The study evaluated the in vitro activity of ceftolozane-tazobactam (C/T) against 94 unique clinical isolates of Enterobacter cloacae complex (ECC). No difference was observed according to the ECC cluster.
KEYWORDS: E. cloacae complex, ECC, ceftolozane, tazobactam, TOL-TAZ, ESBL, AmpC
ABSTRACT
The study evaluated the in vitro activity of ceftolozane-tazobactam (C/T) against 94 unique clinical isolates of Enterobacter cloacae complex (ECC). No difference was observed according to the ECC cluster. The in vitro activity greatly varied depending on the β-lactamase-producing profile: 100%, 67%, and 19% of wild-type, extended-spectrum β-lactamase (ESBL)-producing, and AmpC-overproducing strains, respectively, were susceptible to C/T. The use of C/T could be of interest for the treatment of some infections caused by ESBL-producing AmpC-nonoverexpressing ECC isolates.
TEXT
The species belonging to the Enterobacter genus are responsible for 5 to 10% of infections among patients hospitalized in intensive care units (ICUs) and are primarily due to the members of the Enterobacter cloacae complex (ECC) (1, 2). Actually, ECC is composed of 13 clusters, among which three (C-III, VI, and VIII) are the most frequently recovered from human clinical specimens (3, 4). All ECC members intrinsically harbor a chromosomal ampC gene coding for a cephalosporinase (2, 5–7). Among these third-generation cephalosporin (TGC)-resistant isolates, approximately one-third have acquired plasmid-mediated extended-spectrum β-lactamases (ESBLs), while the remaining two-thirds express high-level production of cephalosporinase (HL-CASE) caused by ampC derepression that results from chromosomal mutations (6).
Ceftolozane-tazobactam (C/T) is a novel TGC combined with a classical inhibitor of β-lactamase (ratio of 2:1), which has recently been approved for the treatment of complicated intra-abdominal and urinary tract infections (8). Although ceftolozane has been developed to be more stable than other TGCs against natural AmpC produced by P. aeruginosa (9), much less is known about its activity against other intrinsically AmpC-producing species, such as ECC. Indeed, previous studies have mainly described the in vitro activity of C/T against Enterobacter spp. with no distinction of species and/or phenotypes of resistance (10–12). In addition, no data are available about the in vitro activity of C/T according to the ECC cluster.
The purpose of the study was to (i) evaluate the in vitro activity of C/T against a collection of ECC clinical isolates, representing relevant clusters and exhibiting various phenotypes of β-lactam susceptibility profiles, and (ii) compare it to those of commonly used β-lactams.
Besides the reference strain of E. cloacae subsp. cloacae ATCC 13047 (belonging to C-XI), a total of 93 ECC clinical isolates (representing 12 clusters) collected from the University Hospital of Caen were included in the study (3). Note that the strains were identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (Microflex LT; Bruker Daltonics, Bremen, Germany), and ECC members were clustered by hsp60 sequencing as previously described (7). MICs of C/T (C was provided by Cubist Pharmaceuticals, and T was purchased from Abcam Biochemicals), piperacillin-tazobactam (TZP), cefotaxime (CTX), ceftriaxone (CRO), ceftazidime (CAZ), cefepime (FEP), ertapenem (ETP), and imipenem (IMP) were determined by the broth microdilution reference method in accordance with EUCAST guidelines (http://www.eucast.org/). ECC isolates were classified into four β-lactam susceptibility phenotypes: wild-type (WT; no resistance to TGCs), ESBL (resistance to at least one TGC with a positive double-disk synergy test), HL-CASE (resistance to at least one TGC with a negative double-disk synergy test and a significant difference in TGC-mediated inhibition with or without 250 mg/liter cloxacillin), and ESBL+HL-CASE (resistance to at least one TGC with a positive double-disk synergy test and a significant difference in TGC-mediated inhibition with or without 250 mg/liter cloxacillin). To confirm the HL-CASE phenotype (especially in isolates producing ESBLs), we quantified the levels of expression of the chromosomal ampC gene by reverse transcription-quantitative PCR using specific primers (see Table S1 in the supplemental material). Total RNAs were extracted as previously described (7). Transcript levels were determined by the ΔΔCT method using the rpoB gene as a housekeeping control gene (Table S1), and the fold change (FC) of expression was calculated between TGC-resistant strains and WT strains of the same cluster. HL-CASE was defined if the FC was higher than 2. ESBLs were characterized as previously described (13–15).
