Urinary tract infections (UTIs) are a tremendous burden on the health care system due to the vast number of infections resulting in antibiotic therapy and/or hospitalization. Additionally, these infections are frequently caused by multidrug-resistant (MDR) organisms, limiting the availability of effective antimicrobials.
KEYWORDS: urinary tract infection, meropenem, nacubactam, ceftazidime-avibactam, Gram-negative bacteria, RG6080, meropenem-nacubactam
ABSTRACT
Urinary tract infections (UTIs) are a tremendous burden on the health care system due to the vast number of infections resulting in antibiotic therapy and/or hospitalization. Additionally, these infections are frequently caused by multidrug-resistant (MDR) organisms, limiting the availability of effective antimicrobials. Nacubactam is a novel non-β-lactam-β-lactamase inhibitor with in vitro activity against class A and class C β-lactamases. Nacubactam is being developed in combination with meropenem, providing broad-spectrum activity in addition to improved stability against common β-lactamases. Here, we utilized a neutropenic murine complicated UTI (cUTI) model to determine the potential clinical utility of meropenem-nacubactam compared with meropenem or nacubactam alone against 10 Klebsiella pneumoniae, Escherichia coli, and Enterobacter cloacae isolates with diverse genotypic and phenotypic profiles, including NDM, KPC, OXA, CTX-M, SHV, and TEM enzyme-producing isolates. Selected isolates had meropenem-nacubactam MICs between 1 and 8 μg/ml. Meropenem-nacubactam demonstrated the greatest in vivo efficacy against 9 of 10 isolates, achieving a ≥3 log reduction from the 48-h control in all isolates tested, including isolates prepared as high inoculums. Nacubactam alone confirmed antibacterial properties, achieving a >1 log reduction against the majority of isolates. The combination of meropenem-nacubactam further enhanced the activity of either agent alone, notably against meropenem-resistant isolates. Against ceftazidime-avibactam-resistant isolates, meropenem-nacubactam demonstrated increased antibacterial kill upwards of 6 log10 CFU in comparison to the 48-h control. Our data support the potential clinical utility of meropenem-nacubactam for cUTI in humans against MDR Enterobacteriaceae, although further clinical data supporting meropenem-nacubactam efficacy are needed.
INTRODUCTION
Urinary tract infections (UTIs) are frequent causes of emergency department visits and hospital admissions, costing the health care system upwards of 3.5 billion dollars (1). Clinical presentations of UTIs can vary, ranging from an uncomplicated UTI treated with oral antibiotics to urosepsis resulting in an intensive care unit (ICU) admission (2, 3). Organisms associated with UTIs include Escherichia coli, Enterococcus spp., Klebsiella spp., Pseudomonas aeruginosa, and Staphylococcus spp. (1). Antimicrobials represent the standard of care for UTIs, albeit treatment has steadily become more difficult due to the increasing prevalence of multidrug-resistant (MDR) organisms, specifically, Gram-negative isolates. Common antimicrobials used for the treatment of UTIs, such as fluoroquinolones and β-lactams, are often no longer effective, with upwards of 50% of urinary tract infections now caused by MDR organisms (4, 5).
Antimicrobial resistance has manifested into a major roadblock for the practice of urology, and the need for new antimicrobial agents is dire (6). Nacubactam (RG6080, OP0595) is a diazabicyclooctanone (non-β-lactam–β-lactamase inhibitor) being developed in combination with meropenem for the treatment of serious Gram-negative bacterial infections. Diazabicyclooctanones have demonstrated inhibition of class A and class C β-lactamases, in addition to minor class D activity (7–10). Nacubactam alone has proven antibacterial activity via PBP2 inhibition of several Enterobacteriaceae isolates (7). Furthermore, studies have shown an enhanced antibacterial effect when the drug was used in combination with β-lactam agents that bind to other PBPs (non-PBP2), hence its development in combination with meropenem (8, 10).
On the basis of the in vitro activity of meropenem-nacubactam against MDR organisms, this agent may have a role in therapy for complicated UTIs (cUTIs). The purpose of this study was to describe the murine pharmacokinetics of meropenem-nacubactam and to evaluate the efficacy of meropenem-nacubactam, compared with the efficacy of meropenem or nacubactam alone, using a neutropenic murine cUTI model against Gram-negative organisms exhibiting variable resistance mechanisms, including NDM and KPC enzyme-producing isolates.
