Host-directed therapy in tuberculosis is a potential adjunct to antibiotic chemotherapy directed at Mycobacterium tuberculosis. Ambroxol, a lead compound, emerged from a screen for autophagy-inducing drugs.
KEYWORDS: autophagy, Mycobacterium tuberculosis
ABSTRACT
Host-directed therapy in tuberculosis is a potential adjunct to antibiotic chemotherapy directed at Mycobacterium tuberculosis. Ambroxol, a lead compound, emerged from a screen for autophagy-inducing drugs. At clinically relevant doses, ambroxol induced autophagy in vitro and in vivo and promoted mycobacterial killing in macrophages. Ambroxol also potentiated rifampin activity in a murine tuberculosis model.
TEXT
Rifampin (Rif) is a mainstay agent for treating tuberculosis (TB) and many nontuberculous mycobacteria (NTM). However, treatments with Rif are long; susceptible TB therapy takes 6 months, and NTM therapy takes 18 to 24 months (1). For TB infection, the standard 600-mg dose may be too low (2, 3), as clinical effects were improved by a high dose of Rif (35 mg/kg daily) (4). For NTM infections, standard 600-mg-daily dosing led to desirable pharmacokinetic outcomes for only 18% of 531 patients (5) and was compounded by high clinical breakpoints (6). Clearly, current Rif therapy can be improved. During a screen of drugs for host-directed therapy for TB, we found several agents that induced autophagy, which is thought to be important in TB control (7, 8) and a mode of action of some TB drugs (9). Autophagy is also important in NTM infections, as inhibition of autophagy by azithromycin predisposes to NTM infection in patients with cystic fibrosis (10). Here, we describe the discovery of our lead compound ambroxol (Amb) and tests of its in vivo autophagy induction in lung and antimycobacterial activity with Rif. Clinically relevant levels of Amb induced autophagy in vitro and in vivo and increased mycobacterial killing in macrophage assays. They also greatly potentiated Rif antimycobacterial activity in vivo. Amb is approved worldwide, and the potential for Amb cotherapy with Rif is discussed.
In vitro microscopy screens (7, 8, 11) and generation of ATG5−/− mice (12) were performed as previously described. In vivo experiments were approved by the University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee. M. tuberculosis Erdman (ATCC 35891) isolates were cultured in 7H10 medium with oleic acid-albumin-dextrose-catalase supplement, and bone marrow-derived macrophages (BMMs) were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, 10 mM HEPES, and 1 mM pyruvate. BMMs from C3H mice (at a multiplicity of infection of 10) or M. tuberculosis Erdman isolates alone were incubated with Amb for 18 h and plated on 7H11 agar plates (after lysis for BMM). For mouse feeding studies, Amb was mixed into bacon-flavored transgenic mouse chow (S3472; BioServ, Flemington NJ) at 100 mg/kg fresh weekly and stored at 4°C. Estimated Amb daily dose was 16 mg/kg body weight. Green fluorescent protein (GFP)-red fluorescent protein (RFP)-LC3B mice (2 per group) were treated with Amb-chow for 0, 4, or 8 days. LC3B puncta were enumerated in lung cryosections in 50 areas. Rif was mixed with water at 6.7 mg/100 ml and chow containing Amb at 100 mg/kg plus or minus pyrazinamide (PZA) at 1,300 mg/kg. Food and water were given ad libitum. Estimated daily drug delivery was Rif 10 mg/kg body weight, PZA 150 mg/kg body weight, and Amb 16 mg/kg body weight. C57BL/6 mice were each infected with 175 CFU of M. tuberculosis Erdman strain using a Glas-Col inhalation exposure system (099C A4212; Terre Haute IN), and 2 weeks after infection, the mice were started on drug treatment. Groups of 6 or 7 were sacrificed after 4 or 6 weeks of drug treatment; lungs were harvested and plated on 7H11 agar plates for CFU and counted after 3 to 4 weeks. Statistics were performed on GraphPad Prism (analysis of variance with Tukey's test) or SigmaPlot (Mann-Whitney test).
