The increasing incidence of multidrug-resistant Mycobacterium tuberculosis strains and the very few drugs available for treatment are promoting the discovery and development of new molecules that could help in the control of this disease. Bacteriocin AS-48 is an antibacterial peptide produced by Enterococcus faecalis and is active against several Gram-positive bacteria.
KEYWORDS: AS-48, antimicrobial peptides, antituberculosis activity, synergism, antimycobacterial agents, bacteriocins, Mycobacterium tuberculosis, intracellular infection
ABSTRACT
The increasing incidence of multidrug-resistant Mycobacterium tuberculosis strains and the very few drugs available for treatment are promoting the discovery and development of new molecules that could help in the control of this disease. Bacteriocin AS-48 is an antibacterial peptide produced by Enterococcus faecalis and is active against several Gram-positive bacteria. We have found that AS-48 was active against Mycobacterium tuberculosis, including H37Rv and other reference and clinical strains, and also against some nontuberculous clinical mycobacterial species. The combination of AS-48 with either lysozyme or ethambutol (commonly used in the treatment of drug-susceptible tuberculosis) increased the antituberculosis action of AS-48, showing a synergic interaction. Under these conditions, AS-48 exhibits a MIC close to some MICs of the first-line antituberculosis agents. The inhibitory activity of AS-48 and its synergistic combination with ethambutol were also observed on M. tuberculosis-infected macrophages. Finally, AS-48 did not show any cytotoxicity against THP-1, MHS, and J774.2 macrophage cell lines at concentrations close to its MIC. In summary, bacteriocin AS-48 has interesting antimycobacterial activity in vitro and low cytotoxicity, so further studies in vivo will contribute to its development as a potential additional drug for antituberculosis therapy.
INTRODUCTION
Mycobacterium tuberculosis is the causal agent of tuberculosis, a disease that mainly affects the lungs and was responsible for 1.6 million deaths in 2016; it is the infectious disease with the highest mortality rate (1). M. tuberculosis is an intracellular pathogen residing in pulmonary macrophages within granulomas, where it is mostly kept in latent forms, the host defense mechanism against this pathogen. Infection progresses into active disease when the host immune system is suppressed and the granulomas cannot contain the bacilli. In this way, coinfection with HIV in tuberculosis patients is one of the key factors behind the increase in the incidence of tuberculosis, predominantly in countries where both diseases are endemic.
Tuberculosis is a curable disease, where the active forms are generally susceptible to diverse antimicrobials, such as the first-line drugs (isoniazid, pyrazinamide, ethambutol [EMB], rifampin, and streptomycin), which are prescribed as standard treatment for tuberculosis. However, the huge incidence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) M. tuberculosis strains (which are not responsive to standard treatment and need to be treated with second-line drugs such as aminoglycosides and fluoroquinolones, among others) has exacerbated the need for developing alternatives for an effective treatment. During 2016, about 490,000 cases of tuberculosis were caused by MDR strains, and 6.2% of them were caused by XDR strains (1).
In the context of drug resistance, not only in respect to tuberculosis but also in connection with many other bacterial pathogens, antimicrobial peptides (AMPs) may have great potential for use in treatment, either by themselves or in combination with other antimicrobials. AMPs are produced by prokaryotic or eukaryotic organisms. They are peptides and proteins, mostly cationic and with an amphiphilic nature, that are ribosomally synthesized. Most of the bacterial lineages can produce AMPs, called bacteriocins, that exhibit antimicrobial activity primarily against those species that are phylogenetically closely related with the producer species. Recently, a classification system of bacteriocins (2) was accepted whereby class I (less than 10 kDa) encompasses all the peptides that undergo enzymatic modification during biosynthesis, providing molecules with uncommon amino acids and structures that have an impact on their properties. Thus, there are lanthipeptides (class Ia), head-to-tail cyclized peptides (class Ib), sactibiotics that are sulfur–α-carbon-containing peptides (class Ic), and linear azol(in)e-containing peptides (class Id). Class II (less than 10 kDa) includes unmodified bacteriocins that do not require enzymes for their maturation; finally, class III consists of heat-labile bacteriocins without modifications that are larger than 10 kDa and with bacteriolytic or nonlytic mechanisms of action.
The prototype of the ribosomally synthesized and posttranslationally modified peptides (RiPPs) is the bacteriocin AS-48, which undergoes head-to-tail cyclization to render a circular molecule (class Ib). All of these cyclized peptides are synthesized as a linear precursor containing a leader sequence with a high molecular weight, and then they are posttranslationally modified. AS-48 is a 70-amino-acid, alpha-helical membrane-interacting peptide produced by Enterococcus faecalis that displays a broad antimicrobial spectrum against Gram-positive and Gram-negative bacteria. The mechanism of AS-48 antibacterial activity involves the accumulation of positively charged molecules at the membrane surface, leading to a disruption of the membrane potential (3, 4). The antimicrobial activity of AS-48 has already been proven in food products (5) and has been observed against numerous Gram-positive bacteria, including Listeria monocytogenes and enterotoxic Staphylococcus aureus (6).
Most of the studies on bacteriocins focus on their potential applications as food preservatives, and only a few of studies are focused on their potential biomedical applications and, specifically, antimicrobial or antituberculosis activity. Activity of nisin and lacticin 3147 has been shown against Mycobacterium strains such as Mycobacterium kansasii, Mycobacterium avium subsp. paratuberculosis, and M. tuberculosis H37Ra; in particular, lacticin 3147 exhibits greater antimycobacterial activity than nisin (7). The antimycobacterial activities of several bacteriocins were found to be similar to the activity of rifampin against M. tuberculosis (8).
In this work, we have investigated the antituberculosis activity of bacteriocin AS-48 alone and in combination with lysozyme and first-line drugs used in the treatment of tuberculosis, both in in vitro cultures and also in M. tuberculosis-infected macrophages. We report that AS-48 is active against M. tuberculosis and other mycobacterial species and synergizes with EMB. Bacteriocin AS-48 was not found to be cytotoxic at the doses required for inhibiting mycobacterial growth. Moreover, AS-48 caused depolarization of mycobacterial membranes and altered morphology of the bacilli. In conclusion, we demonstrate that bacteriocin AS-48 has positive features supporting its potential role in tuberculosis treatment.
RESULTS
Bacteriocin AS-48 showed bactericidal activity against M. tuberculosis complex.
The activity of AS-48 was determined against several mycobacteria, including M. tuberculosis complex reference strains, clinical isolates, and other clinically relevant nontuberculous mycobacteria (NTM).
First, we tested AS-48 activity against M. tuberculosis complex clinical and reference strains. The MICs of AS-48 were quite uniform, between 16 and 64 μg/ml (Table 1; see also Table S1 in the supplemental material), and there was no difference in the MICs of AS-48 between active replicating cells and the nonreplicative strain SS18b of M. tuberculosis. We observed a similar MIC of AS-48 for Mycobacterium smegmatis and other NTM (see below).
TABLE 1.
Mycobacterial strains used and MICs of AS-48 and lysozyme
| Strain | MIC (μg/ml) |
Source or reference | |
|---|---|---|---|
| AS-48 | Lysozyme | ||
| H37Rv | 32–64 | 200–400 | 37 |
| H37Ra | 32 | 400 | 38 |
| BCG Pasteur 1173 | 32 | 200–400 | Laboratory collection |
| Mt103 | 64 | 400 | 39 |
| CDC1551 | 64 | 400 | 40 |
| GC 1237 | 16–32 | 400 | 41 |
| H37Rv phoP | 64 | 400 | 42 |
| SS18b | 32–64 | >1,200 | 43 |
| M. smegmatis mc2155a | 64 | 400 | 44 |
Fast-growing mycobacteria.
