Chloroquine-resistant (CQR) vivax malaria has emerged as a threat to the malaria elimination agenda. The objective of this study was to assess if a combination of chloroquine (CQ) and prochlorperazine was able to reverse CQ resistance of the Plasmodium vivax AMRU-1 strain from Papua New Guinea in infected Aotus monkeys.
KEYWORDS: Plasmodium vivax, chloroquine resistance, resistance reversals, Aotus monkeys, animal models
ABSTRACT
Chloroquine-resistant (CQR) vivax malaria has emerged as a threat to the malaria elimination agenda. The objective of this study was to assess if a combination of chloroquine (CQ) and prochlorperazine was able to reverse CQ resistance of the Plasmodium vivax AMRU-1 strain from Papua New Guinea in infected Aotus monkeys. For this purpose, in two independent experimental drug efficacy trials, a total of 18 Aotus monkeys infected with blood obtained from donor animals were randomly assigned to treatment and control groups and orally administered CQ at 10 mg/kg or prochlorperazine at 20 mg/kg, alone or in combination, for five consecutive days. Reversal of CQR was achieved in animals that received the drug combination, whereas neither drug alone produced cures. This same drug combination reverses CQR in P. falciparum and could be an alternative for treatment in humans with chloroquine-resistant P. vivax infections.
INTRODUCTION
Each year, approximately 216 million new cases of malaria and more than 400,000 deaths occur worldwide (1). Malaria is caused by infection with a protozoan parasite of the genus Plasmodium, of which five species, including Plasmodium falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi, are transmitted to humans by the bites of infected female Anopheles mosquitoes. It is estimated that between 2010 and 2016 the incidence rate of malaria decreased 18% globally; nonetheless, during 2014 to 2016, significant increases were noted, especially in the region of the Americas, where P. vivax infections represent 64% of all cases detected (1). Even if P. falciparum infections are eliminated, it is predicted that P. vivax will remain an important cause of morbidity and mortality outside Africa (2, 3).
The emergence of chloroquine-resistant (CQR) P. vivax is a newly emerging problem of antimalarial drug resistance (4). Since the first description of resistant P. vivax in Papua New Guinea (5), other resistant isolates have been confirmed in Oceania, Asia, and South America (6–10). CQR P. vivax has been treated with artemisinin combination therapies (ACTs) with success (11), and tafenoquine (WR238605), a promising primaquine analog that has been demonstrated to be a potent blood schizonticide for the treatment of chloroquine-resistant P. vivax infections in Aotus monkeys (12), has been submitted for approval by regulatory agencies as a single-dose radical cure treatment (prevention of relapses) in patients 16 years of age and older (13, 14).
The mechanism of CQ resistance in P. vivax is not clearly defined. In P. falciparum, it is known that mutations in the chloroquine resistance transporter pfcrt are the primary determinant of CQ resistance, with mutations in the multidrug resistance gene (pfmdr1) playing a secondary role. Although P. vivax has homologs of pfcrt and pfmdr1, mutations in either gene have not been confirmed to confer CQR in vivax malaria. One of the hallmarks of CQR in P. falciparum is the reversal of resistance in vitro by verapamil, desipramine, and a series of other drugs (15–18). The reversal of CQR in falciparum malaria also has been confirmed in vivo with desipramine and prochlorperazine (PCP) in the Aotus model (15, 19). In this study, we aimed to determine if coadministration of CQ and PCP could reverse CQR in P. vivax in vivo.
(This research was presented at the Vivax Malaria Research: 2002 and Beyond Meeting, 3 to 8 February 2002, Bangkok, Thailand [20].)