RESULTS
In vitro susceptibility.
Meropenem, nacubactam, meropenem-nacubactam, and ceftazidime-avibactam MICs for the 11 isolates used in the growth study are shown in Table 1. These isolates had various phenotypic profiles, with meropenem-nacubactam MICs ranging from 1 to 8 μg/ml. A modal nacubactam MIC was not established for three isolates (EC 492, KP 614, and KP 611). MICs for EC 492 and KP 614 were distributed between 1 and >256 μg/ml and between 2 and >256 μg/ml, respectively. MICs for KP 611 were bimodal at 8 and >256 μg/ml.
TABLE 1.
MICs of meropenem, nacubactam, meropenem-nacubactam, and ceftazidime-avibactam against a collection of 11 Enterobacteriaceae isolatesa
| CAIRD no. | Country of origin | Carbapenemase class | β-Lactamase(s) | MIC |
|||
|---|---|---|---|---|---|---|---|
| CAZ-AVI | Meropenem | Meropenem-Nacubactam (1:1) | Nacubactam | ||||
| KP 593 | Philippines | B | NDM-1; SHV-11; CTX-M-15; OXA-1 | >64 | 64 | 4 | >256 |
| ECL 101 | Vietnam | B | NDM-1; LAP-2; ACT-17; TEM-1 | >64 | 256 | 2 | 1 |
| EC 492 | China | B | NDM-1; CTX-M-3 | >64 | 256 | 1 | 1 to >256c |
| ECL 103 | Turkey | D | OXA-48 | 2 | 16 | 2 | 2 |
| KP 599 | United States | A | KPC-2; SHV-11 | 2 | 512 | 2 | 2b |
| ECL 104 | Colombia | A | KPC-2; CMH-3 | 0.5 | 64 | 1 | >256 |
| KP 604 | Italy | A | KPC-3; TEM-1; SHV-11 | 8 | 512 | 2 | 128 |
| KP 611 | Russia | B | NDM-1; SHV-1; CMY-6; CTX-M-15; TEM-1 | >64 | 128 | 4 | 8 to 256c |
| KP 612 | United Kingdom | D | OXA-48, OXA-1, OXA-10, CTX-M-14, CTX-M-15 | 1 | 32 | 2 | 2 |
| KP 614 | United Kingdom | D | OXA-48, OXA-1, SHV-76, TEM-1, CTX-M-15 | 2 | 128 | 8 | 2 to >256c |
| KP 615 | United Kingdom | A | KPC-3; SHV-11 | 2 | 128 | 1 | 2b |
CAZ-AVI, ceftazidime-avibactam; EC, Escherichia coli; KP, Klebsiella pneumoniae; ECL, Enterobacter cloacae.
Isolates showing trailing or skipped wells with results above the MIC value.
The MIC range is shown where it was not possible to establish a mode.
Pharmacokinetics of meropenem and nacubactam.
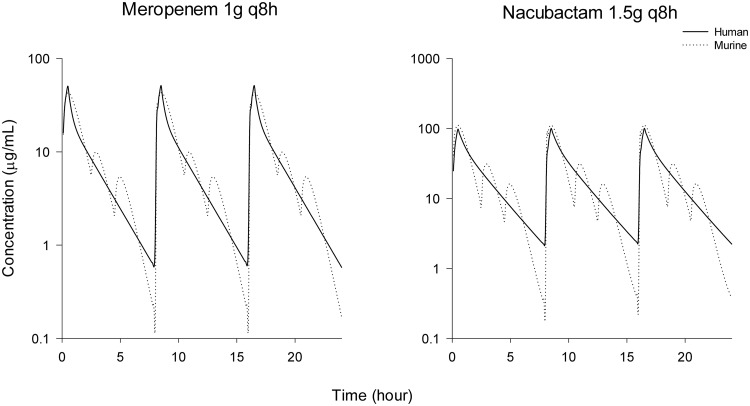
Nacubactam showed maximal concentrations at the first sampling time point (0.5 h) after dosing that declined rapidly afterwards. The plasma concentrations of nacubactam were not substantially affected when it was coadministered with meropenem (∼30%-higher mean exposure in the combination group). Similarly, the plasma concentration profiles of meropenem were not substantially affected by coadministration with nacubactam (∼40%-higher mean exposure in the combination group). The murine pharmacokinetic parameters of meropenem or nacubactam alone and in combination and the parameters of the fitted 2-compartmental models of meropenem and nacubactam are displayed in Tables S1 to S3 in the supplemental material. On the basis of the fitted 2-compartmental models, the dosing regimens were selected to mimic human pharmacokinetics in the mouse. Table 2 demonstrates the fT>MIC exposures of meropenem 1 g given every 8 h (q8h) (0.5-h infusion) and nacubactam 1.5 g q8h (0.5-h infusion) in humans compared with our regimens in mice. The left panel in Fig. 1 illustrates the simulated meropenem dose, revealing that the delivered murine dosing regimen provides a free drug profile similar to that of the human 1 g q8h regimen (11). The right panel in Fig. 1 depicts the nacubactam murine simulated dose in comparison with a human 1.5 g q8h dose.