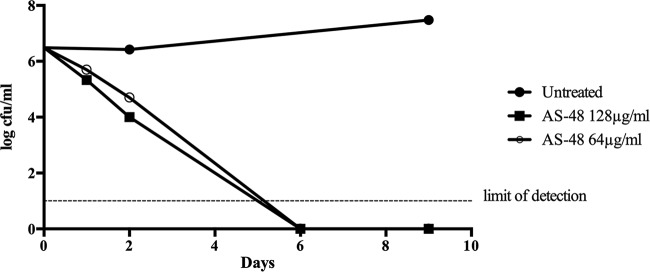
The kill kinetics indicated that bacteriocin AS-48 is bactericidal since concentrations equal to the MIC and 2× MIC were capable of reducing the number of live bacteria in a culture of M. tuberculosis H37Rv. First, after 1 or 2 days of treatment, the number of CFU was reduced by almost 2 logs in comparison with levels in untreated cultures. After 6 days of treatment with 128 or 64 μg/ml of AS-48, no live bacteria could be detected (Fig. 1), and this was found to be statistically significant by a linear regression test (P values of 0.0005 and <0.0001, respectively).
FIG 1.
Kill kinetics of bacteriocin AS-48 at 64 and 128 μg/ml against cultures of M. tuberculosis H37Rv. Untreated cultures were used as controls.
Synergy between lysozyme and nisin was previously observed in Gram-positive bacteria (9, 10). Similarly, we have observed that the presence of lysozyme resulted in an increase in M. tuberculosis susceptibility to AS-48. First, we tested lysozyme activity against mycobacterial strains and found that different mycobacterial strains or species have more variability in the MICs of lysozyme (Tables 1 and S1) than the MIC obtained for AS-48. Notably, the nonreplicating strain SS18b of M. tuberculosis was found to be greatly resistant to lysozyme. Second, we tested the susceptibility of mycobacterial strains to AS-48 in the presence of lysozyme (Table 2). We found that, for most strains, susceptibility to AS-48 greatly increased (between 64- and 16-fold) in the presence of subinhibitory concentrations of lysozyme. In order to quantify the effect of lysozyme on growth inhibition by AS-48, we calculated the fractional inhibitory concentration index (FICI). The results of all strains assayed are indicated in Table 2. For reference strains, maximum synergism was observed for H37Rv and, to a lesser extent, for Mycobacterium bovis BCG, H37Ra, H37Rv phoP, GC 1237 (Beijing genotype), CDC1551, and Mt103. Although technically synergism is defined as an FICI of ≤0.5, we consider the strains Mt103 and HMS 1548, which resulted in FICIs slightly over 0.5, to have weak synergistic interactions. The synergy effect occurred in all of the M. tuberculosis complex strains analyzed.
TABLE 2.
MIC of AS-48 in the presence of different concentrations of lysozyme and the fractional inhibitory concentration index
| Strain | MIC of AS-48 (μg/ml) at the indicated lysozyme concn (μg/ml) |
FICIa | |||||
|---|---|---|---|---|---|---|---|
| 400 | 200 | 100 | 50 | 25 | 12.5 | ||
| H37Rv | <0.03125 | 0.0625 | 1 | 4 | >32 | 0.25 | |
| H37Ra | <0.03125 | 2 | 4 | 16 | >32 | 0.375 | |
| BCG Pasteur | <0.125 | 2 | 16 | >32 | 0.3125 | ||
| Mt103 | <0.125 | 4 | 32 | 32 | 64 | >64 | 0.5625 |
| CDC1551 | <0.125 | 16 | 32 | 64 | >64 | 0.5 | |
| GC 1237 | <0.03125 | 4 | 8 | >32 | 0.375 | ||
| H37Rv phoP | <0.125 | 8 | 32 | 64 | >64 | 0.375 | |
| M. smegmatis mc2155 | <0.125 | 32 | 64 | >64 | 1 | ||
| HMS 1500 | <0.03125 | 8 | >32 | 0.375 | |||
| HMS 1531 | <0.03125 | 0.5 | 4 | 8 | 0.375 | ||
| HMS 1536 | <0.03125 | 8 | >32 | 0.5 | |||
| HMS 1546 | <0.03125 | 2 | 8 | >32 | 0.5 | ||
| HMS 1548 | <0.03125 | 2 | 16 | >32 | 0.5312 | ||
| HMS 1292 | <0.03125 | 4 | 16 | >32 | 0.3125 | ||
| HMS 1278 | <0.03125 | 2 | 8 | >32 | 0.5 | ||
The fractional inhibitory index (FICI) is calculated as follows: FICI = (MICAS-48 in the presence of lysozyme/MICAS-48 alone) + (MIClysozyme in the presence of AS-48/MIClysozyme alone). An FICI of ≤0.5 indicates synergism, a value of 0.5 to 4 indicates no interaction, and a value of >4.0, indicates antagonism.
Bacteriocin AS-48 has variable activity against NTM.
In most NTM, the MIC of AS-48 is the same as that for M. tuberculosis (i.e., 64 μg/ml), with the exception of slow-growing mycobacterial species such as Mycobacterium xenopi, Mycobacterium lentiflavum, and Mycobacterium gordonae. For M. xenopi and M. gordonae, the value of the MIC of AS-48 was <2 μg/ml, and the MIC of lysozyme was <9.375 μg/ml. In contrast, the MIC of AS-48 against M. lentiflavum was higher than 256 μg/ml, and the MIC of lysozyme was also higher than 1,200 μg/ml, indicating that this mycobacterial species is less susceptible to AS-48 and to lysozyme (Table S1).
Synergy test between AS-48 and first-line antituberculosis drugs.
AS-48 has been tested also in combination with the main first-line antituberculosis drugs, EMB, isoniazid, streptomycin, and rifampin. The combination of AS-48 and EMB was the only one to present a synergistic relation, with an FICI of 0.09375, which was the lowest FICI obtained in all the assays (Fig. 2).
FIG 2.
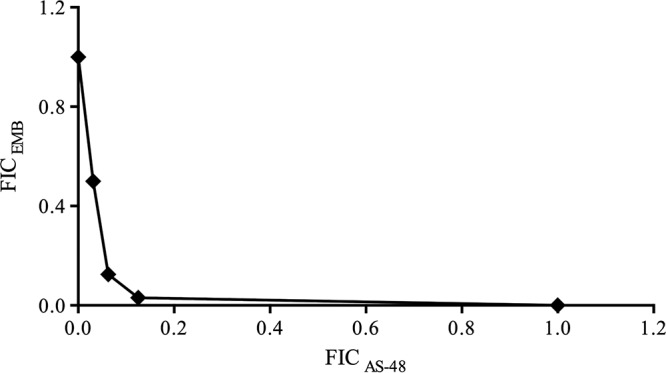
Synergism between AS-48 and EMB in M. tuberculosis H37Rv. For the reference strains CDC1551, Mt103, H37Ra, and H37Rv phoP, the plot had the same shape, indicating that synergy between AS-48 and EMB was very similar to that in H37Rv.
Is AS-48 targeting the mycobacterial membrane?
In order to explore whether bacteriocin AS-48 could be targeting the mycobacterial membrane, we carried out experiments for ethidium bromide accumulation, and we determined the ability of AS-48 for depolarizing the mycobacterial membrane.