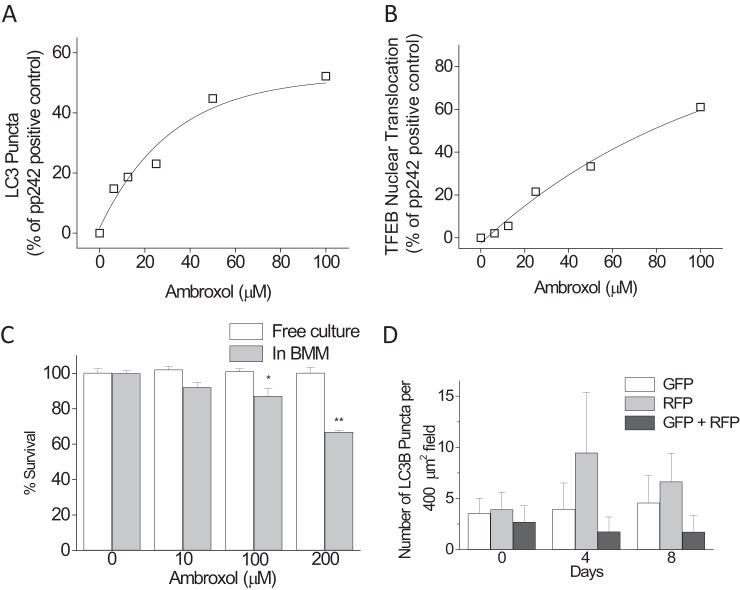
Amb induces autophagy and mycobacterial death in primary macrophages. We used our quantitative high-content microscopy screen to detect induction of autophagy through both cytoplasmic LC3B puncta and transcription factor EB (TFEB) nuclear translocation in primary murine BMMs (11). To standardize assay runs, an inhibitor of mTOR and a powerful autophagy inducer, pp242 (10 μg/ml), was used as positive control; and data were analyzed and presented as the increases in LC3B puncta or TFEB nuclear translocation relative to the pp242 control. Amb showed strong, dose-dependent induction of autophagy (Fig. 1A and B), with effects in LC3B puncta apparent at the low micromolar levels achievable in human plasma (1 to 10 μM) (13). Figure 2 shows LC3B puncta induced in BMMs from both wild-type and autophagy-deficient ATG5−/− mice, showing the need for ATG5 for optimal induction. Amb also induced LC3B puncta by >2-fold in donor-derived primary human peripheral blood monocyte-derived macrophages at 12.5 μM (not shown). Our prior screen identified bromhexine (the prodrug of Amb) as an autophagy inducer in HeLa cells (11), but bromhexine was inactive here, presumably because of a cell-dependent deficit in conversion into Amb (14). We then determined Amb's effects on M. tuberculosis survival in BMMs and free culture (Fig. 1C). Dose-dependent decreases in mycobacterial survival were apparent in BMMs but not in free bacterial culture, suggesting a host-mediated effect, such as autophagy on survival, although Amb at ≥100 μM has been shown to have direct effects on Mycobacterium bovis BCG at pH <7 (15).
FIG 1.
(A) Induction of autophagy in BMMs by ambroxol as determined by LC3B puncta. (B) Induction of autophagy in BMMs by ambroxol as determined by TFEB nuclear translocation. (C) Effects of ambroxol on M. tuberculosis Erdman survival in free culture and when infected into BMMs. Data are means ± SEM of 3 or 4 independent replicates. Tukey's multiple comparison: *, P < 0.05; **, P < 0.01; †, not significant. (D) Effect of ambroxol on autophagy in lung tissue. n = 2 mice/group. Data are means ± SEM.
FIG 2.
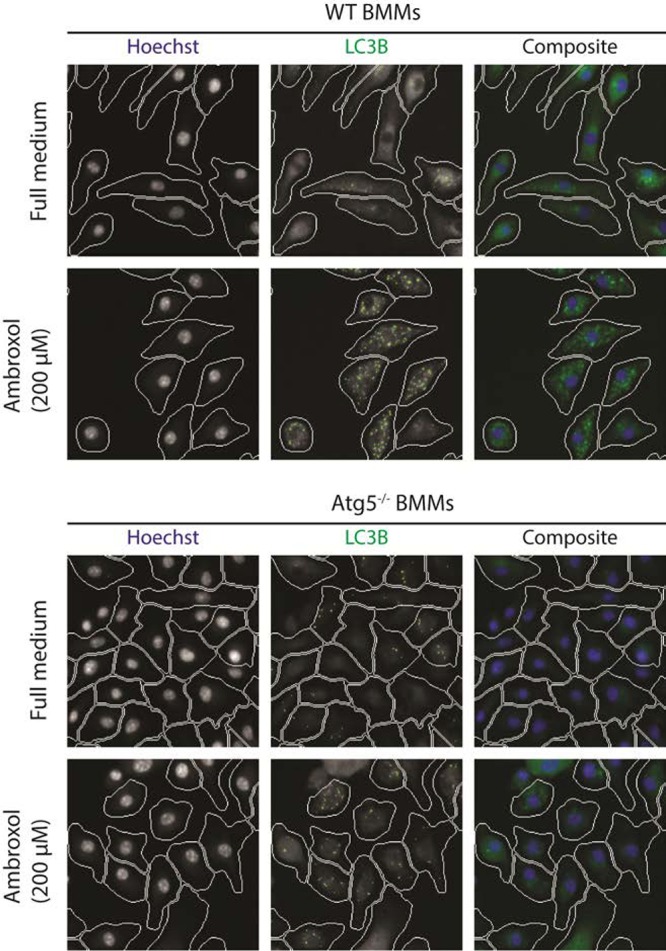
LC3B puncta induced in BMMs from wild-type and autophagy-deficient ATG5−/− mice by 200 μM ambroxol.
