Augmented renal clearance is commonly observed in septic patients and may result in insufficient β-lactam serum concentrations. The aims of this study were to evaluate potential correlations between drug concentrations or total body clearance of β-lactam antibiotics and measured creatinine clearance and to quantify the need for drug dosage adjustments in septic patients with different levels of augmented renal clearance.
KEYWORDS: creatinine clearance, meropenem, ceftazidime, cefepime, piperacillin-tazobactam, critically ill, pharmacokinetics
ABSTRACT
Augmented renal clearance is commonly observed in septic patients and may result in insufficient β-lactam serum concentrations. The aims of this study were to evaluate potential correlations between drug concentrations or total body clearance of β-lactam antibiotics and measured creatinine clearance and to quantify the need for drug dosage adjustments in septic patients with different levels of augmented renal clearance. We reviewed 256 antibiotic measurements (512 drug concentrations) from a cohort of 215 critically ill patients who had a measured creatinine clearance of ≥120 ml/min and who received therapeutic drug monitoring of meropenem, cefepime, ceftazidime, or piperacillin from October 2009 until December 2014 at Erasme Hospital. Population pharmacokinetic (PK) analysis of the data was performed using the Pmetrics software package for R. Fifty-five percent of drug concentrations showed insufficient β-lactam serum concentrations to treat infections due to Pseudomonas aeruginosa. There were significant, yet weak, correlations between measured creatinine clearance and trough concentrations of meropenem (r = −0.21, P = 0.01), trough concentrations of piperacillin (r = −0.28, P = 0.0071), concentrations at 50% of the dosage interval (r = −0.41, P < 0.0001), and total body clearance of piperacillin (r = 0.39, P = 0.0002). Measured creatinine clearance adequately explained changes in drug concentrations in population pharmacokinetic models for cefepime, ceftazidime, and meropenem but not for piperacillin. Therefore, specific PK modeling can predict certain β-lactam concentrations based on renal function but not on absolute values of measured creatinine clearance, easily available for clinicians. Currently, routine therapeutic drug monitoring is required to adjust daily regimens in critically ill patients receiving standard dosing regimens.
INTRODUCTION
Augmented renal clearance (ARC) refers to the enhanced elimination of solutes by the kidneys, and it is common in critically ill patients (1). Reported incidence rates vary significantly (16 to 100%) depending on the patient population studied and the criteria applied (2–8). ARC is usually defined as a creatinine clearance (CLCR) of >130 ml/min/1.73 m2 (1), calculated from either an 8- or 24-h urine collection (5, 9, 10).
In the septic patient, ARC is typically due to an increased cardiac output, resulting in an increased renal blood flow, and therefore an increased glomerular filtration rate. An important consequence of ARC is the increased risk of suboptimal achievement of pharmacokinetic/pharmacodynamic (PK/PD) targets for hydrophilic antibiotics, such as β-lactams, when standard drug regimens are administered (1). Suboptimal PK/PD target achievement may lead to emergence of resistance and/or therapeutic failure. Studies evaluating outcomes in critically ill patients with ARC and receiving β-lactam antibiotics are scarce and have shown conflicting results (8, 11). However, several studies, including an international, multicentric study including 68 intensive care units (ICUs), have demonstrated an association between suboptimal achievement of PK/PD targets and poor outcome in the general critically ill patient population (8, 12–14).
Only four studies have evaluated the relationship between levels of CLCR and β-lactam serum concentrations among patients with ARC. In all four, as CLCR increased, the probability of PK/PD target attainment of piperacillin-tazobactam (TZP), meropenem (MEM), cefepime (FEP), or ceftazidime (CAZ) decreased significantly (11, 15–17). However, the populations studied were small (from 48 to 61 patients), patients without ARC were included, most data came from patients receiving TZP (15, 16), and CLCR was estimated with the Cockcroft-Gault equation (18), an unreliable method to assess renal function in critically ill patients (5, 9). Further analyses are needed to help adjust drug regimens according to the degree of increase in CLCR, similar to the downward dose adjustments in patients with renal failure (19).
Thus, the aims of the present study were (i) to characterize the PKs of several broad-spectrum β-lactams, (ii) to evaluate a potential correlation between the drug concentrations or total body clearance (CL) of β-lactams with measured CLCR (mCLCR), and (iii) to quantify the need for dose adjustment in critically ill patients with different levels of ARC.
RESULTS
Study population.
We evaluated 256 therapeutic drug monitorings (TDMs) in 215 patients, corresponding to 256 measurements of β-lactam serum concentrations before (T0) and 256 measurements 2 h after the initiation of the β-lactam infusion (T2). The TDMs consisted of the following: 11 for FEP (4%), 11 for CAZ (4%), 89 for piperacillin (PIP) (35%), and 145 for MEM (67%). Only 10 of the 512 measured serum concentrations were below the limits of quantification (<1 mg/liter), all measured at T0. The characteristics of the study population are shown in Table 1. Patients were predominantly male, with a median age of 56 years, a median acute physiology and chronic health evaluation (APACHE) II score of 19 on ICU admission, and a median sequential organ failure assessment (SOFA) score of 6 on the day of TDM (20, 21). The most frequent site of infection was the respiratory tract, and the most frequently encountered pathogens were Enterobacteriaceae spp. (43%) (Table 2).
TABLE 1.
