Sepsis is a life-threatening systemic inflammatory condition triggered as a result of an excessive host immune response to infection. In the past, immunomodulators have demonstrated a protective effect in sepsis.
KEYWORDS: polymicrobial sepsis, cytokines, survival, bacterial count
ABSTRACT
Sepsis is a life-threatening systemic inflammatory condition triggered as a result of an excessive host immune response to infection. In the past, immunomodulators have demonstrated a protective effect in sepsis. Azithromycin (a macrolide antibiotic) has immunomodulatory activity and was therefore evaluated in combination with ceftriaxone in a clinically relevant murine model of sepsis induced by cecal ligation and puncture (CLP). First, mice underwent CLP and 3 h later were administered the vehicle or a subprotective dose of ceftriaxone (100 mg/kg of body weight subcutaneously) alone or in combination with an immunomodulatory dose of azithromycin (100 mg/kg intraperitoneally). Survival was monitored for 5 days. In order to assess the immunomodulatory activity, parameters such as plasma and lung cytokine (interleukin-6 [IL-6], IL-1β, tumor necrosis factor alpha) concentrations, the plasma glutathione (GSH) concentration, plasma and lung myeloperoxidase (MPO) concentrations, body temperature, blood glucose concentration, and total white blood cell count, along with the bacterial load in blood, peritoneal lavage fluid, and lung homogenate, were measured 18 h after CLP challenge. Azithromycin in the presence of ceftriaxone significantly improved the survival of CLP-challenged mice. Further, the combination attenuated the elevated levels of inflammatory cytokines and MPO in plasma and lung tissue and increased the body temperature and blood glucose and GSH concentrations, which were otherwise markedly decreased in CLP-challenged mice. Ceftriaxone produced a significant reduction in the bacterial load, while coadministration of azithromycin did not produce a further reduction. Therefore, the survival benefit offered by azithromycin was due to immunomodulation and not its antibacterial action. The findings of this study indicate that azithromycin, in conjunction with appropriate antibacterial agents, could provide clinical benefits in sepsis.
INTRODUCTION
Sepsis is characterized by life-threatening organ dysfunction, in which the proinflammatory cytokines released by host immune cells in response to infection play a major role in pathogenesis (1, 2). In septic patients, there is an imbalance of proinflammatory/anti-inflammatory and oxidant/antioxidant mechanisms, resulting in the uncontrolled production of proinflammatory cytokines (e.g., interleukin-1β [IL-1β], IL-6, and tumor necrosis factor alpha [TNF-α]), oxidative enzymes (e.g., myeloperoxidase [MPO]), and free oxygen species that induce oxidative stress, inflammation, and direct mitochondrial damage, precipitating to organ dysfunction (3, 4, 5). Despite recent advances in our understanding of the pathophysiological mechanism of sepsis and improved antimicrobial therapy, the rate of mortality from sepsis remains frustratingly high (6). Unfortunately, many of the therapeutic options for the management of sepsis proposed over the years have either failed to meet their initial expectations or remained unproved. For instance, cytokine-targeted therapies failed in clinics primarily due to their action on a single inflammatory mediator (7). Therefore, current research employing animal models of sepsis focuses on strategies to minimize the hyperinflammatory response associated with sepsis through the use of agents that exhibit a broader immunomodulatory effect via multiple inflammatory pathways (8).
Macrolides are antibacterial agents, and several reports have also provided evidence of their immunomodulatory activity (9–11). Macrolides inhibit the production of reactive oxygen species, proinflammatory cytokines, and transcription factors of inflammation, such as nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1) (12). They are also known to inhibit expression of adhesion molecules on endothelial cells and, thereby, infiltration of neutrophils from blood to tissues (13). Macrolides have demonstrated a beneficial effect in chronic pulmonary inflammatory conditions, such as diffuse panbronchiolitis, cystic fibrosis, asthma, and bronchiectasis (14, 15). They have also been shown to produce favorable outcomes in critically ill patients with community-acquired bacterial pneumonia (16). Considering the diverse immunomodulatory mechanisms exhibited by macrolides, we sought to explore their beneficial effect for the treatment of sepsis. For this purpose, we opted to evaluate azithromycin, a macrolide antibiotic, in a clinically relevant animal model of sepsis.
Azithromycin was earlier reported to provide a survival benefit in the lipopolysaccharide (LPS)-induced murine sepsis model (17). The present study is the first-ever attempt to evaluate azithromycin in the murine cecal ligation and puncture (CLP) model of polymicrobial sepsis. The CLP model is the gold standard and widely preferred sepsis model, as it closely mimics the clinical condition of human sepsis (18). In the CLP sepsis model, the infection is primarily due to enteric bacteria against which azithromycin lacks activity. As a result, stand-alone azithromycin therapy does not provide a survival benefit. Therefore, CLP-challenged mice were treated with azithromycin in combination with a subprotective dose of the broad-spectrum cephalosporin ceftriaxone, which controls the progression of infection, thereby allowing the study of the immunomodulatory effect of azithromycin.
Initially, azithromycin was evaluated in an LPS-induced sepsis model to identify the immunomodulatory dose to be employed in a CLP-induced sepsis model. The dose was identified on the basis of the survival rate and immunomodulatory activity in terms of the effect on plasma cytokine (IL-1β, IL-6, and tumor necrosis factor alpha), myeloperoxidase (MPO), and glutathione (GSH) concentrations; blood glucose concentrations; body temperature; and total white blood cell (WBC) count. The dose of azithromycin chosen was combined with a subprotective dose of ceftriaxone in the CLP-induced sepsis model, and the survival benefit and immunomodulatory activity were assessed. A group treated with ceftriaxone alone was included as a control for the combination treatment group.
RESULTS
Survival in LPS- or CLP-induced sepsis model.
In the LPS-induced sepsis model, administration of azithromycin intraperitoneally (i.p.) 1 h prior to the administration of a lethal dose of LPS resulted in a dose-dependent improvement in the survival of mice at 24 h. The highest dose of 100 mg/kg of body weight provided 75% survival, whereas the rate of survival in the untreated LPS-challenged group was 12.5% (P < 0.05) (Fig. 1). Since a significant survival benefit was observed with the dose of 100 mg/kg, suggesting the immunomodulatory effect of azithromycin, the same dose was evaluated in the CLP-induced sepsis model. Azithromycin treatment (at 100 mg/kg i.p. 3 h after the CLP procedure) of CLP-challenged mice provided 37.5% protection; in contrast, 75% protection was observed in the LPS-induced sepsis model, indicating that azithromycin might have little or no effect on the infection associated with CLP-induced sepsis. In order to control bacterial growth, azithromycin (100 mg/kg i.p.) was combined with ceftriaxone at a subprotective dose (100 mg/kg subcutaneously [s.c.]). The ceftriaxone dose was identified from an initial survival study in the CLP-induced sepsis model, where it showed only 37.5% protection of mice (data not shown). A log-rank test analysis of the 5-day survival curve for the CLP-induced sepsis model showed that the survival rate for the combination group (75%) was significantly (P < 0.01) higher than that for the CLP control group (0%) and also better than that for the groups treated with azithromycin and ceftriaxone alone (Fig. 2).
