatment and vehicle controls.
Mouse neutropenic i.p. sepsis model.
All animal studies were performed under UK Home Office license P2BC7D240 with local ethical committee clearance. All studies were performed by technicians who completed parts A, B, and C of the UK Home Office personal license course and hold current personal licenses. All experiments were performed in dedicated biohazard 2 facilities (this site holds a certificate of designation).
NOSO-502 was tested against E. coli ATCC BAA-2469 (MIC = 2 μg/ml) in an i.p. sepsis model by Evotec (Manchester, UK). Male CD1/ICR mice, 25 to 30 g (Charles River, UK), were allowed to acclimatize for 11 days. Mice were rendered neutropenic with two intraperitoneal injections of 150 mg/kg of cyclophosphamide 4 days before infection and 100 mg/kg 1 day before infection. Previously prepared frozen stocks of E. coli ATCC BAA-2469 were diluted immediately prior to infection to 6.8 × 107 CFU/ml. Mice were infected by directly administering a 0.5-ml inoculum (3.4 × 107 CFU/mouse) via intraperitoneal injection. Dose formulations of NOSO-502 were prepared in 25 mM PBS. Treatment was initiated 1 h postinfection, and NOSO-502 doses (4, 12, and 24 mg/kg) were administered once by subcutaneous injection. Tigecycline (MIC = 0.5 μg/ml), administered at 40 mg/kg/dose subcutaneously BID, was included as a comparator, and two doses were administered. Animals from the pretreatment groups were euthanized 1 h postinfection, and all remaining mice were euthanized 25 h postinfection. The clinical condition and body weight of all remaining animals were assessed 25 h postinfection or when animals reached the ethical severity endpoint (whichever came first). Mice were anesthetized using 2.5% isoflurane–97.5% oxygen, followed by a pentobarbitone overdose. When mice were deeply unconscious, blood was collected from all animals under terminal cardiac puncture into EDTA blood tubes. In addition, an intraperitoneal wash with sterile PBS (2 ml i.p. injected and 1 ml removed for culture) was collected. Five mice were also euthanized by pentobarbitone overdose to provide a 1-h pretreatment control group. Blood and i.p. wash samples were quantitatively cultured onto cystine lactose electrolyte-deficient (CLED) agar plates and incubated at 37°C for 24 h before colonies were counted. The data from the culture burdens were analyzed using appropriate nonparametric statistical models (Kruskal-Wallis using Conover-Inman to make all pairwise comparisons between groups) with StatsDirect software v.2.7.8 and compared to pretreatment and vehicle controls.
Mouse lung infection model.
All animal experiments were performed under UK Home Office license 40/3644, and with local ethical committee clearance (The University of Manchester AWERB). All experiments were performed by technicians who had completed at least parts 1 to 3 of the Home Office personal license course and held current personal licenses.
NOSO-502 was tested against K. pneumoniae NCTC 13442 (expresses OXA-48 carbapenemase, MIC = 1 μg/ml) in a neutropenic mouse pulmonary infection model by Evotec (Manchester, UK). Male CD-1/ICR mice, 6 to 8 weeks old (Charles River UK), were allowed to acclimatize for 7 days and then rendered neutropenic by i.p. injection of cyclophosphamide (200 mg/kg on day 4 and 150 mg/kg on day 1 before infection). Mice were infected by the intranasal route (∼4 × 106 CFU/mouse) under parenteral anesthesia. At 2 h, 8 h, 14 h, and 20 h postinfection, mice received treatments with NOSO-502 at 2, 6, or 20 mg/kg or with tigecycline at 40 mg/kg administered by the s.c. route in a volume of 10 ml/kg (8 mice per dose). At 2 h postinfection, NOSO-502 was delivered once by the s.c. route at 80 mg/kg in a volume of 10 ml/kg (8 mice). At 2 h postinfection, one infected group was humanely euthanized, and lungs were processed for pretreatment quantitative culture to determine Klebsiella burdens. At 26 h postinfection, all remaining mice were humanely euthanized. Lungs were aseptically removed, homogenized, serially diluted, and plated on CLED agar for CFU titers.
ACKNOWLEDGMENTS
Some of the research leading to these results was conducted as part of the ND4BB ENABLE Consortium and has received support from the Innovative Medicines Initiative Joint Undertaking under grant no. 11583, resources of which are comprised of financial contributions from the European Union's seventh framework program (FP7/2007-2013) and EFPIA companies' in-kind contribution.
We thank Douglas Huseby, Diarmaid Hughes, Sha Cao, Richard Svensson, and Pawel Baranczewski from Uppsala University and Edgars Liepins and Solveiga Grinberga from the Latvian Institute of Organic Synthesis for their contributions.
Footnotes
For a companion article on this topic, see https://doi.org/10.1128/AAC.01067-18.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf Accessed 26 November 2014.