Because long experiment times (8 weeks) make multiple gavage undesirable, we chose to dose Amb mixed into transgenic paste food. No differences were seen in body weight or food consumed between groups of control animals and those fed chow containing 6.25, 30, or 100 mg/kg Amb for 4 days. Chow at 100 mg/kg was estimated to result in 16-mg/kg body weight/day dosing in mice, corresponding to achievable and approved human doses (13). To confirm that our chosen dose of Amb (100 mg/kg food) induced autophagy in lungs when given to mice in vivo, we used RFP-GFP-LC3B knock-in reporter mice, in which the GFP is acid sensitive and the RFP acid resistant, so that the autolysosome becomes enriched in RFP versus GFP on full autophagic flux induction. We tested autophagy induction by monitoring GFP and RFP puncta in lung using cryosections. Although not powered for statistical analysis, large increases in RFP-positive morphometrically discernible puncta were observed with Amb after 4 and 8 days of administration (Fig. 1D). The number of doubly positive (RFP+GFP+) puncta was simultaneously reduced, suggesting that this dose of Amb actively induced autophagy flux.
Two weeks after low-dose infection of C57BL/6 mice with M. tuberculosis Erdman, Rif or PZA at usual mouse dosages were administered alone and in conjunction with Amb for a further 4 or 6 weeks of drug and Amb treatment, and lungs were plated for CFU (Fig. 3). PZA showed no potentiation by Amb and mild antagonism at 4 weeks that resolved by 6 weeks, but there was marked potentiation of the antimycobacterial effects of Rif by Amb at both treatment times.
FIG 3.
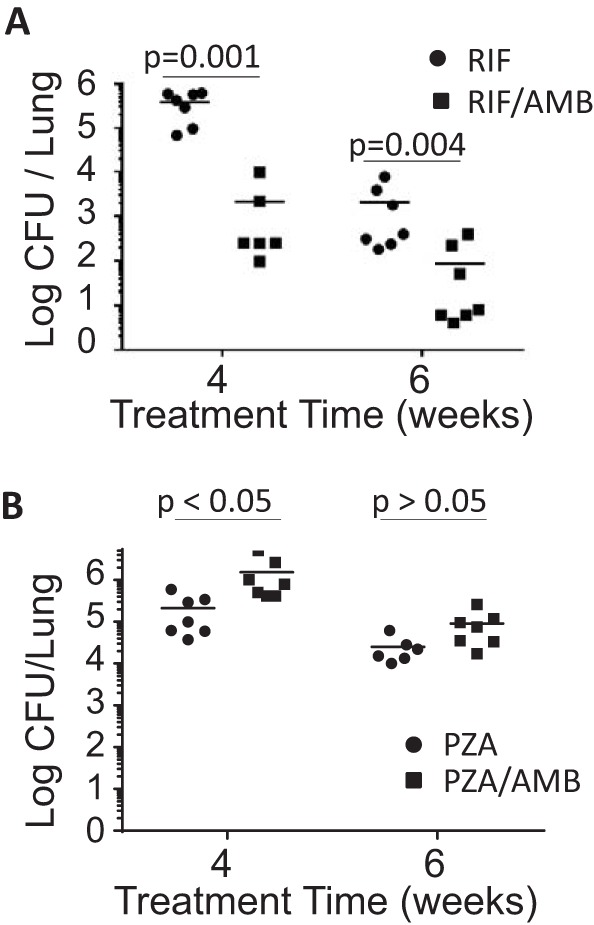
Effect of ambroxol (16 mg/kg body weight daily) on M. tuberculosis killing activity of rifampin 10 mg/kg body weight daily (A) and pyrazinamide 150 mg/kg body weight daily (B) in C57BL/6J mice. Data are means, with each CFU data point shown as a dot. n = 6 or 7 mice/group. Mann-Whitney test was used.
