We tested the in vitro susceptibility of ceftazidime-avibactam and ceftolozane-tazobactam and 13 other antibiotics against 91 Burkholderia cepacia complex (BCC) strains isolated from cystic fibrosis patients since 2012. The highest susceptibility (82%) was found for trimethoprim-sulfamethoxazole.
KEYWORDS: Burkholderia cepacia complex, cystic fibrosis, ceftazidime-avibactam, ceftolozane-tazobactam, in vitro susceptibility
ABSTRACT
We tested the in vitro susceptibility of ceftazidime-avibactam and ceftolozane-tazobactam and 13 other antibiotics against 91 Burkholderia cepacia complex (BCC) strains isolated from cystic fibrosis patients since 2012. The highest susceptibility (82%) was found for trimethoprim-sulfamethoxazole. Eighty-one and 63% of all BCC strains were susceptible to ceftazidime-avibactam and ceftolozane-tazobactam, respectively. For temocillin, ceftazidime, piperacillin-tazobactam, and meropenem, at least 50% of the strains were susceptible. B. stabilis seems to be more resistant than other BCC species.
TEXT
The Burkholderia cepacia complex (BCC) is a group of Gram-negative bacteria that comprise at least 20 closely related opportunistic pathogens (1). Chronic infection with BCC is associated with severe morbidity and mortality in cystic fibrosis (CF) patients (2–4). Treatment of BCC infections requires extensive antibiotic therapy but is hampered by intrinsic resistance to common antibiotics (5–9) and in vivo biofilm formation (10). Currently susceptibility testing is impeded by the shortage of evidence of a relationship between the in vitro susceptibility of antimicrobials and clinical outcome. However, a recent Cochrane Systematic Review concluded that knowledge of in vitro susceptibility can guide clinicians in treating BCC infections (11). Therefore, there is still a need to explore the value of newer antimicrobials for their action against BCC.
Ceftolozane-tazobactam is a cephalosporin with a beta-lactamase inhibitor, and ceftazidime-avibactam is a combination of a cephalosporin and a non-beta-lactam inhibitor of beta-lactamases. Both combinations have the ability to inhibit class A, C, and some class D beta-lactamases (12, 13). These new antimicrobial combinations are approved for treating complicated intra-abdominal and urinary tract infections with multidrug-resistant Enterobacterales and Pseudomonas aeruginosa (14–18).
In this study, we tested the in vitro susceptibility of ceftazidime-avibactam and ceftolozane-tazobactam and 13 other antibiotics to 91 unduplicated BCC isolates from CF patients attending different Belgian hospitals from 2012 to 2016. For identification to species level, recA gene sequence analysis was performed using the method of Spilker et al. (19), with minor modifications (20). Among the 91 BCC isolates, B. multivorans was the most frequently isolated species (55%), followed by B. vietnamiensis (18%), B. cenocepacia (9%), and B. stabilis (9%). B. contaminans (4%), B. lata (3%), and B. cepacia (2%) occurred less frequently.
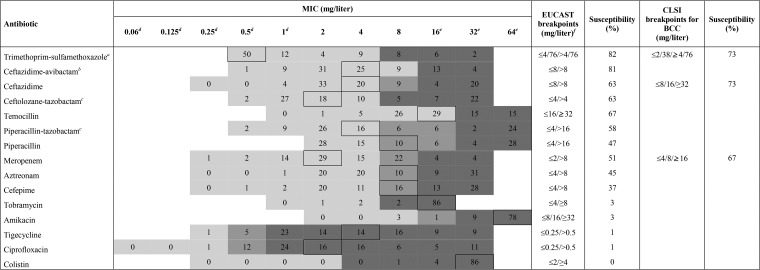
MICs were determined by microdilution in microtiter plates and read on a Sensititre Vizion System (Thermo Scientific). For determination of the in vitro susceptibility (Table 1), EUCAST pharmacokinetic/pharmacodynamics (PK/PD) breakpoints were used, as no EUCAST species-specific breakpoints were available. Breakpoints for aminoglycosides, colistin, and trimethoprim-sulfamethoxazole were based on those from EUCAST for nonfermenters (21). For temocillin, breakpoints described by Fuchs et al. were used (22). These in vitro susceptibilities were compared with those derived from CLSI breakpoints for BCC where available (Table 1).