TABLE 2.
Human and murine fT>MIC exposures of meropenem and nacubactam
| Exposure category | % fT>MIC exposure at MIC (μg/ml) of: |
||||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 16 | 32 | 64 | 128 | |
| Meropenem mousea | 76.25 | 58.75 | 36.25 | 18.75 | 10.00 | 0.00 | 0.00 |
| Meropenem humanb | 67.50 | 50.00 | 32.08 | 16.25 | 6.25 | 0.00 | 0.00 |
| Nacubactam mousec | 82.50 | 77.08 | 65.00 | 42.50 | 20.00 | 12.50 | 0.00 |
| Nacubactam humand | 100.00 | 81.25 | 62.08 | 42.08 | 23.33 | 8.75 | 0.00 |
0 h 50 mg/kg plus 2.5 h 8 mg/kg plus 4.5 h 5 mg/kg q8h.
1 g q8h (infusion length, 0.5 h).
0 h 80 mg/kg plus 2.5 h 20 mg/kg plus 4.5 h 10 mg/kg q8h.
1.5 g q8h (infusion length, 0.5 h).
FIG 1.
Meropenem (left panel) and nacubactam (right panel) humanized pharmacokinetic profiles in a neutropenic murine cUTI model compared to clinical doses (meropenem 1 g q8h, 0.5-h infusion; nacubactam 1.5 g q8h, 0.5-h infusion).
Bacterial density studies.
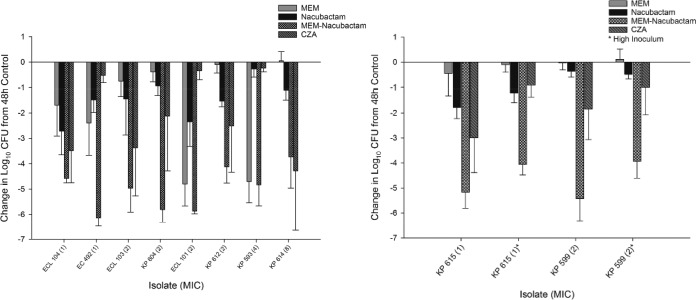
All isolates (7 Klebsiella pneumoniae, 1 E. coli, and 3 Enterobacter cloacae) demonstrated adequate growth (≥1 log10 CFU increase at 48 h) in the neutropenic cUTI model (see Fig. S1 in the supplemental material). The average increase in bacterial density at 48 h was 3.68 ± 0.54 log10 CFU for the 11 isolates in the in vivo growth studies, ensuring that the isolates displayed adequate pathogenicity in this infection model. The results of the bacterial density studies for each isolate are depicted in Fig. 2 (left panel, high inoculum; right panel, low inoculum). Two isolates were tested at high inoculum and low inoculum, and the remainder were tested at low inoculum. The average initial kidney bacterial densities (0 h) were 5.28 ± 0.16 and 4.15 ± 0.16 log10CFU in high- and low-inoculum infections, respectively. The average kidney bacterial densities at 48 h in the control animals were 8.92 ± 0.26 and 8.78 ± 0.53 log10 CFU in high- and low-inoculum infections, respectively. Meropenem-nacubactam achieved ≥3 log reduction from the 48-h control in all isolates tested (MICs of 1 to 8 μg/ml), including isolates prepared as high inoculums. Meropenem-nacubactam achieved an additional 1 log reduction in the setting of a low inoculum compared with high inoculum. Meropenem alone was similar to the 48-h control against 8 of 12 isolates. In 2 meropenem-resistant isolates, meropenem reached a ≥3 log reduction from the 48-h control, despite these being NDM-positive (NDM+) isolates (KP 593, ECL 101), a finding we have observed in previous murine models (12). With the exception of KP 593, EC 492, and ECL 101, nacubactam alone demonstrated greater in vivo efficacy than meropenem alone, producing bacterial reductions of between 0.27 and 2.72 log10 CFU compared with the 48-h control, but was less active than meropenem-nacubactam. Ceftazidime-avibactam achieved reductions of 0.24 to 4.28 log10 CFU, similar to previously reported activity in a murine UTI model (13).
