This open-label, nonrandomized, single-dose, phase 1 study evaluated the pharmacokinetics and safety of murepavadin, a novel peptide antibiotic for the treatment of serious Pseudomonas aeruginosa infections. The study was conducted in 32 subjects of either sex in 4 groups (up to 8 per group) with mild (group 1), moderate (group 2), and severe (group 3) renal function impairment or with normal renal function (group 4).
KEYWORDS: murepavadin, pharmacokinetics, phase 1, renal impairment, safety, tolerability
ABSTRACT
This open-label, nonrandomized, single-dose, phase 1 study evaluated the pharmacokinetics and safety of murepavadin, a novel peptide antibiotic for the treatment of serious Pseudomonas aeruginosa infections. The study was conducted in 32 subjects of either sex in 4 groups (up to 8 per group) with mild (group 1), moderate (group 2), and severe (group 3) renal function impairment or with normal renal function (group 4). The degree of renal impairment of the subjects was classified at screening according to the estimated creatinine clearance (CLCr) according to the Cockcroft-Gault equation. All subjects received a single 2.2-mg/kg of body weight intravenous infusion of murepavadin administered over 3 h. Exposure to murepavadin in plasma increased in subjects with renal function impairment, with the area under the plasma concentration-time curve from zero to infinity (AUC0–∞) increasing about 2.0- to 2.5-fold for subjects with renal function impairment compared to subjects with normal renal function, whereas the increases in maximum observed plasma concentration (Cmax) were about 1.5-fold for subjects with renal function impairment compared to subjects with normal renal function. The total clearance (CL) of murepavadin was lower in all groups of subjects with renal function impairment, with group means ranging from 2.4 liters/h to 3.8 liters/h, compared to 7.0 liters/h in subjects with normal renal function. Accordingly, the terminal elimination half-life (t1/2) prolonged up to 24 h with decreasing renal function compared to 7.7 h in subjects with normal renal function. Murepavadin was well tolerated in all renal function groups. As the elimination of murepavadin is affected by renal function, a dose adjustment is warranted in subjects with impaired renal function. (This paper has been registered at ClinicalTrials.gov under identifier NCT02110459.)
INTRODUCTION
Bacterial resistance has become a major public health problem. In recent years, there have been frequent publications regarding extensively drug-resistant bacteria, while there has been little increase in the development of new antibiotics (1, 2). The currently available options to treat Pseudomonas aeruginosa infections include β-lactam antibiotics (e.g., meropenem), aminoglycosides (e.g., amikacin), quinolones (e.g., levofloxacin), and polymyxins (e.g., colistin). However, P. aeruginosa has an intrinsic resistance to many antibiotics due to high cellular impermeability and efficient drug efflux mechanisms, and the recent increase in the prevalence of multidrug-resistant (MDR) P. aeruginosa infections is particularly threatening in intensive care unit (ICU) settings (3, 4). P. aeruginosa is one of the most common causes of ventilator-associated bacterial pneumonia (VABP) (5), with a prevalence of approximately 25% (6, 7). It is estimated that at least 30% of the P. aeruginosa strains retrieved from respiratory specimens in patients with nosocomial pneumonia are MDR (8). Consequently, the treatment of hospital-acquired bacterial pneumonia (HABP) and VABP caused by P. aeruginosa is becoming more challenging (9), and new treatment options are needed.
Murepavadin (formally known as POL7080) is a pathogen-specific antimicrobial peptidomimetic with a novel nonlytic mechanism of action, the first in class of the outer membrane protein targeting antibiotics (OMPTAs), which is being developed by Polyphor Ltd. (10, 11). Murepavadin functions through a novel mechanism of action by binding to the lipopolysaccharide transport protein D (LptD), an outer membrane protein involved in lipopolysaccharide biogenesis in Gram-negative bacteria (12). By binding to LptD, murepavadin inhibits the lipopolysaccharide (LPS) transport function of LptD and causes lipopolysaccharide alterations in the outer membrane of the bacterium and, ultimately, cell death (13). Nonclinical studies have demonstrated the selective and potent bactericidal antimicrobial activity of murepavadin against P. aeruginosa in vitro, including MDR strains. When tested against over 1,200 P. aeruginosa isolates from the United States, Europe, and China, including MDR isolates, the MIC required to inhibit the growth of 90% of organisms (MIC90) was 0.12 to 0.25 mg/liter (14). Murepavadin had a low propensity to induce resistance in vitro, and the induction of resistance to murepavadin resulted in no cross-resistance to other antibiotics tested (11).
In the first-in-human phase 1 study, following single intravenous (i.v.) dose administration by infusion, the increases in maximum observed plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) were dose proportional from 0.15 mg/kg of body weight to 5 mg/kg of body weight (15). According to compound plasma concentration-time profiles during the terminal elimination period, murepavadin appeared to be cleared from systemic circulation at a rate similar to the glomerular filtration rate. Thus, in the present study, the pharmacokinetics (PK) and safety of single doses of murepavadin were investigated in subjects with mild, moderate, and severe renal function impairment and compared to subjects with normal renal function.
(Parts of this research were previously presented in poster P1308 at the 26th European Congress on Clinical Microbiology and Infectious Diseases [ECCMID], Amsterdam, The Netherlands [16].)
