Scabies is a major and potentially growing public health problem worldwide with an unmet need for acaricidal agents with greater efficacy and improved pharmacological properties for its treatment. The objective of the present study was to assess the efficacy and describe the pharmacokinetics profile of a novel acaricide, afoxolaner (AFX), in a relevant experimental porcine model.
KEYWORDS: scabies, isoxazoline, afoxolaner, ivermectin, acaricide agent, porcine model, parasitology
ABSTRACT
Scabies is a major and potentially growing public health problem worldwide with an unmet need for acaricidal agents with greater efficacy and improved pharmacological properties for its treatment. The objective of the present study was to assess the efficacy and describe the pharmacokinetics profile of a novel acaricide, afoxolaner (AFX), in a relevant experimental porcine model. Twelve pigs were experimentally infested and either treated with 2.5 mg/kg single dose oral AFX (n = 4) or 0.2 mg/kg, two doses 8 days apart, oral ivermectin ([IVM] n = 4) or not treated for scabies (n = 4). The response to treatment was assessed by the reduction of mite counts in skin scrapings as well as clinical and pruritus scores over time. Plasma and skin pharmacokinetics profiles for both AFX and IVM were evaluated. AFX efficacy was 100% at days 8 and 14 posttreatment and remained unchanged until the study end (day 45). IVM efficacy was 86% and 97% on days 8 and 14, respectively, with a few mites recovered at the study end. Clinical and pruritus scores decreased in both treated groups and remained constant in the control group. Plasma mean residence times (MRT) were 7.1 ± 2.4 and 1.1 ± 0.2 days for AFX and IVM, respectively. Skin MRT values were 16.2 ± 16.9 and 2.7 ± 0.5 days for AFX and IVM, respectively. Overall, a single oral dose of AFX was efficacious for the treatment of scabies in experimentally infested pigs and showed remarkably long MRTs in plasma and, notably, in the skin.
INTRODUCTION
Scabies is an epidermal infestation caused by the mite Sarcoptes scabiei in humans (1). It is increasingly recognized as a large and potentially growing public health problem worldwide with a significant burden (2, 3). The prevalence is estimated to be around 100 to 130 million cases/year (4–7). Scabies is often perceived erroneously as causing a simple itch, but over the past decade, studies emphasized its important morbidity, mostly caused by secondary bacterial infections (4). Opportunistic pathogens such as Streptococcus pyogenes (group A streptococcus) and Staphylococcus aureus are commonly associated with human scabies. Especially in tropical and subtropical countries, these can lead to invasive bacterial infections and postinfection complications, such as poststreptococcal glomerulonephritis, acute rheumatic fever, and rheumatic heart disease (8–10). The psychosocial and economic impacts caused by scabies through school absenteeism or a loss of work productivity due to pruritus and lack of sleep are considerable and lead to an exacerbation of poverty in affected populations (5, 11).
The current most accepted medical intervention to treat scabies consists of multiple treatments with either one of four topical agents (5% permethrin, 10% to 25% benzyl benzoate, piperonyl butoxide-synergized pyrethrins, or 0.5% malathion) and/or oral ivermectin (IVM), the only systemic drug approved in some countries (12, 13). The major limitations of these antiparasitic therapies are the absence of 100% cure in the target population, a poor compliance with topical application and repeated treatment schedules, the limited activity against Sarcoptes eggs, and an insufficient half-life to cover the whole 14-day life cycle of the mite (14). The risk of emergence of mite resistance is of growing concern (15, 16), especially with the increasing use of permethrin, esdepallethrin, and IVM for scabies and for other skin diseases in humans (e.g., head lice and rosacea), but also in animal parasitic diseases (17). Thus, there is an unmet need for new acaricide molecules with greater efficacy and improved pharmacological profiles to overcome scabies and its morbidity (3).
New hopes to find an adequate treatment for human scabies are coming from the translation of molecules such as moxidectin from the veterinary field (14). More recently, research has advanced that might give new perspectives for human scabies treatment. The veterinary therapeutic arsenal has been expanded with various effective ectoparasiticides (18). Afoxolaner (AFX), a member of the novel isoxazoline family, is administered orally and shows a great efficacy against fleas, ticks, and mites in dogs (19, 20). AFX inhibits parasite γ-aminobutyric acid (GABA) and glutamate-gated chloride channels. Notably, AFX binds to a site distinct from the binding site of other acaricides-insecticides, among them, the macrocyclic lactones (e.g., IVM) (21). AFX has advantageous pharmacokinetics and toxicity profiles with long-lasting activity (22).
We recently optimized the experimental porcine model developed by Mounsey et al. in 2010 (23) and demonstrated its usefulness for preclinical assessment of drug candidates for the treatment of scabies (24). Here, we assessed drug efficacies and pharmacokinetic profiles of a single oral dose of AFX compared with two oral doses of IVM in experimentally infested pigs.
RESULTS
Study design.
Twelve 3-week-old pigs were randomly assigned to 3 groups in January 2015 and were infested with Sarcoptes scabiei var. suis 2 weeks after their arrival (Fig. 1). Dexamethasone (0.2 mg/kg) was used daily during the entire study to promote the initial infestation and to increase the intensity and duration of the infestation. Pigs were treated in a blinded matter at 9 weeks postinfection, day 0 (D0) at the end of March 2015. The first group of pigs (n = 4) received oral AFX at the dosage of 2.5 mg/kg given once at D0. The second group (n = 4) received oral IVM at the dosage of 0.2 mg/kg twice (on D0 and day 8 [D8]). The third group (n = 4) was a control group and did not receive any treatment against mites. One pig (in the IVM-treated group) died during the study because of a congenital malformation of the digestive tract. The response to treatments was assessed by the reduction of live mite counts in skin scrapings and by the reduction of clinical and pruritus scores at different time points. The endpoint was the complete absence of live mites at day 14 (D14). Plasma and skin pharmacokinetics profiles for both drugs were evaluated.
FIG 1.
Study design showing the three experimental phases. DXM, dexamethasone; D, day; AFX, afoxolaner; IVM, ivermectin.
Pigs assessment at baseline.
