Acinetobacter baumannii has emerged as an important multidrug-resistant nosocomial pathogen. In previous work, we identified a putative MFS transporter, AU097_RS17040, involved in the pathogenicity of A. baumannii (M.
KEYWORDS: Acinetobacter, efflux pumps, quinolones
ABSTRACT
Acinetobacter baumannii has emerged as an important multidrug-resistant nosocomial pathogen. In previous work, we identified a putative MFS transporter, AU097_RS17040, involved in the pathogenicity of A. baumannii (M. Pérez-Varela, J. Corral, J. A. Vallejo, S. Rumbo-Feal, G. Bou, J. Aranda, and J. Barbé, Infect Immun 85:e00327-17, 2017, https://doi.org/10.1128/IAI.00327-17). In this study, we analyzed the susceptibility to diverse antimicrobial agents of A. baumannii cells defective in this transporter, referred to as AbaQ. Our results showed that AbaQ is mainly involved in the extrusion of quinolone-type drugs in A. baumannii.
TEXT
Acinetobacter baumannii is a multidrug-resistant (MDR) pathogen that causes hospital-acquired infections (1). In previous work, we identified a new, putative major facilitator superfamily (MFS) transporter in A. baumannii strain ATCC 17978, AU097_RS17040. This transporter, referred to as AbaQ (A. baumannii quinolone resistance transporter), is involved in surface-associated motility as well as the virulence of A. baumannii (2).
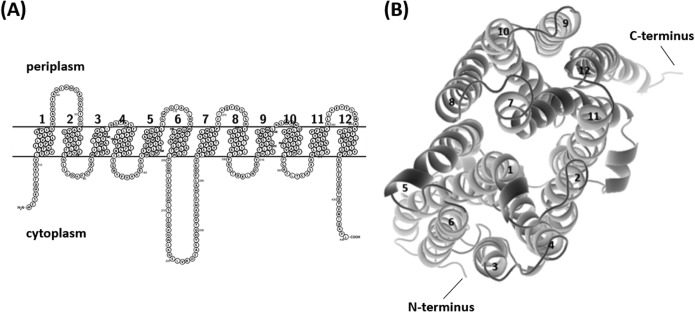
Sequence analysis of AbaQ, annotated as an MFS transporter in A. baumannii strain ATCC 17978 (accession number WP_000345069), indicated an open reading frame (ORF) of 1,305 nucleotides. According to the deduced amino acid sequence, the protein consists of 434 residues and has a molecular mass of 47.8 kDa and a theoretical isoelectric point (pI) of 9.27. On the basis of predictions of its secondary structure and transmembrane topology, AbaQ is composed of 12 α-helical transmembrane segments, with both the N and C termini located in the cytoplasm (Fig. 1A). Support for this structure came from an independent analysis that revealed the three-dimensional (3D) structure of the protein (Fig. 1B). These data indicated that AbaQ is a drug H+ antiporter 1 (DHA1), which differs from DHA2-type MFS drug transporters by the presence of 12 rather than 14 transmembrane segments (3). The predicted product of the abaQ gene exhibited low amino acid identity and similarity (<24% and <38%, respectively) with other MFS transporters involved in drug efflux in A. baumannii (Table 1).
FIG 1.
(A) Prediction of the structure of AbaQ using Protter (10). The putative protein is shown parallel to the cytoplasmic membrane. (B) 3D representation of AbaQ viewed along the plane of the membrane from the periplasmic side using RaptorX Structure Prediction (26) and visualized using PyMOL software (27). The 12 transmembrane α-helices are numbered (1 to 12); both the N and C termini are located in the cytoplasm.
TABLE 1.