RESULTS
Demographics and disposition.
All 32 subjects enrolled in the study received the planned single intravenous dose of murepavadin, completed the study as per protocol, and were subsequently in the PK, tolerability, and safety assessments. The demographic characteristics of the subjects are presented in Table 1. Overall, the study population consisted of 16 females (50%) and 16 males (50%), with a mean (SD) age of 66 (10) years and body mass index of 26.7 (3.4) kg/m2 (Table 1). The male-to-female ratios were 3:5, 4:4, 4:4, and 5:3 for groups 1, 2, 3, and 4, respectively.
TABLE 1.
Summary of demographic data and renal function in safety population at screening
| Parameter |
Data by renal function |
||||
|---|---|---|---|---|---|
| Mild impairment (n = 8) | Moderate impairment (n = 8) | Severe impairment (n = 8) | Normal (n = 8) | All (n = 32) | |
| Age (yr) | |||||
| Mean ± SD | 70 ± 4 | 71 ± 8 | 64 ± 13 | 60 ± 9 | 66 ± 10 |
| Median | 71 | 74 | 70 | 59 | 70 |
| Range | 63–75 | 52–77 | 43–77 | 50–75 | 43–77 |
| Ht (cm) | |||||
| Mean ± SD | 170 ± 5 | 172 ± 5 | 170 ± 6 | 177 ± 7 | 172 ± 6 |
| Median | 168 | 172 | 170 | 176 | 171 |
| Range | 165–180 | 163–180 | 161–184 | 168–188 | 161–188 |
| Wt (kg) | |||||
| Mean ± SD | 75.2 ± 9.4 | 79.8 ± 14.6 | 75.8 ± 7.0 | 86.0 ± 12.4 | 79.2 ± 11.5 |
| Median | 74.1 | 80.1 | 77.8 | 83.3 | 77.8 |
| Range | 62.6–95.1 | 58.2–108.8 | 64.3–86.9 | 72.0–104.0 | 58.2–108.8 |
| Body mass index (kg/m2) | |||||
| Mean ± SD | 26.2 ± 3.9 | 27.0 ± 4.3 | 26.3 ± 2.9 | 27.3 ± 2.7 | 26.7 ± 3.4 |
| Median | 25.1 | 27.4 | 26.2 | 26.4 | 26.3 |
| Range | 22.4–34.9 | 20.1–33.6 | 22.5–30.1 | 24.3–32.2 | 20.1–34.9 |
| Creatinine clearance at screening (ml/min) | |||||
| Mean ± SD | 62.4 ± 4.5 | 41.5 ± 4.2 | 24.9 ± 3.5 | 94.5 ± 12.5 | |
| Median | 61.5 | 40.0 | 25.5 | 93.0 | |
| Range | 54.0–69.0 | 36.0–48.0 | 17.0–29.0 | 81.0–120.0 | |
| eGFR at screening (ml/min/1.73 m2)a | |||||
| Mean ± SD | 71.6 ± 15.3 | 39.5 ± 11.9 | 17.9 ± 2.5 | 81.4 ± 10.3 | |
| Median | 73.0 | 36.0 | 18.0 | 81.0 | |
| Range | 45.0–92.0 | 26.0–56.0 | 15.0–22.0 | 70.0–104.0 | |
| Race (no. [%]) | |||||
| Caucasian | 8 (100) | 8 (100) | 8 (100) | 8 (100) | 32 (100) |
| Sex (no. [%]) | |||||
| Female | 5 (62.5) | 4 (50.0) | 4 (50.0) | 3 (37.5) | 16 (50.0) |
| Male | 3 (37.5) | 4 (50.0) | 4 (50.0) | 5 (62.5) | 16 (50.0) |
eGFR, estimated glomerular filtration rate.
All subjects with renal function impairment (RFI) were receiving medications at baseline for the treatment of their underlying renal disease (e.g., furosemide) and other ongoing diseases (e.g., amlodipine for hypertension, metformin for diabetes mellitus, and simvastatin to prevent hyperlipidemia). No medication was ongoing at baseline in healthy subjects.
Pharmacokinetics.