FIG 1.
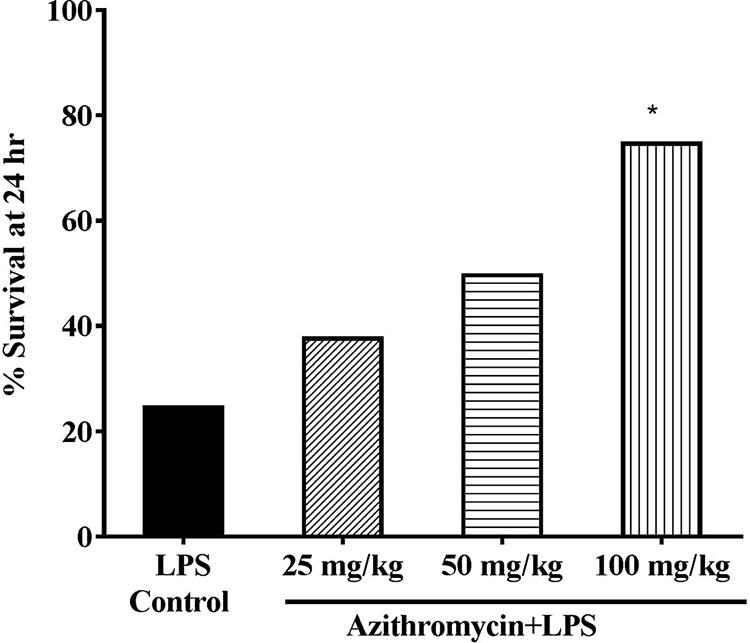
Survival in LPS-induced mouse sepsis model. Vehicle (saline) or azithromycin (25, 50, 100 mg/kg i.p.) was administered to mice (n = 8) 1 h prior to LPS treatment (1,500 μg/mouse i.p.), and survival was monitored for 24 h. Statistical analysis was performed using Fisher's exact test. *, P < 0.05 for significance compared to the LPS control group.
FIG 2.
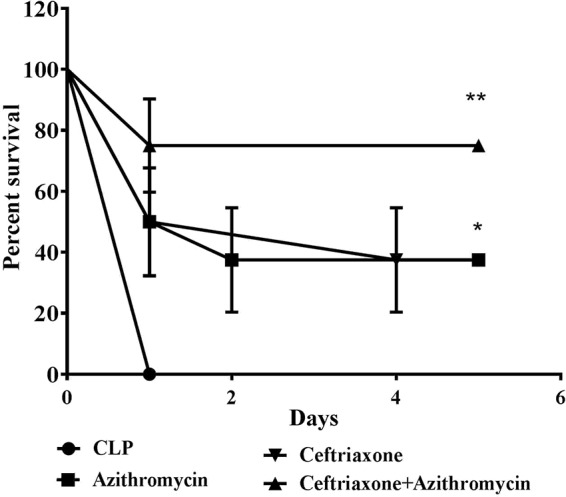
Survival data for mice that underwent CLP. At 3 h after CLP challenge, mice (n = 8) were administered vehicle (saline), ceftriaxone alone (100 mg/kg s.c.), azithromycin alone (100 mg/kg i.p.), or ceftriaxone and azithromycin in combination, and survival was monitored for 5 days. Statistical analysis was performed using the log-rank test. *, P < 0.05 for significance compared to the CLP control group; **, P < 0.01 for significance compared to the CLP control group.
Effect on circulating cytokine levels.
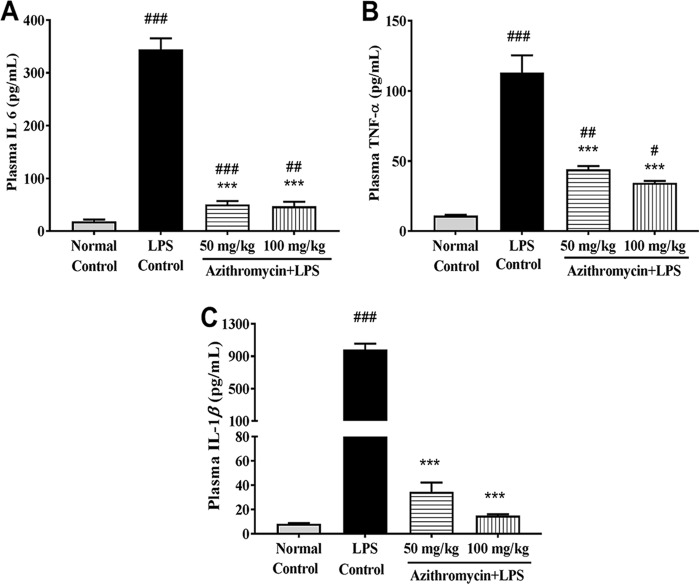
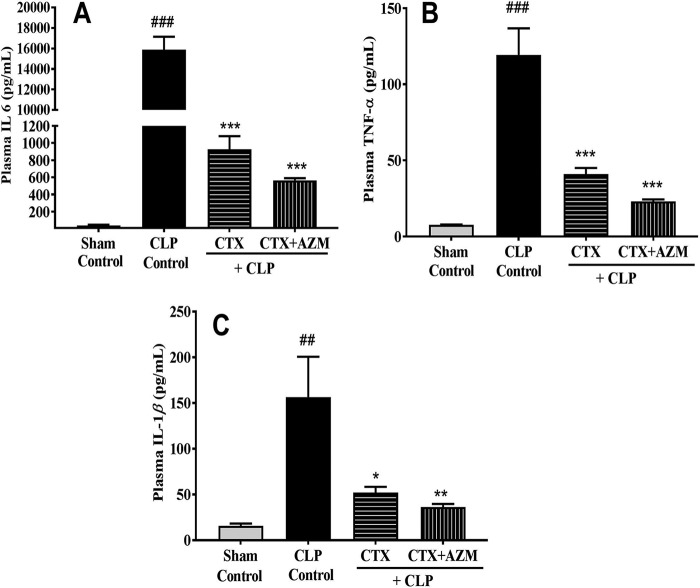
The plasma concentrations of the cytokines IL-6, IL-1β, and TNF-α observed in the LPS-induced sepsis model are illustrated in Fig. 3. As expected, their concentrations increased severalfold in the LPS-treated control group at 18 h post-LPS treatment compared to those in the untreated group. Administration of azithromycin alone to LPS-treated mice produced a dose-dependent reduction in cytokine levels. When it was administered at 100 mg/kg, these cytokines were reduced to levels closer to those in the untreated group. Similarly, CLP-challenged mice showed a statistically significant rise in the plasma cytokine levels compared to those in the sham-treated group after 18 h of CLP. The mean plasma concentrations of IL-6, TNF-α, and IL-1β in the CLP control group were 15,892 ± 1,250.6, 119.3 ± 17.4, and 156.61 ± 43.9 pg/ml, respectively. Interestingly, treatment with ceftriaxone alone significantly reduced the concentrations of all three evaluated cytokines (IL-6, 925 ± 154.5 pg/ml; TNF-α, 41.03 ± 3.9 pg/ml; IL-1β, 52.19 ± 6.2 pg/ml; n = 6). The azithromycin-ceftriaxone combination further marginally reduced their levels (IL-6, 565 ± 25.8 pg/ml; TNF-α, 23.10 ± 1.2 pg/ml; IL-1β, 36.23 ± 3.4 pg/ml; n = 6) (Fig. 4).