Demographic, biological and clinical characteristics of all patients
| Patient characteristica | Value for the group (n = 215)b |
|---|---|
| Demographics | |
| Age (yr) | 57 (42–65) |
| No. of males (%) | 152 (71) |
| No. of female (%) | 63 (29) |
| Body wt (kg) | 73 (62–84) |
| No. of medical admissions (%) | 148 (69) |
| Comorbidities (no. of patients [%]) | |
| Heart disease | 48 (22) |
| COPD/asthma | 47 (22) |
| Diabetes | 45 (21) |
| Immunosuppression | 32 (15) |
| Cancer | 31 (14) |
| Liver cirrhosis | 17 (8) |
| Biological data | |
| Serum creatinine (mg/dl) | 0.6 (0.2–1.0) |
| mCLCR (ml/min) | 179 (148–233) |
| Clinical data | |
| APACHE II score on ICU admission | 19 (14–24) |
| SOFA score on day of TDM | 6 (1–11) |
| ICU LOS (days) | 12 (7–23) |
| ICU 30-day mortality (no. of patients [%]) | 35 (16) |
COPD, chronic obstructive pulmonary disease; mCLCR, measured creatinine clearance; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; ICU, intensive care unit; LOS, length of stay.
Values are medians (IQR) unless otherwise noted.
TABLE 2.
Characteristics of infections and identified pathogens in the study cohort
| Infection parameter | No. (%) |
|---|---|
| Site | |
| Respiratory | 138 (54) |
| Abdomen | 49 (19) |
| Primary bacteremia | 17 (6) |
| Central nervous system | 15 (6) |
| Skin | 9 (3) |
| Catheter | 5 (2) |
| Urinary tract | 5 (2) |
| Mediastinitis | 5 (2) |
| Unknown | 13 (5) |
| Pathogen(s) | |
| Enterobacteriaceae spp. | 110 (43) |
| Pseudomonas aeruginosa | 34 (13) |
| Staphylococcus aureus | 22 (9) |
| Enterococcus spp. | 9 (3) |
| Staphylococcus epidermidis | 7 (3) |
| Acinetobacter spp. | 4 (1) |
| Other | 33 (3) |
| Unidentified | 37 (14) |
| Patients with positive blood cultures | 50 (20) |
Creatinine clearance and insufficient drug concentrations.
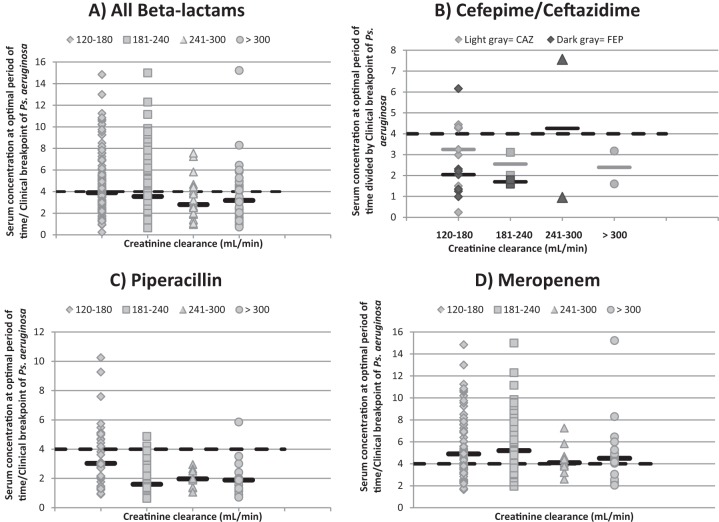
The median mCLCR of the study cohort at the time of TDM was 178 ml/min. The distribution of the TDM values relative to mCLCR is shown in Table 3. The majority of TDMs were performed in patients with mCLCRs varying from 120 to 180 ml/min. Insufficient drug concentrations to treat infections due to Pseudomonas aeruginosa were observed in 141 of these 256 TDMs (55%); the proportion of patients with insufficient concentrations was significantly greater for FEP, CAZ, and PIP than for MEM (82%, 73%, 79% and 37%, respectively; P < 0.001). As mCLCR quartiles increased to an mCLCR of 240 to 300 ml/min, the proportion of patients with insufficient serum concentrations of all β-lactams, of PIP, and of MEM increased (from 49% to 71%, from 58% to 100%, and from 38% to 45%, respectively), as illustrated in Fig. 1. For TDMs of FEP and CAZ, no correlation was observed between proportions of insufficient serum concentrations and the different mCLCR levels; however, only eight TDMs were available for evaluation in patients with mCLCRs of >180 ml/min.
TABLE 3.
Distribution of therapeutic drug monitorings as a function of measured creatinine clearance intervals
| Antibiotic(s)a | No. (%) of TDMs at CLCR of:b |
|||
|---|---|---|---|---|
| 120–180 ml/min | 181–240 ml/min | 241–300 ml/min | >300 ml/min | |
| All | 130 (51) | 67 (26) | 24 (9) | 35 (14) |
| FEP | 7 (32) | 2 (9) | 2 (9) | 0 (0) |
| CAZ | 7 (32) | 2 (9) | 0 (0) | 2 (9) |
| PIP | 43 (48) | 22 (25) | 11 (12) | 13 (15) |
| MEM | 73 (50) | 41 (28) | 11 (8) | 20 (14) |
FEP, cefepime; CAZ, ceftazidime; PIP, piperacillin; MEM, meropenem.