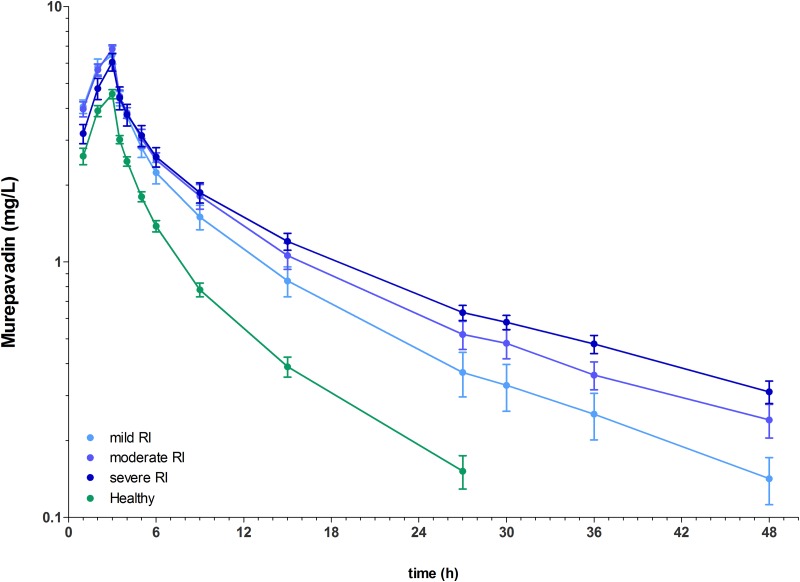
The mean concentration-time profiles for murepavadin in plasma are shown in Fig. 1. The mean concentration profile of the group of healthy subjects had a steeper decline than the profiles of the renal function impairment groups. In healthy subjects, the geometric mean Cmax (4.51 mg/liter) was attained at the end of infusion, the geometric mean terminal elimination half-life (t1/2) was 7.6 h, and the geometric mean area under the plasma concentration-time curve from zero to infinity (AUC0–∞) for healthy subjects was 27.23 h · mg/liter, which was in the range expected from the phase 1 study (14) (Table 2). The variability of the parameters was low, as reflected in the arithmetic coefficient of variation (CV) of ≤30% (Table 2). In the subjects with RFI, the exposure to murepavadin in plasma increased (Table 2). The geometric mean AUC0–∞ in the RFI groups increased with decreasing renal function to 49.66, 60.53, and 69.99 h · mg/liter in mild, moderate, and severe RFI, respectively. The geometric mean Cmax in the RFI groups increased with decreasing renal function to 6.32, 6.78, and 5.92 mg/liter in mild, moderate, and severe RFI, respectively (see Fig. 2). Total clearance (CL) of murepavadin was lower in all groups of subjects with renal function impairment, with group geometric means ranging from 3.3 liters/h (mild RFI) to 2.4 liters/h (severe RFI), compared to 6.9 liters/h in subjects with normal renal function (Table 2). Accordingly, geometric mean t1/2 increased from 13.9 h to 23.1 h with decreasing renal function (Table 2). The longer half-life is consistent with the increase in AUC0–∞.
FIG 1.
Arithmetic mean (SD) plasma concentration-time profiles of murepavadin in healthy subjects (n = 8) and subjects with mild (n = 8), moderate (n = 8), or severe (n = 8) renal function impairment after administration of a single dose of 2.2 mg infused over 3 h on a semilogarithmic scale. Mild RI, mild renal function impairment; moderate RI, moderate renal function impairment; severe RI, severe renal function impairment; healthy, normal renal function.
TABLE 2.
Primary and secondary pharmacokinetic characteristics
| Parameter | Renal impairment group | na | Mean | SD | CV (%)b | Geometric mean | Geometric SD | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|---|---|---|---|
| AUC0–∞ (h · mg/liter) | Mild | 8 | 51.49 | 14.87 | 28.9 | 49.66 | 1.34 | 33.75 | 52.23 | 77.85 |
| Moderate | 8 | 61.84 | 13.61 | 22.0 | 60.53 | 1.25 | 41.19 | 61.55 | 85.32 | |
| Severe | 8 | 71.13 | 12.85 | 18.1 | 69.99 | 1.22 | 47.26 | 74.02 | 85.64 | |
| Healthy | 8 | 27.52 | 42.80 | 15.6 | 27.23 | 1.16 | 23.19 | 26.21 | 33.63 | |
| Cmax (mg/liter) | Mild | 8 | 6.47 | 1.48 | 22.9 | 6.32 | 1.26 | 4.40 | 6.30 | 8.97 |
| Moderate | 8 | 6.82 | 0.74 | 10.9 | 6.78 | 1.12 | 5.54 | 6.96 | 7.89 | |
| Severe | 8 | 6.05 | 1.34 | 22.2 | 5.92 | 1.26 | 4.15 | 6.19 | 8.13 | |
| Healthy | 8 | 4.54 | 0.52 | 11.6 | 4.51 | 1.12 | 3.77 | 4.49 | 5.41 | |
| CL (liters/h) | Mild | 8 | 3.4 | 0.8 | 22.1 | 3.3 | 1.2 | 2.6 | 3.2 | 4.7 |
| Moderate | 8 | 2.9 | 0.3 | 11.7 | 2.9 | 1.1 | 2.1 | 2.9 | 3.2 | |
| Severe | 8 | 2.4 | 0.6 | 26.2 | 2.4 | 1.3 | 1.8 | 2.2 | 3.7 | |
| Healthy | 8 | 7.0 | 1.3 | 19.1 | 6.9 | 1.2 | 5.4 | 6.6 | 8.7 | |
| t1/2 (h) | Mild | 8 | 14.1 | 2.6 | 18.5 | 13.9 | 1.2 | 11.2 | 13.1 | 18.1 |
| Moderate | 8 | 15.9 | 4.0 | 25.2 | 15.5 | 1.3 | 11.6 | 14.9 | 22.0 | |
| Severe | 8 | 24.1 | 7.8 | 32.4 | 23.1 | 1.3 | 18.4 | 20.2 | 39.0 | |
| Healthy | 8 | 7.7 | 1.4 | 18.7 | 7.6 | 1.2 | 5.2 | 7.5 | 10.1 | |
| Vz (liters) | Mild | 8 | 68.5 | 18.3 | 26.7 | 66.5 | 1.3 | 50.9 | 62.1 | 99.7 |
| Moderate | 8 | 65.9 | 17.9 | 27.1 | 63.7 | 1.3 | 39.1 | 62.7 | 91.4 | |
| Severe | 8 | 80.9 | 17.9 | 22.1 | 78.9 | 1.3 | 54.4 | 82.2 | 101.6 | |
| Healthy | 8 | 76.3 | 14.1 | 18.5 | 75.3 | 1.2 | 65.1 | 68.3 | 99.2 |
Number of evaluable subjects.
