Intravenous voriconazole (VRC) is formulated by the incorporation of sulfobutylether-β-cyclodextrin (SBECD), which may accumulate to adversely affect renal function. However, the effect of long-term use of intravenous VRC on renal function is unclear.
KEYWORDS: cumulative dose, intravenous, renal function, sulfobutylether-β-cyclodextrin, voriconazole
ABSTRACT
Intravenous voriconazole (VRC) is formulated by the incorporation of sulfobutylether-β-cyclodextrin (SBECD), which may accumulate to adversely affect renal function. However, the effect of long-term use of intravenous VRC on renal function is unclear. Our retrospective analysis of data confirmed that worsening of renal function was significantly associated with a cumulative dose of intravenous VRC (≥400 mg/kg), suggesting that a higher cumulative dose of intravenous VRC is a risk factor for renal dysfunction.
TEXT
Voriconazole (VRC) is an expanded-spectrum antifungal triazole that is used frequently for prophylaxis and treatment of invasive fungal infections in hematological patients. Because VRC has limited aqueous solubility, the intravenous form is formulated by the incorporation of sulfobutylether-β-cyclodextrin (SBECD), which may accumulate to the point of adversely affecting renal function (1, 2). Therefore, restricted use of intravenous VRC has been recommended for patients with creatinine clearance of <50 ml/min. Meanwhile, long-term use of intravenous VRC is common in hematological patients due to treatment-related gastrointestinal toxicities and prolonged immunosuppression. Several studies demonstrated that the use of intravenous VRC in patients with baseline moderate to severe renal impairment did not lead to acute renal toxicity (3–8). However, the effect of long-term use of intravenous VRC on renal function in patients without baseline severe renal impairment is unclear. Therefore, we analyzed whether use of intravenous VRC affected renal function in hematological patients without baseline severe renal impairment.
We retrospectively examined the medical records of 101 adult patients with hematological disease who received at least 7 days of intravenous VRC at our hospital between May 2009 and March 2017. The Institutional Review Board of The Institute of Medical Science, The University of Tokyo, approved this retrospective study.
Patients with severe renal insufficiency indicated by creatinine clearance of <30 ml/min at baseline were excluded from this study. Patients who died within 14 days after the end of intravenous VRC treatment were also excluded, because renal dysfunction usually occurs as one of multiple organ failures, and the cause of renal dysfunction is not primarily caused by toxicity of VRC treatment in such patients. Sixty-three patients were included in our previous report (9, 10). Changes in serum creatinine levels from baseline to the end of intravenous VRC therapy were defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) 4.0. Worsening of renal function was defined as a >1.5-fold increase from the baseline serum creatinine level, which translates to grade 2 or higher increased serum creatinine levels based on CTCAE 4.0.
The Spearman rank correlation coefficient was calculated to assess the correlation between the cumulative dose of intravenous VRC based on patient body weight and changes in serum creatinine levels. To identify variables affecting worsening of renal function, univariate and multivariate analyses were performed using a logistic regression model. The following variables were taken into consideration: age (≥45 versus <45 years); sex; concomitant use of potentially nephrotoxic drugs, such as aminoglycosides, vancomycin, sulfamethoxazole-trimethoprim, foscarnet, and calcineurin inhibitors; and cumulative dose of intravenous VRC. Variables with a P value of <0.10 in the univariate analysis were included in the multivariate analysis. All statistical analyses were performed in GraphPad Prism 6 for Mac OS X (GraphPad Software, San Diego, CA) or EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria) (11). All P values were two sided, and P values of <0.05 were considered statistically significant.
Clinical characteristics of the patient cohort are summarized in Table 1. The median age of the patients was 47 years (range, 17 to 77 years), and 64 (63%) of the patients were male. The most common disease type was acute myeloid leukemia (44%), and 68 patients (67%) received allogeneic hematopoietic cell transplantation (HCT). The median serum creatinine levels at baseline and at the end of therapy were 0.60 mg/dl (range, 0.26 to 1.49 mg/dl) and 0.70 mg/dl (range, 0.30 to 1.70 mg/dl), respectively. The median duration of intravenous VRC was 27 days (range, 7 to 99 days). The median daily dose of intravenous VRC and cumulative dose of intravenous VRC were 6.88 mg/kg (range, 2.75 to 10.42 mg/kg) and 195 mg/kg (range, 40 to 646 mg/kg), respectively. The median highest serum creatinine level during intravenous VRC treatment was 0.80 mg/dl (range, 0.40 to 2.00 mg/dl).