Creatinine clearance (CLCR) obtained from 24-h urine collections. TDMs, therapeutic drug monitorings.
FIG 1.
Serum concentrations of β-lactams (at optimal period of time divided by clinical breakpoints of Pseudomonas aeruginosa), in function of quartiles of mCLCR, as indicated. Solid horizontal black lines, median serum concentrations; dashed horizontal black lines, pharmacodynamic target of 4× MIC of the clinical breakpoints for Pseudomonas aeruginosa. Serum concentration at optimal period of time is the serum concentration at 40%, 50%, or 70% of the dosage interval of MEM, TZP, or FEP/CAZ, respectively.
Because Enterobacteriaceae spp. were the most frequently documented pathogens in this study, adequacy of drug concentrations to treat infections due to these pathogens was also evaluated. Insufficient drug concentrations to treat infections due to Enterobacteriaceae spp. were observed in 93 of the 256 TDMs (36%): 1/11 (10%) for FEP, 0/11 (0%) for CAZ, 38/89 (43%) for PIP, and 54/145 (37%) for MEM. Once again, as mCLCR quartiles increased to mCLCRs of 241 to 300 ml/min, the proportion of patients with insufficient serum concentrations of all β-lactams, of PIP, and of MEM increased (from 31% to 50%, from 26% to 64%, and from 38% to 45%, respectively).
Creatinine clearance and drug PKs.
Drug PKs are provided in Table 4. No significant correlations were observed between mCLCR and the studied parameters for FEP and CAZ (data not shown). For PIP, we observed a significant, but weak, logarithmic correlation between mCLCR and all the studied parameters, except for half-life (t1/2): between mCLCR and trough concentrations (T0s) of PIP (r = −0.28, P = 0.0071), PIP at 50% of the dosage interval (r = −0.41, P < 0.0001), and CL of PIP (r = 0.39, P = 0.0002) (Fig. 2). Finally, for MEM we also found a significant, but weak, logarithmic correlation between mCLCR and T0, as shown in Fig. 2 (r = −0.21, P = 0.01), but not for the other parameters (data not shown).
TABLE 4.
Pharmacokinetics, elimination constant, and half-life of the different β-lactam antibiotics and measured creatinine clearance
| Parametera | Median value for the parameter (IQR)b |
|||
|---|---|---|---|---|
| FEP (n = 11) | CAZ (n = 11) | PIP (n = 89) | MEM (n = 145) | |
| Serum concn at CT (mg/liter) | 12.7 (10.0–18.5) | 24.9 (12.8–34.1) | 35.2 (23.2–56.2) | 9.1 (6.9–12.9) |
| Serum concn at T0 (mg/liter) | 6.9 (5.0–10.9) | 16.0 (5.7–20.7) | 7.0 (2.8–22.0) | 3.3 (2.0–3.2) |
| Serum concn at T2 (mg/liter) | 40.7 (35.4–48.3) | 42.0 (31.8–59.5) | 65.0 (45.5–83.7) | 12.0 (8.6–17.5) |
| %T >4× MIC | 34.5 (28.1–42.7) | 53.4 (34.8–73.8) | 34.2 (21.5–44.5) | 46.9 (33.0–60.7) |
| mCLCR (ml/min) | 155 (140–199) | 173 (155–226) | 186 (149–249) | 179 (146–231) |
| ke (h−1) | 0.3 (0.2–0.4) | 0.2 (0.2–0.3) | 0.5 (0.3–0.7) | 0.3 (0.2–0.3) |
| Half-life (h) | 2.5 (2.0–3.0) | 3.3 (2.2–4.2) | 2.2 (1.0–1.4) | 2.7 (2.2–3.4) |
CT, serum concentration at optimal period of time (i.e., at 40%, 50%, or 70% of the dosage interval of MEM, TZP, or FEP/CAZ, respectively); T0, trough; T2, 2 h after onset of the β-lactam infusion; %T >4× MIC, percentage of time during which the antibiotic remains above 4× MIC; mCLCR, measured creatinine clearance; ke, elimination constant.
FEP, cefepime; CAZ, ceftazidime; PIP, piperacillin; MEM, meropenem; n, number of TDMs.
FIG 2.
Logarithmic correlations between mCLCR and different parameters. (A) Serum concentrations of PIP at 50% of the dosage interval and mCLCR. (B) Serum trough concentrations of PIP at T0 and mCLCR (ml/min). (C) Total body clearance (CL) of PIP and mCLCR. (D) Serum trough concentrations of MEM at T0 and mCLCR. PIP, piperacillin; mCLCR, measured creatinine clearance; MEM, meropenem.
Population PK analysis.
Each TDM contributed to the analysis. The best models were one-compartment linear models with zero-order input. Other models could not be supported. Between-subject variability was tested during model building for both total body clearance (CL) and volume of distribution (V). The final −2× log likelihood (−2LL) values for the base models for FEP, CAZ, PIP, and MEM were 168.5, 126.6, 1,274, and 1,064, respectively. Population PK parameter estimates are provided in Table 5. There was significant between-subject variability of V and CL for all drugs. The values of between-subject variability with mCLCR (normalized to 130 ml/min) for FEP, CAZ, PIP, and MEM were 24.7%, −1.1%, 5.7%, and −13.8%, respectively (negative values indicate increased variability present). mCLCR improved the models for FEP, CAZ, and MEM and could be retained in the final model. The goodness-of-fit plots for the final models are displayed in Fig. S1 in the supplemental material with r2 correlations between observed and posterior predicted concentrations of the final models of 0.963 (mCLCR covariate supported), 0.994 (mCLCR covariate supported), 0.994 (mCLCR covariate not supported), and 0.996 (mCLCR covariate supported), respectively, as well as little systematic bias evident for any drugs. All other visual predictive checks (VPC) were acceptable and confirmed the goodness of fit of the final model. We found that mCLCR could adequately explain changes in drug CL or concentrations for FEP, CAZ, and MEM but not for PIP.