CV, coefficient of variation.
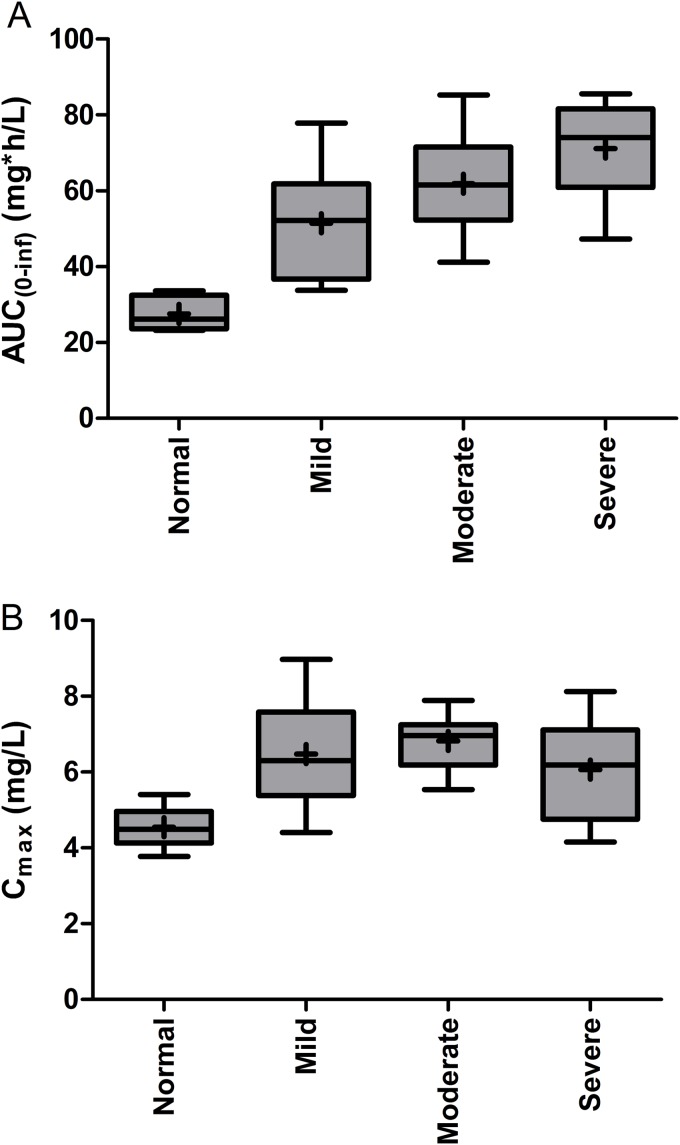
FIG 2.
Boxplots for AUC0–∞ (AUC0–∞) (A) and Cmax (B) of murepavadin in plasma. Mild, moderate, and severe renal function impairment and normal renal function are indicated. Boxplot bottom and top edges represent the 25th and 75th percentiles (difference is interquartile range [IQR]), the horizontal line is the median, the marker is the mean, and the whiskers indicate the minimum and maximum.
The least square mean ratios (90% confidence interval [CI]) of mild RFI to normal renal function were 1.82 (1.51, 2.20) and 1.40 (1.20, 1.64) for AUC0–∞ and Cmax, respectively. The least square mean ratios (90% CI) of moderate RFI to healthy subjects were 2.22 (1.84, 2.69) and 1.50 (1.28, 1.75) for AUC0–∞ and Cmax, and those of severe RFI to healthy subjects were 2.57 (2.12, 3.11) and 1.31 (1.12, 1.53), respectively. Thus, in RFI subjects, the murepavadin AUC0–∞ increased about 2.0- to 2.5-fold compared to subjects with normal renal function (Table 3). With respect to Cmax, the murepavadin exposure increases were about 1.5-fold for subjects RFI compared to subjects with normal renal function. These results were confirmed by visual inspection of the mean plasma concentration-time profiles (Fig. 1).
TABLE 3.
Group comparisons of renal function groups, characterized by CLCr
| Parameter | Intrasubject CV (%)a | Group ratiob | LSmeanc |
Point estimate | 90% confidence interval (%) | |
|---|---|---|---|---|---|---|
| Test | Reference | |||||
| AUC0–∞ (h · mg/liter) | 22.78 | 1/4 | 49.66 | 27.24 | 1.82 | 1.51–2.20 |
| 2/4 | 60.53 | 27.24 | 2.22 | 1.84–2.69 | ||
| 3/4 | 69.99 | 27.24 | 2.57 | 2.12–3.11 | ||
| Cmax (mg/liter) | 18.61 | 1/4 | 6.33 | 4.52 | 1.40 | 1.20–1.64 |
| 2/4 | 6.79 | 4.52 | 1.50 | 1.28–1.75 | ||
| 3/4 | 5.92 | 4.52 | 1.31 | 1.12–1.53 | ||
CV, coefficient of variation.