FIG 3.
Effect of LPS treatment on mouse plasma cytokine IL-6 (A), TNF-α (B), and IL-1β (C) levels. Mice (n = 8) were administered vehicle (saline) or azithromycin (50 and 100 mg/kg i.p.) 1 h prior to LPS challenge (1,000 μg/mouse i.p.), and plasma cytokine levels were measured 18 h after LPS injection. Data are expressed as the mean ± SEM (n = 6). #, P < 0.05 for significance versus the normal control group; ##, P < 0.01 for significance versus the normal control group; ###, P < 0.001 for significance versus the normal control group; ***, P < 0.001 for significance versus the LPS control group.
FIG 4.
Effect of ceftriaxone (CTX) and ceftriaxone (CTX)-azithromycin (AZM) on plasma IL-6 (A), TNF-α (B), and IL-1β (C) levels in mice that underwent CLP. At 3 h after CLP challenge, mice were treated with vehicle, ceftriaxone (100 mg/kg, s.c.), or ceftriaxone (100 mg/kg, s.c.) plus azithromycin (100 mg/kg, i.p.), and the plasma cytokine levels were measured 18 h after CLP (n = 6). Data are expressed as the mean ± SEM (n = 6). ##, P < 0.01 for significance versus the sham-treated group; ###, P < 0.001 for significance versus the sham-treated group; *, P < 0.05 for significance versus the CLP group; **, P < 0.01 for significance versus the CLP group; ***, P < 0.001 for significance versus the CLP group.
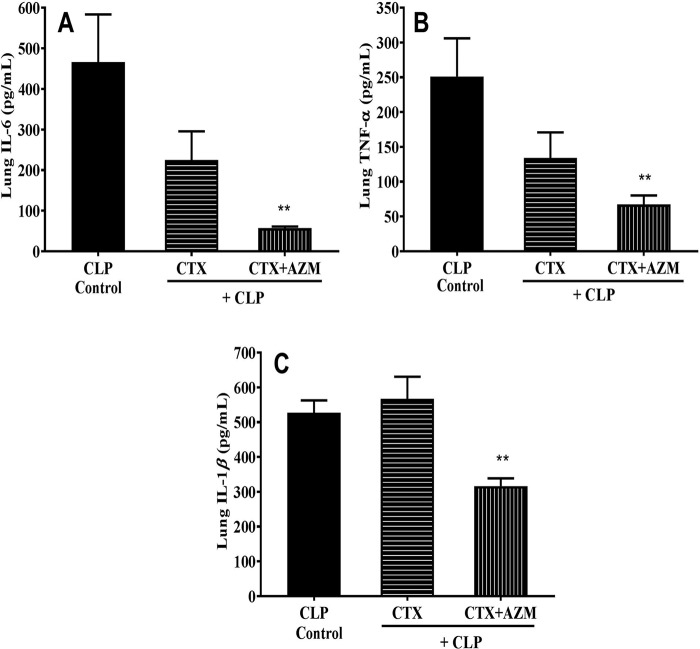
The lung concentrations of IL-6, TNF-α, and IL-1β in CLP-challenged mice (n = 6) were 466, 251.2, and 526.6 pg/ml, respectively, whereas no levels of these cytokines were observed in the sham-treated group (data not shown). Unlike the observation with plasma, ceftriaxone treatment of CLP-challenged mice did not reduce the lung cytokine levels, while the azithromycin-ceftriaxone combination treatment brought about significant reductions (IL-6, 57.06 ± 4.07 pg/ml; TNF-α, 67.17 ± 13.08 pg/ml; IL-1β, 316.53 ± 21.94 pg/ml; n = 6) (Fig. 5).
FIG 5.
Effect of ceftriaxone (CTX) and ceftriaxone plus azithromycin (CTX+AZM) on lung IL-6 (A), TNF-α (B), and IL-1β (C) levels in mice that underwent CLP. At 3 h after CLP challenge, mice were treated with vehicle, ceftriaxone (100 mg/kg s.c.), or ceftriaxone (100 mg/kg s.c.) plus azithromycin (100 mg/kg i.p.), and the plasma cytokine levels were measured 18 h after CLP. Data are expressed as the mean ± SEM (n = 6). **, P < 0.01 for significance versus the CLP group.
Effect on GSH and MPO levels.
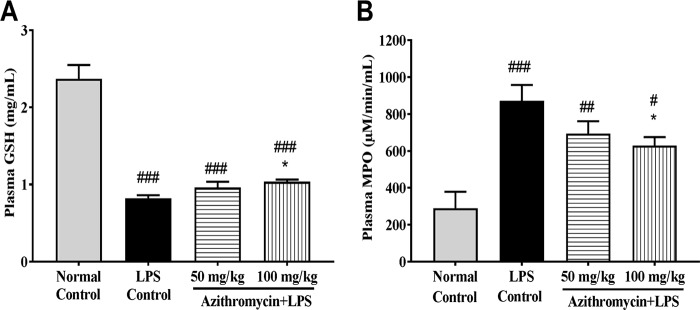
In LPS-treated mice, a significant suppression of plasma GSH levels and a significant elevation in plasma MPO levels compared to those in the untreated group were observed at 18 h. LPS-treated mice administered azithromycin produced a dose-dependent incremental rise in the GSH level and a considerable decrease in MPO levels. However, the effect was significant (P < 0.05) only at 100 mg/kg (Fig. 6A and B).
FIG 6.
Effect of LPS treatment mice on plasma GSH (A) and MPO (B) levels. Mice (n = 8) were administered vehicle (saline) or azithromycin (50 and 100 mg/kg i.p.) 1 h prior to LPS challenge (1,000 μg/mouse i.p.), and the plasma GSH and MPO levels were measured 18 h after LPS injection. Data are expressed as the mean ± SEM (n = 8). #, P < 0.05 for significance versus the normal control group; ##, P < 0.01 for significance versus the normal control group; ###, P < 0.001 for significance versus the normal control group; *, P < 0.05 for significance versus the LPS control group.