Twelve of the 13 clusters were represented in the study (Table S2). Among them, C-III (21%, 20/94), C-VI (20%, 19/94), and C-VIII (28%, 26/94) were predominant, as previously described (Table S2) (4). Note that none of the studied clusters expressing a WT phenotype exhibited an intrinsic resistance to the C/T in spite of the genetic variability of the ampC gene (7).
Among the 94 isolates, four antimicrobial susceptibility phenotypes were distinguished: WT, 34% (32/94); ESBL alone, 10% (9/94); ESBL+HL-CASE, 20% (19/94); and HL-CASE, 36% (34/94) (Table 1 and Table S2). By using the disk method with or without cloxacillin (250 mg/liter), the HL-CASE phenotype was not highlighted in 21% of isolates (4/19) presenting an ESBL+HL-CASE combined phenotype. In contrast, the expression of ampC allowed us to accurately discriminate between all ESBL and ESBL+HL-CASE phenotypes (P < 0.0001) (Fig. 1). Among the 28 isolates expressing an ESBL phenotype (ESBL alone and ESBL+HL-CASE), four genes encoding such β-lactamases were identified: blaCTX-M-15 (17/28; 61%), blaSHV-12 (9/28; 32%), blaCTX-M-9 (2/28; 7%), and blaTEM-15 (1/28; 4%). Note that one isolate coproduced blaCTX-M-15 and blaSHV-12 genes (Table S3). The distribution of ESBLs was similar to that recently described in French E. cloacae isolates (CTX-M-15, 52%; SHV-12, 38%; CTX-M-9, 10%) (16). Besides ESBL production, plasmid-mediated AmpC β-lactamase genes were also identified in two isolates (blaCMY-4 and blaDHA-1), and one strain harbored the acquired OXA-48-like carbapenemase OXA-204 (Table S3).
TABLE 1.
MICs of different β-lactams against a collection of 94 strains (93 clinical isolates and ATCC 13047) of ECC according to resistance phenotypes
| ECC clinical isolate (no.) | MIC (mg/liter) |
EUCAST susceptibility breakpoint (mg/liter) | Susceptible strains (%) | ||
|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | |||
| All (94) | |||||
| Ceftolozane-tazobactam | 1 | 16 | 0.12–128 | ≤1 | 51 |
| Imipenem | 0.25 | 0.5 | 0.12–4 | ≤2 | 99 |
| Ertapenem | 0.25 | 2 | 0.01–32 | ≤0.5 | 70 |
| Cefepime | 0.5 | 16 | 0.03–>256 | ≤1 | 54 |
| Ceftazidime | 64 | 256 | 0.25–>256 | ≤1 | 33 |
| Cefotaxime | 64 | >256 | 0.25–>256 | ≤1 | 34 |