At baseline (D0), pigs were 12 weeks old and their weights ranged from 10.7 to 19.5 kg (mean ± standard deviation [SD], 15.2 ± 2.7 kg). At D0, all 12 pigs were infested with scabies. No statistical difference was found between the three groups in terms of mite count in skin scrapings (P = 0.944) or clinical (P = 0.751) and pruritus (P = 0.893) scores. Mite counts and clinical and pruritus scores at baseline are shown in Fig. 2A, 3, and 4, and Tables S1 and S2 in the supplemental material.
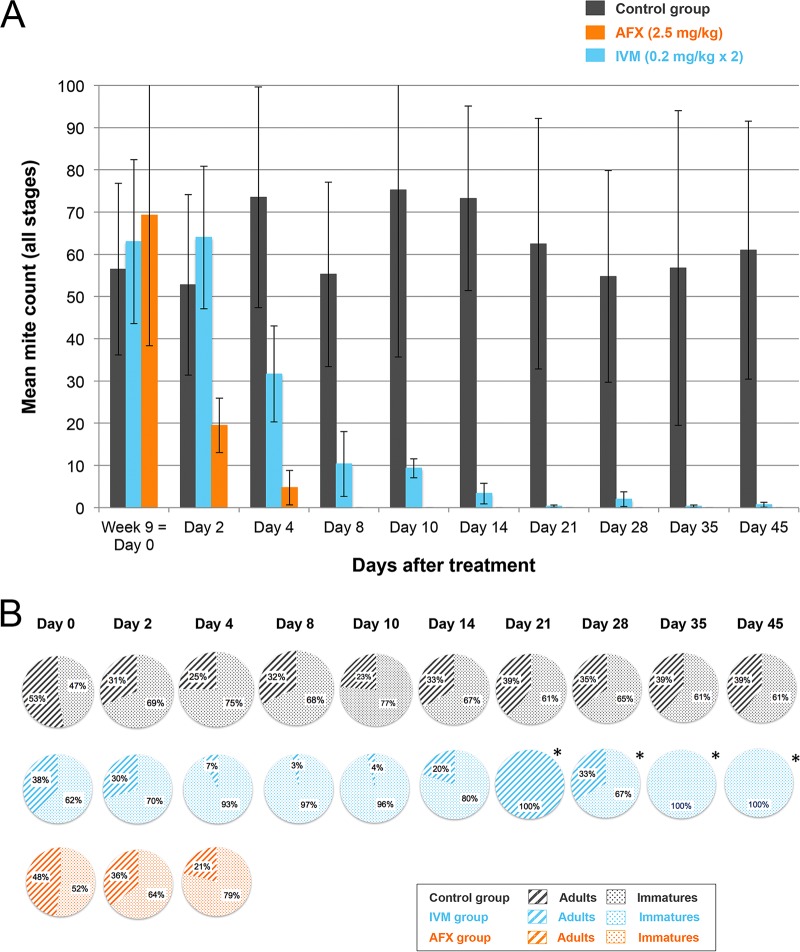
FIG 2.
(A) Mite counts (mean ± SD) for the AFX- and IVM-treated and control pigs over time after treatments from baseline (D0) to the study end. (B) Partition of the different life stages (adult or immature) recovered from the skin scrapings collected from the AFX- and IVM-treated and control pigs over time after treatments from baseline (D0) to the study end. *, less than 10 mites were recovered in total from the skin scrapings; AFX, afoxolaner; IVM, ivermectin.
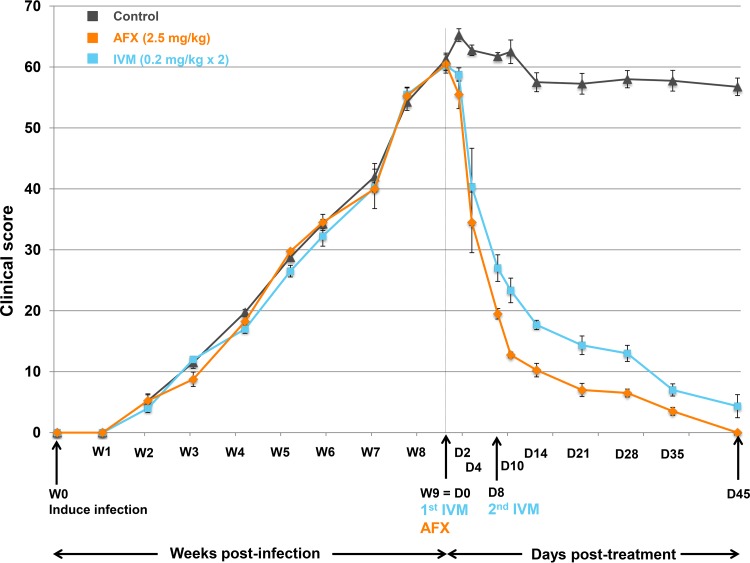
FIG 3.
Clinical scores (means ± SDs) for the AFX- and IVM-treated and control pigs over time from scabies infection to the study end. The manifestation of scabies infestation in the skin areas of five anatomic sites (ears, legs, tail, back, and head) was monitored weekly. Clinical scores are based on the skin surface affected by scabies lesions (scored 0 to 6: 0, 0%; 1, <10%; 2, 10% to 29%; 3, 30% to 49%; 4, 50% to 69%; 5, 70% to 89%; 6, 90% to 100%), intensity of skin erythema (scored 0 to 4: 0, no erythema; 1, mild; 2, moderate; 3, severe; 4, extremely severe), and the encrustment intensity (scored 2× as 0 to 4: 0, no crust; 1, gray to white, thin and irregular 1- to 2-mm crust; 2, 2- to 5-mm crust; 3, gray-brown >5-mm crust; and 4, >5-mm hard crust). The score was calculated for the 5 different anatomic sites and added. W, week; D, day; AFX, afoxolaner; IVM, ivermectin.