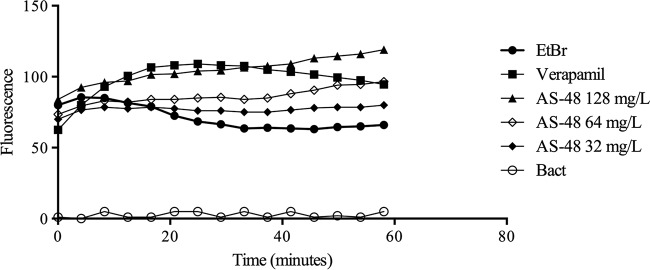
It is assumed that AS-48 forms pores in the membrane (11), thus making the cell more permeable to compounds such as ethidium bromide. Therefore, in an ethidium bromide accumulation assay, we would expect that the fluorescence detected would be higher in the presence of AS-48, reflecting the increased accumulation of ethidium bromide as a result of the effect of AS-48 on the bacterial membrane. We compared the effect of AS-48 with that of verapamil, a well-known efflux inhibitor (12), which produces an increase in the accumulation of ethidium bromide. In the presence of AS-48, fluorescence due to accumulated ethidium bromide increased in a concentration-dependent manner although the kinetics of accumulation was rather distinct from that observed with verapamil (Fig. 3).
FIG 3.
Assay of ethidium bromide (EtBr) accumulation in M. smegmatis cells in the presence of different concentrations of AS-48. VP, verapamil, used as control for the maximum accumulation of ethidium bromide; Bact, negative control with only a bacterial suspension.
The determination of membrane potential was carried out using a BacLight kit according to the manufacturer's instructions using M. smegmatis mc2155 and M. bovis BCG cells. In a representative control experiment, in the untreated cells, 3.4% of cells were depolarized, whereas in the cells treated with the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP), the percentage of depolarized cells was 94.93%. We assessed the effect of AS-48 at several concentrations and for different times of incubation (1 or 24 h). We observed that AS-48 depolarized cells in a concentration-dependent manner, and this effect was much higher in the cells incubated with AS-48 for 24 h than in those tested after 1 h of incubation. Data in Fig. 4 show that the percentage of depolarized cells after 24 h is 67.04% in the presence of 128 μg/ml of AS-48, 26.34% with 64 μg/ml, and 18.68% with 32 μg/ml. Again, this demonstrates that the depolarization of the cells is concentration dependent and increases with time.
FIG 4.
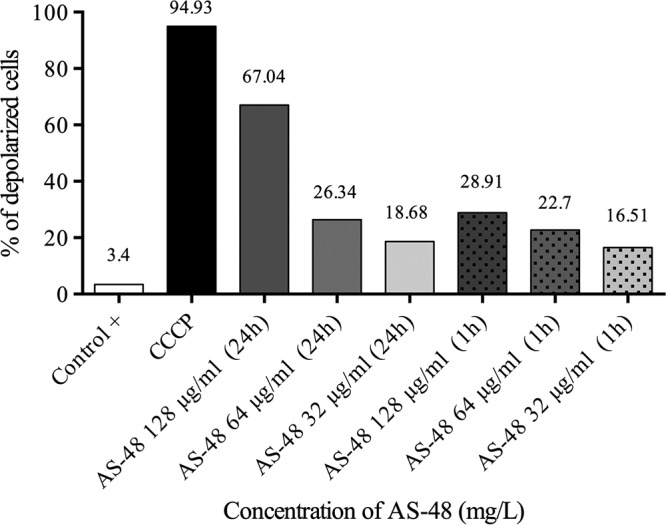
Flow cytometry assay for determination of membrane depolarization. The positive control contained just a bacterial suspension. CCCP, ionophore showing maximum membrane depolarization. The concentration of AS-48 and the time of exposure to AS-48 are shown below each bar.
Antimicrobial concentrations of AS-48 are not cytotoxic for macrophage cell lines.
The cytotoxicity of AS-48 on human and mouse lung macrophage cell lines was carried out by MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] and neutral red assays.
In both protocols, >70% cell viability was observed at concentrations of AS-48 below 128 μg/ml, which is considered noncytotoxic according to standard international protocols (13). Even when slight differences in cell viability were observed below 32 μg/ml of AS-48, they were not significant (Fig. 5). We did not observe a concentration-dependent cytotoxic effect in any of the cell lines assayed. At 128 μg/ml, <40% cell viability was observed (Fig. 5) (P value of <0.0001).
FIG 5.
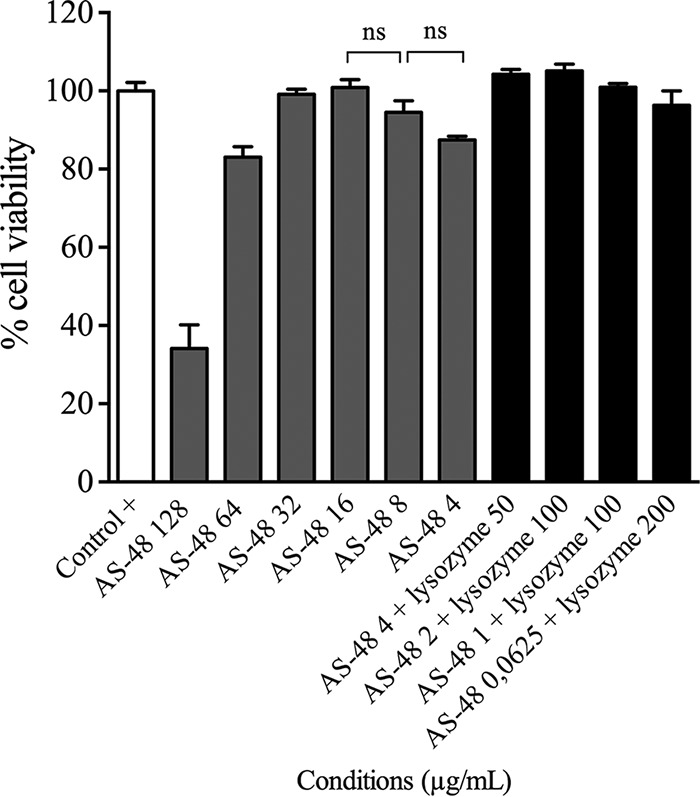
Representative results of the cell viability assays, as determined by neutral red uptake technique in cell cultures of J774.2 murine macrophages. Cell suspensions with no compounds added were used as a positive control. ns, not significant.
Lysozyme cytotoxicity was also analyzed, and, as expected, no cytotoxicity was observed in these cell lines. Finally, we assayed cytotoxicity of some of the synergistic combinations of AS-48 and lysozyme that showed great antimicrobial effect in M. tuberculosis, and we found that these combinations did not cause any cytotoxic effect (Fig. 5).
Treatment with AS-48 results in morphological changes in M. tuberculosis.