TABLE 1.
MIC distribution and in vitro susceptibility of all BCC strains (n = 91) for the 15 tested antibioticsg
Tested breakpoint concentrations were 0.5/9.5, 1/19, 2/38, 4/76, 8/152, 16/304, and 32/608 mg/liter.
Avibactam was at a constant concentration of 4 mg/liter.
Tazobactam was at a constant concentration of 4 mg/liter.
Below or equal to the respective MIC.
Above or equal to the respective MIC.
Temocillin breakpoints from Fuchs et al. (22).
The MIC above the framed number corresponds to the MIC50.
As expected, little or no in vitro activity was noted for amikacin, tobramycin, and colistin (23). Ciprofloxacin and tigecycline also were scarcely active. Using the EUCAST breakpoint for nonfermenters (≤4 and 76 mg/liter, or ≤4/76 mg/liter), the highest in vitro susceptibility (82%) of BCC isolates was found for trimethoprim-sulfamethoxazole, which is in line with other studies (24, 25). However, the CLSI breakpoint for BCC is more stringent (≤2/38 mg/liter), resulting in an in vitro activity of 73%.
In general, MICs varied widely for beta-lactam antibiotics. This variation can be explained by the potential presence of several resistance mechanisms. It has been shown that BCC species contain class A beta-lactamases (like PenA and PenB) with broad-spectrum carbapenemase characteristics, class C beta-lactamases (like AmpC), and class D beta-lactamases. Non-beta-lactamase-mediated resistance mechanisms, like efflux pumps and reduced outer membrane permeability, also play a role in the decreased susceptibility of BCC species (9). Among these beta-lactam antibiotics, the newer antibiotic ceftazidime-avibactam showed the highest in vitro susceptibility (81%). Moreover, adding avibactam to ceftazidime increased its susceptibility by approximately 20%. In the literature, a highly variable ability of avibactam to potentiate ceftazidime activity is observed, suggesting this resistance is not due to beta-lactamase production alone (26, 27). Adding tazobactam to piperacillin only slightly improved (by about 10%) the susceptibility of BCC isolates (58% versus 47%), which was also demonstrated in other studies (28, 29). This limited increase in susceptibility may be because tazobactam does not affect AmpC beta-lactamases, whereas avibactam does. In Europe, temocillin is often used as an orphan drug for treating BCC infection (30). The high in vitro activity (67%) of temocillin against BCC, mainly due to its activity against ESBLs, was confirmed in our study (31–33). However, it remains important to consider that almost 50% of the susceptible strains have a MIC at the pharmacokinetic/pharmacodynamic (PK/PD) breakpoint. For cefepime and aztreonam, we found a susceptibility of about 40%, which is between the susceptibilities from other studies (29, 34). The in vitro susceptibility of meropenem was only 51% and 67%, using EUCAST PK/PD and CLSI BCC breakpoints, respectively, suggesting the presence of carbapenemase production.
Remarkable differences in in vitro susceptibility were noticed between BCC species (see Table S1 in the supplemental material). In contrast to other BCC species, all B. stabilis isolates (n = 8) were resistant to piperacillin-tazobactam, ceftolozane-tazobactam, aztreonam, and meropenem, which suggests the presence of broad-spectrum beta-lactamases. This finding is not described elsewhere, probably because other studies tested none or only a low proportion of B. stabilis strains (24, 25, 29, 34, 35). However, all 8 B. stabilis isolates represented the same sequence type, as determined by multilocus sequence typing (19 and data not shown). We found, similar to Lupo et al. (34), a higher susceptibility to piperacillin-tazobactam, ceftazidime, cefepime, aztreonam, and meropenem for B. multivorans than for B. cenocepacia. Likewise, B. cenocepacia had the highest in vitro susceptibility for ceftazidime-avibactam (88%), ceftolozane-tazobactam (75%), and temocillin (88%), suggesting a higher proportion of beta-lactamase-producing strains in B. cenocepacia isolates. B. cenocepacia isolates are known to contain PenA and PenB, both with broad-spectrum carbapenemase characteristics, and AmpC beta-lactamases, whereas B. multivorans contains PenA beta-lactamases (5, 36).