TABLE 1.
Characteristics of patients and voriconazole treatment
| Characteristic | Value |
|---|---|
| No. of patients | 101 |
| Age (yr) (median [range]) | 47 (17–77) |
| Body weight (kg) (median [range]) | 54.6 (38.6–104.8) |
| Sex (n [%]) | |
| Male | 64 (63) |
| Female | 37 (37) |
| Disease type (n [%]) | |
| Acute myeloid leukemia | 44 (45) |
| Acute lymphoblastic leukemia | 19 (20) |
| Myelodysplastic syndrome | 24 (23) |
| Chronic myelomonocytic leukemia | 4 (5) |
| Non-Hodgkin's lymphoma | 9 (3) |
| Severe aplastic anemia | 1 (1) |
| Serum creatinine at baseline (mg/dl) (median [range]) | 0.60 (0.26–1.49) |
| Duration of intravenous VRC treatment (days) (median [range]) | 27 (7–99) |
| Intravenous VRC daily dose (mg/day) (median [range]) | 6.88 (2.75–10.42) |
| Cumulative dose of intravenous VRC (mg/kg) (median [range]) | 195 (40–646) |
| Indication for intravenous VRC (n [%]) | |
| Prophylaxis | 21 (21) |
| Empirical or preemptive therapy | 80 (79) |
| Concomitant medication (n [%]) | |
| Aminoglycosides | 18 (18) |
| Vancomycin | 63 (62) |
| Sulfamethoxazole-trimethoprim | 15 (15) |
| Foscarnet | 7 (7) |
| Calcineurin inhibitors | 67 (66) |
Overall, a >1.5-fold increase in serum creatinine level from baseline was observed by the end of intravenous VRC treatment in 13 patients (13%). Changes in serum creatinine levels were significantly correlated with the cumulative doses of intravenous VRC (R2 = 0.09; P = 0.001) (Fig. 1). We analyzed the effects of a cumulative dose of VRC on renal function using different thresholds (100, 200, 300, and 400 mg/kg). Among the different doses, univariate analysis confirmed that worsening of renal function at the end of intravenous VRC treatment was significantly associated with the cumulative dose of intravenous VRC (≥400 mg/kg) alone (Table 2). Multivariate analysis showed that worsening of renal function was significantly associated with the cumulative dose of intravenous VRC (≥400 mg/kg) (Table 2). Three months after intravenous VRC treatment, the median serum creatinine level was 0.80 mg/dl (range, 0.36 to 1.50 mg/dl) in 81 evaluable patients, and no patient required renal replacement therapy.
FIG 1.
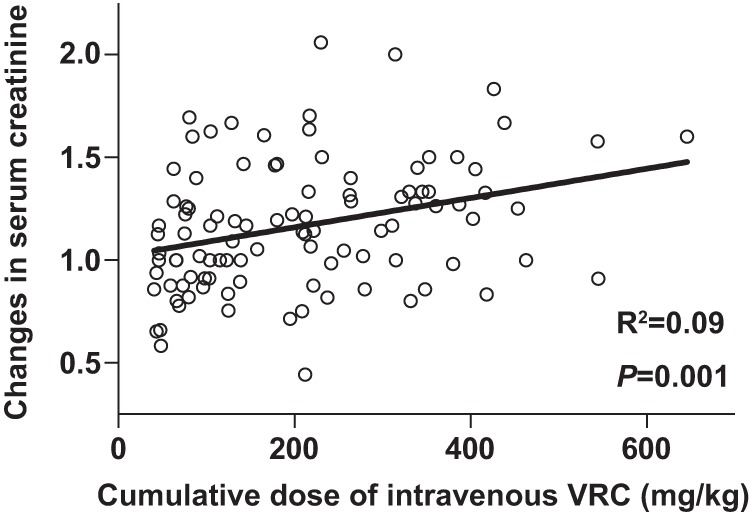
The relationship between cumulative dose of intravenous VRC based on patient body weight and changes in serum creatinine level in hematological patients.