FIG 2.
Mean absolute growth or reduction in log10 CFU counts per milliliter ± standard deviations (SD) from the 48-h control seen with meropenem (MEM), nacubactam, MEM-nacubactam, and ceftazidime-avibactam (CAZ-AVI) in a neutropenic murine cUTI model in (left panel) high-inoculum infections and (right panel) low-inoculum infections.
DISCUSSION
Meropenem-nacubactam provides activity against a broad spectrum of organisms while concurrently inhibiting several enzymes at fault for antimicrobial resistance; therefore, it may be of potential use for the treatment of cUTIs (3, 12). These studies were performed to evaluate the efficacy of humanized exposures of meropenem-nacubactam over 48 h in the neutropenic murine cUTI model against K. pneumoniae, E. coli, and E. cloacae isolates with diverse genotypic and phenotypic profiles, as well as to further compare meropenem-nacubactam efficacy to that of meropenem or nacubactam alone.
Meropenem-nacubactam demonstrated antibacterial activity against Gram-negative isolates with drug MICs between 1 and 8 μg/ml. As expected, the greatest reduction in log10 CFU was observed against isolates with MICs of 1 μg/ml. Accounting for both low- and high-inoculum infections, meropenem-nacubactam was the most effective antimicrobial against 9 of 10 isolates, suggesting that the bacterial burden may not limit the agent's antibacterial efficacy. The activity of the combination of meropenem-nacubactam further exceeded the activity of either agent alone, including against meropenem-resistant isolates.
Historically, antibiotics in the drug development pipeline have sought an FDA indication for the treatment of cUTIs in adults (14–16). The use of a cUTI murine model may predict the potential efficacy of novel antimicrobials in humans (17). Therefore, we consider this an initial step along the drug development pathway, as demonstrated with ceftazidime-avibactam. In a previous UTI model using AmpC- and SHV-producing isolates, administration of ceftazidime-avibactam resulted in bacterial reductions of approximately 2 to 4 log10 CFU from the 48-h control (13, 14). We observed similar but slightly diminished activity of ceftazidime-avibactam against ceftazidime-avibactam-susceptible isolates, which we attribute to the diversity in enzymes produced by the organisms used in this study (NDM, KPC, OXA, CTX-M, SHV, TEM). As expected, ceftazidime-avibactam was not active against isolates with ceftazidime-avibactam MICs of >64 μg/ml. Against these ceftazidime-avibactam-resistant isolates, meropenem-nacubactam demonstrated impressive bacterial kill.
Our study demonstrated the activity of meropenem-nacubactam against a range of β-lactamases, including NDM, KPC, OXA, CTX-M, SHV, and TEM enzymes. The broad inhibition of these enzymes by meropenem-nacubactam is necessitated in current practice. A study in pregnant patients with UTIs demonstrated that 47% of Escherichia coli isolates and 36.9% of K. pneumoniae isolates produced extended-spectrum β-lactamases (ESBLs) (i.e., OXA, CTX-M, SHV, and TEM enzymes) (18). In patients with UTIs caused by KPC-producing Enterobacteriaceae, inactive antibiotics were given 33% of the time, causing a median delay in adequate therapy of 72.5 h (19). In case reports of UTIs caused by K. pneumoniae NDM-1 strains, combination therapy of a carbapenem plus gentamicin or colistin was required (20). The use of gentamicin or colistin potentially increases the risk of adverse events, such as nephrotoxicity (21, 22). Therefore, as the prevalence of these enzymes increases, meropenem-nacubactam might be an attractive therapeutic option if resistance is a concern and/or identified. Albeit, studies generating additional data on meropenem-nacubactam activity against NDM-producing organisms are warranted, as previous in vitro data are limited (9, 10).