Three out of the 91 isolates were multidrug resistant. Twenty-nine (32%) isolates were only susceptible to at least 1 of the 4 antibiotics with highest in vitro susceptibility: trimethoprim-SXT, ceftazidime-avibactam, temocillin, and ceftolozane-tazobactam. Among the 11 strains resistant to trimethoprim-SXT, 4 strains were susceptible to temocillin, and importantly, 3 strains were only susceptible to ceftolozane-tazobactam and ceftazidime-avibactam and 3 strains were only susceptible to ceftazidime-avibactam.
For the treatment of BCC infections with strains resistant to the first-choice treatment, trimethoprim-sulfamethoxazole, there could be a role for the new antimicrobials ceftazidime-avibactam and ceftolozane-tazobactam, sparing the use of meropenem. Our results may help clinicians with the antibiotic treatment of their patients, taking into account previous clinical responses and their own experience. However, more susceptibility studies are required to reach valid specific breakpoints for BCC species, and clinical studies are needed to assess clinical outcomes.
Supplementary Material
ACKNOWLEDGMENTS
We thank the referring laboratories of the following hospitals: GasthuisZusters Antwerpen (GZA; Antwerp, Belgium), Universitair Ziekenhuis Antwerpen (UZA; Antwerp, Belgium), Centre Hospitalier Universitaire Brugmann (CHU Brugmann; Brussels, Belgium), Cliniques Universitaires Saint-Luc (Brussels, Belgium), Hôpital Erasme (Brussels, Belgium), Universitair Ziekenhuis Gent (UZ Gent; Ghent, Belgium), Universitair Ziekenhuis Leuven (UZ Leuven; Leuven, Belgium), Centre Hospitalier Chrétien Clinique Saint-Joseph (CHC Clinique Saint-Joseph; Liège, Belgium), Centre Hospitalier Universitaire de Liège (CHU Liège; Liège, Belgium), Centre Hospitalier Régional de la Citadelle (CHR de la Citadelle; Liège, Belgium), and Centre Hospitalier Universitaire Ambroise Paré (CHU Ambroise Paré; Mons, Belgium).
Part of this work was performed in the framework of the Belgian National Reference Centre for Burkholderia, supported by the Ministry of Social Affairs through a fund within the National Health Insurance System. This funding agency had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
D.P. was member of an advisory board organized by Pfizer PFE Belgium SPRL/BVBA on 3 July 2017. Other authors have no conflict of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00590-18.
REFERENCES
- 1.De Smet B, Mayo M, Peeters C, Zlosnik JE, Spilker T, Hird TJ, LiPuma JJ, Kidd TJ, Kaestli M, Ginther JL, Wagner DM, Keim P, Bell SC, Jacobs JA, Currie BJ, Vandamme P. 2015. Burkholderia stagnalis sp. nov. and Burkholderia territorii sp. nov., two novel Burkholderia cepacia complex species from environmental and human sources. Int J Syst Evol Microbiol 65:2265–2271. doi: 10.1099/ijs.0.000251. [DOI] [PubMed] [Google Scholar]
- 2.Isles A, Maclusky I, Corey M, Gold R, Prober C, Fleming P, Levison H. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr 104:206–210. doi: 10.1016/S0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 3.Tablan OC, Chorba TL, Schidlow DV, White JW, Hardy KA, Gilligan PH, Morgan WM, Carson LA, Martone WJ, Jason JM, Jarvis WR. 1985. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J Pediatr 107:382–387. doi: 10.1016/S0022-3476(85)80511-4. [DOI] [PubMed] [Google Scholar]
- 4.Murray S, Charbeneau J, Marshall BC, LiPuma JJ. 2008. Impact of burkholderia infection on lung transplantation in cystic fibrosis. Am J Respir Crit Care Med 178:363–371. doi: 10.1164/rccm.200712-1834OC. [DOI] [PubMed] [Google Scholar]
- 5.Poirel L, Rodriguez-Martinez JM, Plesiat P, Nordmann P. 2009. Naturally occurring class A β-lactamases from the Burkholderia cepacia complex. Antimicrob Agents Chemother 53:876–882. doi: 10.1128/AAC.00946-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hancock RE. 1998. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin Infect Dis 27(Suppl 1):S93–S99. doi: 10.1086/514909. [DOI] [PubMed] [Google Scholar]