RESULTS
For these studies, we used the CQR AMRU-1 strain of P. vivax (named after the Army Malaria Research Unit in Australia) (5), which was previously shown to infect Aotus monkeys and to be refractory to treatment with CQ at doses that clear and cure CQ-sensitive P. falciparum and P. vivax infections (12, 21, 22). We conducted two experiments and had experimental groups with CQ treatment alone (10 mg/kg/day), PCP alone (20/mg/kg/day), and CQ-PCP combinations (CQ at 10 mg/kg/day and PCP at 20 mg/kg/day), as well as untreated controls (Table 1). All treatments were given orally (per os) once a day for 5 days. The drug doses used in this study were based upon those in previous studies of PCP reversal of CQ resistance in Aotus monkeys infected with P. falciparum (22).
TABLE 1.
Detailed activity of prochlorperazine (PCP) and chloroquine (CQ) against infections of the chloroquine-resistant AMRU-1 strain of Plasmodium vivax in Aotus monkeys
| Expt no. and group no. (mean ± SD days from final drug to clearance) | Monkey ID | Drug regimen |
Response of parasitemia to drug |
No. of days from final drug to: |
No. of days negative | ||||
|---|---|---|---|---|---|---|---|---|---|
| Druga | Daily dose (mg/kg) | None | Suppressed | Cleared | Clearance | Recrudescence | |||
| Expt 1 | |||||||||
| Group 1 (22 ± 19) | 12914 | CQ | 10 | X | 8 | 55 | |||
| 12911 | X | 43 | 52 | 11 | |||||
| 12906 | X | 14 | 49 | ||||||
| Group 2 (11 ± 17) | 12894 | CQ | 10 | X | 1 | 63 | |||
| 12900 | PCP | 20 | X | 1 | 63 | ||||
| 12940 | X | 31 | 35 | ||||||
| Control (14 ± 0) | 12910 | Vehicle | X | 14 | 49 | ||||
| 12943 | X | 14 | 49 | ||||||
| Expt 2 | |||||||||
| Group 1 (13 ± 11) | 12865 | CQ | 10 | X | 24 | 76 | |||
| 12866 | X | 19 | 30 | 11 | |||||
| 12904 | X | 12 | 20 | 8 | |||||
| Group 2 (7 ± 0) | 12882 | PCP | 20 | X | 7 | 93 | |||
| 12870 | X | 7 | 30 | 23 | |||||
| 12876 | X | 7 | 14 | 7 | |||||
| Group 3 (2 ± 0) | 12875 | CQ | 10 | X | 2 | 98 | |||
| 12903 | PCP | 20 | X | 2 | 98 | ||||
| 12880 | X | 2 | 78 | ||||||
| Control | 12869 | Vehicle | X | 7 | |||||
CQ, chloroquine; PCP, prochlorperazine.
P. vivax AMRU-1 infected Aotus monkeys treated with CQ alone demonstrated marked resistance to CQ, with parasitemia in 5 of 6 animals not responding to 5 days of treatment (Fig. 1a and Fig. 2a). Only one Aotus monkey infected with CQR AMRU-1 had suppression of parasitemia during CQ treatment, yet the infection recrudesced 3 days after clearance. These results confirm that the AMRU-1 strain of P. vivax is CQR in vivo in Aotus monkeys.
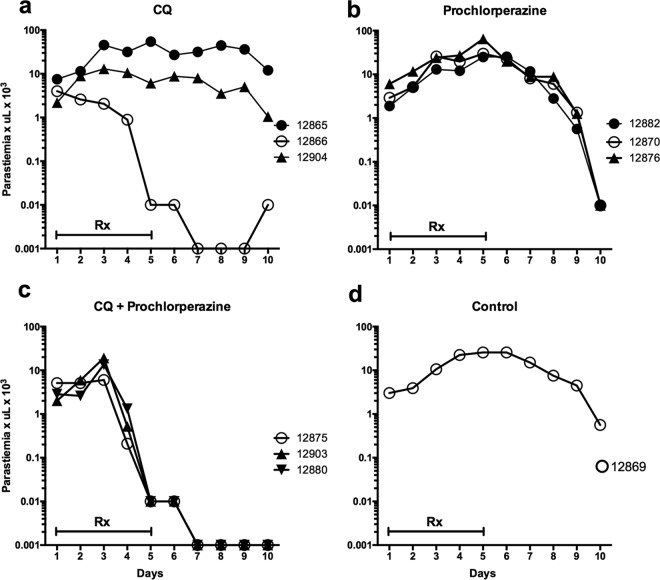
FIG 1.