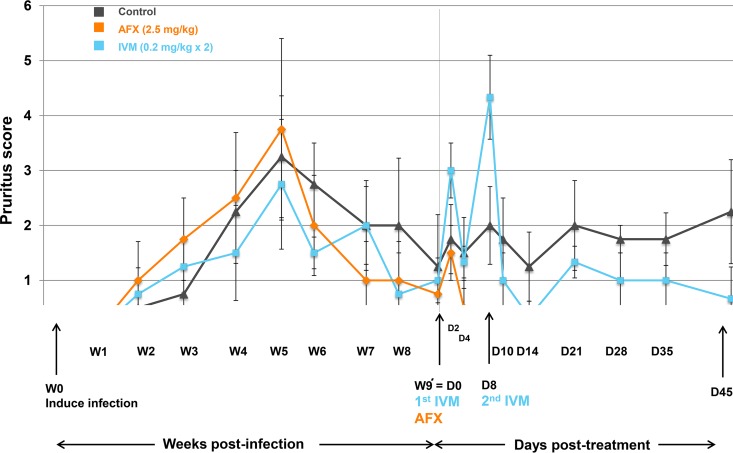
FIG 4.
Pruritus scores (means ± SDs) for the AFX- and IVM-treated and control pigs over time from scabies infestation induction to the study end. D, day; W, week; AFX, afoxolaner; IVM, ivermectin.
Parasitological assessment of drug efficacy.
On D14, the endpoint, all AFX-treated pigs and one of three IVM-treated pigs were mite free. The progression of the parasite burden in the AFX- or IVM-treated and control pigs after treatments from baseline (D0) to the study end (D45) is presented in Fig. 2A. The partition of the parasite population between immature and adult live stages detected in the scrapings is presented in Fig. 2B. The percentage efficacy of the treatment and the percentage reduction in the number of live mites in skin scrapings over time are shown in Table S1. On D14, the drug efficacy was 100% in the AFX-treated pigs compared to 95.4% (range, 87.7% to 98.6%) in IVM-treated pigs, and the percentage reduction of the mite count was 100% in AFX-treated pigs compared to 94.7% (range, 82.7% to 100%) in the IVM-treated pigs. From D8 posttreatment to the study end, not a single mite was detected in the scrapings of the AFX-treated pigs. In contrast, among the IVM-treated pigs, one pig was still infested with live mites at the end of the study. In all animals of the untreated control group, the mite count remained constant until the end of the study. After treatment, the numbers of mites over time in both treated groups were statistically different from the count in the control group (P = 0.0001) and statistically different from each other (P = 0.045).
After treatment, the large majority of eggs retrieved from scrapings of all three cohorts hatched in the incubator (37°C, 90% relative humidity). At baseline (prior to treatment), 12 eggs from each cohort were incubated, and all except one in the control group and one in the AFX-treated group hatched. At D2 posttreatment, hatching was observed for 10 of 10 eggs, 7 of 8 eggs, and 8 of 8 eggs from the AFX, IVM-treated, and control groups, respectively. At D4 posttreatment, hatching was observed for 1 of 1 egg, 3 of 3 eggs, and 12 of 12 eggs from the AFX, IVM-treated, and control groups, respectively. At D8 and D14 posttreatment, no eggs were found in scrapings from the AFX-treated and the IVM-treated animals. All the eggs from the control group hatched.
Clinical assessment of drug efficacy.
The mean clinical scores over time in the three groups are presented in Fig. 3 and Table S2. Clinical lesions disappeared completely in all AFX-treated pigs, whereas two of three IVM-treated pigs still had lesions at the end of the study, albeit with 93% improvement (Table S2). After treatment, the mean clinical scores of both treated groups were statistically different from those of the control group (P = 0.0001) and statistically different from each other (P = 0.023). No clinical signs of drug intolerance were noticed during the 50-day period of observation after administration of the two drugs. Side effects due to steroid long-term administration were mild (increase of the appetite and hairiness).
Straight after treatment (D2), an increase of pruritus was observed in both treated groups, followed by a decrease of the pruritus score (Fig. 4). A second peak was observed in the IVM-treated group at D8, just after the second administration of IVM. After treatment, the mean pruritus scores of both treated groups were statistically different from those of the control group (P = 0.0001) but not statistically different from each other (P = 0.566).
Plasma and skin drug levels in AFX- and IVM-treated groups.
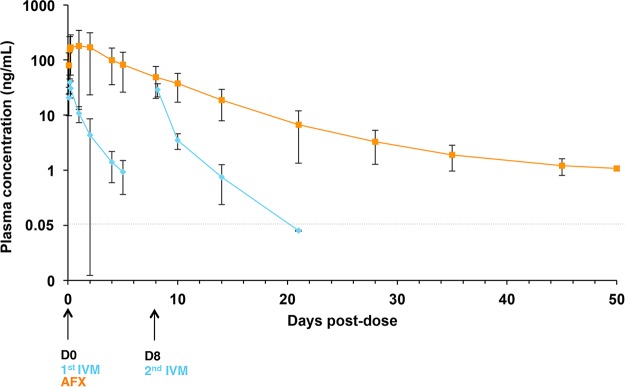
Pharmacokinetics after a single oral dose of AFX and double oral doses of IVM were determined in plasma and in skin (Table 1). In the plasma, the highest concentrations of both drugs were detectable within 2 h after oral administration and declined in a linear manner over time (Fig. 5). There was a high individual variability of AFX plasma concentration the first days postadministration, certainly due to drug absorption variability. Nevertheless, AFX was detectable until the study end (D50), whereas IVM was barely detectable 5 (D5) or 6 (D14) days after the first or second oral administration, respectively. Less than half a day postadministration, the AFX plasma maximum concentration of drug [Cmax] was 4.4-fold higher than that of IVM. The AFX area under the concentration-time curve [AUC] was ∼32-fold larger than that for IVM. AFX exhibited a long mean residence time [MRT] in plasma, with a mean MRT value of 7.1 ± 2.4 days. For IVM the value was 1.1 ± 0.2 days.
TABLE 1.
Pharmacological parameters for AFX and IVM in plasma and in skin after oral administration to scabies-infested pigsa
| Sample type | Cmax (ng/ml)b | Tmax (days)c | AUCt-last (days · ng/ml)d | MRTC-last (days)e |
|---|---|---|---|---|
| Plasma | ||||
| AFX | 196 ± 160.2 | 0.4 ± 0.4 | 1,217.5 ± 751.5 | 7.1 ± 2.4 |
| IVM | 44.2 ± 11.6f | 0.2 ± 0.0 | 38.7 ± 17.9 | 1.1 ± 0.2f |
| Skin | ||||
| AFX | 1,909.2 ± 962.4 | 1.25 ± 0.5 | 11,159.3 ± 6,158.4 | 16.2 ± 16.9 |
| IVM | 72.0 ± 40.2f | 1.0 ± 0.0 | 186.2 ± 104.8 | 2.7 ± 0.5f |
Values are presented as means ± SDs of AFX and IVM in the plasma and in the skin of pigs following oral intake.