MFS transporters described in A. baumannii
| MFSa | Accession no. | Main antimicrobial exported | Identity (%)b | Similarity (%)b | Reference |
|---|---|---|---|---|---|
| AmvA | ACQ82816 | Erythromycin | 17.8 | 31.0 | 7 |
| TetA | AAO38186 | Tetracycline | 17.6 | 28.5 | 18 |
| TetB | AFV67369 | Minocycline | 19 | 33.8 | 19 |
| CraA | ABO13543 | Chloramphenicol | 19 | 36.9 | 20 |
| FloR | AQT19056 | Chloramphenicol | 16.9 | 28.2 | 21 |
| CmlA | AMD83513 | Chloramphenicol | 17.5 | 28.3 | 22 |
| AbaF | ABO11759 | Fosfomycin | 23.7 | 37.6 | 23 |
| EmrB | ABO12199 | Colistin | 17.2 | 30.7 | 24 |
| AedC | ABO11341 | Tetracycline-chloramphenicol | 18.5 | 32.7 | 25 |
MFS, Major facilitator superfamily.
With respect to AbaQ of A. baumannii strain ATCC 17978, using the Basic Local Alignment Search Tool ([BLAST] http://www.ncbi.nlm.nih.gov) and Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/).
To determine whether a lack of AbaQ alters antimicrobial susceptibilities, the responses of two A. baumannii abaQ mutants obtained in previous work (2) to antimicrobials of different classes were tested. Compared to the wild-type (WT) parental strain, A. baumannii ATCC 17978, the abaQ mutant had 2- to 4-fold higher susceptibilities to trimethoprim and novobiocin (Table 2). The highest susceptibilities (approximately 8- to 32-fold) occurred in response to the quinolone-type antibiotics ciprofloxacin, levofloxacin, and nalidixic acid (Table 2). In contrast, the mutant and its WT parent did not differ in their susceptibilities to β-lactams (meropenem and ampicillin), aminoglycosides (amikacin and gentamicin), macrolides (erythromycin), and polymyxins (colistin) or to other antimicrobials (chloramphenicol, tetracycline, minocycline, and rifampin) (Table 2). To further corroborate the relevance of this MFS in other A. baumannii strains, we analyzed the abaQ knockout mutant derived from A. baumannii strain MAR002, a biofilm-hyperproducing strain recently isolated from a wound sample collected from a patient at the Hospital del Mar in Barcelona (2, 4, 5). Assays of the mutant indicated that inactivation of the AbaQ homologue in A. baumannii MAR002 (99% amino acid identity) also caused the highest reduction (approximately 8- to 32-fold) in the MICs of quinolone-type antibiotics (data not shown).
TABLE 2.
MICs of various antimicrobials in wild-type A. baumannii ATCC 17978 and the abaQ mutant derivative
| Antimicrobial | MIC (mg/liter) |
Fold changea | MIC (mg/liter) |
Fold changeb | ||
|---|---|---|---|---|---|---|
| WT | AbaQ− | AbaQ− plus pET-RAØ | AbaQ− plus pET-RA+AbaQ | |||
| Ciprofloxacin | 0.25 | 0.0078 | 32 | 0.0078 | 0.25 | 32 |
| Levofloxacin | 0.125 | 0.0078 | 16 | 0.0078 | 0.125 | 16 |
| Nalidixic acid | 8 | 1 | 8 | 1 | 8 | 8 |
| Trimethoprim | 16 | 4 | 4 | 4 | 16 | 4 |
| Novobiocin | 8 | 4 | 2 | 4 | 8 | 2 |
| Meropenem | 2 | 2 | 1 | 2 | 2 | 1 |
| Ampicillin | 16 | 16 | 1 | 16 | 16 | 1 |
| Amikacin | 1 | 1 | 1 | 1 | 1 | 1 |
| Gentamicin | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 1 |
| Erythromycin | 4 | 4 | 1 | 4 | 4 | 1 |
| Colistin | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 1 |
| Chloramphenicol | 16 | 16 | 1 | 16 | 16 | 1 |
| Tetracycline | 4 | 4 | 1 | 4 | 4 | 1 |
| Minocycline | 0.062 | 0.062 | 1 | 0.062 | 0.062 | 1 |
| Rifampin | 4 | 4 | 1 | NAc | NA | NA |
Ratio of the MICs of the wild type (WT) versus abaQ mutant derivative (AbaQ−).