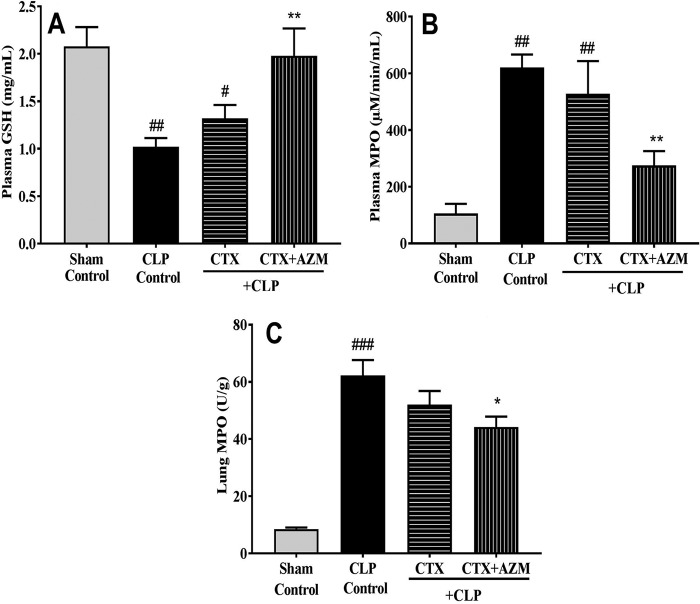
Similar to the findings with the LPS-induced sepsis model, the plasma GSH level was significantly reduced while the plasma MPO level was significantly increased in the CLP control group compared to the sham-treated group (GSH concentration, 1.02 ± 0.1 mg/ml in the CLP control group versus 2.08 ± 0.2 mg/ml in the sham-treated group [P < 0.01]; MPO concentration, 620.64 ± 45.9 μM/min/ml in the CLP control group versus 105.84 ± 33.8 μM/min/ml in the sham-treated group [P < 0.01]; n = 8). Ceftriaxone treatment did not cause a significant change in the GSH and MPO levels compared to those in the CLP control group, while the combination treatment group had significantly elevated GSH levels (1.98 ± 0.3 mg/ml; P < 0.01; n = 8) and decreased MPO levels (275.13 ± 50.4 μM/min/ml; P < 0.01; n = 8) compared to those in the CLP control group (Fig. 7A and B). In lung tissue, MPO levels in CLP-challenged mice increased significantly compared to those in the sham-treated group (60.27 versus 8.51 U/g of tissue; P < 0.001; n = 8). Ceftriaxone did not have any effect on the lung tissue MPO level (52.04 U/g), while the ceftriaxone-azithromycin combination treatment considerably reduced its level (44.20 U/g) (P < 0.05; n = 8) compared to that in the CLP control group (Fig. 7C).
FIG 7.
Effect of ceftriaxone (CTX) and ceftriaxone plus azithromycin (CTX+AZM) on plasma GSH (A), plasma MPO (B), and lung MPO (C) levels in mice that underwent CLP. Vehicle (saline), ceftriaxone (100 mg/kg s.c.), or ceftriaxone (100 mg/kg s.c.) plus azithromycin (100 mg/kg i.p.) was administered 3 h after CLP, and plasma GSH and plasma and lung MPO levels were measured after 18 h of CLP. Data are expressed as the mean ± SEM (n = 8). #, P < 0.05 for significance versus the sham-treated group; ##, P < 0.01 for significance versus the sham-treated group; ###, P < 0.001 for significance versus the sham-treated group; *, P < 0.05 for significance versus the CLP group; **, P < 0.01 for significance versus the CLP group.
Effect on blood glucose levels, body temperature, and total WBC count.
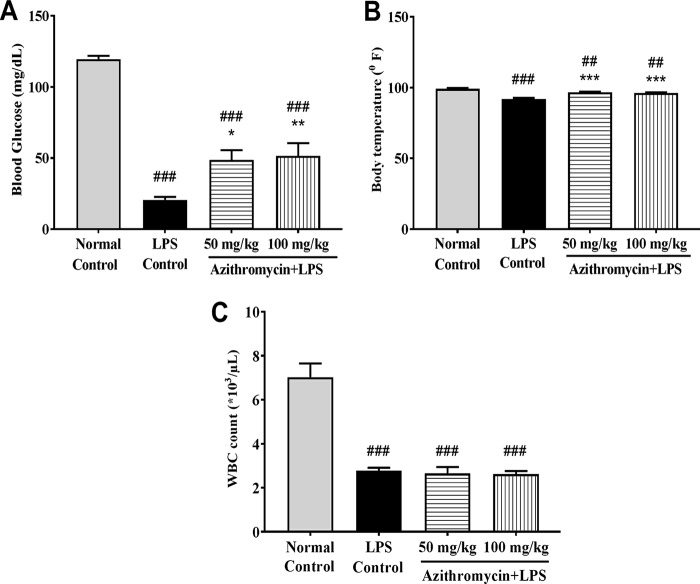
In the LPS-induced sepsis model, the mean blood glucose level and body temperature in the untreated group were 119.5 ± 2.4 mg/dl and 99.2 ± 0.7°F, respectively, and these dropped significantly to 20.67 ± 2.1 mg/dl (P < 0.001; n = 6) and 92.0 ± 0.7°F (P < 0.001; n = 6), respectively, in the LPS-challenged group. Azithromycin (100 mg/kg i.p.) treatment resulted in a significant reversal in the blood glucose level (51.5 ± 9.0 mg/dl; P < 0.001; n = 6) and body temperature (96.28 ± 0.4°F; P < 0.01; n = 6) compared to those in the LPS control group (Fig. 8A and B). The WBC count was significantly (P < 0.001; n = 6) reduced in the LPS control group (2.77 × 103 ± 0.14 × 103/μl) compared to the untreated group (7.02 × 103 ± 0.63 × 103/μl), and this reduction was not reversed by treatment with azithromycin (Fig. 8C).
FIG 8.
Effect of LPS treatment on blood glucose concentration (A), body temperature (B), and WBC count (C). Mice (n = 8) were administered azithromycin (50 and 100 mg/kg i.p.) or vehicle (saline) 1 h prior to LPS treatment (1,000 μg/mouse, i.p.), and the body temperature, blood glucose concentration, and WBC count were determined 18 h after LPS injection. Data are expressed as the mean ± SEM (n = 6). ##, P < 0.01 for significance compared to the normal control group; ###, P < 0.001 for significance compared to the normal control group; *, P < 0.05 for significance compared to the LPS control group; **, P < 0.01 for significance compared to the LPS control group; ***, P < 0.001 for significance compared to the LPS control group.