TABLE 5.
Population PK parameter estimates of the different β-lactam antibiotics from the one-compartment model
| Drug (n)a |
Vb |
CLc |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean (liters) | CV (%) | Variance (liters) | Median (liters) | Mean (liters/h) | CV (%) | Variance (liters/h) | Median (liters/h) | |
| FEP (11) | 47.8 | 15.7 | 56.5 | 50.0 | 18.8 | 106 | 401.2 | 10.1 |
| CAZ (11) | 63.4 | 44.4 | 790.9 | 47.1 | 13.1 | 68.9 | 81.1 | 10.0 |
| PIP (89) | 56.3 | 30.4 | 293.2 | 49.6 | 26.8 | 52.4 | 197.1 | 25.9 |
| MEM (145) | 77.2 | 41.4 | 1,020.3 | 76.0 | 15.0 | 56.7 | 72.2 | 13.5 |
FEP, cefepime; CAZ, ceftazidime; PIP, piperacillin; MEM, meropenem; n, number of TDMs.
V, volume of distribution in the central compartment; CV, coefficient of variation.
CL, total body clearance.
DISCUSSION
In this study, we found that β-lactam serum concentrations were insufficient to treat infections due to Pseudomonas aeruginosa in the majority of septic patients with ARC. This was particularly significant for the patients with very high mCLCR (>180 ml/min) values. Linear and/or logarithmic correlations between mCLCR and drug concentrations and between mCLCR and CL of β-lactams were weak or absent. Specific population PK modeling could predict concentrations of FEP, CAZ, and MEM (but not of PIP) based on renal function but not based on absolute values of mCLCR. Therefore, a simple upward dose adjustment of these β-lactam antibiotics cannot be proposed on the basis of only mCLCR. These results highlight the complexity of drug PKs in septic patients.
The majority of TDMs were performed in patients with an mCLCR between 120 and 180 ml/min. In agreement with other studies on ARC, patients were young (6, 8, 22, 23) and predominantly male (8), with low plasma creatinine concentrations and relatively low severity-of-illness scores (6, 8, 15).
There is currently no consensus concerning the optimal PD targets for β-lactams in infected, critically ill patients. Indeed, few papers on the PKs of β-lactams in critically ill patients report MIC data or clinical outcome (24). Therefore, the clinical efficacy of different PD targets has not been robustly assessed. Nevertheless, we aimed for a PD target of >4× the MIC of the pathogen at the end of the optimal period of time (CT), corresponding to 70% of the dose interval for FEP/CAZ, 50% for PIP, and 40% for MEM, based on results from in vitro (25–27), animal in vivo (28), and clinical studies. Indeed, microbiological success has been predicted in patients with Gram-negative infections when FEP concentrations were maintained at 4× to 6× the MIC of the infecting pathogen (19). Another study has shown that unbound MEM trough serum concentrations at 5× the MIC of the infecting pathogen for patients with lower respiratory infections was predictive of microbiological and clinical success (12).
Antimicrobial treatment of sepsis is often initiated empirically when pathogens and MICs are unknown. We therefore aimed to treat infections due to Pseudomonas aeruginosa, a pathogen frequently responsible for infections in the ICU setting and associated with high mortality rates (29). However, we found that the most frequently documented pathogens in our study were Enterobacteriaceae spp. Although adequacy of drug concentrations was better in the treatment of infections due to Enterobacteriaceae spp. instead of those due to Pseudomonas aeruginosa, one out of three patients still had insufficient β-lactam serum concentrations to attain PK/PD targets.
We report, for the first time, a significantly greater proportion of insufficient concentrations of FEP, CAZ PIP, than of MEM for treatment of infections due to Pseudomonas spp. in patients with ARC. Indeed, in a prospective PK study, Carlier et al. reported that in 60 critically ill patients with apparently normal renal function (43 patients received TZP, and only 17 received MEM), no significant differences in pharmacodynamic target attainment were observed between antibiotics (15). In another study, De Waele et al. prospectively randomized critically ill patients with apparent normal kidney function to receive TDM-guided or standard therapy (MEM or TZP); baseline PK/PD target attainment for the entire cohort was similar for TZP and MEM (20/28, or 71.4%, and 6/13, or 46.2%, respectively; P = 0.12) (30). Huttner et al. also performed a prospective observational PK study in 100 critically ill patients with CLCRs of >60 ml/min who received MEM, TZP, FEP, or imipenem/cilastatin. Subtherapeutic concentrations of MEM and PIP were observed in 90% and 61% of trough samples, respectively, but ARC was present in 9/11 patients who received MEM and in only 21/33 patients who received TZP (11). In all three of these studies, fewer patients received MEM than TZP, not all patients had ARC, and CLCR was estimated using the Cockcroft-Gault equation, yielding results that often differ significantly from measured values (5, 9). Whether our findings are due to drug characteristics or to the choice in target concentrations and whether this would translate into a more “effective” (i.e., less risk of underdosing) therapy with MEM than with TZP in critically ill patients need to be further evaluated in other studies.