Parasitemia courses of Aotus monkeys infected with the chloroquine-resistant AMRU-1 strain of P. vivax treated with chloroquine (CQ) at 10 mg/kg (a), prochlorperazine (PCP) at 20 mg/kg (b), or CQ and PCP in combination (c). Parasitemia in an untreated control (d). Negative parasitemia data are plotted at 0.001. Rx, day of drug dosing.
FIG 2.
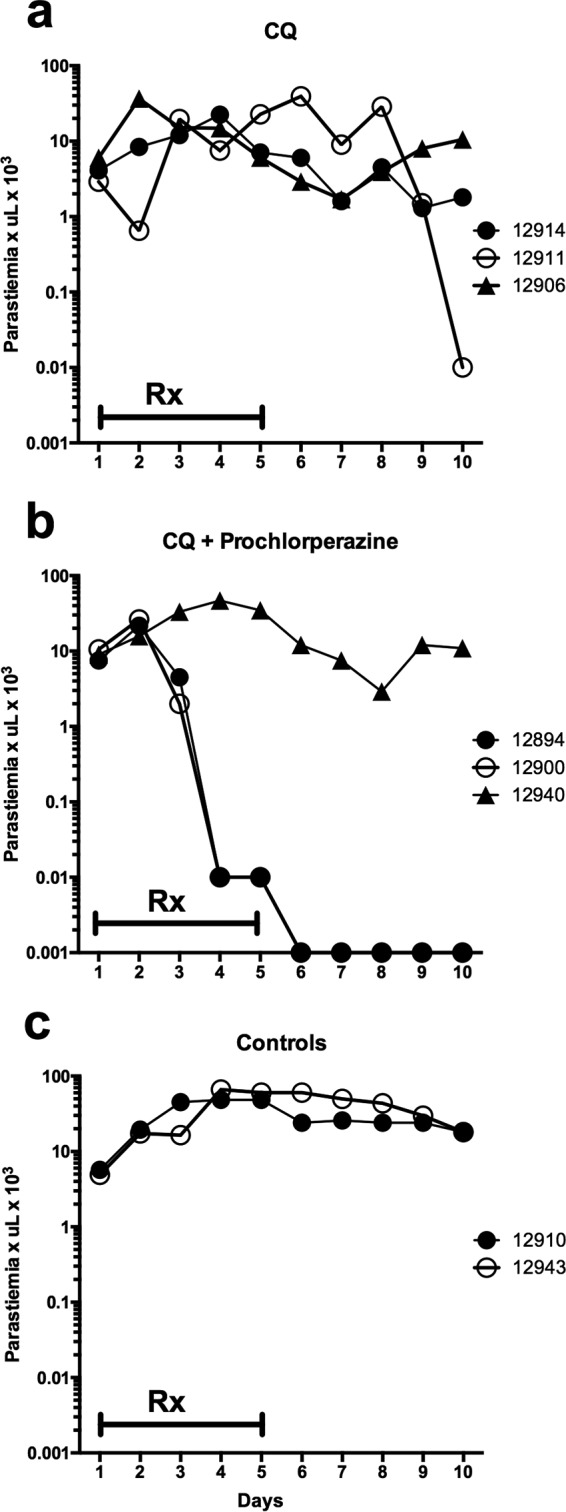
Parasitemia courses of Aotus monkeys infected with the chloroquine-resistant AMRU-1 strain of P. vivax treated with chloroquine at 10 mg/kg alone (a) or in combination with prochlorperazine (PCP; 20 mg/kg) (b) and controls (c). Negative parasitemia data are plotted at 0.001. Rx, day of drug dosing.