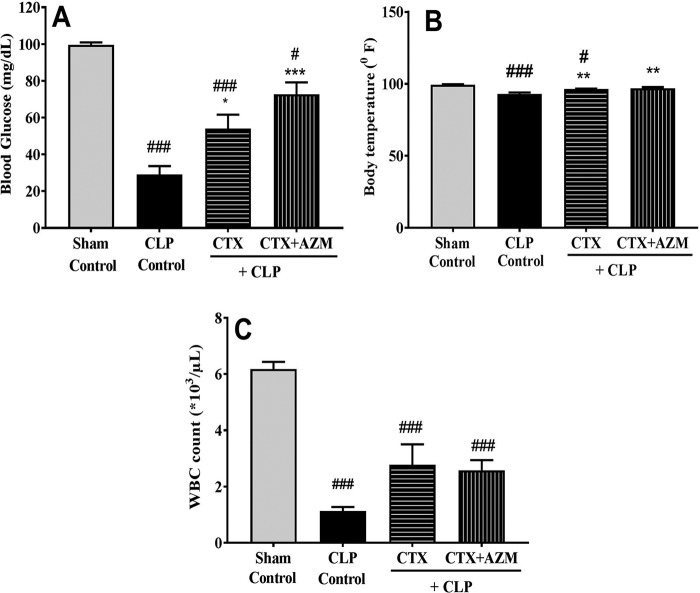
In the CLP-induced sepsis model, CLP treatment caused a significant reduction in the blood glucose concentration (from 99.55 ± 3.3 to 29.0 ± 13.0 mg/dl; P < 0.001; n = 6) and body temperature (from 99.33 ± 0.4 to 92.97 ± 1.1°F; P < 0.001; n = 6) compared to those in the sham-treated group. Treatment with ceftriaxone significantly reversed the blood glucose concentration (54.0 ± 7.6 mg/dl, P < 0.05; n = 6) as well as the body temperature (96.33 ± 0.4°F; P < 0.01; n = 6) in the CLP-challenged mice. Azithromycin in combination with ceftriaxone caused a further elevation in the blood glucose concentration (72.7 ± 6.5 mg/dl; P < 0.01; n = 6) compared to that in the group receiving ceftriaxone alone, but the animals exhibited no change in body temperature (97.0 ± 0.5°F; P < 0.01; n = 6) (Fig. 9A and B).
FIG 9.
Effect of ceftriaxone (CTX) and ceftriaxone (CTX) plus azithromycin (AZM) on blood glucose concentration (A), body temperature (B), and WBC count (C) in mice that underwent CLP. At 3 h after CLP challenge, mice (n = 8) were administered vehicle (saline), ceftriaxone (100 mg/kg s.c.), or ceftriaxone (100 mg/kg s.c.) plus azithromycin (100 mg/kg i.p.), and 18 h later, the WBC count, blood glucose concentration, and body temperature were determined. Data are expressed as the mean ± SEM (n = 6). #, P < 0.05 for significance versus the control group; ###, P < 0.001 for significance versus the control group; *, P < 0.05 for significance versus the CLP group; **, P < 0.01 for significance versus the CLP group; ***, P < 0.001 for significance versus the CLP group.
With respect to the total WBC count, it was significantly lower in CLP-challenged mice than in the sham-treated group (1.13 × 103 ± 0.14 × 103/μl versus 6.18 × 103 ± 0.25 × 103/μl; P < 0.001; n = 6). Treatment with ceftriaxone alone or treatment with ceftriaxone in combination with azithromycin did not significantly reverse the WBC count (Fig. 9C).
Effect on bacterial clearance.
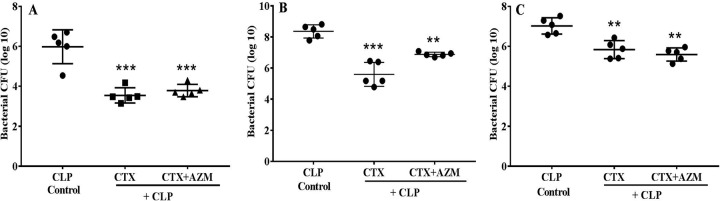
In order to determine whether the protective effect of the combination treatment in the CLP model was due to synergistic antimicrobial activity, the number of bacterial CFU in the blood, peritoneal lavage fluid, and lung homogenate was estimated 18 h after CLP challenge. Ceftriaxone produced a significant reduction in the bacterial count in blood, lung homogenate, and peritoneal lavage fluid, while azithromycin in combination with ceftriaxone provided no further reduction compared to that in the CLP control group. This observation suggests that azithromycin did not have a synergistic effect in this polymicrobial sepsis model (Fig. 10).
FIG 10.
Effect of ceftriaxone (CTX) and ceftriaxone (CTX) plus azithromycin (AZM) on the bacterial count in blood (A), peritoneal lavage fluid (B), and lung tissue (C) in mice that underwent CLP. At 3 h after CLP, mice (n = 6) were treated with ceftriaxone (100 mg/kg s.c.) or ceftriaxone (100 mg/kg s.c.) plus azithromycin (100 mg/kg i.p.), and the bacterial counting was done 18 h after CLP challenge. Data are expressed as the mean ± SEM (n = 5). **, P < 0.01 for significance versus the CLP control group; ***, P < 0.001 for significance versus the CLP control group.
DISCUSSION
In the present study, azithromycin was evaluated in combination with ceftriaxone in a clinically relevant animal model of sepsis (18). As azithromycin lacks antibacterial activity against the enteric bacteria which lead to mortality in mice, the LPS-induced sepsis model was used to identify the immunomodulatory dose of azithromycin that was later employed in the CLP-induced sepsis model. Treatment with an azithromycin dose of 100 mg/kg, which led to 75% survival of mice in the LPS sepsis model, was chosen as the immunomodulatory dose and is in the therapeutic range of azithromycin (19). As expected, the same dose did not provide equivalent protection in the CLP-induced sepsis model, suggesting its inability to stop the progression of infection. In order to investigate the survival benefit of azithromycin, it was necessary to combine it with ceftriaxone at a dose that by itself did not provide a survival benefit but that in combination helped to control the spread of infection, thereby enabling assessment of the survival benefit of azithromycin. Treatment with ceftriaxone at a dose of 100 mg/kg led to only 37.5% survival, even though it produced a significant reduction in the bacterial load. Azithromycin in combination with ceftriaxone was able to provide a protection rate comparable to that observed with azithromycin alone in the LPS-induced sepsis model, thus experimentally demonstrating its immunomodulatory effect in the CLP-induced sepsis model. Such an effect was not based on antibacterial synergy between these two agents, as evidenced from a lack of an effect of azithromycin on the bacterial load. These observations suggest that for a survival benefit in the CLP model of sepsis, it is important to manage both infection and inflammation (20).