Cmax, maximum plasma concentration.
Tmax, time to maximum plasma concentration.
AUCt-last, area under the plasma curve concentration-time curve from time zero after first administration to the last sampling time point with a measurable concentration.
MRTC-last, mean residence time.
P < 0.001 versus. AFX (compared with a nonparametric Mann-Whitney test).
FIG 5.
Mean concentration-time profiles (means ± SDs, ng/ml) in plasma following oral administration of AFX or IVM in scabies-infested pigs. Concentrations measured at hour 2, 4, 6, and 24 of D0 for AFX and IVM and on D2, 4, 5, 8 (4 h after the second IVM dose), 10, 14, 21, 28, 35, 45, and 50 posttreatment are depicted. D, day; AFX, afoxolaner; IVM, ivermectin.
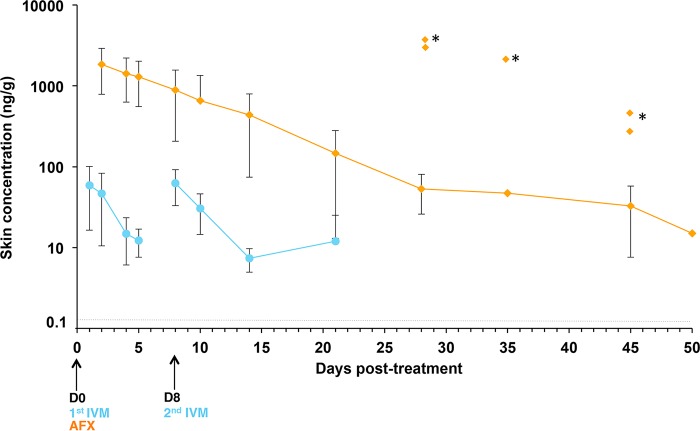
Both drugs reached the skin compartment on D1 postadministration. There were good correlations between plasma and skin concentrations for the two drugs (r = 0.855 and r = 0.804 for AFX and IVM, respectively). Both drugs accumulated at high concentrations in the skin (Fig. 6), and the Cmax values were 9.7-fold and 1.6-fold higher than those measured in plasma for AFX and IVM, respectively. Consequently, for both drugs, the skin exposure based on AUC and Cmax values was greater than the corresponding parameters in plasma, indicating marked distribution into the target tissue (i.e., the skin), especially for AFX. The calculation of tissue/plasma AUC ratios indicated that exposure relative to plasma was high for AFX in skin (ratio, 9.2) compared to IVM (ratio, 4.8). Interestingly, AFX showed a strong persistence in the skin with a mean MRT value of 16.2 ± 16.9 days, much higher than that for ivermectin, with a MRT of 2.7 ± 0.5 days.
FIG 6.
Mean concentration-time profiles (means ± SDs, ng/g) in skin following oral administration of AFX or IVM in scabies-infested pigs. Concentrations measured on D1, 2, 4, 5, 8 (4 h after the second IVM dose), 10, 14, 21, 28, 35, 45, and 50 posttreatment are depicted. *, outlier measured concentrations. D, day; AFX, afoxolaner; IVM, ivermectin.
DISCUSSION
Scabies mites cannot be maintained or propagated in vitro away from their host for more than a few days (25). Therefore, the establishment of a surrogate experimental porcine scabies model (23) provides real potential to conduct translational preclinical and pharmacokinetics studies with a new drug candidate. Although costly, pigs represent the ideal host to model human scabies. Porcine sarcoptic mange features are very similar to scabies in humans (26), and pigs have unsurpassed similarities in skin anatomy, physiology, and immunology (27). We recently showed that this experimental porcine model was useful for the preclinical assessment of drug candidates (24). Our first preclinical study using this model showing that moxidectin was more efficient than the regular IVM-based treatment has served as the baseline for a rational strategy to conduct larger high-powered efficacy studies in humans (24). Even though the number of pigs involved in these pilot studies is limited, the cohort size can be considered a representative sample for a proof of concept.
In this preclinical study, we demonstrated a better efficacy of a single dose (2.5 mg/kg) of AFX than of two doses of IVM (0.2 mg/kg) against a scabies infestation in pigs. AFX achieved a complete and fast parasitological and clinical cure. This was not the case in the IVM-treated group, where two of three pigs were not cured at the 14-day posttreatment endpoint. In fact, one pig of three was still infested with live mites at the end of the study. Laboratory or field studies looking at the efficacy of AFX (28) or other isoxazolines such as fluralaner or sarolaner (29–31) against S. scabiei infestation in dogs were recently completed. In naturally infested dogs (n = 10), Beugnet et al. showed that oral AFX dosed at 2.5 mg/kg also achieved a 100% efficacy on the basis of mite counts at D28 and D56 posttreatment. Clinical scores declined to 80% in the AFX-treated dogs versus 50% in the control group (28). The general observations of the present study were strictly comparable to those of our first trial (24) and successfully replicated previous Australian reports (23, 32, 33) with regard to the development of disease, strengthening the use of this robust experimental model for drug development.
The population structure of the mites recovered from the skin scrapings from the pigs differed in the different treatment groups (Fig. 2B). Before the administration of treatments, we observed a homogenous partition of the immature versus adult stages in all animals. This population structure remained constant in untreated pigs. Four days after the first IVM oral administration, there was a dramatic decrease of the number of adult mites, presumably killed by the drug, and an increased proportion of immature stages, which corresponded to newly hatched mites from the eggs at the time of treatment. This can be explained by the absence of ovicidal activity of IVM and in accordance with the life cycle of S. scabiei, as previously proposed by Currie and McCarthy (34). In contrast, in AFX treated-pigs, a rapid and definitive decrease in the mite count was observed. To date, there are no studies investigating the ovicidal activity of AFX. While our sampling protocol did not aim for isolating large numbers of eggs, the data set nevertheless indicates that both drugs have limited ovicidal activity.