Amb significantly induced autophagy in vitro, and we delineated an effective and clinically relevant dose of Amb for inducing autophagy in vivo in mouse lung. Amb potentiated only rifampin, although the reasons are unclear. However, in addition to autophagy potentiating Rif killing, Rif levels are most likely increased in lung tissue after Amb treatment. In a rat study, 15 mg/kg of Amb given intravenously pretreatment significantly increased Rif levels in lung tissue at 2 and 4 h after administration, (16), increasing half-life by almost 3-fold. Accordingly, the area under the curve/MIC of Rif drives efficacy (17). Amb addition to Rif-containing drug regimens for TB and NTM infections might significantly improve patient outcomes. However, Amb is metabolized by cytochrome P450 3A4 (Cyp3A4) (18), which is strongly induced by rifamycins in humans but not in mice (19). Although high-dose Amb has been used clinically and may overcome Cyp3A4 induction by Rif (20–22), appropriate deuteration of Amb is also predicted to decrease Cyp3A4 sensitivity (23). Amb deuteration, rather than higher doses, may prevent interpatient variability in Amb exposure due to polymorphism in Cyp3A4 induction (24) and Cyp3A4 activity (25). In summary, Amb improved TB killing through an unexpected combination of host-directed effects, i.e., induction of autophagy in the lung and improved Rif pharmacokinetics.
ACKNOWLEDGMENTS
This work was supported by NIH grants from NIAID, UH2AI122313 to V.D., G.S.T., J.J.E., and P.S. and R01AI111935 to V.D., and center grant P20GM121176 from NIGMS to V.D.
REFERENCES
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, Subcommittee ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 2.Diacon AH, Patientia RF, Venter A, van Helden PD, Smith PJ, McIlleron H, Maritz JS, Donald PR. 2007. Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob Agents Chemother 51:2994–2996. doi: 10.1128/AAC.01474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Ingen J, Aarnoutse RE, Donald PR, Diacon AH, Dawson R, Plemper van Balen G, Gillespie SH, Boeree MJ. 2011. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis 52:e194–9. doi: 10.1093/cid/cir184. [DOI] [PubMed] [Google Scholar]
- 4.Boeree MJ, Heinrich N, Aarnoutse R, Diacon AH, Dawson R, Rehal S, Kibiki GS, Churchyard G, Sanne I, Ntinginya NE, Minja LT, Hunt RD, Charalambous S, Hanekom M, Semvua HH, Mpagama SG, Manyama C, Mtafya B, Reither K, Wallis RS, Venter A, Narunsky K, Mekota A, Henne S, Colbers A, van Balen GP, Gillespie SH, Phillips PPJ, Hoelscher M, Pan ACEA Consortium. 2017. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 17:39–49. doi: 10.1016/S1473-3099(16)30274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Ingen J, Egelund EF, Levin A, Totten SE, Boeree MJ, Mouton JW, Aarnoutse RE, Heifets LB, Peloquin CA, Daley CL. 2012. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med 186:559–565. doi: 10.1164/rccm.201204-0682OC. [DOI] [PubMed] [Google Scholar]
- 6.Schon T, Chryssanthou E. 2017. Minimum inhibitory concentration distributions for Mycobacterium avium complex—towards evidence-based susceptibility breakpoints. Int J Infect Dis 55:122–124. doi: 10.1016/j.ijid.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Deretic V, Singh S, Master S, Harris J, Roberts E, Kyei G, Davis A, de Haro S, Naylor J, Lee HH, Vergne I. 2006. Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism. Cell Microbiol 8:719–727. doi: 10.1111/j.1462-5822.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. 2004. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 9.Kim JJ, Lee HM, Shin DM, Kim W, Yuk JM, Jin HS, Lee SH, Cha GH, Kim JM, Lee ZW, Shin SJ, Yoo H, Park YK, Park JB, Chung J, Yoshimori T, Jo EK. 2012. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe 11:457–468. doi: 10.1016/j.chom.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Renna M, Schaffner C, Brown K, Shang S, Tamayo MH, Hegyi K, Grimsey NJ, Cusens D, Coulter S, Cooper J, Bowden AR, Newton SM, Kampmann B, Helm J, Jones A, Haworth CS, Basaraba RJ, DeGroote MA, Ordway DJ, Rubinsztein DC, Floto RA. 2011. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J Clin Invest 121:3554–3563. doi: 10.1172/JCI46095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauhan S, Ahmed Z, Bradfute SB, Arko-Mensah J, Mandell MA, Won Choi S, Kimura T, Blanchet F, Waller A, Mudd MH, Jiang S, Sklar L, Timmins GS, Maphis N, Bhaskar K, Piguet V, Deretic V. 2015. Pharmaceutical screen identifies novel target processes for activation of autophagy with a broad translational potential. Nat Commun 6:8620. doi: 10.1038/ncomms9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo EF, Dekonenko A, Arko-Mensah J, Mandell MA, Dupont N, Jiang S, Delgado-Vargas M, Timmins GS, Bhattacharya D, Yang H, Hutt J, Lyons CR, Dobos KM, Deretic V. 2012. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc Natl Acad Sci U S A 109:E368–E376. doi: 10.1073/pnas.1115729109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaida W, Klinder K, Arndt K, Weiser T. 2005. Ambroxol, a Nav1.8-preferring Na(+) channel blocker, effectively suppresses pain symptoms in animal models of chronic, neuropathic and inflammatory pain. Neuropharmacology 49:1220–1227. doi: 10.1016/j.neuropharm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Maegawa GHB, Tropak MB, Buttner JD, Rigat BA, Fuller M, Pandit D, Tang LI, Kornhaber GJ, Hamuro Y, Clarke JTR, Mahuran DJ. 2009. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J Biol Chem 284:23502–23516. doi: 10.1074/jbc.M109.012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grange JM, Snell NJ. 1996. Activity of bromhexine and ambroxol, semi-synthetic derivatives of vasicine from the Indian shrub Adhatoda vasica, against Mycobacterium tuberculosis in vitro. J Ethnopharmacol 50:49–53. doi: 10.1016/0378-8741(95)01331-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Yang C, Tu W. 2009. Effect of ambroxol on the transport of rifampicin into pulmonary. Jiangsu Med J 35:1486–1488. [Google Scholar]
- 17.Gumbo T, Louie A, Deziel MR, Liu W, Parsons LM, Salfinger M, Drusano GL. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother 51:3781–3788. doi: 10.1128/AAC.01533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishiguro N, Senda C, Kishimoto W, Sakai K, Funae Y, Igarashi T. 2000. Identification of CYP3A4 as the predominant isoform responsible for the metabolism of ambroxol in human liver microsomes. Xenobiotica 30:71–80. doi: 10.1080/004982500237839. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. 1998. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 102:1016. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baranwal AK, Murthy AS, Singhi SC. 2015. High-dose oral ambroxol for early treatment of pulmonary acute respiratory distress syndrome: an exploratory, randomized, controlled pilot trial. J Trop Pediatr 61:339–350. doi: 10.1093/tropej/fmv033. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Wang L, Wang H, Zhang H. 2015. Perioperative lung protection provided by high-dose ambroxol in patients with lung cancer. Cell Biochem Biophys 73:281–284. doi: 10.1007/s12013-015-0590-z. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Yao G, Zhu X. 2012. High-dose ambroxol reduces pulmonary complications in patients with acute cervical spinal cord injury after surgery. Neurocrit Care 16:267–272. doi: 10.1007/s12028-011-9642-4. [DOI] [PubMed] [Google Scholar]
- 23.Timmins GS. 2014. Deuterated drugs: where are we now? Expert Opin Ther Pat 24:1067–1075. doi: 10.1517/13543776.2014.943184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niemi M, Kivisto KT, Diczfalusy U, Bodin K, Bertilsson L, Fromm MF, Eichelbaum M. 2006. Effect of SLCO1B1 polymorphism on induction of CYP3A4 by rifampicin. Pharmacogenet Genomics 16:565–568. doi: 10.1097/01.fpc.0000215070.52212.0e. [DOI] [PubMed] [Google Scholar]
- 25.Hesselink DA, van Schaik RH, van der Heiden IP, van der Werf M, Gregoor PJ, Lindemans J, Weimar W, van Gelder T. 2003. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther 74:245–254. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]