Previous studies have demonstrated that the proinflammatory cytokines TNF-α, IL-1β, and IL-6 are the key contributors to the pathogenesis of sepsis (21, 22). In CLP-challenged mice, the plasma levels of IL-6, IL-β, and TNF-α significantly increased by 18 h. Treatment with ceftriaxone alone caused a significant decline in the cytokine levels, and no further significant drop was observed when it was used in combination with azithromycin. Therefore, we could not assess the contribution of azithromycin to the reduction of the plasma cytokine levels without the confounding effect of ceftriaxone. The decline in the plasma cytokine levels produced by ceftriaxone could be the result of its antibacterial activity. It should be noted that in the LPS-induced sepsis model, azithromycin alone elicited a significant decline in the levels of these cytokines in plasma.
However, in lung tissues, only a subdued effect of ceftriaxone on both the bacterial count and the measured cytokine levels was seen, enabling assessment of the effect of azithromycin in the presence of ceftriaxone. This subdued effect of ceftriaxone could be attributed to lower lung concentrations of ceftriaxone, as this drug is highly plasma protein bound (23). Compared to treatment with ceftriaxone alone, the addition of azithromycin remarkably suppressed the levels of these cytokines in lung tissue, thus demonstrating its immunomodulatory effect.
The immunomodulatory effect of azithromycin was further observed in the LPS- and CLP-induced sepsis models through a significant reversal of the plasma MPO and GSH levels. As part of the innate immune response, during a normal infection, MPO is secreted by activated neutrophils, which help in bacterial killing. However, in sepsis, there is an LPS-mediated abnormal hyperproduction of MPO, a potent oxidative enzyme which causes the peroxidation of cell membrane lipids and a decrease in the levels of the antioxidant GSH, leading to tissue dysfunction (24–27). The present study demonstrated that azithromycin could reverse this imbalance in the oxidant and antioxidant response and potentially minimize tissue injury in sepsis. It should be noted that therapy with ceftriaxone alone did not reverse the plasma MPO and GSH levels in the CLP sepsis model. Therefore, the effect of the combination treatment was primarily due to azithromycin. In lung tissue, we could measure only the MPO concentration, and unlike the findings in plasma, treatment with azithromycin in combination with ceftriaxone produced only a marginal reversal of the MPO concentration. Further investigation is required to understand this phenomenon.
LPS- and CLP-challenged mice experienced a significant reduction in the WBC count compared to that in the sham-treated group, possibly due to the immunosuppression observed during the later phase of sepsis. A similar decrease in the leukocyte count was also noted in previous publications (28, 29). In both models, azithromycin neither elevated nor further suppressed the WBC count, which may suggest that it has no immunosuppressive property.
Sepsis is reported to produce a hypodynamic state by reducing the body temperature and blood glucose concentration (30). Therefore, these parameters were measured in all the groups. As expected, mice subjected to LPS treatment or CLP challenge showed a significant reduction in the blood glucose concentration and body temperature, while mice treated with azithromycin alone in the endotoxemia model, with ceftriaxone alone, and with ceftriaxone in combination with azithromycin in the CLP challenge model exhibited a significant reversal of these parameters. The involvement of the excessive release of IL-1β and TNF-α in the pathogenesis of hypothermia and hypoglycemia during sepsis has been postulated previously (22, 31). Reversal of these parameters could be a result of inhibition of these cytokines in plasma. However, treatment with the combination had a relatively better effect in reversing the blood glucose level than treatment with ceftriaxone alone in the CLP model.
This is the first-ever study in which azithromycin in combination with ceftriaxone, through its immunomodulatory property, produced a survival benefit in a murine CLP-induced sepsis model.
However, due to the acute nature of the infection in the present model, the beneficial role of repeated doses of azithromycin could not be evaluated. The study results form the basis for undertaking further nonclinical and clinical investigations of azithromycin for its therapeutic role in the management of sepsis. As azithromycin exhibits a narrow spectrum of antibacterial activity, it is imperative to use a potent antibiotic to which the infecting pathogen is susceptible. Clinically, the early phase of sepsis is characterized by an excessive proinflammatory response, while the later phase is associated with immunosuppression (32, 33). The present study showed the survival benefit of the combination of azithromycin and ceftriaxone, which were administered early during the progression of sepsis. In the clinical setting, mortality occurs at the later phase of sepsis; therefore, early intervention with antibiotics in combination with immunomodulatory agents, such as azithromycin, is likely to provide a better outcome. Moreover, the azithromycin dose employed in mice, 100 mg/kg, is within the therapeutic scope of the drug.
The findings of this study indicate that combination therapy with azithromycin and potent antibacterial agents has the potential to improve survival in critically ill patients with bacterial infection.
MATERIALS AND METHODS
All the experimental protocols were approved by the Institutional Animals Ethics Committee (IAEC) of the Wockhardt Research Centre, Aurangabad, Maharashtra, India. Female Swiss albino mice weighing 25 to 30 g were used for the entire study. Mice were housed in cages, with standard rodent feed and drinking water being provided ad libitum. The light cycle was controlled automatically (lights on at 7:00 a.m. and off at 7:00 p.m.), and the room temperature was regulated at a temperature of 18 to 22°C with 40 to 70% humidity.
LPS-induced sepsis model.
The purpose of evaluating azithromycin in this model was to determine its immunomodulatory dose in our experimental setup. LPS derived from Escherichia coli serotype O127:B8 (Sigma-Aldrich) was dissolved in saline and injected intraperitoneally (i.p.) in a fixed volume of 0.25 ml to induce sepsis in mice. Two different LPS doses were used: a lethal dose of 1,500 μg/mouse for the survival study, in which monitoring was done for up to 24 h, and a sublethal dose of 1,000 μg/mouse for estimation of the body temperature, blood glucose concentration, total white blood cell (WBC) count, and plasma cytokine, myeloperoxidase (MPO), and glutathione (GSH) levels.
Treatment groups for the LPS-induced sepsis model.
For the survival experiment, at 1 h prior to administration of a lethal dose of LPS, vehicle (saline) or azithromycin (Azithral; Alembic Pharmaceutical) at a dose of 25, 50, or 100 mg/kg i.p. was administered to mice (n = 8) only once. From this survival study, two effective doses of azithromycin were selected to further assess the biochemical and physiological parameters in mice exposed to a sublethal dose of LPS.
CLP-induced sepsis model.
The CLP procedure was performed as described previously (34). Mice were anesthetized with a mixture of ketamine and xylazine (100 and 10 mg/kg i.p., respectively), and the abdomen was disinfected with iodine. A small midabdominal incision was made to expose the cecum. A distended portion of the cecum just distal to the ileocecal valve was isolated and ligated with a 3-0 silk suture in a manner not to disrupt bowel continuity. The ligated portion of the cecum was punctured twice with an 18-gauge needle. To ensure the patency of the punctured portion, the cecum was gently squeezed until a small quantity of feces extruded through it. The cecum was placed back in the abdomen, and the incision was closed with sutures. The mice were then resuscitated with 1 ml of saline injected subcutaneously and returned to their cages with free access to feed and water. Sham-operated controls were treated in an identical manner, but without ligation and puncture. Survival was assessed twice a day for 5 days. In a separate study, parameters like body temperature, blood glucose concentration, total WBC count, and plasma GSH, cytokine, and MPO levels in plasma and lung tissue, along with the bacterial count in blood, peritoneal lavage fluid, and lung homogenate, were measured 18 h after CLP challenge.