Drug uptake into the skin and stability under its physiological conditions are further factors that may contribute to the difference in efficacy of the two treatments. As previously reported (24), we observed a relatively short duration of the effectiveness of IVM, matching with the presence of the drug measured in plasma and skin compartments. Hence, newly hatched mites may not have been killed, confirming the importance of the second administration of IVM. Accordingly, for maximum efficacy, the second IVM dose should be given between days 7 and 10, as soon as all eggs have hatched but before newly hatched mites have time to mate and produce a new generation of eggs. To optimize the interval of consecutive IVM treatments, additional studies about egg survival and hatchability in the presence of IVM are required.
The pharmacokinetics profile of AFX orally administered at a dose of 2.5 mg/kg exhibited a long elimination profile, with the drug present for approximately 7 days in plasma and 16 days in skin, i.e., ∼7-fold longer than that of IVM. Previous investigations in dogs with AFX orally administered at the same dosage demonstrated similar results. AFX was rapidly absorbed (around 2 to 4 h), with a high initial plasma peak (Cmax, 1,655 ± 332 ng/ml). The terminal plasma half-life was remarkably long at up to 15 days (15.5 ± 7.8 d) (22), consistent with the lipophilic and unionized properties of the small AFX molecule, and with its high affinity to plasma proteins shown in dogs (>99%) (22). Our study is the first to address AFX pharmacology parameters in the skin, and further studies are needed to investigate in which layer of the skin AFX is accumulating.
Data in pigs can provide interesting insight for projection and comparison with human pharmacokinetics (35). We found here that AFX and IVM both potentially have no ovicidal activity. In contrast to IVM, it seems that due to the long plasma and the skin persistence of AFX at an effective dose, newly hatched mites are killed and the parasitic life cycle is completely interrupted. Indeed, AFX could be given as a single dose, thereby conferring a major advantage of ensuring better treatment adherence, a determining factor for drug efficacy in resource-poor communities where scabies is endemic.
AFX is considered a safe drug. So far, no adverse clinical signs have been observed in previous studies in dogs, even after six oral administrations with up to 5 times the maximum exposure dose (22, 36). Mammalian chloride channels of rat brain cells showed no significant response to isoxazolines in binding assays (37), indicating that the binding site (NCA-II) of these channels to AFX is either not present or of low sensitivity (38).
In summary, AFX demonstrated high efficacy in treating scabies in this preclinical study in pigs, and combined with an interesting pharmacokinetics profile, it guarantees long-lasting activity, ensuring a convenient dosing as a unique oral administration.
MATERIALS AND METHODS
Experimentally infested pigs.
All procedures were approved by our Institutional Animal Care and Use Committee, Comité d'éthique pour l'expérimentation animale, Anses/EnvA/Université Paris-Est Créteil, France (approval no. 02515.03). The animals were handled in accordance with guidelines established by the French and European regulations for the care and use of animals for scientific purposes (articles R.214-87 to 214-137 du Code Rural et de la Pêche Maritime, Décret 2013-118, and European Directive 2010/63/UE). The ARRIVE guidelines were used to design and report the study (39). Procedures were in accordance with the method described by Mounsey et al. (23) and optimized by Bernigaud et al. (24).
Twelve 3-week old female Sus scrofa domesticus (large white breed) pigs from the same pig farm (Gambais, France) were housed at the Centre de Recherche Biomédicale in the Ecole Nationale Vétérinaire d'Alfort, France (http://www.vet-alfort.fr/). The mean ± SD weight at arrival was 7.15 ± 0.64 kg. Pigs were healthy and initially free of sarcoptic mange, and they had never received any antiparasitic treatments. At their arrival, drawing lots randomly allocated pigs into three groups (n = 4). To reduce stress and to acclimate the animals, the pigs were housed 2 weeks before starting the study in small groups of the same sex. Pigs where placed in similar experimental climate-controlled units by group (temperature, 21 ± 2°C; humidity, 50% ± 10%; surface area, 12 m2). Environmental enrichment included wood shavings on concrete floors that were cleaned once daily. Feed was given once a day, and tap water was continuously provided. A 12/12-h light/dark cycle was maintained (on at 7 a.m. and off at 7 p.m.). A physical examination of each animal by a veterinarian was performed twice a week before treatment and daily after treatment for general health conditions, ascertained management according to animal welfare standards. Care was taken to reduce stress or pain in the pigs. Invasive procedures such as the collection of blood samples and skin biopsy were kept to a minimum and performed under a short-term mild sedation, using a mixture of 0.2 ml/kg chlorhydrate of ketamine (Ketamine 1000; Virbac, Carros, France) and 0.02 ml/kg of xylazine (Rompun 2%; Bayer Healthcare, Loos, France) given by a single intramuscular injection. The synthetic glucocorticoid immune-suppressant dexamethasone (Fagron SAS, Thiais, France) was used to promote the initial infestation and increase the intensity and duration of the infestation. A daily oral dosage of 0.2 mg/kg dexamethasone was administered. Dexamethasone treatment was initiated 1 week prior to infestation and continued during the entire study period. The infestation was accomplished by directly introducing mite-infected skin crusts deep into the ear canals of the pigs. Crusts were obtained from a previous cohort of pigs initially infected with crusts of naturally infected pigs coming from a farm in Brittany (Dominique Dreau, Saint-Allouestre, France) (24). Crusts were collected in the morning and pigs were inoculated on the same day. Crusts were dissected into small pieces (approximately 0.5 cm2) containing between 600 and 800 mites. During the procedure, the pigs were put under mild sedation for 15 min to prevent the dislodgement of the crusts by agitation and to ensure successful infestation.
Drugs.
AFX (Nexgard; Merial/Boehringer, Inc., Lyon, France) was a 68-mg, soft beef-flavored chew (for dogs weighing between 10.1 and 25 kg). IVM was the human formulation (Stromectol; MSD France, Courbevoie, France) provided as 3-mg tablets. Pigs were weighed on day 0 to calculate the dose of treatment required. The pigs were fed their normal ration of food immediately after drug administration. Pigs were hand pilled to ensure accurate and complete dosing. Researchers involved in performing assessments and observations did not administer the treatments to pigs. No other acaricide or endectocide treatment was used throughout the study.