- 7.Papp-Wallace KM, Taracila MA, Gatta JA, Ohuchi N, Bonomo RA, Nukaga M. 2013. Insights into beta-lactamases from Burkholderia species, two phylogenetically related yet distinct resistance determinants. J Biol Chem 288:19090–19102. doi: 10.1074/jbc.M113.458315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns JL, Wadsworth CD, Barry JJ, Goodall CP. 1996. Nucleotide sequence analysis of a gene from Burkholderia (Pseudomonas) cepacia encoding an outer membrane lipoprotein involved in multiple antibiotic resistance. Antimicrob Agents Chemother 40:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes KA, Schweizer HP. 2016. Antibiotic resistance in Burkholderia species. Drug Resist Updat 28:82–90. doi: 10.1016/j.drup.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sajjan US, Yang JH, Hershenson MB, LiPuma JJ. 2006. Intracellular trafficking and replication of Burkholderia cenocepacia in human cystic fibrosis airway epithelial cells. Cell Microbiol 8:1456–1466. doi: 10.1111/j.1462-5822.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 11.Horsley A, Jones AM, Lord R. 2016. Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database Syst Rev 2016:CD009529. doi: 10.1002/14651858.CD009529.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Durand-Reville TF, Lahiri S, Thresher J, Livchak S, Gao N, Palmer T, Walkup GK, Fisher SL. 2013. Kinetics of avibactam inhibition against class A, C, and D beta-lactamases. J Biol Chem 288:27960–27971. doi: 10.1074/jbc.M113.485979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhanel GG, Chung P, Adam H, Zelenitsky S, Denisuik A, Schweizer F, Lagace-Wiens PR, Rubinstein E, Gin AS, Walkty A, Hoban DJ, Lynch JP III, Karlowsky JA. 2014. Ceftolozane/tazobactam: a novel cephalosporin/beta-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs 74:31–51. doi: 10.1007/s40265-013-0168-2. [DOI] [PubMed] [Google Scholar]
- 14.Lagace-Wiens P, Walkty A, Karlowsky JA. 2014. Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of Gram-negative bacterial infections. Core Evid 9:13–25. doi: 10.2147/CE.S40698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C. 2013. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, phase II trial. J Antimicrob Chemother 68:1183–1192. doi: 10.1093/jac/dks523. [DOI] [PubMed] [Google Scholar]
- 16.Vazquez JA, Gonzalez Patzan LD, Stricklin D, Duttaroy DD, Kreidly Z, Lipka J, Sable C. 2012. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin 28:1921–1931. doi: 10.1185/03007995.2012.748653. [DOI] [PubMed] [Google Scholar]
- 17.Livermore DM, Mushtaq S, Meunier D, Hopkins KL, Hill R, Adkin R, Chaudhry A, Pike R, Staves P, Woodford N, BSAC Resistance Surveillance Standing Committee. 2017. Activity of ceftolozane/tazobactam against surveillance and “problem” Enterobacteriaceae, Pseudomonas aeruginosa and non-fermenters from the British Isles. J Antimicrob Chemother 72:2278–2289. doi: 10.1093/jac/dkx136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig WA, Andes DR. 2013. In vivo activities of ceftolozane, a new cephalosporin, with and without tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae, including strains with extended-spectrum beta-lactamases, in the thighs of neutropenic mice. Antimicrob Agents Chemother 57:1577–1582. doi: 10.1128/AAC.01590-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spilker T, Baldwin A, Bumford A, Dowson CG, Mahenthiralingam E, LiPuma JJ. 2009. Expanded multilocus sequence typing for burkholderia species. J Clin Microbiol 47:2607–2610. doi: 10.1128/JCM.00770-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peeters C, Zlosnik JE, Spilker T, Hird TJ, LiPuma JJ, Vandamme P. 2013. Burkholderia pseudomultivorans sp. nov., a novel Burkholderia cepacia complex species from human respiratory samples and the rhizosphere. Syst Appl Microbiol 36:483–489. doi: 10.1016/j.syapm.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 21.European Committee on Antimicrobial Susceptibility Testing. 2018. Breakpoint tables for interpretation of MICs and zone diameters, version 8.0. http://www.eucast.org/clinical_breakpoints/.