There were limitations to this study. As described previously, the direct inoculation method was used to establish a cUTI in our model (17). A variety of physiological factors can cause a cUTI in human, notably an ascending infection or bacterial translocation. While there may be physiological differences between mice and humans in the development of infection, we have previously demonstrated the clinical translatability of this model to human outcomes (17). Additionally, urine concentrations of nacubactam were not assessed. We developed our pharmacokinetic studies on the basis of plasma concentrations given the lack of availability of human urine nacubactam concentrations. The impressive reduction in the kidney's bacterial burden demonstrated adequate meropenem-nacubactam kidney penetration for cUTI infections.
In conclusion, the humanized exposure profile of meropenem-nacubactam was effective against meropenem- and ceftazidime-avibactam-resistant Enterobacteriaceae in a murine cUTI model. The observed efficacy of meropenem-nacubactam in this cUTI model has potential translatability to humans; therefore, the data may be of clinical use as the rates of infections caused by MDR bacteria in cUTIs continue to grow (3).
MATERIALS AND METHODS
Antimicrobial test agents.
Nacubactam (RG6080) vials (lot 510-015-4097-01, n = 2; lot 603-028-4097-01, n = 4) were supplied by F. Hoffmann-La Roche for in vitro and in vivo testing. The vials were stored refrigerated at +4°C and were protected from light during storage and throughout solution preparation and administration. Nacubactam powder was reconstituted to a 20 mg/ml concentration with 20 ml of sterile 0.9% normal saline solution (Hospira, Inc., Lake Forest, IL). Subsequent dilutions in sterile 0.9% normal saline solution were made to attain final concentrations that would deliver doses of 80, 20, and 10 mg/kg of body weight based on the mean weight of the population of study mice. Nacubactam was administered by subcutaneous (SC) injections of 0.2 ml.
Commercially available meropenem 500-mg vials (lot 0009D55; Fresenius Kabi USA, LLC) and ceftazidime-avibactam 2.5-g vials (lot 0309; Forest Pharmaceuticals, Inc.) were acquired from Cardinal Health, Inc. Meropenem vials were reconstituted and diluted with 0.9% normal saline solution (Hospira, Inc., Lake Forest, IL) to achieve doses of 50, 8, and 5 mg/kg. Ceftazidime-avibactam vials were reconstituted and diluted with 0.9% normal saline solution (Hospira, Inc., Lake Forest, IL) to achieve a dose of 25/6.25 mg/kg, as described in a previous UTI model (13). All doses were administered as 0.2-ml SC injections.
Microbiology—isolates and antimicrobial susceptibility studies.
A total of 30 Gram-negative isolates were provided by F. Hoffmann-La Roche. KP 615 was ordered from Public Health England (https://www.phe-culturecollections.org.uk). Isolates were stored at the Center for Anti-Infective Research and Development (CAIRD) at Hartford Hospital. Genotypes were previously identified by F. Hoffmann-La Roche. All isolates were screened using in vitro methods to determine suitable resistance phenotypes. The MICs of ceftazidime-avibactam, meropenem, nacubactam, and meropenem-nacubactam (1:1) were determined in triplicate for all test organisms by broth microdilution as described by Clinical and Laboratory Standards Institute (CLSI) guidelines (23). The modal MIC was reported. In instances where a modal MIC could not be determined, the MIC range was reported. From the 31 isolates, 11 were used in the initial in vivo growth studies to confirm adequate bacterial growth over 48 h. From the 11 isolates, 10 isolates were selected for the in vivo efficacy portion of this study. These isolates included a variety of Enterobacteriaceae species such as Klebsiella sp., Enterobacter sp., and E. coli isolates. Final isolates were selected to represent diverse genotypic and phenotypic profiles with limited susceptibility to comparator antibiotics.
Animal infection model. (i) Laboratory animals.
Specific-pathogen-free female ICR mice weighing 20 to 22 g were obtained from Envigo RMS, Inc. (Indianapolis, IN). The animals were allowed to acclimate for a minimum of 48 h before commencement of experimentation and were provided food and water ad libitum. The protocol was reviewed and approved by the Institutional Animal Care and Use Committee at Hartford Hospital. Mice were rendered transiently neutropenic by injection of cyclophosphamide intraperitoneally (i.p.) at doses of 150 mg/kg of body weight at 4 days before inoculation and 100 mg/kg of body weight at 1 day before inoculation. Mice were anesthetized during the inoculation period with ketamine/xylazine/acepromazine 100/10/3 mg/kg administered intraperitoneally. Buprenorphine 0.05 mg/kg was administered intraperitoneally prior to anesthesia induction and surgical incision and every 6 to 12 h for 48 h (17).