Treatment of P. vivax-infected Aotus monkeys with PCP alone (20 mg/kg/day) did not significantly affect parasitemia during treatment, although parasitemia began to decrease on day 9 (4 days after treatment ended) in all 3 animals treated with 20 mg/kg/day of PCP alone (Fig. 1b). Interestingly, the untreated control animal (Fig. 1d) in the same experiment also showed a decrease in parasitemia by day 10 of the study. In the second experiment (Fig. 2), the untreated controls maintained high levels of parasitemia through day 10. The untreated controls and the animals treated with CQ or PCP alone were treated with mefloquine as rescue therapy on day 10, as stipulated in the Institutional Animal Care and Use Committee (IACUC) protocol.
Five of 6 animals treated with the combination of CQ and PCP exhibited a marked decrease in parasitemia (Fig. 1c and 2b). The first decreases in parasitemia were observed on days 3 and 4 of the study, and parasitemia declined rapidly thereafter until clearance was observed on day 6 or 7. In the animals that cleared infection following treatment with CQ-PCP, no recrudescence was observed for up to days 65 to 98 of the study, and they were considered cured (Table 1). The treatment with CQ-PCP combinations thus produced cures, whereas treatment with CQ or PCP alone was ineffective against CQR P. vivax.
DISCUSSION
Reversal of CQ resistance in vivo using resistance reversers was demonstrated for the first time in 1988 by Bitonti et al. when CQ was administered in combination with desipramine to Aotus monkeys infected with CQR P. falciparum (16). Additional studies have shown that it is possible to achieve in vivo reversal of CQ resistance by the coadministration of prochlorperazine and chloroquine, as evidenced by infection cure (22). Historic use of inexpensive antihistamine drugs, such as chlorpheniramine and promethazine, for the treatment or prevention of CQ-associated pruritus or as antiemetics suggest that the combination is safe and effective when used at standard dosages (18). Recent clinical trials have already demonstrated successful cure rates of 81 to 96% in patients with CQ-resistant uncomplicated malaria (23). Although not widely adopted for use, the potential remains for a combination of CQ with a resistance reversal drug to increase therapeutic responses in areas with CQR falciparum malaria.
Notwithstanding that CQR vivax malaria was first reported in 1989 (5), and these resistant infections have become quite prevalent in Indonesia (24), there remains a lack of understanding of the mechanism of CQR in P. vivax. Putative resistance genes pvcrt-0 and pvmdr1 have been investigated, yet the data are not yet convincing that either plays a central role in CQR in P. vivax. In P. falciparum, the in vitro reversal of resistance with verapamil phenotype was used to map the pfcrt locus and to identify the mutations that confer CQR in P. falciparum. In this study, we aimed to assess if the phenotypic reversal of CQR by PCP in vivo with P. falciparum (22) could be achieved with CQR P. vivax. Our data clearly show evidence for reversal of CQR in P. vivax by PCP combined with CQ. Although 1 of 6 animals treated with the combination did not respond as expected, we did not measure the blood levels of CQ or PCP, and thus the lack of response could be due to inadequate drug exposure.
The results of this study demonstrate for the first time a phenotypic similarity between CQR in P. falciparum and P. vivax, namely, the ability of PCP coadministration to overcome CQR in an in vivo model of disease. As noted from previous studies in the Aotus model, PCP is the most potent drug demonstrated to reverse CQR in P. falciparum in vivo, and PCP produces a similar effect in vitro (22). Interestingly, an ex vivo phenotypic study with CQR P. vivax was not able to demonstrate a reduction in CQ 50% inhibitory concentrations (IC50s) with several CQ reversal agents, including verapamil; unfortunately, this study did not include PCP, so we do not know if synergy happens in vitro as well (25).