Group 1, mild renal function impairment; group 2, moderate renal function impairment; group 3, severe renal function impairment; group 4, normal renal function.
LSmean, least square mean.
Safety and tolerability.
No deaths, serious adverse events, or adverse events (AEs) that led to study discontinuation were reported. Eleven treatment-emergent adverse events (TEAEs) were reported for 6 of the 32 subjects (18.8%), with 7 events being of mild intensity and 4 events being of moderate intensity (Table 4). The highest incidence of TEAEs was in group 3 (severe renal function impairment), with 9 TEAEs reported for 4 subjects (50%). The lowest incidence was in group 2 (moderate renal function impairment), in which no TEAEs were reported. Most TEAEs (number of AEs [F], 9) were not related to murepavadin. Two TEAEs (fatigue and oral paresthesia), both of mild intensity, were considered to be related to murepavadin, and for 1 TEAE (headache) of mild intensity, a relationship was considered unlikely. All TEAEs were observed only once. All TEAEs were resolved at the end of the study. One event (melena) was further investigated by gastroscopy. Local tolerability at the infusion site was good. Only a few subjects reported mild to moderate reactions, with mild (n = 2) to moderate (n = 1) erythema in 3 of the 32 subjects treated. The TEAE and tolerability profiles are in line with the observations reported in the single- and multiple-dose studies (15). Safety laboratory parameters, vital signs, and electrocardiogram (ECG) parameters showed no medically relevant changes associated with murepavadin. Overall, an infusion of 2.2 mg/kg murepavadin was considered safe and well tolerated in subjects with mild to severe renal function impairment.
TABLE 4.
Frequency of TEAEs by system organ class and preferred terma
| System organ class/preferred term | Data by renal function |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild impairment (n = 8) |
Moderate impairment (n = 8) |
Severe impairment (n = 8) |
Normal (n = 8) |
Total (n = 32) |
|||||||||||
| F | No. of subjects with AEs | % subjects with AEs | F | No. of subjects with AEs | % subjects with AEs | F | No. of subjects with AEs | % subjects with AEs | F | No. of subjects with AEs | % subjects with AEs | F | No. of subjects with AEs | % subjects with AEs | |
| Total TEAEs | 2 | 2 | 25.0 | 0 | 0 | 0 | 9 | 4 | 50 | 2 | 1 | 12.5 | 14 | 8 | 25.0 |
| Gastrointestinal disorders | 3 | 2 | 25 | 3 | 2 | 6.3 | |||||||||
| Erosive gastritis | 1 | 1 | 12.5 | 1 | 1 | 3.1 | |||||||||
| Melena | 1 | 1 | 12.5 | 1 | 1 | 3.1 | |||||||||
| Oral paresthesia | 1 | 1 | 12.5 | 1 | 1 | 3.1 | |||||||||
| General disorders and administration site conditions | 2 | 2 | 25.0 | 1 | 1 | 12.5 | 1 | 1 | 12.5 | 4 | 4 | 12.5 | |||
| Infusion site erythema | 1 | 1 | 12.5 | 1 | 1 | 12.5 | 1 | 1 | 12.5 | 3 | 3 | 9.4 | |||
| Fatigue | 1 | 1 | 12.5 | 1 | 1 | 3.1 | |||||||||
| Investigations | 6 | 3 | 37.5 | 6 | 3 | 9.4 | |||||||||
| Blood potassium decreased | 1 | 1 | 12.5 | 1 | 1 | 3.1 | |||||||||
| Hematocrit decreased | 1 | 1 | 12.5 | 1 | 1 | 3.1 | |||||||||
| Hemoglobin decreased | 1 | 1 | 12.5 | 1 | 1 | 3.1 | |||||||||
| Neutrophil count increased | 1 | 1 | 12.5 | 1 | 1 | 3.1 | |||||||||
| Red blood cell count decreased | 1 | 1 | 12.5 | 1 | 1 | 3.1 | |||||||||
| White blood cell count increased | 1 | 1 | 12.5 | 1 | 1 | 3.1 | |||||||||
| Nervous system disorders | 1 | 1 | 12.5 | 1 | 1 | 3.1 | |||||||||
| Headache | 1 | 1 | 12.5 | 1 | 1 | 3.1 | |||||||||
Adverse events were coded using MedDRA version 14.0. F, number of AEs.
DISCUSSION
In the current study, a single 3-h i.v. infusion of 2.2 mg/kg of murepavadin was generally well tolerated in all subjects, and no safety concern related to murepavadin was raised. Two AEs were reported in this study as being related to murepavadin (fatigue and oral paresthesia), both of which were considered mild in intensity. The oral paresthesia started 1 h 10 min after the start of infusion and lasted 4 h 50 min. All AEs resolved without sequelae before the end of the study, and no AE or serious AE led to study discontinuation.