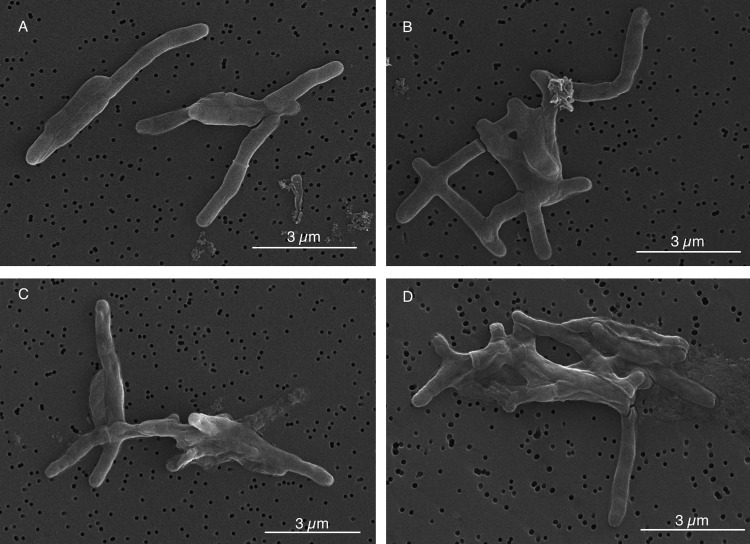
We treated M. tuberculosis cells with AS-48, along with untreated cells as a control, and samples were taken at several time points for microscopic observation. We found that at short time exposures, cells were affected by AS-48 (Fig. 6): the morphology of cells treated with a concentration of AS-48 equal to the MIC after 48 h and 72 h was rougher than that of the control.
FIG 6.
Scanning electron microscopy images of M. tuberculosis H37Rv under different conditions. (A and B) Positive control after 48 and 72 h of growth, respectively. (C and D) Cells treated with 64 μg/ml of AS-48 for 48 and 72 h, respectively.
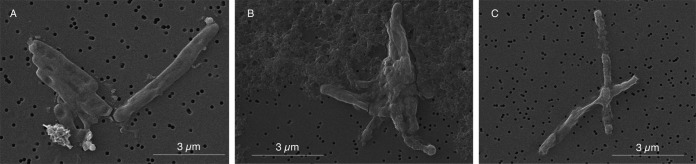
Moreover, we assayed the combination of 8 μg/ml of AS-48 with 25 μg/ml of lysozyme and analyzed cells at 24, 48, and 96 h after treatment. When bacterial cells were treated with a synergistic combination of AS-48 and lysozyme, they exhibited greater morphological damage (Fig. 7) than cells treated with AS-48 alone (Fig. 6).
FIG 7.
Scanning electron microscopy images of M. tuberculosis H37Rv treated with a synergistic combination of 8 μg/ml of AS-48 and 25 μg/ml of lysozyme after treatment for 24 h (A) 48 h (B), and 96 h (C).
Synergy between EMB and AS-48 in the infected macrophages.
In the model of Raw 264.7 cells infected with M. tuberculosis H37Rv-green fluorescent protein (GFP), we observed low FICI values (Table 3) ranging from 0.5 and 0.18, which is indicative of synergism. These FICI values were similar to those obtained in the in vitro antimycobacterial assays. The combination of 2 μg/ml AS-48 and 2 μg/ml EMB resulted in the lowest FICI value. Other combinations also resulted in synergistic FICI values but to a lesser extent (Table 3). The determination of viable bacteria after treatment with EMB and AS-48 resulted in a reduction of CFU counts between 90 and 99.9% in comparison with totals in untreated controls. The efficacy of AS-48 for killing intracellular pathogens was previously proven by Abengózar et al. (14) when they showed the anti-leishmanial activity of AS-48 in Raw 264.7 infected macrophages. Despite the fact that the infection mechanism is different between Leishmania and mycobacteria, these experiments show that AS-48 is active against pathogens residing intracellularly in macrophages.
TABLE 3.
Intracellular MICs and FICs of AS-48 and EMB alone or in combination and FICIsa
| MIC (μg/ml) |
FIC |
FICIb | ||
|---|---|---|---|---|
| AS-48 | EMB | AS-48 | EMB | |
| 32 | ||||
| 16 | 0.125 | 0.5 | 0.007 | 0.50 |
| 8 | 0.5 | 0.25 | 0.03 | 0.28 |
| 2 | 2 | 0.06 | 0.12 | 0.18 |
| 16 | ||||
Values were determined in Raw 264.7 cells infected with M. tuberculosis H37Rv-GFP. EMB, ethambutol.
The fractional inhibitory index (FICI) is calculated as follows: FICI = (MICAS-48 in the presence of ethambutol/MICAS-48 alone) + (MICethambutol in the presence of AS-48/MICethambutol alone). An FICI of ≤0.5 indicates synergism, a value of 0.5 to 4 indicates no interaction, and a value of >4.0 indicates antagonism.
DISCUSSION
During recent years, we have witnessed a decline in the number of deaths and incidence of tuberculosis globally; however, recent reports estimate that 600,000 people had MDR or rifampin-resistant (RR) tuberculosis in 2016, which represents a notable increase over the previous year (1). Drug-based treatment for MDR or RR tuberculosis is available only for 20% of these patients, and it is much longer, more expensive, and more toxic than treatment of drug-susceptible tuberculosis. More worryingly, the success rate of MDR tuberculosis treatment is around 50%. Altogether, these facts clearly mean that there is an imperative need for researching new drugs against tuberculosis and, more specifically, against MDR and RR tuberculosis.
The pipeline of new antituberculosis drugs in clinical development includes just seven new compounds (petromanid, delpazolid, SQ-109, GSK-3036656, Q-203, PBTZ-169, and OPC-167832), which, although promising, seems to be insufficient for the treatment of tuberculosis since it requires at least three drugs to be administered simultaneously (15).
Natural or synthetic peptides are emerging as a promising source of novel antimicrobials (16). Currently, there are two AMPs in phase 2 of clinical development (murepavadin and brilacidin) for treating, respectively, Pseudomonas aeruginosa and S. aureus infections. As with most AMPs, both target the bacterial membrane, an interesting new target for treating persistent infections such as tuberculosis (17). Other membrane-targeting compounds have been shown to be effective in vitro against Clostridium difficile (18). The interest in the possible application of AMPs in tuberculosis treatment has arisen since it was demonstrated that a high proportion of AMPs active against P. aeruginosa also showed activity against M. tuberculosis (19). Recently, the activity of antimicrobial peptides against several species of mycobacteria has been reviewed (20).
Bacteriocins are AMPs produced by certain genera of bacteria, such as lactic acid bacteria. Previously, antimicrobial activity of bacteriocin AS-48 has been reported (4). In this study, we report the activity of bacteriocin AS-48 against M. tuberculosis.
Susceptibility of M. tuberculosis strains to bacteriocin AS-48 ranged from 16 to 64 μg/ml, including reference strains and several clinical isolates of M. tuberculosis selected by their different spoligotype and restriction fragment length polymorphism (RFLP) profiles. We found that AS-48 is bactericidal against M. tuberculosis. Several other bacteriocins have been reported as effective against M. tuberculosis in in vitro assays (7, 21–23): colistin has a MIC of 16 μg/ml (24), and nisin showed a MIC of >60 μg/ml against M. tuberculosis H37Ra (7). The authors described variants of nisin with an enhanced antimicrobial activity (22) against M. tuberculosis as well as other naturally occurring bacteriocins such as lacticin 3147 (MIC90 of 7.5 μg/ml) (7). Bacteriocins capable of strongly inhibiting growth of M. tuberculosis at a concentration similar to that of rifampin have also been described (8).
The success in the improvement of antituberculosis activity of nisin derivatives (22) prompted us to explore whether variants of AS-48 (altered in residues important for maturation, conformation stability, etc.) could have reduced MICs in comparison with the MIC of wild-type AS-48. However, none of these variants resulted in a significant change of their MICs against M. tuberculosis (data not shown), similar to what has been described for other bacterial species (4).