(ii) Direct inoculation complicated urinary tract infection model.
A previously published direct inoculation model (13, 17) was used to establish a cUTI in mice. Bacterial isolates were stored frozen at −80°C in skim milk. Two transfers of the bacterial isolate onto Trypticase soy agar with 5% sheep blood (Becton, Dickinson and Co., Sparks, MD) were performed, and the agar was placed into an incubator at 37°C. After 18 to 24 h of incubation of the second transfer, a bacterial suspension of approximately 107 CFU/ml was prepared in sterile saline solution. Further dilutions were prepared in sterile saline solution to achieve a specific target inoculum (high inoculum, 105.5 log10 CFU/ml; low inoculum, 104 log10 CFU/ml). High inoculums were used to assess the antibacterial efficacy in infections with large bacterial burdens. Low inoculums were used to reflect previously published cUTI methodology (13). Two isolates, KP 599 and KP 615, were prepared as both high and low inoculums. The remaining isolates were prepared as low inoculums. Final inoculum concentrations were confirmed by serial dilution and plating techniques. The area over each kidney (flanks) was shaved and prepped for surgical incision. A volume of 0.05 ml of a predetermined concentration of the bacterial isolate was injected directly into each kidney. The skin was closed with 4.0 Vicryl suture materials with an RB-1 needle. A period of 3 h after inoculation was required to establish infection.
(iii) Single-dose pharmacokinetic study.
The purpose of this study was to describe the pharmacokinetics and develop humanized exposures of meropenem 1 g q8h (30-min infusion), nacubactam 1.5 g q8h (30-min infusion), and meropenem-nacubactam 2.5 g q8h (30-min infusion) in infected ICR mice (11). The nacubactam human pharmacokinetic data were provided by F. Hoffmann-La Roche (clinical trial registration no. NCT021134834). Mice were prepared for the specific infection model as described above. Three hours after bacterial inoculation, groups of 21 mice (3 mice per time point, 7 sampling times) were administered 0.2-ml single doses of 400, 150, and 400 to 150 mg/kg of meropenem, nacubactam, and meropenem-nacubactam, respectively. At 7 time points (0.0833, 0.25, 0.5, 1, 2, 4, and 7 h) after dosing, groups of 3 mice were euthanized by CO2 exposure followed by blood collection via intracardiac puncture and, ultimately, cervical dislocation. Blood samples were collected in vials containing lithium heparin. The blood was centrifuged, and 100 μl of the plasma was transferred into polypropylene tubes. These tubes were stored at −80°C until analysis via high-performance liquid chromatography.
The pharmacokinetic analysis was carried out by F. Hoffmann-La Roche using the following methods. Phoenix 64 (WinNonlin 6.4, NLME 1.3) was used to carry out noncompartmental analysis (NCA) for determination of the pharmacokinetic parameters as well as to fit compartmental models. The NCA was based on the mean data of the composite profile per individual dosing group. Cmax (maximum concentration of drug in serum) and Tmax (time to maximum concentration of drug in serum) data were determined directly from the measured composite plasma concentration-time profiles. AUC(0-tlast) data were calculated as the area under the curve from the time of dosing (t = 0 h) to the last measurable concentration by the linear trapezoidal rule. AUC(0–inf) was calculated as AUC from t = 0 h extrapolated to infinity, based on the last observed concentration (Clastobs) [AUC(0–inf) = AUC(0–tlast) + Clastobs/λZ]. The terminal half-life λZ was estimated by linear regression of time versus log concentration. CL/F was calculated as dose/AUC(0–inf). Phoenix 64 (WinNonlin 6.4, NLME 1.3) was used also to carry out exploratory compartmental model fitting. Murine doses were created to simulate free time above MIC (fT>MIC) values similar to those of humans.
(iv) Neutropenic bacterial in vivo growth studies.
The purpose of these studies was to establish the extent of growth of the bacterial isolates over 48 h in the neutropenic cUTI model. For each of the 11 bacterial isolates, two groups of 3 animals each were inoculated as described above. After inoculation, one group for each bacterial isolate was euthanized by CO2 exposure followed by cervical dislocation to demonstrate the establishment of infection. At 48 h, one group for each bacterial isolate was sacrificed. The kidneys were removed and homogenized in normal saline solution. Serial dilutions were plated on an appropriate agar media for determination of CFU counts per milliliter. A minimum of a 1 log10 CFU/ml increase in bacterial count at 48 h was required to include the isolate in the efficacy studies.