Following intravenous administration to mice, rats, rabbits, and monkeys, dose linearity was observed for the maximum observed plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC). In these animals, murepavadin was thought to follow a biphasic clearance pattern consistent with a two-compartment model. The volume of distribution after intravenous (i.v.) administration in these organisms indicates that murepavadin distributes into the aqueous phase but also to some extent into body tissue, and systemic plasma clearance (CL) values were similar to the species-specific glomerular filtration rates (GFRs) (10). Similarly, in the phase 1 study in healthy volunteers, murepavadin appears to be cleared from systemic circulation at glomerular filtration rates (15). However, in experimental animals and humans, the fraction of the administered murepavadin dose excreted unchanged into urine was low (<6%). Thus, glomerular filtration of the unchanged precursor was suggested to only be one part of the overall elimination process, while proteolytic degradation might be the other principal cause of murepavadin elimination (15, 17). Proteolytic degradation is dependent on the substrate concentration, whereas glomerular filtration is not. Thus, at high murepavadin concentration, proteolytic elimination seems to contribute substantially to the clearance. When the drug concentration then considerably drops below the enzyme(s) substrate affinity, glomerular filtration becomes the predominant elimination route for the drug. Thus, reduced kidney function should lead to an increase in Cmax and the AUC of murepavadin (17).
In the present study, the impact of different degrees of renal function impairment on the PK of murepavadin was evaluated for the use of this novel antibacterial in patients with nosocomial pneumonia due to P. aeruginosa. Renal impairment increased the exposure to murepavadin in a manner related to the severity of renal function impairment. These results suggest that in patients with moderately or severely impaired renal function, the dose of murepavadin may need to be adjusted.
MATERIALS AND METHODS
Study design.
This open-label, nonrandomized, monocenter, single-intravenous-dose, phase 1 study was performed at Clinical Research Services (CRS) Kiel GmbH (Kiel, Germany) between 17 April 2013 and 1 July 2015. In accordance with the Declaration of Helsinki, the German Drug Law (Arzneimittelgesetz), the German Good Clinical Practice decree, and the Note for Guidance on Good Clinical Practice, the study was presented to the Independent Ethics Committee (IEC) of the “Ärztekammer Schleswig-Holstein” (chairman, Gerhard Hintze) and the Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM). The opinion of the IEC and the authorization of the BfArM were obtained prior to any study-related procedures. Written informed consent was obtained from subjects prior to participation in the study.
Renal function was classified at screening by the estimated CLCr according to the Cockcroft-Gault equation. Eight subjects were assigned to one of the following treatment groups according to renal function impairment (RFI): group 1, mild (CLCr, 50 to 80 ml/min); group 2, moderate (CLCr, 30 to <50 ml/min); group 3, severe (CLCr, <30 ml/min; and group 4, an age-matched cohort with normal renal function (CLCr, >80 ml/min). For safety reasons, a sentinel group of 2 to 3 subjects with mild and moderate RFI was treated first. After completion of the mild and moderate impairment groups, an interim analysis was performed to decide on the recruitment of subjects with severe RFI based on comparison with currently available pharmacokinetic data from healthy volunteers of the first-in-humans study (14).
Subject selection.
Eligible subjects were males age ≥18 and ≤79 years and females of non-childbearing potential age ≥18 and ≤79 years. Additional inclusion criteria were a body weight within the body mass index range of 19.0 to 35.0 kg/m2 for all subjects to exclude underweight subjects and subjects from obesity class 2 and above, and either normal (CLCr, >80 ml/min) or impaired (CLCr, ≤80 ml/min) renal function. For subjects with renal impairment, there must have been no clinically significant change in disease status within at least 1 month prior to study entry. Healthy subjects had to be in a good health in the opinion of the study physician, as determined by medical history, electrocardiogram (ECG), vital signs, physical examination, and clinical laboratory tests. Key exclusion criteria were excess xanthine consumption (more than 5 cups of coffee or equivalent per day), smoking more than 10 cigarettes a day, consumption of more than 28 units (males) or more than 21 units (females) of alcohol per week or a significant history of alcoholism or drug/chemical abuse, or strenuous exercise within 72 h before admission to the study center. Subjects were excluded if they had any history of hypersensitivity to the investigational medicinal product, any clinically significant neurological, gastrointestinal, hepatic, cardiovascular, psychiatric, respiratory, metabolic, endocrine, hematological, or other major disorders, hepatic disease, any condition associated with intravascular volume depletion, a history of seizures or epilepsy, ECG abnormalities, significant allergies requiring intranasal or systemic corticosteroids during any time of the year, a history of any anaphylactic reaction, a positive test for human immunodeficiency virus (HIV) antibodies, or acute hepatitis B or C infection.
Administration of study drug.