Ratio of the MICs for the abaQ mutant carrying the pET-RA+AbaQ plasmid (complemented mutant) versus the abaQ mutant carrying the empty pET-RA plasmid (pET-RAØ).
NA, not applicable (the pET-RA plasmid carries rifampin resistance).
To complement the mutants, the abaQ gene, including its own promoter, was amplified from the genome of A. baumannii strain ATCC 17978 using the AbaQFXbaI and AbaQRXbaI oligonucleotides (5′-ACTGTCTAGAGGAATATCACAGCTTGCAGCG and 5′-ACTGTCTAGATTACAAAGGCTTTTGAATATTC, respectively), cloning them into the XbaI restriction site of the pET-RA vector (6). Complementation of the abaQ-mutant derived from A. baumannii ATCC 17978 completely restored antimicrobial susceptibility to the same levels determined in the WT parental strain (Table 2). The recovery of the WT phenotype was also observed in the complemented abaQ MAR002 mutant derivative (data not shown). These results provided clear evidence of the critical role of AbaQ in the efflux of quinolone-type antibiotics in A. baumannii.
To assess whether AbaQ confers resistance to quinolones through an active efflux mechanism, the MIC of ciprofloxacin in the presence of the efflux pump inhibitor carbonyl cyanide 3-chlorophenylhydrazone ([CCCP] Sigma) was determined in A. baumannii strains carrying (WT and AbaQ− plus pET-RA+AbaQ) or lacking (AbaQ− and AbaQ− plus pET-RAØ) the abaQ gene. The addition of CCCP (20 mg/liter) decreased the ciprofloxacin MIC 16-fold in AbaQ-carrying cells (from 0.25 to 0.015 mg/liter) but only 4-fold in cells lacking the abaQ gene (from 0.0078 to 0.0019 mg/liter). The addition of CCCP alone did not inhibit bacterial cell growth in any of the A. baumannii strains, indicating that the above results were not due to the toxicity of CCCP itself. Accordingly, when ampicillin, which is not a substrate of the AbaQ transporter (Table 2), was used instead of ciprofloxacin, there was no difference in the antimicrobial susceptibilities in the presence or absence of CCCP in any of the cultured A. baumannii strains (data not shown). These results unequivocally demonstrated that the abaQ gene product confers decreased susceptibility to quinolones by encoding an active efflux transporter.
The majority of the MFS transporters involved in drug-efflux described thus far in A. baumannii mediate chloramphenicol efflux, with a smaller number mediating the efflux of other antimicrobials, such as erythromycin, tetracycline, minocycline, fosfomycin, and colistin (Table 1). Among the latter group of transporters is AmvA, which participates in the efflux of erythromycin and different classes of disinfectants, detergents, and dyes but also confers modest resistance to quinolone-type antimicrobials (7). In amvA mutant strains, susceptibilities to both ciprofloxacin and norfloxacin were 2-fold higher than in the WT strain, whereas the susceptibility to nalidixic acid was unchanged (7). Other transporters belonging to different families and involved in the extrusion of a wide range of antimicrobials, including quinolones, have been described in A. baumannii: AdeABC (8), AdeDE (9), AdeFGH (10), AdeIJK (11), AdeM (12), and AdeT (13), all belonging to the resistance/nodulation/division (RND) superfamily; AbeM, AbeM2, and AbeM4 transporters, belonging to the multiple antimicrobial toxin extrusion (MATE) family (12, 14); and AbeS, belonging to the small multidrug resistance (SMR) family (15). No role in quinolone efflux has been detected in the only ATP-binding cassette (ABC) transporter described so far in A. baumannii (A1S_1535) (16) or in any of the transporters that make up the most recently discovered family of efflux pumps: the proteobacterial antimicrobial compound efflux (PACE) transporters (17).
To our knowledge, AbaQ, which is widely present in A. baumannii clinical isolates and involved in both surface-associated motility and virulence (2), is the first MFS efflux pump shown to play an important role in the extrusion of quinolone-type antimicrobials in this MDR nosocomial pathogen.