The data presented in this study demonstrate that a combination of CQ and PCP reverses CQ resistance of the P. vivax AMRU-1 strain in infected Aotus monkeys. Neither drug effects cure when used alone. This drug combination could be an alternative for treatment in humans with CQ-resistant P. vivax infections, and the resistance reversal phenotype may assist with efforts to identify the molecular basis for CQR in P. vivax.
MATERIALS AND METHODS
Animals.
Twenty male and female Aotus lemurinus lemurinus karyotype VIII and IX monkeys (26), consisting of 18 experimental and two P. vivax-infected blood donors, were maintained in the animal facility of the Gorgas Memorial Institute in Panama City, Panama. The animals were housed and cared for as described previously (19, 27). The weight of the monkeys when inoculated with parasites ranged from 749 to 1,002 g.
Parasites.
The CQ-resistant P. vivax AMRU-1 strain was originally isolated from an Australian soldier infected in Papua New Guinea in 1989 (5) and was successfully adapted by serial passage to Panamanian Aotus l. lemurinus monkeys, which served as a suitable model for drug evaluation studies (12, 21).
Parasitemia determination and follow up.
Giemsa thick blood smears were prepared from all animals by a prick in the marginal ear vein and were examined daily beginning the day after inoculation, until parasitemia was cleared and for at least 7 days thereafter. Blood films were then examined twice a week up to 100 days after treatment when, if negative, infection was considered cured. Parasitemia was enumerated by the Earle-Perez technique and expressed as number of parasites/microliter (28). Parasitemia outcomes were defined as described previously (29–31): suppressed, if parasitemia persisted but reduced to less than one-fiftieth of control; cleared, if parasitemia became negative by 12 days after patency and remained negative for 7 days (undetectable by microscopy after 5 min of examination); and cured, if parasitemia cleared and remained negative for 100 days after end of treatment. If parasitemia reached >150,000 parasites/μl, hematocrit (HTO %) dropped to 50% below baseline, or platelets reached <50,000/μl prior to day 28 postinfection (p.i.), the animals were rescue treated with a single oral dose of 20 mg/kg of mefloquine (MQ).
Drug preparation and administration.
Stock solutions of CQ (WR1544BM;AR20613) and prochlorperazine (PCP) (WR280001AC;BN3106) in distilled water were prepared at appropriate concentrations of drug base and maintained at 4°C during the course of treatment. CQ and PCP doses are expressed as mg/kg of base drug. Drugs were administered over 5 days in equally divided doses once a day by gastric intubation in a volume of 7 ml, followed by a 7-ml rinse with distilled water.
Experimental design: experiment 1.
Each of eight Aotus monkeys (749 to 950 g) were randomized by weight and sex into two groups of three monkeys each, and two controls and were inoculated intravenously (i.v.) in the saphenous vein with 5 × 104 parasitized erythrocytes of the AMRU-1 strain of P. vivax, obtained from the femoral vein of a donor monkey as described previously (12). When parasitemia reached ∼5 × 103 parasites/μl of blood, the animals were treated for 5 days as follows: group 1, comprised of Aotus 12914, 12911, and 12906, received chloroquine (CQ) at 10 mg/kg; group 2, comprised of Aotus 12894, 12900, and 12940, was treated with PCP at 20 mg/kg plus CQ at 10 mg/kg; and the control group, consisting of Aotus 12910 and 12943, received the drug vehicle only.
Experimental design: experiment 2.
Each of 10 Aotus monkeys (753 to 1,002 g) were randomized by weight and sex into three groups of three monkeys each and one control and were inoculated i.v. in the saphenous vein with 5 × 106 parasitized erythrocytes of the AMRU-1 strain of P. vivax, as above. When parasitemia reached ∼5 × 103 parasites/μl of blood, the animals were treated for 5 days as follows: group 1, comprised of Aotus 12865, 12866, and 12904, received CQ at 10 mg/kg; group 2, comprised of Aotus 12882, 12870, and 12876, was treated with PCP at 20 mg/kg; group 3, consisting of Aotus 12875, 12903, and 12880, received PCP at 20 mg/kg plus CQ at 10 mg/kg; and the control group, consisting of Aotus 12869, received the drug vehicle only.