Treatment group for the CLP-induced sepsis model.
From the initial survival studies performed in mice that had undergone CLP (data not shown), it was determined that a subprotective dose of ceftriaxone (100 mg/kg s.c.) provided protection in 37.5% of mice. The survival study included a sham-treated group and mice that had undergone CLP treated with vehicle (saline), ceftriaxone (100 mg/kg s.c.), azithromycin (100 mg/kg i.p.), or both ceftriaxone and azithromycin in combination. For the estimation of biochemical and other parameters, the groups included were sham-treated controls and mice that had undergone CLP treated with vehicle (saline), ceftriaxone (100 mg/kg s.c.), and ceftriaxone (100 mg/kg s.c.) plus azithromycin (100 mg/kg i.p.). The drug treatments were done only once and consisted of a single dose administered 3 h after CLP.
Experimental protocol.
For estimation of biochemical and other parameters, 16 mice were included in each group. Mice were made septic by treatment either with LPS or by the CLP technique, and 18 h later the body temperature was measured. Six mice were bled through the tail vein for glucose concentration estimation, followed by the collection of blood, which was placed in EDTA-containing tubes, through the retroorbital sinus for determination of the total WBC count. To obtain plasma for estimation of cytokine, MPO, and GSH levels, the remaining mice (n = 8) were bled through the retroorbital sinus and the blood was placed in heparinized tubes. In addition, lungs harvested from CLP-challenged mice were rinsed with saline, dried, and weighed. Lungs from six mice were homogenized with chilled saline and centrifuged at 15,000 rpm for 10 min at 4°C to obtain a supernatant for cytokine measurements. Lungs from eight other mice were homogenized with 50 mM potassium phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide, sonicated, and centrifuged to obtain a supernatant for MPO estimation.
Measurements. (i) Estimation of body temperature, blood glucose concentration, and total WBC count.
The rectal temperature and blood glucose concentration were measured using a digital thermometer (CareTouch) and a calibrated Bayer Contour TS glucometer, respectively. The total white blood cell count was determined from EDTA-containing blood samples using a Sysmex hematology analyzer.
(ii) Estimation of plasma and lung cytokine levels.
Tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-1β levels were estimated using commercially available enzyme-linked immunosorbent assays (ELISAs), according to the manufacturer's instructions (R & D Systems Inc., USA).
(iii) Measurement of MPO activity in plasma and lung.
MPO activity was determined by the o-dianisidine method, with modification for 96-well plates. The assay mixture consisted of 30 μl of 0.1 M phosphate buffer (pH 6), 30 μl of 0.01 M hydrogen peroxide (Merck), 20 μl plasma and lung supernatant, 170 μl deionized water, and 50 μl of freshly prepared 0.02 M o-dianisidine (Sigma-Aldrich) solution. The o-dianisidine solution was added last, and the absorbance at 460 nm was recorded each minute for a duration of 10 min using a spectrophotometer (SpectraMax Plus 384 Microplate Reader). MPO activity was expressed in micromolar per minute per milliliter of plasma and in units per gram of tissue.
(iv) Determination of plasma GSH content.
The GSH content was determined using Ellman's reagent [5,5′-dithiobis(2-nitrobenzoic acid) (DTNB)]. The reaction mixture consisted of 50 μl of clear plasma, 250 μl of 0.1 M phosphate buffer (pH 6), and 25 μl of DTNB reagent (4 mg/ml dissolved in 1% sodium citrate). This mixture was incubated for 10 min at 37°C, and the absorbance at 412 nm was read using a spectrophotometer. The GSH concentration was determined using a standard curve constructed with different concentrations of reduced l-glutathione (Sigma-Aldrich).
(v) Evaluation of bacterial clearance.
The bacterial load in blood, lung homogenate, and peritoneal lavage fluid of CLP-challenged mice was determined following vehicle and drug treatments. In brief, mice were anesthetized with ketamine-xylazine after 18 h of CLP. The blood samples were collected through the retroorbital sinus and placed in heparinized tubes, the contents of the tubes were mixed, and the tubes were placed on an ice bath. The mice were then injected with 2 ml sterile saline intraperitoneally, and the abdomen was gently massaged, cleansed with 70% alcohol, and cut open to expose the peritoneal cavity to collect the peritoneal lavage fluid and lungs. Lung homogenate (20%) was prepared in sterile saline. Later, the peritoneal lavage fluid, blood, and lung homogenate (100 μl) were placed on ice and serially diluted with sterile saline. Ten microliters of each diluted sample was placed on Trypticase soy agar plates (BD Biosciences, San Diego, CA) and incubated at 37°C for 24 h. The numbers of bacterial colonies were then counted and expressed as the number of CFU per milliliter of blood and peritoneal lavage fluid and the number of CFU per milligram of lung tissue.
Statistics.
Survival data were analyzed using the log rank test and Fisher's exact test. Data are represented as the mean ± standard error of the mean (SEM). Differences among groups were assessed using one-way analysis of variance (ANOVA), followed by Dunnett's multiple-comparison post hoc test. A probability (P) value of <0.05 was considered significant. All statistical analyses were performed using GraphPad Prism statistical software (version 5).