TABLE 2.
Univariate and multivariate analyses of variables affecting renal function
| Variable | Analysisa |
|||
|---|---|---|---|---|
| Univariate |
Multivariateb |
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age ≥45 years | 0.68 (0.21–2.20) | 0.52 | ||
| Male | 0.64 (0.20–2.05) | 0.44 | ||
| Cumulative VRC dose | ||||
| ≥100 mg/kg | 2.43 (0.51–11.7) | 0.26 | ||
| ≥200 mg/kg | 1.83 (0.56–6.05) | 0.31 | ||
| ≥300 mg/kg | 1.87 (0.56–6.33) | 0.31 | ||
| ≥400 mg/kg | 5.14 (1.26–21.0) | 0.02 | 5.28 (1.13–24.7) | 0.03 |
| Concomitant medication | ||||
| Aminoglycosides | 0.82 (0.17–4.06) | 0.80 | ||
| Vancomycin | 3.81 (0.80–18.2) | 0.09 | 2.59 (0.47–14.1) | 0.27 |
| Sulfamethoxazole-trimethoprim | 0.44 (0.05–3.66) | 0.44 | ||
| Foscarnet | 6.30 (1.23–32.3) | 0.02 | 4.36 (0.75–25.3) | 0.10 |
| Calcineurin inhibitors | 7.20 (0.90–57.9) | 0.06 | 3.83 (0.42–34.3) | 0.22 |
HR, hazard ratio; CI, confidence interval.
Multivariate analysis was performed using a cumulative VRC dose of ≥400 mg/kg and concomitant use of vancomycin, foscarnet, and calcineurin inhibitors.
Previous studies showed that use of intravenous VRC was not associated with renal dysfunction in patients with baseline moderate to severe renal impairment in several different clinical contexts (3–8). However, the median duration of intravenous VRC treatment in the previous studies was much shorter than that in our study (3–7). Indeed, intravenous SEBCD administered at ≥160 mg/kg causes dose-dependent changes in renal tubular vacuolation in animal models treated for 1 to 6 months (1), but there are no data evaluating the effect of cumulative doses of SBECD on renal function in humans. Because the formulation of intravenous VRC contains 16 mg of SBECD for every 1 mg of VRC (12), our data suggest that accumulation of >6,400 mg of SBECD may contribute to renal dysfunction in humans.
Several potentially nephrotoxic drugs, such as aminoglycosides, vancomycin, sulfamethoxazole-trimethoprim, foscarnet, and calcineurin inhibitors, are commonly used to treat hematological patients, particularly those undergoing allogeneic HCT. Two-thirds of the patients in our study received allogeneic HCT. Therefore, >60% of the patients also used calcineurin inhibitors. In fact, a higher cumulative dose and longer duration of calcineurin inhibitor treatment are also associated with renal toxicity (13, 14). In addition, our data showed that concomitant use of foscarnet was significantly associated with renal dysfunction in univariate analysis. These findings suggest that concomitant use of potentially nephrotoxic drugs and intravenous VRC may contribute to the renal dysfunction in patients without baseline renal impairment in our cohort.
Our study has several limitations. First, the cause of renal dysfunction in hematological patients can be multifocal, which may contribute to the significant but weak correlation between the cumulative dose of intravenous VRC and changes in serum creatinine levels in our study. Therefore, further studies are required to clarify the relationship between the long-term use of intravenous VRC and renal function in patients without baseline renal impairment in several different clinical contexts. Second, we did not evaluate the correlation between the cumulative dose of intravenous VRC, serum concentration of SBECD, and renal histopathological changes in patients with or without renal impairment, because biopsy samples were unavailable. Therefore, the exact role of SBECD accumulation in development of renal dysfunction remains unclear in this study. Third, the severity of fungal infection, for which the VRC was used, was not evaluated. The severity of infection may affect the longer duration of intravenous VRC treatment, which might also be independently associated with worsening renal dysfunction.
In conclusion, although our study was a retrospective single-institute analysis and the number of patients was small, our results demonstrated that a higher cumulative dose of intravenous VRC was a risk factor for renal dysfunction in hematological patients without baseline renal impairment.
ACKNOWLEDGMENTS
We thank all of the physicians and staff at the hospital in this study.