We have also explored susceptibility of other mycobacterial species to AS-48 and found that for some species (M. smegmatis, Mycobacterium fortuitum, and Mycobacterium mucogenicum, three fast-growing mycobacteria) the MIC of AS-48 did not change significantly from that of M. tuberculosis. Carroll et al. (22) also described a rather uniform susceptibility of nisin derivatives between M. tuberculosis, M. kansasii, and M. avium. For other mycobacterial species (slow-growing and chromogenic species), however, we obtained either very low MICs (<2 μg/ml for M. xenopi and Mycobacterium gordonae), or we could not detect any inhibition of growth up to 256 μg/ml (M. lentiflavum).
Lysozyme is an enzyme present in respiratory tract secretions as part of the body's defense mechanisms against microbes. It has been reported that the combination of lysozyme and bacteriocins such as nisin results in a synergistic effect against many bacterial pathogens such as C. difficile (25). This synergistic effect of lysozyme has also been described for AS-48 (10). Lysozyme acting on the cell wall could facilitate the access of AS-48 into the bacteria and increase its bactericidal effect. Through synergy tests, we have found that AS-48 strongly synergizes with lysozyme, as has been reported for other bacterial species (10).
From the early dates of antituberculosis treatment, it became evident that monotherapy results in the selection of drug-resistant strains, so a combination therapy was established as a gold standard for antituberculosis treatment, with decreased probabilities to develop resistance. Thus, for any new antituberculosis agent, it is interesting to investigate not only its own antimicrobial activity but also its interactions with other drugs in use against tuberculosis. We have investigated the interactions between AS-48 and first-line antituberculosis drugs by using a checkerboard assay, considered an accurate drug combination analysis method (26) that has been used for demonstrating synergism of drugs and drug candidates in M. tuberculosis (27, 28). We found that there is synergism between AS-48 and EMB. In fact, the synergism between AS-48 and EMB was stronger than that of AS-48 and lysozyme. We can hypothesize that EMB, by inhibiting biosynthesis of arabinogalactan in the cell wall, could facilitate the access of AS-48 to its potential target, the mycobacterial membrane. The work by Kalita et al. (29) reported synergism between the antimicrobial human neutrophil peptide 1 (HNP-1) and the antituberculosis drugs isoniazid and rifampin and, hence, proposed the use of this AMP as an adjuvant in antituberculosis chemotherapy.
We conducted two experiments aimed at investigating the potential effect of AS-48 on the cell membrane. On the one hand, by determining the proportion of depolarized cells after AS-48 treatment, we found that this effect was both concentration and time dependent. On the other hand, in ethidium bromide accumulation assays, we monitored a time course increase in fluorescence and also an AS-48 concentration-dependent effect. Thus, we have demonstrated by using two independent methods that AS-48 acts on mycobacterial membranes.
In the intracellular M. tuberculosis growth assays in macrophages, the combination of AS-48 and EMB readily showed strong synergism, and the FICI values obtained were similar to the ones found in in vitro cultures. However, when we tested the interaction between AS-48 and lysozyme in the intracellular model of infection, no synergistic effect was observed under any of the conditions tested, in contrast with what we have observed in in vitro cultures. We can speculate either that lysozyme could not get inside the macrophages and thus could not reach the intracellular bacteria residing in the phagosome or that it could have been degraded by the macrophage.
In summary, we have reported here the antituberculosis activity in vitro of circular bacteriocin AS-48 on both extracellular and intracellular bacteria. Interestingly, we have found that in both systems, AS-48 strongly synergizes with EMB, hence opening the way to further explore the interaction of other AMPs with antituberculosis drugs and their future potential use as adjuvants of current antituberculosis treatments or as part of a new, alternative therapeutic regimen.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Mycobacterial strains used in this work for in vitro tests are listed in Table 1 (see also Table S1 in the supplemental material). All strains were cultured in Middlebrook 7H9 medium (Difco) supplemented with 10% albumin-dextrose-catalase (ADC) (BD) and 0.05% Tween 80. For drug susceptibility testing, Tween 80 was replaced by 0.5% glycerol. Cultures were incubated at 37°C in 25-cm2 flasks. Cultures on solid medium were performed in Middlebrook 7H10 agar (Difco) supplemented with 10% ADC and 0.05% Tween 80.
For infection assays, a recombinant strain of M. tuberculosis H37Rv (ATCC 27294) constitutively expressing a green fluorescent protein (H37Rv-GFP) was used as a reporter for the intracellular mycobacterial replication assay (30). This strain was grown in Middlebrook 7H9 medium supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC; Difco), 0.05% Tween 80 (Sigma-Aldrich), 0.5% glycerol (Euromedex), and 50 μg/ml hygromycin B (Invitrogen). Cultures were maintained at 37°C under static conditions for up to 14 days to reach the exponential phase of bacterial growth before being used for the intracellular mycobacterial replication assay.
Cell cultures.
The following cell lines were used: murine macrophages MHS (Health Protection Agency [HPA] Culture Collections 95090612), J774.2 (European Collection of Authenticated Cell Cultures [ECACC] 85011428), Raw 264.7 (TIB-71), and human monocytes THP-1 (ECACC 88081201). MHS cells were cultured in Dulbecco's modified Eagle's medium (DMEM), and the rest were cultivated in RPMI 1640 medium with GlutaMAX (Gibco) and containing 10% heat-inactivated fetal bovine serum (FBS; Gibco). When cells were to be used for cytotoxicity assays, antibiotics (penicillin, streptomycin, and ciprofloxacin) were added to cell precultures. Cells were cultured in a controlled 5% CO2 atmosphere at 37°C.
MIC.
Serial broth microdilutions were made to determine the MIC of AS-48, which was purified to homogeneity from the E. faecalis UGRA10 strain (31) according to Ananou et al. (32), lysozyme (L3790; Sigma-Aldrich), and other antimicrobials (isoniazid, moxifloxacin, streptomycin, and EMB [Sigma-Aldrich]).
Dilutions of compounds were made in 100 μl of culture medium for mycobacterial strains in sterile 96-well polypropylene flat-bottom plates. Wells were inoculated with 1 × 105 CFU in 100 μl and incubated at 37°C for 7 days for slow-growing mycobacteria or for 4 days for fast-growing species. Then, 30 μl of 0.1 mg/ml filter-sterilized resazurin (Sigma-Aldrich) was added. The results were revealed after 48 h in the case of slow-growing mycobacteria or after 24 h for fast-growing species. Positive and negative controls were added in all the experiments, and moxifloxacin (Sigma) at 0.5 mg/ml was used in all the assays as a control (33).
Kill kinetics.
We determined the kill kinetics in order to find out whether the antituberculosis effect of bacteriocin AS-48 could be bactericidal or bacteriostatic. For this, Mycobacterium tuberculosis H37Rv was grown in culture medium until exponential phase, then diluted to 106 CFU/ml, and separated in three flasks. Bacteriocin AS-48 was added to cultures to give final concentrations of 64 and 128 μg/ml, and the third culture was left untreated as a control. All cultures were incubated at 37°C. After 1, 2, 6, and 9 days of incubation, samples of 100 μl were taken, and serial 1:10 dilutions in phosphate-buffered saline (PBS) supplemented with tyloxapol (a nonionic surfactant) at 0.1% was added to avoid bacterial clumping. Dilutions were plated on Middlebrook 7H10 agar supplemented with ADC, in triplicate, and plates were incubated for 3 weeks at 37°C. The numbers of CFU for each time point and culture were determined and plotted as log CFU versus time, and results were analyzed for significance by a linear regression test.