Parasitological and clinical assessment and data scoring.
The first experimental phase was the progression of the scabies infestation for 9 weeks after infection. At week 9 postinfection (day 0), the treatments were administered. The second experimental phase was the assessment of drug efficacy and the pharmacokinetics study from day 0 to day 50 after treatment. Figure 1 illustrates the study design. The primary outcome was based on the reduction in the numbers of live mites counted in the skin scrapings after treatment. The endpoint was the complete absence of live mites at day 14 posttreatment. Mites were collected and counted in skin scrapings, taken on day 0 (just before treatment) and subsequently on days 2, 4, 8, 10, 14, 21, 28, 35, and 45 posttreatment to estimate the percentage efficacy of treatment and the percentage of mite count reduction. Skin scrapings were obtained from each pig; around 0.2 g of crusts were scraped using a scalpel blade from the ears until blood seeped from the abrasion. Samples were examined in a petri dish within 2 h after collection. Under a light heat source, mites were encouraged to crawl out of the crusts. The mites were examined under a stereomicroscope (SMZ645; Nikon). Only live mites were counted, and the numbers of mites at life stages (adult or immature stages) were noted. Immature stages included larvae and nymphs.
A clinical score (Fig. 3) was used based on the skin surface affected by scabies lesions (scale from 0 to 6: 0, 0%; 1, <10%; 2, 10% to 29%; 3, 30% to 49%; 4, 50% to 69%; 5, 70% to 89%; 6, 90% to 100%), the intensity of the erythema of the skin (from 0 to 4: 0, no erythema; 1, mild; 2, moderate; 3, severe; 4, extremely severe), and the intensity of the encrustment (from 0 to 4: 0, no crust; 1, gray to white, thin and irregular 1-to 2-mm crust; 2, 2- to 5-mm crust; 3, gray-brown >5-mm crust; and 4, >5-mm hard crust). The score was calculated for five anatomic sites (ears, legs, tail, back, and head) and added up. Clinical examination and scoring of animals were carried out weekly after infestation and on day 0 (just before treatment) and subsequently on days 2, 4, 8, 10, 14, 21, 28, 35, and 45 posttreatment. All animals were individually examined. Photographs were taken of each pig.
Pigs were observed weekly for 15 min to record pruritus. Movements in response to pruritus, such as flapping of the ears, rubbing on a surface, and scratching ears with a posterior leg, were recorded. The scoring of pruritus was carried out after infestation and on day 0 (just before treatment) and subsequently on days 2, 4, 8, 10, 14, 21, 28, 35, and 45 posttreatment. All animals were individually examined.
To estimate the hatchability of the eggs, eggs were collected from the skin scrapings taken at day 0 (just before treatment) and subsequently on days 2, 4, 8, and 14 posttreatment. Each time, 10 eggs were collected from each group in a sterile plastic petri dish. The eggs were placed in an incubator at 37°C with 90% relative humidity and observed in 24 h intervals.
Afoxolaner and ivermectin pharmacokinetics analysis.
Blood samples were collected by jugular vein puncture in heparinized tubes (BD Vacutainer; BD-Plymouth, UK) on day 0 (just before treatment) and subsequently on hours 2, 4, 6, and 24, and days 2, 4, 5, 8 (4 h after the second administration of drug), 10, 14, 21, 28, 35, 45, and 50 posttreatment. Plasma samples were prepared by centrifuging blood samples at 2,000 × g for 10 min. Skin biopsy specimens were made by using a standard 5-mm-diameter punch biopsy tool (KAI Europe, GmbH, Germany) to extract a piece of epidermis and dermis from the neck regions of the pigs on day 0 (just before treatment) and subsequently on days 1, 2, 4, 5, 8 (4 h after the second administration of drug), 10, 14, 21, 28, 35, 45, and 50 posttreatment. Plasma and tissue samples were stored at −20°C until drug analysis. IVM concentrations were measured in plasma and skin by high-performance liquid chromatography (HPLC) with fluorescence detection using a procedure previously described and validated (24, 40). The procedure was performed in the Toxalim laboratory, INRA, Toulouse, France. AFX concentrations were measured in plasma and skin by liquid chromatography-mass spectrometry (LC-MS) in the Merial/Boehringer laboratories in Missouri (plasma analyses) and New Jersey (skin analyses), USA. The extracted analyses were chromatographed by reverse-phase HPLC and quantified by a triple quadrupole mass spectrometer system using the electrospray interface (41, 42). For IVM concentrations, the linearity was similar in the plasma and in the skin (r = 0.99 over a 0.1- to100-ng/ml concentration range), and the limits of quantitation (LOQs) were 0.05 ng/ml in the plasma and 0.1 ng/g in the skin (24). For AFX concentrations, the lower LOQ was 1 ng/ml in plasma and in skin. The pharmacokinetics parameters were determined using a noncompartmental analysis (Kinetica computer program, version 4.2; InnaPhase, Philadelphia, PA). The area under the concentration-time curve (AUC) and the mean residence time (MRT) were calculated from the time of administration to the time of the last measurable concentration (tlast), using the arithmetic trapezoidal rule. The peak plasma concentration (Cmax) and time of peak plasma concentration (Tmax) were read from the plotted concentration versus time for each pig.
Statistical analysis.
The nonparametric Kruskal-Wallis test was used to compare the groups at baseline. The primary outcome was based on the reduction in the number of live mites counted in skin scrapings following treatment. The percentage efficacy was calculated according to the following formula: efficacy (%) = [(C − T)/C] × 100, where C was the arithmetic mean number of live mites for the control group and T was the arithmetic mean number of live mites for the treated group for each time point. The percentage reduction of the mite count was calculated according to the formula: reduction (%) = [(Mpre − Mpost)/Mpre] × 100, where Mpre was the arithmetic mean number of live mites at baseline (day 0) and Mpost was the arithmetic mean number of live mites posttreatment (days 2, 4, 8, 10, 14, 21, 28, 35, and 45). The decrease over time in mite count and in clinical and pruritus scores within each group of pigs was tested for significance (P < 0.05) by repeated measures in a mixed model with a robust variance estimate using STATA version 12 software. We used a negative binomial regression model to assess the relationship between parasites (variable to explain), treatments, and time (43). Pharmacokinetics parameters obtained in the different groups were compared by a nonparametric Mann-Whitney test at a significance level of P < 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the French Society of Dermatology (research grant 2014-1). C.B. learned the Australian model with a grant from the Fondation René Touraine pour la Dermatologie. F.F. was supported by the Fund of the China Scholarship Council.