Ethical statement.
The experimental protocol “Reversal of chloroquine resistance with the coadministration of prochlorperazine and chloroquine against infections of chloroquine resistant (CQR) AMRU-1 strain of Plasmodium vivax in Aotus lemurinus lemurinus monkeys” was approved and registered at the Instituto Conmemorativo Gorgas de Estudios de la Salud (ICGES) – Institutional Animal Care and Use Committee (CIUCAL) under accession number 1999/01. It was conducted in accordance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals (32), and the laws and regulations of the Republic of Panama. The general animal use protocol for the contract “Evaluation of Drug and Vaccine Candidates in the Human Malaria/Aotus Monkey Model” (award DAMD17-96-C-6051), was approved by the U.S. Army Medical Research and Materiel Command (Fort Detrick, Maryland, USA).
ACKNOWLEDGMENTS
This work was done with the financial support of the U.S. Army Medical Research and Materiel Command (award DAMD17-96-C-6051). The views, opinions, and/or findings contained herein are those of the authors and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation.
We gratefully acknowledge the following individuals: Gloria Cisneros, Frank Durham, William Otero, and Lionel Martinez for technical assistance and Maritza Brewer for secretarial assistance. At Promed S.A., we thank Ginés Sánchez and Gladys Calvino for administrative assistance and Jose Camilo Marín, Temistocles Lao, Roberto Rojas, and the animal caretakers for the care and handling of animals. We also acknowledge the support of the Sistema Nacional de Investigación (SNI) of the Secretaria Nacional de Ciencia Tecnología e Innovación (SENACYT) and the Gorgas Memorial Institute, Republic of Panama in the publication of this article.
N.O. III, W.K.M., and D.E.K. planned and conducted the experiments, analyzed the data, and wrote the paper.
REFERENCES
- 1.WHO. 2017. World malaria report 2017. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Vitor-Silva S, Siqueira AM, de Souza Sampaio V, Guinovart C, Reyes-Lecca RC, de Melo GC, Monteiro WM, Del Portillo HA, Alonso P, Bassat Q, Lacerda MV. 2016. Declining malaria transmission in rural Amazon: changing epidemiology and challenges to achieve elimination. Malar J 15:266. doi: 10.1186/s12936-016-1326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabinovich RN, Drakeley C, Djimde AA, Hall BF, Hay SI, Hemingway J, Kaslow DC, Noor A, Okumu F, Steketee R, Tanner M, Wells TNC, Whittaker MA, Winzeler EA, Wirth DF, Whitfield K, Alonso PL. 2017. malERA: an updated research agenda for malaria elimination and eradication. PLoS Med 14:e1002456. doi: 10.1371/journal.pmed.1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Liang Y, Jie Z, Wu S, Wang H. 2015. Global extent of chloroquine-resistant Plasmodium vivax. Lancet Infect Dis 15:630. doi: 10.1016/S1473-3099(15)00016-X. [DOI] [PubMed] [Google Scholar]
- 5.Rieckmann KH, Davis DR, Hutton DC. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183–1184. [DOI] [PubMed] [Google Scholar]
- 6.Whitby M, Wood G, Veenendaal JR, Rieckmann K. 1989. Chloroquine-resistant Plasmodium vivax. Lancet ii:1395. [DOI] [PubMed] [Google Scholar]
- 7.Whitby M. 1997. Drug resistant Plasmodium vivax malaria. J Antimicrob Chemother 40:749–752. doi: 10.1093/jac/40.6.749. [DOI] [PubMed] [Google Scholar]