Synergy test.
The synergy assay blends gradients of two compounds to evaluate their activities and the interactions between them. In fresh medium for culturing mycobacteria, solutions at 4-fold the highest concentration to be tested were prepared for each compound. Then, both compounds were serially 2-fold diluted in sterile 96-well flat bottom plates; one of the compounds was diluted along the columns, and the other was diluted in the rows. The first column would then have just one of the compounds, and the last row would have the other; the rest of the plate would be a matrix of both gradients. Finally, wells were inoculated as described above and incubated and treated with resazurin as in a conventional MIC assay.
FIC index analysis.
The fractional inhibitory concentration (FIC) index allows objective identification of any interaction between two compounds against a particular bacterial strain, indicating whether there is synergy, no interaction, or antagonism.
In the 96-well plate, the first column shows the MIC of one of the compounds (X), and the last row shows the MIC of the second compound (Y). For each well in which there is no growth next to a well with growth, the FIC must be calculated using the following formulas: FICX = MICX in the presence of Y/MICX alone and FICY = MICY in the presence X/MICY alone.
Both values, together with other points of inhibition, can be represented graphically, and a concave line will be indicative of synergy. Alternatively, the addition of FICX and FICY indicates the FICI value. An FICI value up to 0.5 indicates synergy, a value between 0.5 and 4 indicates that there is no interaction between the compounds, and a value over 4 indicates antagonism (34, 35).
Ethidium bromide accumulation assay.
The ethidium bromide assay assessed the capacity to accumulate ethidium bromide at increasing concentrations of bacteriocin AS-48. M. smegmatis was cultivated until the culture reached an optical density at 600 nm (OD600) of 0.6 to 0.8 under the conditions mentioned above. The culture was centrifuged, and the pellet was washed in PBS supplemented with 0.05% Tween 80; then, the OD600 was adjusted to 0.8 in the same buffer further supplemented with 0.4 % glucose. Ethidium bromide was used at 1 μg/ml combined with inhibitory and subinhibitory concentrations of AS-48. Verapamil at 75 μg/ml was used as a positive control showing the highest accumulation of ethidium bromide. A control experiment was done with PBS-Tween 80 buffer and no bacterial inoculum to set the values of fluorescence of the compounds by themselves. Other controls were done without ethidium bromide. Accumulation of ethidium bromide was measured by recording fluorescence at 530-nm excitation and 590-nm detection for 1 h (12).
Cytotoxicity assays.
An MTT assay and neutral red assay (36) were used to determine AS-48 cytotoxicity in MHS and J774.2 murine macrophages and THP-1 human macrophages. Cells were cultured in 96-well flat-bottom plates with the appropriate medium. THP-1 monocytes were differentiated to macrophages by using 10 ng/ml of phorbol 12-myristate 13-acetate (PMA) (P1585-1MG; Sigma-Aldrich) for 72 h. The concentrations used for cytotoxicity assays were 15,000 cells/well (MHS) and 20,000 cells/well (J774.2) for mouse lung macrophages and 50,000 cells/well (THP-1) for human lung macrophages. They were cultured for 24 h, and then the compounds were added dissolved in fresh culture medium. Absorbance was measured in an MTT assay at 570 and 650 nm and by fluorescence in the case of neutral red at 530- and 645-nm excitation and emission wavelengths, respectively. Percent cell viability in comparison with that of untreated controls was determined, and treatments were considered to be cytotoxic or not according to the International Organization for Standardization (13). To check the lack of differences between samples treated with lower concentrations of AS-48, we performed one-way analysis of variance (ANOVA) tests.
Analysis of the bacterial membrane potential.
M. smegmatis and M. bovis BCG membrane potentials were measured by a BacLight bacterial membrane potential kit (B34950; Thermo Fisher); integrity of the membrane potential results in fluorescence due to the accumulation of DiOC2 (3) inside bacterial cells. Bacterial cells were treated with several concentrations of AS-48 for 24 h to assess its effect on membrane potential. The samples were analyzed by flow cytometry.
Samples for SEM.
The effect of AS-48 on the morphology of M. tuberculosis H37Rv was analyzed by scanning electron microscopy (SEM). SEM images were acquired using an SEM Inspect F50 (FEI Company, Eindhoven, The Netherlands). Bacterial cultures treated with AS-48 at 64 μg/ml or with 8 μg/ml of AS-48 plus 25 μg/ml of lysozyme were diluted to 105 CFU/ml, and samples were first fixed with glutaraldehyde. Then, samples were washed three times with 10× PBS, filtered through a 0.1-μm-pore-size filter (0.1-μm VCTP Isopore membrane filter; Millipore), and then dehydrated with a graded ethanol series and kept in 100% ethanol. Finally, ethanol was evaporated at room temperature, and samples were covered with a thin layer of metal (Pt; 15 nm) and examined at 15,000 eV and different magnifications.
M. tuberculosis macrophage infection assay.
Cultures of H37Rv-GFP were maintained with stirring for 3 days before infection to ensure that bacterial cells were in exponential growth. Then, bacterial cells were washed twice with PBS without Mg2+ and Ca2+, followed by a third wash with RPMI 1640 medium supplemented with 10% FBS (RPMI-FBS), and centrifuged at 70 × g for 2 min to pellet bacterial clumps; the supernatant corresponded to a homogenous suspension of bacteria. Bacterial titer was determined by measuring the optical density at 600 nm and by measuring enhanced GFP (EGFP) fluorescence on a Victor Multilabel Counter (PerkinElmer); the sample was further diluted in RPMI-FBS medium prior to infection. Raw 264.7 macrophages were used for the intracellular mycobacterial replication assay and harvested by using Versene (Life Technologies). Macrophages were prepared at a concentration of 5 × 105 cells/ml, infected at a multiplicity of infection (MOI) of 1:2 with H37Rv-GFP, and incubated in RPMI-FBS cell culture medium at 37°C with stirring at 120 rpm. After 2 h, cells were centrifuged at 160 × g for 5 min, resuspended in the same volume of fresh medium containing 50 μg/ml of amikacin to kill extracellular bacteria, and incubated for 1 h at 37°C with shaking at 120 rpm. Cells were washed and resuspended in the same volume of fresh medium. Infected cells were seeded in 384-well plates (Greiner Bio-One) at 2 × 104 cells/well containing compound (AS-48, EMB, and lysozyme diluted in water). Plates were incubated for 5 days at 37°C in 5% CO2. Infected cells were stained for 30 min with Syto60 dye (Invitrogen) at a final concentration of 5 μM. Finally, images were acquired (as described below), and then cells were lysed with Dulbecco's PBS (DPBS)–0.1% Triton X-100 buffer for 1 min to release intracellular bacteria. The number of viable bacterial cells was determined by serially plating dilutions on Middlebrook 7H11 (Difco) agar plates, supplemented with 0.5% glycerol, 10% OADC, and 50 μg/ml hygromycin B. After 3 weeks of incubation at 37°C in 5% CO2, the numbers of viable bacteria (CFU) were calculated.
Image acquisition and analysis.