- 2.Zhang Y, Wang Q, Yin Y, Chen H, Jin L, Gu B, Xie L, Yang C, Ma X, Li H, Li W, Zhang X, Liao K, Man S, Wang S, Wen H, Li B, Guo Z, Tian J, Pei F, Liu L, Zhang L, Zou C, Hu T, Cai J, Yang H, Huang J, Jia X, Huang W, Cao B, Wang H. 2018. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE Network. Antimicrob Agents Chemother 62:e01882-. doi: 10.1128/AAC.00529-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pantel L, Florin T, Dobosz-Bartoszek M, Racine E, Sarciaux M, Serri M, Houard J, Campagne JM, Marcia de Figueiredo R, Gaudriault S, Givaudan A, Forst S, Aumelas A, Cotteaux-Lautard C, Bolla JM, Vingsbo Lundberg C, Huseby D, Hughes D, Vázquez-Laslop N, Mankin AS, Polikanov YS, Gualtieri M. 2018. Odilorhabdins, a class of potent antibacterial agents, cause miscoding by binding at a new ribosomal site. Mol Cell 70:83–94. doi: 10.1016/j.molcel.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Cheng YH, Lin TL, Lin YT, Wang JT. 2018. A putative RND-type efflux pump, H239_3064, contributes to colistin resistance through CrrB in Klebsiella pneumoniae. J Antimicrob Chemother 73:1509–1516. doi: 10.1093/jac/dky054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright MS, Suzuki Y, Jones MB, Marshall SH, Rudin SD, van Duin D, Kaye K, Jacobs MR, Bonomo RA, Adams MD. 2015. Genomic and transcriptomic analyses of colistin-resistant clinical isolates of Klebsiella pneumoniae reveal multiple pathways of resistance. Antimicrob Agents Chemother 59:536–543. doi: 10.1128/AAC.04037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Firsov AA, Vostrov SN, Shevchenko AA, Cornaglia G. 1997. Parameters of bacterial killing and regrowth kinetics and antimicrobial effect examined in terms of area under the concentration-time curve relationships: action of ciprofloxacin against Escherichia coli in an in vitro dynamic model. Antimicrob Agents Chemother 41:1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang JX, Kaeslin G, Ranall MV, Blaskovich MA, Becker B, Butler MS, Little MH, Lash LH, Cooper MA. 2015. Evaluation of biomarkers for in vitro prediction of drug-induced nephrotoxicity: comparison of HK-2, immortalized human proximal tubule epithelial, and primary cultures of human proximal tubular cells. Pharmacol Res Perspect 3:e00148. doi: 10.1002/prp2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidyasagar A, Wilson NA, Diamali A. 2012. Heat shock protein 27 (HSP27): biomarker of disease and therapeutic target. Fibrogenesis Tissue Repair 5:7. doi: 10.1186/1755-1536-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty AT. 2012. The in vitro micronucleus assays. Methods Mol Biol 817:121–141. doi: 10.1007/978-1-61779-421-6_7. [DOI] [PubMed] [Google Scholar]
- 10.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, Venditti M, Bordi E, Capozzi D, Balice MP, Tarasi A, Parisi G, Lappa A, Carattoli A, Petrosillo N. 2013. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect 19(1):E23–E30. doi: 10.1111/1469-0691.12070. [DOI] [PubMed] [Google Scholar]
- 11.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 12.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P. 2015. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 13.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falcone M, Russo A, Iacovelli A, Restuccia G, Ceccarelli G, Giordano A, Farcomeni A, Morelli A, Venditti M. 2016. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Microbiol Infect 22:444–450. doi: 10.1016/j.cmi.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Simmonds A, Nelson B, Eiras DP, Loo A, Jenkins SG, Whittier S, Calfee DP, Satlin MJ, Kubin CJ, Furuya EY. 2016. Combination regimens for treatment of carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother 60:3601–3607. doi: 10.1128/AAC.03007-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kontopidou F, Giamarellou H, Katerelos P, Maragos A, Kioumis I, Trikka-Graphakos E, Valakis C, Maltezou HC. 2014. Infections caused by carbapenem-resistant Klebsiella pneumoniae among patients in intensive care units in Greece: a multi-centre study on clinical outcome and therapeutic options. Clin Microbiol Infect 20:117–123. doi: 10.1111/1469-0691.12341. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trecarichi EM, Pagano L, Martino B, Candoni A, Di Blasi R, Nadali G, Fianchi L, Delia M, Sica S, Perriello V, Busca A, Aversa F, Fanci R, Melillo L, Lessi F, Del Principe MI, Cattaneo C, Tumbarello M. 2016. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am J Hematol 91:1076–1081. doi: 10.1002/ajh.24489. [DOI] [PubMed] [Google Scholar]
- 19.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. 2011. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 17:1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 20.Falcone M, Paterson D. 2016. Spotlight on ceftazidime/avibactam: a new option for MDR Gram-negative infections. J Antimicrob Chemother 71:2713–2722. doi: 10.1093/jac/dkw239. [DOI] [PubMed] [Google Scholar]