Subjects received the murepavadin intravenous infusion in the morning of day 1 after a light breakfast. A standardized lunch and dinner were given was given at 4 h and 10 h after start of infusion. Based on the single- and multiple-ascending-dose phase 1 study (15), both Cmax and AUC were predicted to increase with decreased glomerular filtration rate (GFR). A prediction of plasma levels for subjects with decreased GFR (50 or 30 ml/min with mild or moderate RFI, respectively) was calculated for a dose of 2.2 mg/kg infused over 3 h. This dose was expected to result in a Cmax and AUC0–∞ below, i.e., approximately 55% of, the Cmax and AUC observed in the highest dose groups of the phase 1 study. Thus, subjects with RFI and healthy subjects received a single dose of 2.2 mg/kg murepavadin as a 3-h intravenous infusion to ensure a Cmax below that previously observed, as well as to enable a direct comparison to the phase 1 study (15). However, it should be noted that all subsequent clinical studies were conducted with a 2-h infusion (ClinicalTrials.gov identifiers NCT03409679, NCT02165332, NCT02165293, NCT02165332, NCT02897869, NCT02096315, and NCT02096328).
Sample collection and bioanalysis.
For all subjects, blood samples were taken predose and 1, 2, 3, 3.5, 4, 5, 6, 9, 15, and 27 h after the start of infusion. For subjects with renal impairment, additional samples were taken at 30, 36, and 48 h, and at 72 h for subjects with severe renal function impairment. The quantitative determination of murepavadin concentrations in plasma was carried out at Charles River Laboratories (Tranent, UK). Plasma concentrations of murepavadin were determined using a validated liquid chromatography-tandem mass spectrometry with electrospray ionization assay. Samples were extracted by protein precipitation in 0.1% formic acid in acetonitrile-dimethyl sulfoxide (acetonitrile-DMSO) (75/25 [vol/vol]). After 5 min of centrifugation at 3,500 rpm, 10 μl supernatant was injected on a Waters ultraperformance liquid chromatography (UPLC) system at 65°C equipped with a Waters Acquity BEH C18 (2.1 by 50 mm, 1.7-μm particle size) column, running at a flow rate of 500 μl/min. An AB Sciex API 5000 mass spectrometer, TurboIonSpray source (positive mode), with Analyst (version 1.6.2) software was used to monitor mass transition (±0.2 for each mass) by selected reaction monitoring (m/z 518.9→m/z 115.1 [M+3H]3+). The method was validated in the range of 10 to 2,000 ng/ml, with a lower limit of quantification (LLOQ) of 10 ng/ml using a 0.1-ml volume. The assay accuracy and precision values for murepavadin were 3.3 to 9.5%. The primary endpoints were Cmax and AUC0–∞. Secondary endpoints included half-life (t1/2), total body clearance of drug from plasma (CL), and apparent volume of distribution during the terminal phase (Vz).
Pharmacokinetic assessments.
The plasma PK parameters of murepavadin were derived by noncompartmental analysis (Phoenix WinNonlin version 6.4; Certara USA, Princeton, NJ, USA) of the concentration-time profiles. Pharmacokinetic parameters were calculated based on actual sampling times. At time points in the lag time between time zero and the first quantifiable concentration, concentrations below the LLOQ were calculated as zero. All other concentrations below the LLOQ were not used in calculations.
The measured individual plasma concentrations of murepavadin were used to directly obtain the maximum observed plasma concentration (Cmax) and time to Cmax (tmax). The area under the plasma concentration-time curve from 0 until the last quantifiable concentration (AUCt) was calculated according to the linear trapezoidal rule, using the measured concentration-time values above the LLOQ. The area under the plasma concentration-time curve from zero to infinity (AUC0–∞) was calculated by combining AUCt and AUCextra. AUCextra represents an extrapolated value obtained by Ct/λz, where Ct is the last plasma concentration above the LLOQ and λz represents the terminal elimination rate constant determined by linear regression of log-transformed concentration data after the time of maximum concentration. The t1/2 of murepavadin was calculated as follows: t1/2 = ln2/λz.
For descriptive statistics, values below the LLOQ were excluded from any calculations. Descriptive statistics of plasma concentrations were calculated if at least 2/3 of the individual data points had been measured at equal or above the LLOQ.
Safety.
Safety monitoring included adverse events (AEs), vital signs, ECG, safety laboratory tests (clinical chemistry, hematology, coagulation, urinalysis, serology, i-STAT for bedside calcium, physical examination, and local and overall tolerability).
Statistical analysis.
The planned sample size of 6 evaluable subjects per renal function impairment group and at least 8 evaluable subjects in the group of subjects with normal renal function was based on empirical considerations but was judged to be adequate to obtain reliable results meeting the objectives of this study, in line with the FDA and European Medicines Agency (EMA) guidelines. Statistical analyses were performed using the SAS software, version 9.3 (SAS Institute, Inc., Cary, NC, USA). The differences between groups of subjects with impaired renal function and healthy subjects were analyzed for the pharmacokinetic parameters Cmax and AUC0–∞ of murepavadin with an analysis of variance (ANOVA) model on the log-transformed values, with the renal function group as a fixed effect. The mean group ratios, that is, mild/normal, moderate/normal, and severe/normal, were estimated for each parameter, and 90% confidence intervals (CIs) for the estimates were given.
ACKNOWLEDGMENTS
We acknowledge the excellent support of Larissa Zimmer from CRS and the medical writing and editorial assistance of K. E. D. Coan in the preparation of this paper.