The funders had no role in study design, data collection and interpretation, or decision to submit the work for publication. We thank Dominique Dreau for providing help with the pig model and Françoise Foulet for advice on the study. We also thank Vanessa Dore and Sébastien Perrot for the pill formulation of dexamethasone.
C.B. has received a research grant from MSD France and research support from Bioderma Laboratoire Dermatologique and Codexial Dermatologie to help establish and develop the scabies experimental porcine model in France. F. Botterel has received lecture fees and a research grant form MSD France. O.C. has received drugs donated free of charge for research from Codexial Dermatologie and MSD France and lecture fees from Zambon Laboratoire, Codexial Dermatologie, and MSD France. F. Beugnet, A.J.M., and B.T. are employees of Merial/Boehringer; they were involved with pharmacokinetics analysis of afoxolaner only. Merial (part of Boehringer Ingelheim) had no role in the study design, data collection and interpretation, or decision to submit the work for publication. No other potential conflict of interest relevant to this article was reported.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02334-17.
REFERENCES
- 1.Chosidow O. 2006. Clinical practices. Scabies N Engl J Med 354:1718–1727. doi: 10.1056/NEJMcp052784. [DOI] [PubMed] [Google Scholar]
- 2.Chosidow O, Fuller LC. 2017. Scratching the itch: is scabies a truly neglected disease? Lancet Infect Dis 17:1220–1221. doi: 10.1016/S1473-3099(17)30469-3. [DOI] [PubMed] [Google Scholar]
- 3.WHO. 2017. 10th meeting of the Strategic and Technical Advisory Group for Neglected Tropical Diseases. WHO, Geneva, Switzerland. [Google Scholar]
- 4.Engelman D, Kiang K, Chosidow O, McCarthy J, Fuller C, Lammie P, Hay R, Steer A, Members Of The International Alliance for the Control of Scabies. 2013. Toward the global control of human scabies: introducing the International Alliance for the Control of Scabies. PLoS Negl Trop Dis 7:e2167. doi: 10.1371/journal.pntd.0002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, Marks R, Naldi L, Weinstock MA, Wulf SK, Michaud C, Murray JLC, Naghavi M. 2014. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Investig Dermatol 134:1527–1534. doi: 10.1038/jid.2013.446. [DOI] [PubMed] [Google Scholar]
- 6.Karimkhani C, Dellavalle RP, Coffeng LE, Flohr C, Hay RJ, Langan SM, Nsoesie EO, Ferrari AJ, Erskine HE, Silverberg JI, Vos T, Naghavi M. 2017. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol 153:406–412. doi: 10.1001/jamadermatol.2016.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karimkhani C, Colombara DV, Drucker AM, Norton SA, Hay R, Engelman D, Steer A, Whitfeld M, Naghavi M, Dellavalle RP. 2017. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis 17:1247–1254. doi: 10.1016/S1473-3099(17)30483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid HF, Bassett DC, Gaworzewska E, Colman G, Poon-King T. 1990. Streptococcal serotypes newly associated with epidemic post-streptococcal acute glomerulonephritis. J Med Microbiol 32:111–114. doi: 10.1099/00222615-32-2-111. [DOI] [PubMed] [Google Scholar]
- 9.Hoy WE, White AV, Dowling A, Sharma SK, Bloomfield H, Tipiloura BT, Swanson CE, Mathews JD, McCredie DA. 2012. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int 81:1026–1032. doi: 10.1038/ki.2011.478. [DOI] [PubMed] [Google Scholar]
- 10.Steer AC, Jenney AWJ, Kado J, Batzloff MR, La Vincente S, Waqatakirewa L, Mulholland EK, Carapetis JR. 2009. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis 3:e467. doi: 10.1371/journal.pntd.0000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez M-G, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, et al. 2012. Years lived with disability (YLDs) for 1,160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strong M, Johnstone P. 2007. Interventions for treating scabies. Cochrane Database Syst Rev 2007:CD000320. doi: 10.1002/14651858.CD000320.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. 2017. European guideline for the management of scabies. J Eur Acad Dermatol Venereol 31:1248–1253. doi: 10.1111/jdv.14351. [DOI] [PubMed] [Google Scholar]
- 14.Mounsey KE, Bernigaud C, Chosidow O, McCarthy JS. 2016. Prospects for moxidectin as a new oral treatment for human scabies. PLoS Negl Trop Dis 10:e0004389. doi: 10.1371/journal.pntd.0004389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Currie BJ, Harumal P, McKinnon M, Walton SF. 2004. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clin Infect Dis 39:e8–e12. doi: 10.1086/421776. [DOI] [PubMed] [Google Scholar]
- 16.Mounsey KE, Holt DC, McCarthy JS, Currie BJ, Walton SF. 2009. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol 145:840–841. doi: 10.1001/archdermatol.2009.125. [DOI] [PubMed] [Google Scholar]
- 17.Chosidow O, Giraudeau B. 2012. Topical ivermectin–a step toward making head lice dead lice? N Engl J Med 367:1750–1752. doi: 10.1056/NEJMe1211124. [DOI] [PubMed] [Google Scholar]
- 18.Weber T, Selzer PM. 2016. Isoxazolines: a novel chemotype highly effective on ectoparasites. ChemMedChem 11:270–276. doi: 10.1002/cmdc.201500516. [DOI] [PubMed] [Google Scholar]