- 8.Soto J, Toledo J, Gutierrez P, Luzz M, Llinas N, Cedeno N, Dunne M, Berman J. 2001. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am J Trop Med Hyg 65:90–93. doi: 10.4269/ajtmh.2001.65.90. [DOI] [PubMed] [Google Scholar]
- 9.Khan A, Godil FJ, Naseem R. 2016. Chloroquine-resistant Plasmodium vivax in Pakistan: an emerging threat. Lancet Glob Health 4:e790. doi: 10.1016/S2214-109X(16)30251-0. [DOI] [PubMed] [Google Scholar]
- 10.Baird JK. 2004. Chloroquine resistance in Plasmodium vivax. Antimicrob Agents Chemother 48:4075–4083. doi: 10.1128/AAC.48.11.4075-4083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grigg MJ, William T, Menon J, Barber BE, Wilkes CS, Rajahram GS, Edstein MD, Auburn S, Price RN, Yeo TW, Anstey NM. 2016. Efficacy of artesunate-mefloquine for chloroquine-resistant Plasmodium vivax malaria in Malaysia: an open-label, randomized, controlled trial. Clin Infect Dis 62:1403–1411. doi: 10.1093/cid/ciw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obaldia N 3rd, Rossan RN, Cooper RD, Kyle DE, Nuzum EO, Rieckmann KH, Shanks GD. 1997. WR 238605, chloroquine, and their combinations as blood schizonticides against a chloroquine-resistant strain of Plasmodium vivax in Aotus monkeys. Am J Trop Med Hyg 56:508–510. doi: 10.4269/ajtmh.1997.56.508. [DOI] [PubMed] [Google Scholar]
- 13.Walsh DS, Looareesuwan S, Wilairatana P, Heppner DG Jr, Tang DB, Brewer TG, Chokejindachai W, Viriyavejakul P, Kyle DE, Milhous WK, Schuster BG, Horton J, Braitman DJ, Brueckner RP. 1999. Randomized dose-ranging study of the safety and efficacy of WR 238605 (tafenoquine) in the prevention of relapse of Plasmodium vivax malaria in Thailand. J Infect Dis 180:1282–1287. doi: 10.1086/315034. [DOI] [PubMed] [Google Scholar]
- 14.MMV. 28 November 2017. GSK submits US regulatory application for single-dose tafenoquine for Plasmodium vivax malaria. MMV, Geneva, Switzerland: https://www.mmv.org/newsroom/press-releases/gsk-submits-us-regulatory-application-single-dose-tafenoquine-plasmodium. [Google Scholar]
- 15.Martin RE, Marchetti RV, Cowan AI, Howitt SM, Broer S, Kirk K. 2009. Chloroquine transport via the malaria parasite's chloroquine resistance transporter. Science 325:1680–1682. doi: 10.1126/science.1175667. [DOI] [PubMed] [Google Scholar]
- 16.Bitonti AJ, Sjoerdsma A, McCann PP, Kyle DE, Oduola AM, Rossan RN, Milhous WK, Davidson DE Jr. 1988. Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science 242:1301–1303. doi: 10.1126/science.3057629. [DOI] [PubMed] [Google Scholar]
- 17.Milhous W, Kyle D. 1998. Introduction to the modes of action and mechanisms of resitance to antimalarials. In Sherman IW. (ed), Malaria: parasite biology, pathogenesis and protection. ASM Press, Washington, DC. [Google Scholar]
- 18.Oduola AM, Sowunmi A, Milhous WK, Brewer TG, Kyle DE, Gerena L, Rossan RN, Salako LA, Schuster BG. 1998. In vitro and in vivo reversal of chloroquine resistance in Plasmodium falciparum with promethazine. Am J Trop Med Hyg 58:625–629. doi: 10.4269/ajtmh.1998.58.625. [DOI] [PubMed] [Google Scholar]
- 19.Obaldia N III, Otero W, Marin C, Aparicio J, Cisneros G. 2011. Long-term effect of a simple nest-box on the reproductive efficiency and other life traits of an Aotus lemurinus lemurinus monkey colony: an animal model for malaria research. J Med Primatol 40:383–391. doi: 10.1111/j.1600-0684.2011.00489.x. [DOI] [PubMed] [Google Scholar]