Neutropenic bacterial density studies—antibiotic efficacy.
The purpose of these studies was to assess the in vivo activity of each humanized treatment regimen over a 2-day (48-h) dosing period against 10 Gram-negative isolates as described above. The predetermined regimens of each treatment were studied in groups of 6 mice over 48 h for each bacterial isolate. Meropenem was dosed at 50 mg/kg at 0 h, 8 mg/kg at 2.5 h, and 5 mg/kg at 4.5 h; this cycle was repeated every 8 h. Similarly, nacubactam was dosed at 80 mg/kg, 20 mg/kg, and 10 mg/kg at 0, 2.5, and 4.5 h; this cycle was repeated every 8 h. Ceftazidime-avibactam was dosed at 25/6.25 mg/kg at 0, 4, 20, and 28 h. Control animals received sterile normal saline solution in the same volume, route, and schedule as the most frequently dosed drug regimen. For each isolate tested, 6 untreated mice (12 kidneys) were used as the 0-h controls and 6 additional mice (receiving normal saline solution) were used as the 48-h controls. After inoculation, one group of 6 mice for each bacterial isolate was euthanized by CO2 exposure followed by cervical dislocation. At 48 h after the initiation of dosing, a group of 6 animals from each treatment and a group of 6 mice in a control group were euthanized by CO2 exposure followed by cervical dislocation. After sacrifice, the kidney(s) was removed and homogenized in normal saline solution. Serial dilutions were plated on an appropriate agar media for determination of CFU counts per milliliter. While assessment from the starting inoculum (0 h) is frequently done as a conservative estimate of activity, we determined the change in bacterial density from the 48-h growth control, as this methodology has been utilized previously for the assessment of ceftazidime-avibactam in cUTI using the same dosing methods (13).
Supplementary Material
ACKNOWLEDGMENTS
We thank Jennifer Tabor-Rennie, Debora Santini, Elizabeth Cyr, Christina Sutherland, Kimelyn Greenwood, Sean Stainton, Safa Abuhussain, Kamilia Abdelraouf, and Mordechai Grupper from the Center for Anti-Infective Research and Development, Hartford, CT, for their assistance in conducting the study.
This study was conducted using funding from F. Hoffmann-La Roche (Basel, Switzerland). D.P.N. has received research funding from F. Hoffmann-La Roche (Basel, Switzerland). C.B. and C.Z. are employees of F. Hoffmann-La Roche (Basel, Switzerland). This project has been funded in whole or in part with federal funds from the Department of Health and Human Services, the Office of the Assistant Secretary for Preparedness and Response, the Biomedical Advanced Research and Development Authority, under OT number HHS0100201600038C. The rest of us have no conflicts of interest to disclose.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02596-17.
REFERENCES
- 1.Steiger SN, Comito RR, Nicolau DP. 2017. Clinical and economic implications of urinary tract infections. Expert Rev Pharmacoecon Outcomes Res 17:377–383. doi: 10.1080/14737167.2017.1358618. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B. 2014. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am 28:1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. 2015. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jena J, Sahoo RK, Debata NK, Subudhi E. 2017. Prevalence of TEM, SHV, and CTX-M genes of extended-spectrum β-lactamase-producing Escherichia coli strains isolated from urinary tract infections in adults. 3 Biotech 7:244. doi: 10.1007/s13205-017-0879-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shakya P, Shrestha D, Maharjan E, Sharma VK, Paudyal R. 2017. ESBL Production among E. coli and Klebsiella spp. causing urinary tract infection: a hospital based study. Open Microbiol J 11:23–30. doi: 10.2174/1874285801711010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandil H, Cramp E, Vaghela T. 2016. Trends in antibiotic resistance in urologic practice. Eur Urol Focus 2:363–373. doi: 10.1016/j.euf.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Papp-Wallace KM, Bonomo RA. 2016. New β-lactamase inhibitors in the clinic. Infect Dis Clin North Am 30:441–464. doi: 10.1016/j.idc.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morinaka A, Tsutsumi Y, Yamada M, Suzuki K, Watanabe T, Abe T, Furuuchi T, Inamura S, Sakamaki Y, Mitsuhashi N, Ida T, Livermore DM. 2015. OP0595, a new diazabicyclooctane: mode of action as a serine β-lactamase inhibitor, antibiotic and β-lactam ‘enhancer’. J Antimicrob Chemother 70:2779–2786. doi: 10.1093/jac/dkv166. [DOI] [PubMed] [Google Scholar]