| Ceftriaxone | 128 | >256 | 0.25–>256 | ≤1 | 34 |
| Piperacillin-tazobactam | 64 | 256 | 2–256 | ≤8 | 37 |
| Wild type (32) | |||||
| Ceftolozane-tazobactam | 0.25 | 0.5 | 0.12–0.5 | ≤1 | 100 |
| Imipenem | 0.25 | 0.5 | 0.12–0.5 | ≤2 | 100 |
| Ertapenem | 0.06 | 0.12 | 0.01–0.25 | ≤0.5 | 100 |
| Cefepime | 0.03 | 0.06 | 0.03–0.06 | ≤1 | 100 |
| Ceftazidime | 0.5 | 1 | 0.25–2 | ≤1 | 97 |
| Cefotaxime | 0.5 | 1 | 0.25–1 | ≤1 | 100 |
| Ceftriaxone | 0.5 | 1 | 0.25–1 | ≤1 | 100 |
| Piperacillin-tazobactam | 2 | 4 | 2–8 | ≤8 | 100 |
| ESBL alone (9) | |||||
| Ceftolozane-tazobactam | 1 | 2 | 0.25–4 | ≤1 | 67 |
| Imipenem | 0.25 | 0.5 | 0.12–0.5 | ≤2 | 100 |
| Ertapenem | 0.125 | 0.5 | 0.03–1 | ≤0.5 | 89 |
| Cefepime | 4 | 256 | 0.06–64 | ≤1 | 44 |
| Ceftazidime | 64 | 128 | 32–128 | ≤1 | 0 |
| Cefotaxime | 256 | >256 | 4–>256 | ≤1 | 0 |
| Ceftriaxone | 256 | >256 | 2–>256 | ≤1 | 0 |
| Piperacillin-tazobactam | 64 | 128 | 8–128 | ≤8 | 22 |
| ESBL+HL-CASE (19) | |||||
| Ceftolozane-tazobactam | 8 | 128 | 1–128 | ≤1 | 11 |
| Imipenem | 0.5 | 1 | 0.25–4 | ≤2 | 95 |
| Ertapenem | 0.5 | 8 | 0.12–32 | ≤0.5 | 53 |
| Cefepime | 4 | 256 | 0.12–>256 | ≤1 | 11 |
| Ceftazidime | 128 | 256 | 32–>256 | ≤1 | 0 |
| Cefotaxime | 256 | >256 | 64–>256 | ≤1 | 0 |
| Ceftriaxone | 256 | >256 | 128–>256 | ≤1 | 0 |
| Piperacillin-tazobactam | 128 | 256 | 32–>256 | ≤8 | 0 |
| HL-CASE (34) | |||||
| Ceftolozane-tazobactam | 4 | 16 | 0.25–32 | ≤1 | 24 |
| Imipenem | 0.25 | 0.5 | 0.12–1 | ≤2 | 100 |
| Ertapenem | 1 | 2 | 0.03–4 | ≤0.5 | 47 |
| Cefepime | 2 | 8 | 0.12–16 | ≤1 | 35 |
| Ceftazidime | 128 | 256 | 2–>256 | ≤1 | 0 |
| Cefotaxime | 256 | >256 | 16–>256 | ≤1 | 0 |
| Ceftriaxone | 256 | >256 | 32–>256 | ≤1 | 0 |
| Piperacillin-tazobactam | 128 | 256 | 8–256 | ≤8 | 3 |
FIG 1.
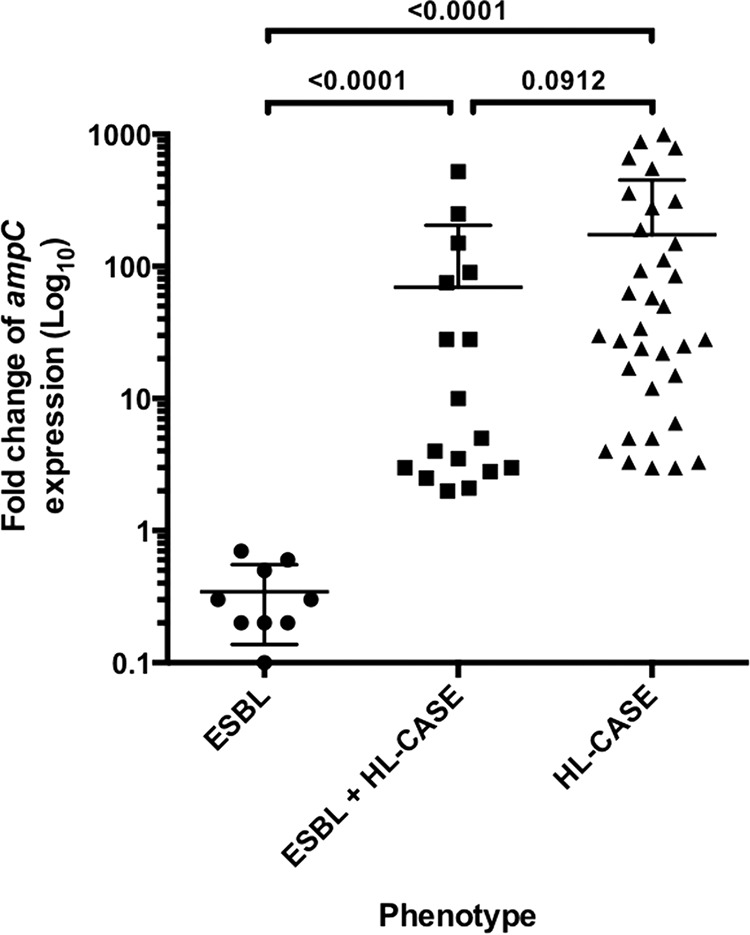
Fold change of expression of the ampC chromosomal gene according to the resistant phenotype: production of an ESBL, AmpC overproduction (HL-CASE), and ESBL+HL-CASE. The fold change (expressed as log10 values) was calculated between resistant strains and wild-type strains of the same cluster. HL-CASE was defined if the fold change was higher than 2.