- 20.Obaldia N, Kyle D, Milhous W. 2002. Reversal of chloroquine resistance with the co-administration of prochlorperazine and chloroquine against infections of the chloroquine resistant (CQR) AMRU-1 strain of P. vivax in Aotus lemurinus lemurinus monkeys, p 56. Vivax Malaria Research: 2002 and Beyond Meeting, 3 to 8 February 2002, Bangkok, Thailand. [Google Scholar]
- 21.Cooper RD. 1994. Studies of a chloroquine-resistant strain of Plasmodium vivax from Papua New Guinea in Aotus and Anopheles farauti s.l. J Parasitol 80:789–795. doi: 10.2307/3283259. [DOI] [PubMed] [Google Scholar]
- 22.Kyle DE, Milhous WK, Rossan RN. 1993. Reversal of Plasmodium falciparum resistance to chloroquine in Panamanian Aotus monkeys. Am J Trop Med Hyg 48:126–133. doi: 10.4269/ajtmh.1993.48.126. [DOI] [PubMed] [Google Scholar]
- 23.Bagla P. 1997. Malaria fighters gather at site of early victory. Science 277:1437–1438. doi: 10.1126/science.277.5331.1437. [DOI] [PubMed] [Google Scholar]
- 24.Price RN, von Seidlein L, Valecha N, Nosten F, Baird JK, White NJ. 2014. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis 14:982–991. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirjanata G, Handayuni I, Prayoga P, Leonardo L, Apriyanti D, Trianty L, Wandosa R, Gobay B, Kenangalem E, Poespoprodjo JR, Noviyanti R, Kyle DE, Cheng Q, Price RN, Marfurt J. 2017. Plasmodium falciparum and Plasmodium vivax demonstrate contrasting chloroquine resistance reversal phenotypes. Antimicrob Agents Chemother 61:e00355-17. doi: 10.1128/AAC.00355-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma NS, Rossan RN, Kelley ST, Harper JS, Bedard MT, Jones TC. 1978. Banding patterns of the chromosomes of two new karyotypes of the owl monkey, Aotus, captured in Panama. J Med Primatol 7:146–155. doi: 10.1159/000459804. [DOI] [PubMed] [Google Scholar]
- 27.Obaldia N., 3rd 1991. Detection of Klebsiella pneumoniae antibodies in Aotus l. lemurinus (Panamanian owl monkey) using an enzyme linked immunosorbent assay (ELISA) test. Lab Anim 25:133–141. doi: 10.1258/002367791781082603. [DOI] [PubMed] [Google Scholar]
- 28.Earle W, Perez M. 1931. Enumeration of parasites in the blood of malarial patients. J Lab Clin Med 19:1124–1130. [Google Scholar]
- 29.Schmidt LH. 1978. Plasmodium falciparum and Plasmodium vivax infections in the owl monkey (Aotus trivirgatus). III. Methods employed in the search for new blood schizonticidal drugs. Am J Trop Med Hyg 27:718–737. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt LH. 1978. Plasmodium falciparum and Plasmodium vivax infections in the owl monkey (Aotus trivirgatus). II. Responses to chloroquine, quinine, and pyrimethamine. Am J Trop Med Hyg 27:703–717. doi: 10.4269/ajtmh.1978.27.703. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt LH. 1978. Plasmodium falciparum and Plasmodium vivax infections in the owl monkey (Aotus trivirgatus). I. The courses of untreated infections. Am J Trop Med Hyg 27:671–702. doi: 10.4269/ajtmh.1978.27.671. [DOI] [PubMed] [Google Scholar]
- 32.National Research Council. 1996. Care and use of laboratory animals. National Research Council, National Academies Press, Washington, DC: https://www.nap.edu/catalog/5140/guide-for-the-care-and-use-of-laboratory-animals. [Google Scholar]