In view of the large number of patients identified in this study with insufficient serum concentrations to attain PK/PD targets, optimization of treatment must be considered. Because β-lactam antibiotics are time dependent and because the majority have a short half-life, prolonged or continuous infusions of these drugs may theoretically improve PK/PD target attainment in cases of ARC by ensuring that serum antibiotic concentrations remain above the MIC of the infecting pathogen during a longer period of time (1). However, even when prolonged infusions of standard total daily doses of β-lactam antibiotics are administered in these circumstances, PK/PD attainment may still remain suboptimal (15). Hence, an increased dosage may be necessary.
Unfortunately, we are not yet able to provide a potential drug regimen adjustment based only on mCLCR. Indeed, only weak correlations were observed between mCLCR and proportions of insufficient β-lactams, between mCLCR and PIP serum concentrations, between mCLCR and CL of PIP, and between mCLCR and T0 of MEM. For FEP and CAZ, no correlations were found between increasing values of ARC, proportion of insufficient drug levels, drug levels at different time points, or CL. Finally, for all β-lactams studied, no correlations were found between mCLCR and the half-life of these antibiotics. Despite these observations, specific PK modeling could predict drug concentrations of CAZ, FEP, and MEM but not of PIP based on renal function but not based on the absolute mCLCR value, suggesting several points. First, no simple upward dose adjustment can be proposed based only on mCLCR, a measure that is easily available to clinicians. Second, at extreme values of mCLCR (e.g., >300 ml/min), there may no longer be a linear relationship between drug concentrations and mCLCR. Third, mCLCR may not reflect precisely the true renal elimination of hydrophilic drugs such as β-lactams. The measure of CLCR with 24-h urine collections informs us on the glomerular filtration rate of the kidney, but it does not account for tubular reabsorption or secretion of drugs, which may be altered in the critically ill patient (1). This may explain why even with PK modeling, mCLCR could not be retained as a covariable to predict serum concentrations of PIP. Indeed, PIP has a well-described nonlinear renal clearance with a strong element of tubular secretion (31). Fourth, the measured total drug concentration of β-lactams may not allow for a good evaluation of the renal drug CL as it is the free fraction of the drug that is eliminated by the kidney (19). Fifth, other confounding factors may affect the PK of β-lactams in critically ill patients with ARC, such as changes in V, or nonrenal elimination of the drug (19), such as biliary clearance mechanisms, particularly in the case of PIP (32). Indeed, significant between-individual variability in values of V of all four antibiotics was observed in our cohort of patients.
This study has some limitations. First, this is a retrospective study performed in a single hospital. However, the data were systematically collected, and the monocentric nature of the study may increase some homogeneity in patient management. Second, the impact of antibiotic concentrations on outcome and the adequacy of increased dosage regimens after TDM were not evaluated. Third, the free fraction of β-lactams was not measured because the measurements were not routinely available in daily clinical practice. Fourth, the possible effect of significant variations in mCLCR was not accounted for. However, in a retrospective study on 56 septic patients, changes in mCLCR did not predict β-lactam serum concentration variations (17). Fifth, the sampling regimen was defined for TDM-based dose optimization rather than being designed for PK analysis, so the results may not be optimal for estimating PK parameters although we found that each of the drug's models performed adequately. Finally, we did not propose any new dosage regimens.
Unfortunately, our results indicate that no simple upward dose adjustment can be proposed on the basis of mCLCR alone. Even if new dosage regimens can be obtained from dose simulations based on population PK models for FEP, CAZ, and MEM, they will still need to be validated in the clinical setting. In the meantime, three case reports illustrate the potential benefits of TDM-guided therapy in three septic patients with ARC where daily doses of 8 to 12 g per day of MEM were needed to cure these patients of their infections (33, 34). De Waele et al. also showed in a randomized controlled trial in critically ill patients with apparently normal renal function that TDM-guided therapy of MEM and TZP allowed for better PK/PD target attainment than non-TDM-guided therapy. Furthermore, standard dosage regimens had to be increased by up to 100% to allow patients to attain the optimal PK/PD targets (30). Without performing TDM, these extremely high dosage regimens could not be administered. Therefore, currently we recommend, when possible, TDM-guided therapy to optimize PK/PD target attainment in critically ill patients and particularly in those at risk of ARC.
MATERIALS AND METHODS
Study design.
In our 35-bed Department of Intensive Care at Erasme Hospital (Brussels, Belgium), daily 24-h urine collections via an indwelling catheter and daily determination of serum creatinine concentrations are performed routinely, allowing for mCLCR to be calculated on a daily basis, according to the following formula: mCLCR = (urinary creatinine × urine volume)/(serum creatinine × duration of urine collection), where serum and urinary creatinine concentrations are measured in milligrams/deciliter, urine volume is measured in milliliters, and duration of collection is in minutes. The mCLCR of the day corresponds to a urine collection that was initiated at 8 a.m. on the previous day and collected for 24 h. The serum and urinary creatinine concentration values used to calculate mCLCR are those measured at the end of the urine collection. Therapeutic drug monitoring (TDM) of broad-spectrum β-lactam antibiotics (FEP, CAZ, PIP, and MEM) has also been performed routinely in septic patients since October 2009.