For the 32 isolates with a WT phenotype, all were categorized as susceptible for all tested β-lactams, except for one strain that was not susceptible to CAZ (MIC of 2 mg/liter), according to EUCAST breakpoints (Table 1). MICs of C/T ranged from 0.12 to 0.5 mg/liter with MIC50 and MIC90 at 0.25 and 0.5 mg/liter, respectively (Table 1). These MIC values were identical to MIC50 (0.25 mg/liter) and MIC90 (0.5 mg/liter) values published for ceftazidime-susceptible Enterobacter strains (12, 17).
For the nine isolates expressing an ESBL phenotype, all were resistant to TGCs (CTX, CRO, and CAZ), while TZP and FEP retained activity against 22% and 44% of strains, respectively (Table 1). Six isolates (67%) were categorized as susceptible to C/T, with MICs between 0.25 and 4 mg/liter (Table 1). MIC50 and MIC90 were at 1 and 2 mg/liter, which is similar to values (2 and 4 mg/liter, respectively) reported in a previous study on 15 ESBL-producing Enterobacter strains (18). Also, a recent study reported 85% (40/47) of Enterobacter isolates were susceptible to C/T (19). This is in accordance with the facts that tazobactam inhibits most class A β-lactamases (including ESBLs) and that C/T remains active against >80% of ESBL-producing Escherichia coli clinical isolates (10–12, 17).
All 53 isolates showing an HL-CASE phenotype, including 19 that coproduced an ESBL, were categorized as resistant to TGCs (CTX, CRO, and CAZ), and only 19% were susceptible to C/T (Table 1). The percentages of susceptible strains were comparable between ESBL+HL-CASE and HL-CASE isolates for TZP (0 versus 3%), ETP (53 versus 47%), and IMP (95 versus 100%) but different for FEP (11 versus 35%) (Table 1). MIC50 and MIC90 of C/T were higher for ECC isolates with an ESBL+HL-CASE phenotype (8 and 128 mg/liter, respectively) than those for HL-CASE strains (4 and 16 mg/liter, respectively) (Table 1). Consequently, eight isolates (24%) were categorized as susceptible to C/T among HL-CASE isolates, whereas only two (11%) remained susceptible to the combination in the group of ESBL+HL-CASE strains (Table 1). Compared to ESBL producers, this poorer activity of C/T against HL-CASE ECC isolates is due to the fact that tazobactam is not effective against AmpC β-lactamases (8). In this subgroup (HL-CASE ECC), the percentage of strains inhibited by ≤1 mg/liter (corresponding to the EUCAST breakpoint) of C/T varied between 14 and 36% (10–12, 17), which is similar to our results. Surprisingly, for the two studies where resistance mechanisms were specified (12, 19), 50 to 75% of HL-CASE strains remained susceptible to C/T, which is much higher than proportions reported here. Interestingly, 30% (28/94) of ECC isolates were not susceptible to ETP (including one not susceptible to IMP), of which only two were susceptible to C/T (MICs of 1 mg/liter), suggesting that C/T is not a good option for the treatment of infections caused by non-carbapenemase-producing enterobacterial isolates showing reduced carbapenem susceptibility.
In summary, there is no difference in β-lactamase-producing profiles for C/T according to the ECC cluster. In contrast, the in vitro activity of C/T greatly varies depending on the β-lactam susceptibility profile.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by internal funding.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00675-18.