- 19.Kunkle BN, Drag MD, Chester TS, Larsen DL. 2014. Assessment of the onset of action of afoxolaner against existing adult flea (Ctenocephalides felis) infestations on dogs. Vet Parasitol 201:204–206. doi: 10.1016/j.vetpar.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Otranto D. 2014. Nexgard. Afoxolaner, a new oral insecticide-acaricide to control fleas and ticks in dogs. Editorial. Vet Parasitol 201:177–178. doi: 10.1016/j.vetpar.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 21.Shoop WL, Hartline EJ, Gould BR, Waddell ME, McDowell RG, Kinney JB, Lahm GP, Long JK, Xu M, Wagerle T, Jones GS, Dietrich RF, Cordova D, Schroeder ME, Rhoades DF, Benner EA, Confalone PN. 2014. Discovery and mode of action of afoxolaner, a new isoxazoline parasiticide for dogs. Vet Parasitol 201:179–189. doi: 10.1016/j.vetpar.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Letendre L, Huang R, Kvaternick V, Harriman J, Drag M, Soll M. 2014. The intravenous and oral pharmacokinetics of afoxolaner used as a monthly chewable antiparasitic for dogs. Vet Parasitol 201:190–197. doi: 10.1016/j.vetpar.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Mounsey K, Ho M-F, Kelly A, Willis C, Pasay C, Kemp DJ, McCarthy JS, Fischer K. 2010. A tractable experimental model for study of human and animal scabies. PLoS Negl Trop Dis 4:e756. doi: 10.1371/journal.pntd.0000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernigaud C, Fang F, Fischer K, Lespine A, Aho LS, Dreau D, Kelly A, Sutra J-F, Moreau F, Lilin T, Botterel F, Guillot J, Chosidow O. 2016. Preclinical study of single-dose moxidectin, a new oral treatment for scabies: efficacy, safety, and pharmacokinetics compared to two-dose ivermectin in a porcine model. PLoS Negl Trop Dis 10:e0005030. doi: 10.1371/journal.pntd.0005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arlian LG, Runyan RA, Achar S, Estes SA. 1984. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol 11:210–215. doi: 10.1016/S0190-9622(84)70151-4. [DOI] [PubMed] [Google Scholar]
- 26.Van Neste DJ, Staquet MJ. 1986. Similar epidermal changes in hyperkeratotic scabies of humans and pigs. Am J Dermatopathol 8:267–273. doi: 10.1097/00000372-198606000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Meyer W, Schwarz R, Neurand K. 1978. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr Probl Dermatol 7:39–52. doi: 10.1159/000401274. [DOI] [PubMed] [Google Scholar]
- 28.Beugnet F, de Vos C, Liebenberg J, Halos L, Larsen D, Fourie J. 2016. Efficacy of afoxolaner in a clinical field study in dogs naturally infested with Sarcoptes scabiei. Parasite 23:26. doi: 10.1051/parasite/2016026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taenzler J, Liebenberg J, Roepke RKA, Frénais R, Heckeroth AR. 2016. Efficacy of fluralaner administered either orally or topically for the treatment of naturally acquired Sarcoptes scabiei var. canis infestation in dogs. Parasit Vectors 9:392. doi: 10.1186/s13071-016-1670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero C, Heredia R, Pineda J, Serrano JA, Mendoza GD, Trápala P, Cordero AM. 2016. Efficacy of fluralaner in 17 dogs with sarcoptic mange. Vet Dermatol 27:353–e88. doi: 10.1111/vde.12363. [DOI] [PubMed] [Google Scholar]
- 31.Becskei C, De Bock F, Illambas J, Cherni JA, Fourie JJ, Lane M, Mahabir SP, Six RH. 2016. Efficacy and safety of a novel oral isoxazoline, sarolaner (Simparica), for the treatment of sarcoptic mange in dogs. Vet Parasitol 222:56–61. doi: 10.1016/j.vetpar.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Mounsey KE, Murray HC, Bielefeldt-Ohmann H, Pasay C, Holt DC, Currie BJ, Walton SF, McCarthy JS. 2015. Prospective study in a porcine model of Sarcoptes scabiei indicates the association of Th2 and Th17 pathways with the clinical severity of scabies. PLoS Negl Trop Dis 9:e0003498. doi: 10.1371/journal.pntd.0003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swe PM, Zakrzewski M, Kelly A, Krause L, Fischer K. 2014. Scabies mites alter the skin microbiome and promote growth of opportunistic pathogens in a porcine model. PLoS Negl Trop Dis 8:e2897. doi: 10.1371/journal.pntd.0002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Currie BJ, McCarthy JS. 2010. Permethrin and ivermectin for scabies. N Engl J Med 362:717–725. doi: 10.1056/NEJMct0910329. [DOI] [PubMed] [Google Scholar]
- 35.Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. 2012. The pig: a model for human infectious diseases. Trends Microbiol 20:50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drag M, Saik J, Harriman J, Larsen D. 2014. Safety evaluation of orally administered afoxolaner in 8-week-old dogs. Vet Parasitol 201:198–203. doi: 10.1016/j.vetpar.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Ozoe Y, Asahi M, Ozoe F, Nakahira K, Mita T. 2010. The antiparasitic isoxazoline A1443 is a potent blocker of insect ligand-gated chloride channels. Biochem Biophys Res Commun 391:744–749. doi: 10.1016/j.bbrc.2009.11.131. [DOI] [PubMed] [Google Scholar]
- 38.Casida JE. 2015. Golden age of RyR and GABA-R diamide and isoxazoline insecticides: common genesis, serendipity, surprises, selectivity, and safety. Chem Res Toxicol 28:560–566. doi: 10.1021/tx500520w. [DOI] [PubMed] [Google Scholar]
- 39.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lifschitz A, Virkel G, Sallovitz J, Sutra JF, Galtier P, Alvinerie M, Lanusse C. 2000. Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle. Vet Parasitol 87:327–338. doi: 10.1016/S0304-4017(99)00175-2. [DOI] [PubMed] [Google Scholar]
- 41.Lifschitz A, Virkel G, Imperiale F, Sutra JF, Galtier P, Lanusse C, Alvinerie M. 1999. Moxidectin in cattle: correlation between plasma and target tissues disposition. J Vet Pharmacol Ther 22:266–273. doi: 10.1046/j.1365-2885.1999.00222.x. [DOI] [PubMed] [Google Scholar]
- 42.Lespine A, Alvinerie M, Sutra J-F, Pors I, Chartier C. 2005. Influence of the route of administration on efficacy and tissue distribution of ivermectin in goat. Vet Parasitol 128:251–260. doi: 10.1016/j.vetpar.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 43.Hilbe JM. 2011. Negative binomial regression, 2nd ed Cambridge University Press, New York, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.