We reviewed data from all adult patients included in an institutional database of β-lactam TDMs from October 2009 to December 2014. This database contains the following information: the date and time TDM was performed, the drug regimen administered, and the β-lactam serum concentrations obtained. Study inclusion criteria were the following: (i) diagnosis of sepsis or septic shock according to standard criteria (35), (ii) therapy with a standard dosage regimen of a broad-spectrum β-lactam (FEP/CAZ, 2 g every 8 h [q8h]; TZP, 4.5 g q6h; or MEM, 1 g q8h), and (iii) mCLCR of≥120 ml/min (so that all patients close to the threshold of ARC could be included in the study) on the day of TDM. Incomplete TDMs (missing measured drug concentrations at T0 or T2) were excluded, as were TDMs performed on patients with burns or cystic fibrosis and those who were treated with extracorporeal membrane oxygenation. Patients could be included more than once. The study protocol was approved by the local Ethics Committee, who waived the need for informed consent in view of the retrospective nature of the study.
Data collection.
We recorded demographic data, comorbidities, biological data, primary reason for ICU admission, source of infection, pathogens responsible for the infection(s), use of vasopressors, use of mechanical ventilation, number of days of β-lactam therapy, the day of TDM, and the duration of ICU stay and the 30-day ICU mortality. Severity of disease was determined with the APACHE II score (20) at ICU admission and with the SOFA score (21) on the day of TDM.
Measurement of β-lactam serum concentrations.
β-Lactam concentrations were measured on two blood samples (3 ml each), one taken right before (T0) and the other one 2 h after (T2) the onset of a 30-min infusion. Samples were kept on ice and sent directly to the clinical chemistry laboratory; after centrifugation at 3,000 rpm at 4°C for 10 min, the supernatant was removed and analyzed. The serum concentrations of the four β-lactams were determined using high-performance liquid chromatography connected to UV spectrophotometry (HPLC-UV). Technical details have been described previously (36). For patients receiving TZP, only PIP concentrations were measured. The lower and upper limits of quantification for each analyzed β-lactam were 1 and 200 mg/liter, respectively. The coefficient of variation for all four β-lactam antibiotics was ≤7.6% for mean concentrations varying from 1 to 200 mg/liter. If a measured serum concentration was below the limits of quantification, the concentration used for PK analysis was 0.5 mg/liter.
PK analyses.
The following equation was used to estimate the serum concentrations of the drug at one given time: ln Ct = −ket + ln C0, assuming that the steady state was reached, considering the exponential elimination of drugs, and that sampling (T0 and T2) was performed during the elimination phase. Ct is the measured serum concentration at the specified time t, C0 is the virtual serum concentration at the beginning of the elimination phase, and ke is the elimination constant. The half-life (t1/2) could be calculated using the following equation: t1/2 = 0.693/ke.
The concentration-time data for FEP, CAZ, MEM, and PIP were also subject to a population pharmacokinetic analysis with the nonparametric adaptive grid (NPAG) algorithm within the freely available Pmetrics software package for R (Laboratory of Applied Pharmacokinetics and Bioinformatics, Los Angeles, CA) (37). mCLCR was evaluated as a clinically relevant and physiologically plausible covariate. Covariate selection was performed using a stepwise linear regression from R and Bayesian posterior parameters. mCLCR was entered into the model and statistically tested by use of the −2LL values. If inclusion of mCLCR resulted in a statistically significant improvement in the −2LL values (P < 0.05) and/or improved the goodness-of-fit plots, then it was retained in the final model.
The goodness of fit of the model was assessed by linear regression, with an observed-predicted (both population- and individual-predicted concentrations) plot, coefficients of determination, and −2LL values. Predictive performance was based on mean prediction error (bias) and the mean bias-adjusted squared prediction error (imprecision) of the population and individual prediction models. The internal validity of the population pharmacokinetic model was assessed by the bootstrap resampling method (n = 1,000) and normalized prediction distribution errors (NPDEs) (38). Using the VPC method, parameters obtained from the distribution of predicted concentrations were plotted with the observed concentrations. NPDE plots were checked for normal distribution characteristics and trends in the data errors.
Definitions for adequacy of β-lactam serum concentrations.
All TDMs were stratified into four groups according to mCLCR ranges: 120 to ≤180 ml/min, 181 to ≤240 ml/min, 241 to ≤300 ml/min, and >300 ml/min. Because β-lactam antibiotics are time dependent, the PD index that best describes their efficacy is the time the unbound concentration of the antibiotic remains above the MIC of the infecting pathogen (fT>MIC). In vitro studies have shown that bactericidal effects are obtained when antibiotic concentrations remain above the MIC of the pathogen during 70%, 50%, and 40% of the dosing interval for cephalosporins, penicillins, and carbapenems, respectively (25, 26). Furthermore, maximal killing rates for β-lactam antibiotics are observed at 4× to 6× the MIC of pathogens (27). Therefore, for each TDM, the drug concentration at the end of the optimal period of time (CT) was calculated, corresponding to 70% of the dose interval for FEP/CAZ, 50% for PIP, and 40% for MEM. A CT of ≤4× MIC was defined as an insufficient serum concentration, and a CT of >4× MIC was defined as adequate. We used clinical breakpoints for Pseudomonas aeruginosa, as defined by the EUCAST as our target MICs: 8 mg/liter for FEP/CAZ, 16 mg/liter for PIP, and 2 mg/liter for MEM (39). Adequate CTs for treating infections due to Pseudomonas aeruginosa were therefore >32 mg/liter for FEP/CAZ, >64 mg/liter for PIP, and >8 mg/liter for MEM.