We have no competing financial interests to declare.
A.T. received research grants from Pfizer for research other than directory for this work.
REFERENCES
- 1.Luke DR, Tomaszewski K, Damle B, Schlamm HT. 2010. Review of the basic and clinical pharmacology of sulfobutylether-beta-cyclodextrin (SBECD). J Pharm Sci 99:3291–3301. doi: 10.1002/jps.22109. [DOI] [PubMed] [Google Scholar]
- 2.Turner RB, Martello JL, Malhotra A. 2015. A worsening renal function in patients with baseline renal impairment treated with intravenous voriconazole: a systematic review. Int J Antimicrob Agents 46:362–366. doi: 10.1016/j.ijantimicag.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Kwon JC, Park C, Han S, Yim DS, Choi JK, Cho SY, Lee HJ, Park SH, Choi SM, Choi JH, Yoo JH, Lee DG, Lee JW. 2016. Therapeutic drug monitoring and safety of intravenous voriconazole formulated with sulfobutylether β-cyclodextrin in haematological patients with renal impairment. Mycoses 59:644–651. doi: 10.1111/myc.12517. [DOI] [PubMed] [Google Scholar]
- 4.Lilly CM, Welch VL, Mayer T, Ranauro P, Meisner J, Luke DR. 2013. Evaluation of intravenous voriconazole in patients with compromised renal function. BMC Infect Dis 13:14. doi: 10.1186/1471-2334-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neofytos D, Lombardi LR, Shields RK, Ostrander D, Warren L, Nguyen MH, Thompson CB, Marr KA. 2012. Administration of voriconazole in patients with renal dysfunction. Clin Infect Dis 54:913–921. doi: 10.1093/cid/cir969. [DOI] [PubMed] [Google Scholar]
- 6.Abel S, Allan R, Gandelman K, Tomaszewski K, Webb DJ, Wood ND. 2008. Pharmacokinetics, safety and tolerance of voriconazole in renally impaired subjects: two prospective, multicentre, open-label, parallel-group volunteer studies. Clin Drug Investig 28:409–420. doi: 10.2165/00044011-200828070-00002. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Lerma F, Allepuz-Palau A, Garcia MP, Angeles Leon M, Navarro A, Sanchez-Ruiz H, Iruretagoyena JR, Luque-Gomez P, Voriconazole Study Group in Critically Ill Patients. 2008. Impact of intravenous administration of voriconazole in critically ill patients with impaired renal function. J Chemother 20:93–100. doi: 10.1179/joc.2008.20.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Oude Lashof AM, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Schlamm HT, Rex JH, Kullberg BJ. 2012. Safety and tolerability of voriconazole in patients with baseline renal insufficiency and candidemia. Antimicrob Agents Chemother 56:3133–3137. doi: 10.1128/AAC.05841-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasu T, Konuma T, Kato S, Kurokawa Y, Takahashi S, Tojo A. 2017. Serum C-reactive protein levels affect the plasma voriconazole trough levels in allogeneic hematopoietic cell transplant recipients. Leuk Lymphoma 58:2731–2733. doi: 10.1080/10428194.2017.1300897. [DOI] [PubMed] [Google Scholar]
- 10.Yasu T, Konuma T, Kato S, Kurokawa Y, Takahashi S, Tojo A. 2016. Different effects of lansoprazole and rabeprazole on the plasma voriconazole trough levels in allogeneic hematopoietic cell transplant recipients. Ann Hematol 95:1845–1851. doi: 10.1007/s00277-016-2782-z. [DOI] [PubMed] [Google Scholar]
- 11.Kanda Y. 2013. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfizer, Inc. 2017. Vfend package insert. Pfizer, Inc., New York, NY: http://www.pfizer.com/files/products/uspi_vfend.pdf. [Google Scholar]
- 13.Falkenhain ME, Cosio FG, Sedmak DD. 1996. Progressive histologic injury in kidneys from heart and liver transplant recipients receiving cyclosporine. Transplantation 62:364–370. doi: 10.1097/00007890-199608150-00011. [DOI] [PubMed] [Google Scholar]
- 14.Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Chapman JR, Allen RD. 2004. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation 78:557–565. [DOI] [PubMed] [Google Scholar]