- 22.Fuchs PC, Barry AL, Thornsberry C, Jones RN. 1985. Interpretive criteria for temocillin disk diffusion susceptibility testing. Eur J Clin Microbiol 4:30–33. doi: 10.1007/BF02148656. [DOI] [PubMed] [Google Scholar]
- 23.Jassem AN, Zlosnik JE, Henry DA, Hancock RE, Ernst RK, Speert DP. 2011. In vitro susceptibility of Burkholderia vietnamiensis to aminoglycosides. Antimicrob Agents Chemother 55:2256–2264. doi: 10.1128/AAC.01434-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fehlberg LC, Nicoletti AG, Ramos AC, Rodrigues-Costa F, de Matos AP, Girardello R, Marques EA, Gales AC. 2016. In vitro susceptibility of Burkholderia cepacia complex isolates: comparison of disk diffusion, Etest, agar dilution, and broth microdilution methods. Diagn Microbiol Infect Dis 86:422–427. doi: 10.1016/j.diagmicrobio.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Mazer DM, Young C, Kalikin LM, Spilker T, LiPuma JJ. 2017. In vitro activity of ceftolozane-tazobactam and other antimicrobial agents against Burkholderia cepacia complex and Burkholderia gladioli. Antimicrob Agents Chemother 61:e00766-17. doi: 10.1128/AAC.00766-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papp-Wallace KM, Becka SA, Zeiser ET, Ohuchi N, Mojica MF, Gatta JA, Falleni M, Tosi D, Borghi E, Winkler ML, Wilson BM, LiPuma JJ, Nukaga M, Bonomo RA. 2017. Overcoming an extremely drug resistant (XDR) pathogen: avibactam restores susceptibility to ceftazidime for Burkholderia cepacia complex isolates from cystic fibrosis patients. ACS Infect Dis 3:502–511. doi: 10.1021/acsinfecdis.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mushtaq S, Warner M, Livermore DM. 2010. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother 65:2376–2381. doi: 10.1093/jac/dkq306. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Chen Y, Tabibi S, Alba L, Garber E, Saiman L. 2007. Antimicrobial susceptibility and synergy studies of Burkholderia cepacia complex isolated from patients with cystic fibrosis. Antimicrob Agents Chemother 51:1085–1088. doi: 10.1128/AAC.00954-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbott FK, Milne KE, Stead DA, Gould IM. 2016. Combination antimicrobial susceptibility testing of Burkholderia cepacia complex: significance of species. Int J Antimicrob Agents 48:521–527. doi: 10.1016/j.ijantimicag.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Committee for Orphan Medicinal Products. 26 June 2014. Public summary of positive opinion for orphan designation of temocillin sodium. European Medicines Agency, London, United Kingdom: www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2009/10/WC500005689.pdf Accessed 16 May 2018. [Google Scholar]
- 31.Van Acker H, Van Snick E, Nelis HJ, Coenye T. 2010. In vitro activity of temocillin against planktonic and sessile Burkholderia cepacia complex bacteria. J Cyst Fibros 9:450–454. doi: 10.1016/j.jcf.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Lekkas A, Gyi KM, Hodson ME. 2006. Temocillin in the treatment of Burkholderia cepacia infection in cystic fibrosis. J Cyst Fibros 5:121–124. doi: 10.1016/j.jcf.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez M, Nelson M, Kelly JE, Elward A, Morley SC. 2014. Successful use of temocillin as salvage therapy for cervical osteomyelitis secondary to multidrug-resistant Burkholderia cepacia. J Pediatric Infect Dis Soc 3:77–80. doi: 10.1093/jpids/pis110. [DOI] [PubMed] [Google Scholar]
- 34.Lupo A, Isis E, Tinguely R, Endimiani A. 2015. Clonality and antimicrobial susceptibility of Burkholderia cepacia complex isolates collected from cystic fibrosis patients during 1998-2013 in Bern, Switzerland. New Microbiol 38:281–288. [PubMed] [Google Scholar]
- 35.Everaert A, Coenye T. 2016. Effect of beta-lactamase inhibitors on in vitro activity of beta-lactam antibiotics against Burkholderia cepacia complex species. Antimicrob Resist Infect Control 5:44. doi: 10.1186/s13756-016-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang J, Kim HS. 2015. Cell wall recycling-linked coregulation of AmpC and PenB beta-lactamases through ampD mutations in Burkholderia cenocepacia. Antimicrob Agents Chemother 59:7602–7610. doi: 10.1128/AAC.01068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.