REFERENCES
- 1.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K. 2009. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329. [DOI] [PubMed] [Google Scholar]
- 2.Mezzatesta ML, Gona F, Stefani S. 2012. Enterobacter cloacae complex: clinical impact and emerging antibiotic resistance. Future Microbiol 7:887–902. doi: 10.2217/fmb.12.61. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann H, Roggenkamp A. 2003. Population genetics of the nomenspecies Enterobacter cloacae. Appl Environ Microbiol 9:5306–5318. doi: 10.1128/AEM.69.9.5306-5318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morand PC, Billoet A, Rottman M, Sivadon-Tardy V, Eyrolle L, Jeanne L, Tazi A, Anract P, Courpied JP, Poyart C, Dumaine V. 2009. Specific distribution within the Enterobacter cloacae complex of strains isolated from infected orthopedic implants. J Clin Microbiol 8:2489–2495. doi: 10.1128/JCM.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacoby GA. 2009. AmpC beta-lactamases. Clin Microbiol Rev 22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Lim YM. 2005. Prevalence of derepressed ampC mutants and extended-spectrum beta-lactamase producers among clinical isolates of Citrobacter freundii, Enterobacter spp., and Serratia marcescens in Korea: dissemination of CTX-M-3, TEM-52, and SHV-12. J Clin Microbiol 43:2452–2455. doi: 10.1128/JCM.43.5.2452-2455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guérin F, Isnard C, Cattoir V, Giard JC. 2015. Complex regulation pathways of AmpC-mediated β-lactam resistance in Enterobacter cloacae complex. Antimicrob Agents Chemother 59:7753–7761. doi: 10.1128/AAC.01729-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Duin D, Bonomo RA. 2016. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 63:234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhanel GG, Chung P, Adam H, Zelenitsky S, Denisuik A, Schweizer F, Lagacé-Wiens PR, Rubinstein E, Gin AS, Walkty A, Hoban DJ, Lynch JP III, Karlowsky JA. 2014. Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs 74:31–51. doi: 10.1007/s40265-013-0168-2. [DOI] [PubMed] [Google Scholar]
- 10.Pfaller MA, Bassetti M, Duncan LR, Castanheira M. 2017. Ceftolozane/tazobactam activity against drug-resistant Enterobacteriaceae and Pseudomonas aeruginosa causing urinary tract and intraabdominal infections in Europe: report from an antimicrobial surveillance programme (2012–15). J Antimicrob Chemother 72:1386–1395. doi: 10.1093/jac/dkx009. [DOI] [PubMed] [Google Scholar]
- 11.Sader HS, Farrell DJ, Castanheira M, Flamm RK, Jones RN. 2014. Antimicrobial activity of ceftolozane/tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011–12). J Antimicrob Chemother 69:2713–2722. doi: 10.1093/jac/dku184. [DOI] [PubMed] [Google Scholar]
- 12.Tato M, García-Castillo M, Bofarull AM, Cantón R. 2015. CENIT Study Group. In vitro activity of ceftolozane/tazobactam against clinical isolates of Pseudomonas aeruginosa and Enterobacteriaceae recovered in Spanish medical centres: Results of the CENIT study. Int J Antimicrob Agents 46:502–510. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet R, Sampaio JL, Chanal C, Sirot D, De Champs C, Viallard JL, Labia R, Sirot J. 2000. A novel class A extended-spectrum beta-lactamase (BES-1) in Serratia marcescens isolated in Brazil. Antimicrob Agents Chemother 44:3061–3068. doi: 10.1128/AAC.44.11.3061-3068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Champs C, Chanal C, Sirot D, Baraduc R, Romaszko JP, Bonnet R, Plaidy A, Boyer M, Carroy E, Gbadamassi MC, Laluque S, Oules O, Poupart MC, Villemain M, Sirot J. 2004. Frequency and diversity of class A extended-spectrum beta-lactamases in hospitals of the Auvergne, France: a 2 year prospective study. J Antimicrob Chemother 54:634–639. doi: 10.1093/jac/dkh395. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Pérez FJ, Hanson ND. 2002. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 40:2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robin F, Beyrouthy R, Bonacorsi S, Aissa N, Bret L, Brieu N, Cattoir V, Chapuis A, Chardon H, Degand N, Doucet-Populaire F, Dubois V, Fortineau N, Grillon A, Lanotte P, Leyssene D, Patry I, Podglajen I, Recule C, Ros A, Colomb-Cotinat M, Ponties V, Ploy MC, Bonnet R. 2017. Inventory of extended-spectrum-β-lactamase-producing Enterobacteriaceae in France as assessed by a multicenter study. Antimicrob Agents Chemother 61:e01911-16. doi: 10.1128/AAC.01911-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shortridge D, Pfaller MA, Castanheira M, Flamm RK. 2018. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. hospitals (2013–2016) as part of the surveillance program: program to assess ceftolozane-tazobactam susceptibility. Microb Drug Resist 24:563–577. doi: 10.1089/mdr.2017.0266. [DOI] [PubMed] [Google Scholar]
- 18.Melchers MJ, van Mil AC, Mouton JW. 2015. In vitro activity of ceftolozane alone and in combination with tazobactam against extended-spectrum-β-lactamase-harboring Enterobacteriaceae. Antimicrob Agents Chemother 59:4521–4525. doi: 10.1128/AAC.04498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livermore DM, Mushtaq S, Meunier D, Hopkins KL, Hill R, Adkin R, Chaudhry A, Pike R, Staves P, Woodford N. 2017. BSAC Resistance Surveillance Standing Committee. Activity of ceftolozane/tazobactam against surveillance and “problem” Enterobacteriaceae, Pseudomonas aeruginosa and non-fermenters from the British Isles. J Antimicrob Chemother 72:2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