Images were acquired on an automated fluorescent confocal microscope (Opera; PerkinElmer), using a 20× water immersion lens. Syto60-labeled cells were detected using a 640-nm excitation laser coupled with a 690/70-nm detection filter, and GFP-labeled bacteria were detected using a 488-nm laser coupled with a 540/75-nm detection filter. A series of six images was taken per well, and each one was analyzed using the Columbus system (version 2.5.1; PerkinElmer) image analysis software. Briefly, the images were processed to determine the output image of GFP mask and remove background in that channel. Then, the output images corresponding to the Syto60 values were processed similarly to assess morphological parameters of cells. Properties of intensity and morphology were filtered to select the correct nuclear population. Spots corresponding to the bacteria infecting the cells were detected, and bacterial number and the area in pixels were determined for each cell. The intracellular bacterial growth was quantified by the total intracellular bacterial area (pixels) per well. In order to consider a cell infected, the number of bacteria within the cell area was set as ≥1. Efficiency of infection and effectivity of treatment with compounds were determined in terms of the percentage of infected cells and mean M. tuberculosis area per infected cell.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ana Belén Gómez for technical support in the culture of eukaryotic cells and Nacho Aguiló for help in the interpretation of cytometry flow experiments and for helpful discussion on the manuscript. We thank Ainhoa Lucía for support with the cytotoxicity assays. We thank Alexandre Vandeputte and Isabelle Ricard for technical expertise in the infection assays.
This work was supported by grants from the Spanish Government (SAF2013-48971-C2-2-R to J.A.A. and SAF2013-48971-C2-1-R to M.M.). C.A.-P. is a recipient of a predoctoral fellowship (BES-2014-067962) from the Spanish Government and was funded by EMBO Short Term Fellowships (number 6986). P.B., N.D., and O.-R.S. received financial support from the European Community (ERC-STG INTRACELLTB grant number 260901 and MM4TB grant number260872), the EMBO Young Investigator Program, the Agence Nationale de la Recherche (ANR-10-EQPX-04-01), the Feder (12001407 [D-AL] Equipex Imaginex BioMed), and the Région Nord Pas de Calais (convention number 12000080).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00359-18.
REFERENCES
- 1.World Health Organization. 2017. Global tuberculosis report 2017. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2.Alvarez-Sieiro P, Montalban-Lopez M, Mu D, Kuipers OP. 2016. Bacteriocins of lactic acid bacteria: extending the family. Appl Microbiol Biotechnol 100:2939–2951. doi: 10.1007/s00253-016-7343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sánchez-Barrena MJ, Martínez-Ripoll M, Gálvez A, Valdivia E, Maqueda M, Cruz V, Albert A. 2003. Structure of bacteriocin AS-48: from soluble state to membrane bound state. J Mol Biol 334:541–549. doi: 10.1016/j.jmb.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Hidalgo M, Montalban-Lopez M, Cebrian R, Valdivia E, Martinez-Bueno M, Maqueda M. 2011. AS-48 bacteriocin: close to perfection. Cell Mol Life Sci 68:2845–2857. doi: 10.1007/s00018-011-0724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abriouel H, Lucas R, Omar NB, Valdivia E, Galvez A. 2010. Potential applications of the cyclic peptide enterocin AS-48 in the preservation of vegetable foods and beverages. Probiotics Antimicrob Proteins 2:77–89. doi: 10.1007/s12602-009-9030-y. [DOI] [PubMed] [Google Scholar]
- 6.Ananou S, Valdivia E, Martinez Bueno M, Galvez A, Maqueda M. 2004. Effect of combined physico-chemical preservatives on enterocin AS-48 activity against the enterotoxigenic Staphylococcus aureus CECT 976 strain. J Appl Microbiol 97:48–56. doi: 10.1111/j.1365-2672.2004.02276.x. [DOI] [PubMed] [Google Scholar]
- 7.Carroll J, Draper LA, O'Connor PM, Coffey A, Hill C, Ross RP, Cotter PD, O'Mahony J. 2010. Comparison of the activities of the lantibiotics nisin and lacticin 3147 against clinically significant mycobacteria. Int J Antimicrob Agents 36:132–136. doi: 10.1016/j.ijantimicag.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Sosunov V, Mischenko V, Eruslanov B, Svetoch E, Shakina Y, Stern N, Majorov K, Sorokoumova G, Selishcheva A, Apt A. 2007. Antimycobacterial activity of bacteriocins and their complexes with liposomes. J Antimicrob Chemother 59:919–925. doi: 10.1093/jac/dkm053. [DOI] [PubMed] [Google Scholar]
- 9.Chun W, Hancock RE. 2000. Action of lysozyme and nisin mixtures against lactic acid bacteria. Int J Food Microbiol 60:25–32. doi: 10.1016/S0168-1605(00)00330-5. [DOI] [PubMed] [Google Scholar]
- 10.Ananou S, Rivera S, Madrid MI, Maqueda M, Martínez-Bueno M, Valdivia E. 2018. Application of enterocin AS-48 as biopreservative in eggs and egg fractions: synergism through lysozyme. LWT Food Sci Technol 89:409–417. doi: 10.1016/j.lwt.2017.11.018. [DOI] [Google Scholar]
- 11.Gonzalez C, Langdon GM, Bruix M, Galvez A, Valdivia E, Maqueda M, Rico M. 2000. Bacteriocin AS-48, a microbial cyclic polypeptide structurally and functionally related to mammalian NK-lysin. Proc Natl Acad Sci U S A 97:11221–11226. doi: 10.1073/pnas.210301097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues L, Viveiros M, Ainsa JA. 2015. Measuring efflux and permeability in mycobacteria. Methods Mol Biol 1285:227–239. doi: 10.1007/978-1-4939-2450-9_13. [DOI] [PubMed] [Google Scholar]
- 13.International Organization for Standardization. 2009. Biological evaluation of medical devices. Part 5: tests for in vitro cytotoxicity. ISO 10993-5:2009 International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 14.Abengozar MA, Cebrian R, Saugar JM, Garate T, Valdivia E, Martinez-Bueno M, Maqueda M, Rivas L. 2017. Enterocin AS-48 as evidence for the use of bacteriocins as new leishmanicidal agents. Antimicrob Agents Chemother 61:e02288-16. doi: 10.1128/AAC.02288-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. 2017. Antibacterial agents in clinical development. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 16.da Cunha NB, Cobacho NB, Viana JF, Lima LA, Sampaio KB, Dohms SS, Ferreira AC, de la Fuente-Nunez C, Costa FF, Franco OL, Dias SC. 2017. The next generation of antimicrobial peptides (AMPs) as molecular therapeutic tools for the treatment of diseases with social and economic impacts. Drug Discov Today 22:234–248. doi: 10.1016/j.drudis.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurdle JG, O'Neill AJ, Chopra I, Lee RE. 2011. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol 9:62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X, Cherian PT, Lee RE, Hurdle JG. 2013. The membrane as a target for controlling hypervirulent Clostridium difficile infections. J Antimicrob Chemother 68:806–815. doi: 10.1093/jac/dks493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramon-Garcia S, Mikut R, Ng C, Ruden S, Volkmer R, Reischl M, Hilpert K, Thompson CJ. 2013. Targeting Mycobacterium tuberculosis and other microbial pathogens using improved synthetic antibacterial peptides. Antimicrob Agents Chemother 57:2295–2303. doi: 10.1128/AAC.00175-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutsmann T. 2016. Interaction between antimicrobial peptides and mycobacteria. Biochim Biophys Acta 1858:1034–1043. doi: 10.1016/j.bbamem.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Carroll J, O'Mahony J. 2011. Anti-mycobacterial peptides: made to order with delivery included. Bioeng Bugs 2:241–246. doi: 10.4161/bbug.2.5.16229. [DOI] [PubMed] [Google Scholar]