ACKNOWLEDGMENT
We thank the Wockhardt Research Centre for granting permission to perform these studies.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. 2016. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavaillon JM, Adib-Conquy M, Fitting C, Adrie C, Payen D. 2003. Cytokine cascade in sepsis. Scand J Infect Dis 35:535–544. doi: 10.1080/00365540310015935. [DOI] [PubMed] [Google Scholar]
- 3.Harsha N, Shuyu P, Cuk-Seong K. 2018. Role of mitochondrial oxidative stress in sepsis. Acute Crit Care 33:65–72. doi: 10.4266/acc.2018.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Bi J, Liu S, Pang Q, Zhang R, Wang S, Liu C. 2017. 5-HT drives mortality in sepsis induced by cecal ligation and puncture in mice. Mediators Inflamm 2017:6374283. doi: 10.1155/2017/6374283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steckert AV, de Castro AA, Quevedo J, Dal-Pizzol F. 2014. Sepsis in the central nervous system and antioxidant strategies with N-acetylcysteine, vitamins and statins. Curr Neurovasc Res 11:83–90. doi: 10.2174/1567202610666131211111012. [DOI] [PubMed] [Google Scholar]
- 6.Dolin HH, Papadimos TJ, Stepkowski S, Chen X, Pan ZK. 2018. A novel combination of biomarkers to herald the onset of sepsis prior to the manifestation of symptoms. Shock 49:364–370. doi: 10.1097/SHK.0000000000001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remick DG. 2003. Cytokine therapeutics for the treatment of sepsis: why has nothing worked? Curr Pharm Des 9:75–82. doi: 10.2174/1381612033392567. [DOI] [PubMed] [Google Scholar]
- 8.June LR. 2014. Putting the brakes on inflammation to control sepsis. Sci Transl Med 6:233ec73. doi: 10.1126/scitranslmed.3009254. [DOI] [Google Scholar]
- 9.Giamarellos-Bourboulis EJ. 2008. Macrolide beyond the conventional antimicrobial: a class of potent immunomodulators. Int J Antimicrob Chemother 31:12–20. doi: 10.1016/j.ijantimicag.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Labro MT. 1998. Anti-inflammatory activity of macrolides: a new therapeutic potential? J Antimicrob Chemother 41(Suppl B):37–46. doi: 10.1093/jac/41.suppl_2.37. [DOI] [PubMed] [Google Scholar]
- 11.Feldman C, Anderson R. 2009. Non-antimicrobial activity of macrolides: therapeutic potential in chronic inflammatory airway disorders. S Afr J Infect Dis 24:21–26. [Google Scholar]
- 12.Kanoh S, Rubin BK. 2010. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terao H, Asano K, Kanai K, Kyo Y, Watanabe S, Hisamitsu T, Suzaki H. 2003. Suppressive activity of macrolide antibiotics on nitric oxide production by lipopolysaccharide stimulation in mice. Mediators Inflamm 12:195–202. doi: 10.1080/09629350310001599620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magdalena B, Orszulak-Michalak D. 2014. Immunomodulatory and anti-inflammatory properties of macrolides. Curr Issues Pharm Med Sci 27:61–64. [Google Scholar]
- 15.Schultz MJ. 2004. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis. J Antimicrob Chemother 54:21–28. doi: 10.1093/jac/dkh309. [DOI] [PubMed] [Google Scholar]
- 16.Garcia Vazquez E, Mensa J, Martinez JA, Marcos MA, Puig J, Ortega M, Torres A. 2005. Lower mortality among patients with community-acquired pneumonia treated with a macrolide plus a beta-lactam agent versus a beta-lactam agent alone. Eur J Clin Microbiol Infect Dis 24:190–195. doi: 10.1007/s10096-005-1295-9. [DOI] [PubMed] [Google Scholar]
- 17.Ivetic Tkalcevic V, Bosnjak B, Hrvacic B, Bosnar M, Marjanovic N, Ferencic Z, Situm K, Culic O, Parnham MJ, Erakovic V. 2006. Anti-inflammatory activity of azithromycin attenuates the effects of lipopolysaccharide administration in mice. Eur J Pharmacol 539:131–138. doi: 10.1016/j.ejphar.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 18.Dejager L, Pinheiro I, Dejonckheere E, Libert C. 2011. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 19:198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Yoshioka D, Kajiwara C, Ishii Y, Umeki K, Hiramatsu K, Kadota J, Tateda K. 2016. Efficacy of β-lactam-plus-macrolide combination therapy in a mouse model of lethal pneumococcal pneumonia. Antimicrob Agents Chemother 60:6146–6154. doi: 10.1128/AAC.01024-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sha T, Iizawa Y, Ii M. 2011. Combination of imipenem and TAK-242, a Toll-like receptor 4 signal transduction inhibitor, improves survival in a murine model of polymicrobial sepsis. Shock 35:205–209. doi: 10.1097/SHK.0b013e3181f48942. [DOI] [PubMed] [Google Scholar]
- 21.Takala A, Nupponen I, Kylanpaa-Back ML, Repo H. 2002. Markers of inflammation in sepsis. Ann Med 34:614–623. doi: 10.1080/078538902321117841. [DOI] [PubMed] [Google Scholar]
- 22.Jaffer U, Wade RG, Gourlay T. 2010. Cytokines in the systemic inflammatory response syndrome: a review. HSR Proc Intensive Care Cardiovasc Anesth 2:161–175. [PMC free article] [PubMed] [Google Scholar]
- 23.Sauve C, Azoulay-Dupuis E, Moine P, Darras-Joly C, Rieux V, Carbon C, Bédos JP. 1996. Efficacies of cefotaxime and ceftriaxone in a mouse model of pneumonia induced by two penicillin- and cephalosporin-resistant strains of Streptococcus pneumoniae. Antimicrob Agents Chemother 40:2829–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kothari N, Keshari RS, Bogra J, Kohli M, Abbas H, Malik A, Dikshit M, Barthwal MK. 2011. Increased myeloperoxidase enzyme activity in plasma is an indicator of inflammation and onset of sepsis. J Crit Care 26:435.e1–e7. doi: 10.1016/j.jcrc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Schrijver IT, Kemperman H, Roest M, Kesecioglu J, de Lange DW. 2017. Myeloperoxidase can differentiate between sepsis and non-infectious SIRS and predicts mortality in intensive care patients with SIRS. Intensive Care Med Exp 5:1–9. doi: 10.1186/s40635-016-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villa P, Saccani A, Sica A, Ghezzi P. 2002. Glutathione protects mice from lethal sepsis by limiting inflammation and potentiating host defense. J Infect Dis 185:1115–1120. doi: 10.1086/340042. [DOI] [PubMed] [Google Scholar]
- 27.Ali KC, Murat Y, Akgun O, Fehmi O, Zekai H, Oner M, Elif C, Fadime A, Halis S. 2011. The effects of montelukast on antioxidant enzymes and proinflammatory cytokines on the heart, liver, lungs, and kidneys in a rat model of cecal ligation and puncture-induced sepsis. Sci World J 11:1341–1356. doi: 10.1100/tsw.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Liu H, Jia Z, Olsen C, Litwin S, Guan G, Yang T. 2010. Nitro-oleic acid protects against endotoxin-induced endotoxemia and multiorgan injury in mice. Am J Physiol Renal Physiol 298:F754–F762. doi: 10.1152/ajprenal.00439.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kassim M, Mansor M, Al-Abd N, Yusoff KM. 2012. Gelam honey has a protective effect against lipopolysaccharide (LPS)-induced organ failure. Int J Mol Sci 13:6370–6381. doi: 10.3390/ijms13056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyde SR, Stith RD, McCallum RE. 1990. Mortality and bacteriology of sepsis following cecal ligation and puncture in aged mice. Infect Immun 58:619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogel SN, Henricson BE, Neta R. 1991. Roles of interleukin-1 and tumor necrosis factor in lipopolysaccharide-induced hypoglycemia. Infect Immun 59:2494–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hotchkiss RS, Monneret F, Payen D. 2013. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jinwook S, Mirim J. 2017. Potential immunotherapeutics for immunosuppression in sepsis. Biomol Ther (Seoul) 25:569–577. doi: 10.4062/biomolther.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samuel E, Douglas C, Jean N, Gerald B, David N, Daniel R. 1999. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun 67:6603–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]