As a post hoc analysis, we also analyzed adequacy of serum concentrations to treat infections due to Enterobacteriaceae spp. because these were the most frequently documented pathogens in our study. We once again used clinical breakpoints for Enterobacteriaceae spp., as defined by EUCAST: 1 mg/liter for FEP/CAZ, 8 mg/liter for PIP, and 2 mg/liter for MEM (39). Adequate CTs for treating infections due to Enterobacteriaceae spp. were therefore >4 mg/liter for FEP/CAZ, >32 mg/liter for PIP, and >8 mg/liter for MEM.
Statistical analysis.
Statistical analyses were performed using the software package SPSS, version 24.0, for Windows NT (SPSS, Inc., Chicago, IL, USA). Descriptive statistics were computed for all study variables; discrete variables were expressed as counts (percentage), and continuous variables were expressed as means ± standard deviations or medians (interquartile range [IQR]). Categorical data were compared using a chi-square test or Fisher's exact test, as appropriate, and continuous variables were compared using a Mann-Whitney U test. We looked for a relationship between mCLCR and drug concentrations at different time points (T0, T2, and the predefined optimal periods of time for each β-lactam), between mCLCR and T >4× MIC, between mCLCR and CL, and between mCLCR and half-life for all drugs. The Spearman's correlation coefficient (r) was used to determine linear correlation as appropriate. Association between variables was tested by simple regression analysis and the coefficient of determination (R2) in case of nonlinear correlation. All tests were two-tailed, and a P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
J.A.R. acknowledges funding from the Australian National Health and Medical Research Council for a Centre of Research Excellence (APP1099452) and Practitioner Fellowship (APP1117065). No funding was received to carry out this study.
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02534-17.
REFERENCES
- 1.Sime FB, Udy AA, Roberts AA. 2015. Augmented renal clearance in critically ill patients: etiology, definition, and implications for beta-lactam dose optimization. Curr Opin Pharmacol 24:1–6. doi: 10.1016/j.coph.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 2.De Waele JJ, Dumoulin A, Janssen A, Hoste EA. 2015. Epidemiology of augmented renal clearance in mixed ICU patients. Minerva Anestesiol 81:1079–1085. [PubMed] [Google Scholar]
- 3.Udy AA, Baptista JP, Lim NL, Joynt GM, Jarrett P, Wockner L, Boots RJ, Lipman J. 2014. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit Care Med 42:520–527. doi: 10.1097/CCM.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 4.Adnan S, Ratnam S, Kumar S, Paterson D, Lipman J, Roberta J, Udy AA. 2014. Select critically ill patients at risk of augmented renal clearance: experience in a Malaysian intensive care unit. Anaesth Intensive Care 42:715–722. [DOI] [PubMed] [Google Scholar]
- 5.Baptista JP, Neves M, Rodrigues L, Teixeira L, Pinho J, Pimentel J. 2014. Accuracy of the estimation of glomerular filtration rate within a population of critically ill patients. J Nephrol 27:403–410. doi: 10.1007/s40620-013-0036-x. [DOI] [PubMed] [Google Scholar]
- 6.Udy AA, Roberts JA, Shorr AF, Boots RJ, Lipman J. 2013. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: identifying at-risk patients. Crit Care 17:R35. doi: 10.1186/cc12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May CC, Arora S, Parli SE, Fraser JF, Bastin MT, Cook AM. 2015. Augmented renal clearance in patients with subarachnoid hemorrhage. Neurocrit Care 23:374–379. doi: 10.1007/s12028-015-0127-8. [DOI] [PubMed] [Google Scholar]
- 8.Claus BO, Hoste EA, Colpaert K, Robays H, Decruyenaere J, De Waele JJ. 2013. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J Crit Care 28:695–700. doi: 10.1016/j.jcrc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Udy AA, Morton FJ, Nguyen-Pham S, Jarrett P, Lassig-Smith M, Stuart J, Dunlop R, Starr T, Boots RJ, Lipman J. 2013. A comparison of CKD-EPI estimated glomerular filtration rate and measured creatinine clearance in recently admitted critically ill patients with normal plasma creatinine concentrations. BMC Nephrol 14:250. doi: 10.1186/1471-2369-14-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grootaert V, Willems L, Debveye Y, Meyfroidt G, Spriet I. 2012. Augmented renal clearance in the critically ill: how to assess kidney function. Ann Pharmacother 46:952–959. doi: 10.1345/aph.1Q708. [DOI] [PubMed] [Google Scholar]
- 11.Huttner A, Von Dach E, Renzoni H, Huttner BD, Affaticati M, Pagani L, Daali Y, Pugin J, Karmime A, Fathi M, Lew D, Harbarth S. 2015. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int J Antimicrob Agents 45:385–392. doi: 10.1016/j.ijantimicag.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Du X, Kuti JL, Nicolau DP. 2007. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother 51:1725–1730. doi: 10.1128/AAC.00294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Koukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J. 2014. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 15.Carlier M, Carrette S, Roberts JA, Stove V, Verstraete A, Hoste E, Depuydt P, Decruyenaere J, Lipman J, Wallis SC, De Waele JJ. 2013. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: does augmented renal clearance affect pharmacokinetic/pharmacodyamic target attainment when extended infusions are used? Crit Care 17:R84. doi: 10.1186/cc12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Udy AA, Lipman J, Jarrett P, Klein K, Wallis SC, Patel K, Kirkpatrick CM, Kruger PS, Paterson DL, Roberts MS, Roberts JA. 2015. Are standard doses of piperacillin sufficient for critically ill patients with augmented renal clearance? Crit Care 19:28. doi: 10.1186/s13054-015-0750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casu GS, Hites M, Jacobs F, Cotton F, Wolff F, Beumier M, De Backer D, Vincent JL, Taccone FS. 2013. Can changes in renal function predict variations in β-lactam concentrations in septic patients? Int J Antimicrob Agents 42:422–428. doi: 10.1016/j.ijantimicag.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 19.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL. 2014. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knaus W, Draper E, Wagner DP, Zimmerman JE. 1985. Apache II: a severity disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Vincent JL, Moreno R, Takala J, Wilatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. 1996. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710. [DOI] [PubMed] [Google Scholar]
- 22.Campassi ML, Gonzalez MC, Masevicius FD, Vazquez AR, Moseinco M, Navarro NC, Previgliano L, Rubatto NP, Benites MH, Estenssoro E, Dubin A. 2014. Augmented renal clearance in critically ill patients: incidence, associated factors, and effects on vancomycin treatment. Rev Brasil Terapia Intensiva 26:13–20. (In Portuguese.) doi: 10.5935/0103-507X.20140003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. 2010. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet 49:1–16. doi: 10.2165/11318140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Delattre IK, Taccone FS, Jacobs F, Hites M, Dugernier T, Spapen H, Laterre PF, Wallemacq PE, Van Bambeke F, Tulkens P. 2017. Optimizing β-lactams treatment in critically ill patients using pharmacokinetics/pharmacodynamics targets: are first conventional doses effective? Expert Rev Anti Infect Ther 15:677–688. doi: 10.1080/14787210.2017.1338139. [DOI] [PubMed] [Google Scholar]
- 25.Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 17:479–501. doi: 10.1016/S0891-5520(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 26.Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of “bug and drug.” Nat Rev Microbiol 2:289–300. [DOI] [PubMed] [Google Scholar]
- 27.Craig WA, Ebert SC. 1990. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis Suppl 74:63–70. [PubMed] [Google Scholar]
- 28.Tam VH, McKinnon PS, Akins RL, Rybak MJ, Drusano GL. 2002. Pharmacodynamics of cefepime in patients with Gram-negative infections. J Antimicrobial Chemother 50:425–428. doi: 10.1093/jac/dkf130. [DOI] [PubMed] [Google Scholar]
- 29.Shorr AF. 2009. Review of studies of the impact on Gram-negative bacterial resistance on outcomes in the intensive care unit. Crit Care Med 37:1463–1469. doi: 10.1097/CCM.0b013e31819ced02. [DOI] [PubMed] [Google Scholar]
- 30.De Waele JJ, Carrette S, Carlier M, Stove V, Boelens J, Claeys G, Leroux-Roels I, Hoste E, Depuydt P, Decruyenaere J, Verstraete AG. 2014. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: a randomised controlled trial. Intensive Care Med 40:380–387. doi: 10.1007/s00134-013-3187-2. [DOI] [PubMed] [Google Scholar]
- 31.Felton TW, Hope WW, Lomaestro BM, Butterfield JM, Kwa AL, Drusano GL, Lodise TP. 2012. Population pharmacokinetics of extended-infusion piperacillin-tazobactam in hospitalized patients with nosocomial infections. Antimicrob Agents Chemother 56:4087–4094. doi: 10.1128/AAC.00521-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westphal JF, Brogard JM, Caro-Sampara F, Adloff M, Blicklé JF, Monteil H, Jehl F. 1997. Assessment of biliary tract excretion of piperacillin-tazobactam in humans. Antimicrob Agents Chemother 41:1636–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taccone FS, Cotton F, Roisin S, Vinvent JL, Jacobs F. 2012. Optimal Meropenem concentrations to treat multi-drug resistant Pseudomonas aeruginosa septic shock. Antimicrob Agents Chemother 56:2129–2131. doi: 10.1128/AAC.06389-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tröger U, Drust A, Martens-Lobenhoffer J, Taney I, Braun-Dullaeus RC, Bode-Böger SM. 2012. Decreased meropenem levels in Intensive Care Unit patients with augmented renal clearance: benefit of therapeutic drug monitoring. Int J Antimicrob Agents 40:370–372. doi: 10.1016/j.ijantimicag.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolff F, Deprez G, Seyler L, Taccone F, Hites M, Gulbis B, Vincent JL, Jacobs F, Cotton F. 2013. Rapid quantification of six β-lactams to optimize dosage regimens in severely septic patients. Talanta 103:153–160. doi: 10.1016/j.talanta.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modelling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Comets E, Brendel K, Mentre F. 2008. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed 90:154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 39.European Committee on Antimicrobial Susceptibility Testing. 2017. Breakpoint tables for interpretation of MICs and zone diameters, version 7.1. European Committee on Antimicrobial Susceptibility Testing, Basel, Switzerland: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.