This study was funded by Polyphor Ltd., Allschwil, Switzerland.
A. Wach, C. Zwingelstein, and G. E. Dale are employees of Polyphor Ltd.
REFERENCES
- 1.Depuydt PO, Vandijck DM, Bekaert MA, Decruyenaere JM, Blot SI, Vogelaers DP, Benoit DD. 2008. Determinants and impact of multidrug antibiotic resistance in pathogens causing ventilator-associated-pneumonia. Crit Care 12:R142. doi: 10.1186/cc7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 3.Martin-Loeches I, Torres A, Rinaudo M, Terraneo S, de Rosa F, Ramirez P, Diaz E, Fernández-Barat L, Li Bassi GL, Ferrer M. 2015. Resistance patterns and outcomes in intensive care unit (ICU)-acquired pneumonia. Validation of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) classification of multidrug resistant organisms. J Infect 70:213–222. doi: 10.1016/j.jinf.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Luna CM, Rodriguez-Noriega E, Bavestrello L, Guzman-Blanco M. 2014. Gram-negative infections in adult intensive care units of Latin America and the Caribbean. Crit Care Res Pract 2014:480463. doi: 10.1155/2014/480463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramírez-Estrada S, Borgatta B, Rello J. 2016. Pseudomonas aeruginosa ventilator-associated pneumonia management. Infect Drug Resist 9:7–18. doi: 10.2147/IDR.S50669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones RN. 2010. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 51(Suppl 1):S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 7.Kollef MH, Chastre J, Fagon JY, Francois B, Niederman MS, Rello J, Torres A, Vincent JL, Wunderink RG, Go KW, Rehm C. 2014. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit Care Med 42:2178–2187. doi: 10.1097/CCM.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 8.Micek ST, Wunderink RG, Kollef MH, Chen C, Rello J, Chastre J, Antonelli M, Welte T, Clair B, Ostermann H, Calbo E, Torres A, Menichetti F, Schramm GE, Menon V. 2015. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Crit Care 19:219. doi: 10.1186/s13054-015-0926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassetti M, Welte T, Wunderink RG. 2016. Treatment of Gram-negative pneumonia in the critical care setting: is the beta-lactam antibiotic backbone broken beyond repair? Crit Care 20:19. doi: 10.1186/s13054-016-1197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luther A, Moehle K, Chevalier E, Dale G, Obrecht D. 2017. Protein epitope mimetic macrocycles as biopharmaceuticals. Curr Opin Chem Biol 38:45–51. doi: 10.1016/j.cbpa.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Loeches I, Dale GE, Torres A. 2018. Murepavadin: a new antibiotic class in the pipeline. Expert Rev Anti Infect Ther 16:259–268. doi: 10.1080/14787210.2018.1441024. [DOI] [PubMed] [Google Scholar]
- 12.Srinivas N, Jetter P, Ueberbacher BJ, Werneburg M, Zerbe K, Steinmann J, Van der Meijden B, Bernardini F, Lederer A, Dias RLA, Misson PE, Henze H, Zumbrunn J, Gombert FO, Obrecht D, Hunziker P, Schauer S, Ziegler U, Kach A, Eberl L, Riedel K, DeMarco SJ, Robinson JA. 2010. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science 327:1010–1013. doi: 10.1126/science.1182749. [DOI] [PubMed] [Google Scholar]
- 13.Werneburg M, Zerbe K, Juhas M, Bigler L, Stalder U, Kaech A, Ziegler U, Obrecht D, Eberl L, Robinson JA. 2012. Inhibition of lipopolysaccharide transport to the outer membrane in Pseudomonas aeruginosa by peptidomimetic antibiotics. Chembiochem 13:1767–1775. doi: 10.1002/cbic.201200276. [DOI] [PubMed] [Google Scholar]
- 14.Sader HS, Dale GE, Rhomberg PR, Flamm RK. 2018. Antimicrobial activity of murepavadin against clinical isolates of Pseudomonas aeruginosa from the United States, Europe, and China. Antimicrob Agents Chemother 62:e00311-. doi: 10.1128/AAC.00311-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wach A, Dembowsky K, Dale GE. 2018. Pharmacokinetics and safety of intravenous murepavadin infusion in healthy adult subjects administered as single and multiple ascending doses. Antimicrob Agents Chemother 62:e02355-17. doi: 10.1128/AAC.02355-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halabi A, Petersen-Sylla M, Sommerville K, Wach A, Dembowsky K, Rangaraju M, Dale GE. 2016. Pharmacokinetics and safety of POL7080 in subjects with impaired renal function, poster P1308. 26th European Congress on Clinical Microbiology and Infectious Diseases (ECCMID), 9 to 12 April 2016, Amsterdam, The Netherlands. [Google Scholar]
- 17.Wach A, Sennhauser J, Kern F, Ehmer P, Douglas G, Beni L, Zwingelstein C, Dale GE. 2017. Catabolism and excretion of the anti-pseudomonal peptidomimetic murepavadin (POL7080) in humans, abstr P2748. Abstr 27th European Congress on Clinical Microbiology and Infectious Diseases (ECCMID), 22 to 25 April 2017, Vienna, Austria. [Google Scholar]