- 21.Castanheira M, Huband MD, Mendes RE, Flamm RK. 2017. Meropenem-vaborbactam tested against contemporary Gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant Enterobacteriaceae. Antimicrob Agents Chemother 61:e00567-17. doi: 10.1128/AAC.00567-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livermore DM, Mushtaq S, Warner M, Woodford N. 2016. In vitro activity of eravacycline against carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii. Antimicrob Agents Chemother 60:3840–3844. doi: 10.1128/AAC.00436-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassetti M, Peghin M, Pecori D. 2016. The management of multidrug-resistant Enterobacteriaceae. Curr Opin Infect Dis 29:583–594. doi: 10.1097/QCO.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 24.van Duin D, Doi Y. 2017. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 8:460–469. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viale P, Giannella M, Lewis R, Trecarichi EM, Petrosillo N, Tumbarello M. 2013. Predictors of mortality in multidrug-resistant Klebsiella pneumoniae bloodstream infections. Expert Rev Anti Infect Ther 11:1053–1063. doi: 10.1586/14787210.2013.836057. [DOI] [PubMed] [Google Scholar]
- 26.Tängdén T, Giske CG. 2015. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: clinical perspectives on detection, treatment and infection control. J Intern Med 277:501–512. doi: 10.1111/joim.12342. [DOI] [PubMed] [Google Scholar]
- 27.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantón R, Akóva M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen Ø, Seifert H, Woodford N, Nordmann P, European Network on Carbapenemases. 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18:413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 29.Petrosillo N, Giannella M, Lewis R, Viale P. 2013. Treatment of carbapenem-resistant Klebsiella pneumoniae: the state of the art. Expert Rev Anti Infect Ther 11:159–177. doi: 10.1586/eri.12.162. [DOI] [PubMed] [Google Scholar]
- 30.Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. 2014. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 20:862–872. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 31.van Duin D, Kaye KS, Neuner EA, Bonomo RA. 2013. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis 75:115–120. doi: 10.1016/j.diagmicrobio.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giacobbe DR, Del Bono V, Trecarichi EM, De Rosa FG, Giannella M, Bassetti M, Bartoloni A, Losito AR, Corcione S, Bartoletti M, Mantengoli E, Saffioti C, Pagani N, Tedeschi S, Spanu T, Rossolini GM, Marchese A, Ambretti S, Cauda R, Viale P, Viscoli C, Tumbarello M. 2015. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: results from a multicenter case-control-control study. Clin Microbiol Infect 21(12):1106.e1–1106.e8. doi: 10.1016/j.cmi.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Humes HD. 1988. Aminoglycoside nephrotoxicity. Kidney Int 33:900–911. doi: 10.1038/ki.1988.83. [DOI] [PubMed] [Google Scholar]
- 34.Moore RD, Smith CR, Lipsky JJ, Mellits ED, Lietman PS. 1984. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann Intern Med 100:352–357. doi: 10.7326/0003-4819-100-3-352. [DOI] [PubMed] [Google Scholar]
- 35.Kelesidis T, Falagas ME. 2015. The safety of polymyxin antibiotics. Expert Opin Drug Saf 14:1687–1701. doi: 10.1517/14740338.2015.1088520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falagas ME, Kasiakou SK. 2006. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 10(1):R27. doi: 10.1186/cc3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nation RL, Velkov T, Li J. 2014. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis 59:88–94. doi: 10.1093/cid/ciu213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galløe AM, Graudal N, Christensen HR, Kampmann JP. 1995. Aminoglycosides: single or multiple daily dosing? A meta-analysis on efficacy and safety. Eur J Clin Pharmacol 48:39. [DOI] [PubMed] [Google Scholar]
- 39.Iannini PB. 2002. Cardiotoxicity of macrolides, ketolides and fluoroquinolones that prolong the QTc interval. Expert Opin Drug Saf 1(2):121–128. doi: 10.1517/14740338.1.2.121. [DOI] [PubMed] [Google Scholar]
- 40.Fosgerau K, Hoffmann T. 2015. Peptide therapeutics: current status and future directions. Drug Discov Today 20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Kaspar AA, Reichert JH. 2013. Future direction for peptide therapeutics development. Drug Discov Today 18:807–817. doi: 10.1016/j.drudis.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard— 10th ed CLSI document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 43.Clinical and Laboratory Standards Institute. 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. CLSI document M26-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 44.Xia M, Huang R, Witt KL, Southall N, Fostel J, Cho MH, Jadhav A, Smith CS, Inglese J, Portier CJ, Tice RR, Austin CP. 2008. Compound cytotoxicity profiling using quantitative high-throughput screening. Environ Health Perspect 116:284–291. doi: 10.1289/ehp.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathes C. 2006. QPatch: the past, present and future of automated patch clamp. Expert Opin Ther Targets 10:230–241. [DOI] [PubMed] [Google Scholar]
- 46.Diaz D, Scott A, Carmichael P, Shi W, Costales C. 2007. Evaluation of an automated in vitro micronucleus assay in CHO-K1 cells. Mutat Res 630:1–13. doi: 10.1016/j.mrgentox.2007.02.006. [DOI] [PubMed] [Google Scholar]