- 22.Carroll J, Field D, O'Connor PM, Cotter PD, Coffey A, Hill C, Ross RP, O'Mahony J. 2010. Gene encoded antimicrobial peptides, a template for the design of novel anti-mycobacterial drugs. Bioeng Bugs 1:408–412. doi: 10.4161/bbug.1.6.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montalban-Lopez M, Sanchez-Hidalgo M, Valdivia E, Martinez-Bueno M, Maqueda M. 2011. Are bacteriocins underexploited? Novel applications for old antimicrobials. Curr Pharm Biotechnol 12:1205–1220. doi: 10.2174/138920111796117364. [DOI] [PubMed] [Google Scholar]
- 24.van Breda SV, Buys A, Apostolides Z, Nardell EA, Stoltz AC. 2015. The antimicrobial effect of colistin methanesulfonate on Mycobacterium tuberculosis in vitro. Tuberculosis (Edinb) 95:440–446. doi: 10.1016/j.tube.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Chai C, Lee KS, Imm GS, Kim YS, Oh SW. 2017. Inactivation of Clostridium difficile spore outgrowth by synergistic effects of nisin and lysozyme. Can J Microbiol 63:638–643. doi: 10.1139/cjm-2016-0550. [DOI] [PubMed] [Google Scholar]
- 26.Jia J, Zhu F, Ma X, Cao Z, Cao ZW, Li Y, Li YX, Chen YZ. 2009. Mechanisms of drug combinations: interaction and network perspectives. Nat Rev Drug Discov 8:111–128. doi: 10.1038/nrd2683. [DOI] [PubMed] [Google Scholar]
- 27.Makarov V, Lechartier B, Zhang M, Neres J, van der Sar AM, Raadsen SA, Hartkoorn RC, Ryabova OB, Vocat A, Decosterd LA, Widmer N, Buclin T, Bitter W, Andries K, Pojer F, Dyson PJ, Cole ST. 2014. Towards a new combination therapy for tuberculosis with next generation benzothiazinones. EMBO Mol Med 6:372–383. doi: 10.1002/emmm.201303575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lechartier B, Hartkoorn RC, Cole ST. 2012. In vitro combination studies of benzothiazinone lead compound BTZ043 against Mycobacterium tuberculosis. Antimicrob Agents Chemother 56:5790–5793. doi: 10.1128/AAC.01476-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalita A, Verma I, Khuller GK. 2004. Role of human neutrophil peptide-1 as a possible adjunct to antituberculosis chemotherapy. J Infect Dis 190:1476–1480. doi: 10.1086/424463. [DOI] [PubMed] [Google Scholar]
- 30.Christophe T, Jackson M, Jeon HK, Fenistein D, Contreras-Dominguez M, Kim J, Genovesio A, Carralot JP, Ewann F, Kim EH, Lee SY, Kang S, Seo MJ, Park EJ, Skovierova H, Pham H, Riccardi G, Nam JY, Marsollier L, Kempf M, Joly-Guillou ML, Oh T, Shin WK, No Z, Nehrbass U, Brosch R, Cole ST, Brodin P. 2009. High content screening identifies decaprenyl-phosphoribose 2′ epimerase as a target for intracellular antimycobacterial inhibitors. PLoS Pathog 5:e1000645. doi: 10.1371/journal.ppat.1000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cebrian R, Banos A, Valdivia E, Perez-Pulido R, Martinez-Bueno M, Maqueda M. 2012. Characterization of functional, safety, and probiotic properties of Enterococcus faecalis UGRA10, a new AS-48-producer strain. Food Microbiol 30:59–67. doi: 10.1016/j.fm.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Ananou S, Muñoz A, Gálvez A, Martínez-Bueno M, Maqueda M, Valdivia E. 2008. Optimization of enterocin AS-48 production on a whey-based substrate. Int Dairy J 18:923–927. doi: 10.1016/j.idairyj.2008.02.001. [DOI] [Google Scholar]
- 33.Palomino JC, Martin A, Camacho M, Guerra H, Swings J, Portaels F. 2002. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 46:2720–2722. doi: 10.1128/AAC.46.8.2720-2722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 35.European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). 2000. Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin Microbiol Infect 6:503–508. doi: 10.1046/j.1469-0691.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- 36.Repetto G, del Peso A, Zurita JL. 2008. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3:1125–1131. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- 37.Cole ST, Barrell BG. 1998. Analysis of the genome of Mycobacterium tuberculosis H37Rv. Novartis Found Symp 217:160–172. doi: 10.1002/0470846526.ch12. [DOI] [PubMed] [Google Scholar]
- 38.Lee JS, Krause R, Schreiber J, Mollenkopf HJ, Kowall J, Stein R, Jeon BY, Kwak JY, Song MK, Patron JP, Jorg S, Roh K, Cho SN, Kaufmann SH. 2008. Mutation in the transcriptional regulator PhoP contributes to avirulence of Mycobacterium tuberculosis H37Ra strain. Cell Host Microbe 3:97–103. doi: 10.1016/j.chom.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalo Asensio J, Maia C, Ferrer NL, Barilone N, Laval F, Soto CY, Winter N, Daffe M, Gicquel B, Martin C, Jackson M. 2006. The virulence-associated two-component PhoP-PhoR system controls the biosynthesis of polyketide-derived lipids in Mycobacterium tuberculosis. J Biol Chem 281:1313–1316. doi: 10.1074/jbc.C500388200. [DOI] [PubMed] [Google Scholar]
- 40.Fleischmann RD, Alland D, Eisen JA, Carpenter L, White O, Peterson J, DeBoy R, Dodson R, Gwinn M, Haft D, Hickey E, Kolonay JF, Nelson WC, Umayam LA, Ermolaeva M, Salzberg SL, Delcher A, Utterback T, Weidman J, Khouri H, Gill J, Mikula A, Bishai W, Jacobs WR, Venter JC, Fraser CM. 2002. Whole-genome comparison of Mycobacterium tuberculosis clinical and laboratory strains. J Bacteriol 184:5479–5490. doi: 10.1128/JB.184.19.5479-5490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caminero JA, Pena MJ, Campos-Herrero MI, Rodriguez JC, Garcia I, Cabrera P, Lafoz C, Samper S, Takiff H, Afonso O, Pavon JM, Torres MJ, van Soolingen D, Enarson DA, Martin C. 2001. Epidemiological evidence of the spread of a Mycobacterium tuberculosis strain of the Beijing genotype on Gran Canaria Island. Am J Respir Crit Care Med 164:1165–1170. doi: 10.1164/ajrccm.164.7.2101031. [DOI] [PubMed] [Google Scholar]
- 42.Chesne-Seck ML, Barilone N, Boudou F, Gonzalo Asensio J, Kolattukudy PE, Martin C, Cole ST, Gicquel B, Gopaul DN, Jackson M. 2008. A point mutation in the two-component regulator PhoP-PhoR accounts for the absence of polyketide-derived acyltrehaloses but not that of phthiocerol dimycocerosates in Mycobacterium tuberculosis H37Ra. J Bacteriol 190:1329–1334. doi: 10.1128/JB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M, Sala C, Hartkoorn RC, Dhar N, Mendoza-Losana A, Cole ST. 2012. Streptomycin-starved Mycobacterium tuberculosis 18b, a drug discovery tool for latent tuberculosis. Antimicrob Agents Chemother 56:5782–5789. doi: 10.1128/AAC.01125-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snapper SB, Melton RE, Mustafa S, Kieser T, Jacobs WR Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol 4:1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.