- 9.Livermore DM, Warner M, Mushtaq S, Woodford N. 2016. Interactions of OP0595, a novel triple-action diazabicyclooctane, with β-lactams against OP0595-resistant Enterobacteriaceae mutants. Antimicrob Agents Chemother 60:554–560. doi: 10.1128/AAC.02184-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Docquier JD, Mangani S. 2018. An update on β-lactamase inhibitor discovery and development. Drug Resist Updat 36:13–29. doi: 10.1016/j.drup.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Kuti JL, Nightingale CH, Nicolau DP. 2006. Population pharmacokinetic analysis and dosing regimen optimization of meropenem in adult patients. J Clin Pharmacol 46:1171–1178. doi: 10.1177/0091270006291035. [DOI] [PubMed] [Google Scholar]
- 12.MacVane SH, Crandon JL, Nichols WW, Nicolau DP. 2014. Unexpected in vivo activity of ceftazidime alone and in combination with avibactam against New Delhi metallo-β-lactamase-producing Enterobacteriaceae in a murine thigh infection model. Antimicrob Agents Chemother 58:7007–7009. doi: 10.1128/AAC.02662-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borgonovi M, Miossec C, Lowther J. 2007. The efficacy of ceftazidime combined with NXL104, a novel β-lactamase inhibitor, in a mouse model of kidney infections induced by β-lactamase producing Enterobacteriaceae, poster P794. Abstr 17th Eur Soc Clin Microbiol Infect Dis (ECCMID), Munich, Germany. [Google Scholar]
- 14.U.S. Food and Drug Administration. 2014. Microbiology review. Drug approval package: Avycaz. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206494Orig1s000MicroR.pdf Updated 12 March 2015 Accessed 26 January 2016.
- 15.U.S. Food and Drug Administration. 2017. Microbiology review. Drug approval package: Vabomere. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209776lbl.pdf Accessed 23 October 2017.
- 16.U.S. Food and Drug Administration. 2014. Microbiology review. Drug approval package: Zerbaxa. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206829lbl.pdf Accessed 23 October 2017.
- 17.Monogue ML, Nicolau DP. 24 October 2017. Translational efficacy of humanized exposures of cefepime, ertapenem, and levofloxacin against extended-spectrum-β-lactamase-producing Escherichia coli in a murine model of complicated urinary tract infection. Antimicrob Agents Chemother doi: 10.1128/AAC.01329-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizvi M, Khan F, Shukla I, Malik A, Shaheen. 2011. Rising prevalence of antimicrobial resistance in urinary tract infections during pregnancy: necessity for exploring newer treatment options. J Lab Physicians 3:98–103. doi: 10.4103/0974-2727.86842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander BT, Marschall J, Tibbetts RJ, Neuner EA, Dunne WM Jr, Ritchie DJ. 2012. Treatment and clinical outcomes of urinary tract infections caused by KPC-producing Enterobacteriaceae in a retrospective cohort. Clin Ther 34:1314–1323. doi: 10.1016/j.clinthera.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkowski P, Ciszek M, Dobrzaniecka K, Sańko-Resmer J, Łabuś A, Grygiel K, Grochowiecki T, Młynarczyk G, Pączek L. 2016. Successful treatment of urinary tract infection in kidney transplant recipients caused by multiresistant Klebsiella pneumoniae producing New Delhi metallo-beta-lactamase (NDM-1) with strains genotyping. Transplant Proc 48:1576–1579. doi: 10.1016/j.transproceed.2016.01.060. [DOI] [PubMed] [Google Scholar]
- 21.Poulikakos P, Tansarli GS, Falagas ME. 2014. Combination antibiotic treatment versus monotherapy for multidrug-resistant, extensively drug-resistant, and pandrug-resistant Acinetobacter infections: a systematic review. Eur J Clin Microbiol Infect Dis 33:1675–1685. doi: 10.1007/s10096-014-2124-9. [DOI] [PubMed] [Google Scholar]
- 22.Mingeot-Leclercq MP, Tulkens PM. 1999. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother 43:1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. CLSI document M100-S17. 27. CLSI, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.