ACKNOWLEDGMENTS
We thank Joan Ruiz (UAB) and Susana Escribano (UAB) for their excellent technical assistance, as well as our UAB students Paula García and Daniel Quiñones for their helpful support. We also thank the Germán Bou lab for the A. baumannii MAR002 strain.
This study was supported by grant BIO2016-77011-R from the Ministerio de Economía y Competitividad.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
J.A. is a Serra Húnter Fellow, Generalitat de Catalunya, Barcelona, Spain.
REFERENCES
- 1.Lynch J, Zhanel G, Clark N. 2017. Infections due to Acinetobacter baumannii in the ICU: treatment options. Semin Respir Crit Care Med 38:311–325. doi: 10.1055/s-0037-1599225. [DOI] [PubMed] [Google Scholar]
- 2.Pérez-Varela M, Corral J, Vallejo JA, Rumbo-Feal S, Bou G, Aranda J, Barbé J. 2017. Mutants in the β-subunit of the RNA polymerase impairing the surface-associated motility and virulence of Acinetobacter baumannii. Infect Immun 85:e00327-17. doi: 10.1128/IAI.00327-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulsen IT, Brown MH, Skurray RA. 1996. Proton-dependent multidrug efflux systems. Microbiol Rev 60:575–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Álvarez-Fraga L, López M, Merino M, Rumbo-Feal S, Tomás M, Bou G, Poza M. 2015. Draft genome sequence of the biofilm-hyperproducing Acinetobacter baumannii clinical strain MAR002. Genome Announc 3:e00824-15. doi: 10.1128/genomeA.00824-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Álvarez-Fraga L, Pérez A, Rumbo-Feal S, Merino M, Vallejo JA, Ohneck EJ, Edelmann RE, Beceiro A, Vázquez-Ucha JC, Valle J, Actis LA, Bou G, Poza M. 2016. Analysis of the role of the LH92_11085 gene of a biofilm hyper-producing Acinetobacter baumannii strain on biofilm formation and attachment to eukaryotic cells. Virulence 7:443–455. doi: 10.1080/21505594.2016.1145335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aranda J, Poza M, Pardo BG, Rumbo S, Rumbo C, Parreira JR, Rodriguez-Velo P, Bou G, Rodríguez-Velo P, Bou G. 2010. A rapid and simple method for constructing stable mutants of Acinetobacter baumannii. BMC Microbiol 10:279. doi: 10.1186/1471-2180-10-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajamohan G, Srinivasan VB, Gebreyes WA. 2010. Molecular and functional characterization of a novel efflux pump, AmvA, mediating antimicrobial and disinfectant resistance in Acinetobacter baumannii. J Antimicrob Chemother 65:1919–1925. doi: 10.1093/jac/dkq195. [DOI] [PubMed] [Google Scholar]
- 8.Magnet S, Courvalin P, Lambert T. 2001. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob Agents Chemother 45:3375–3380. doi: 10.1128/AAC.45.12.3375-3380.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chau S-L, Chu Y-W, Houang ETS. 2004. Novel resistance-nodulation-cell division efflux system AdeDE in Acinetobacter genomic DNA group 3. Antimicrob Agents Chemother 48:4054–4055. doi: 10.1128/AAC.48.10.4054-4055.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coyne S, Rosenfeld N, Lambert T, Courvalin P, Périchon B. 2010. Overexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 54:4389–4393. doi: 10.1128/AAC.00155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damier-Piolle L, Magnet S, Bremont S, Lambert T, Courvalin P. 2008. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob Agents Chemother 52:557–562. doi: 10.1128/AAC.00732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eijkelkamp BA, Hassan KA, Paulsen IT, Brown MH. 2011. Development of a high-throughput cloning strategy for characterization of Acinetobacter baumannii drug transporter proteins. J Mol Microbiol Biotechnol 20:211–219. doi: 10.1159/000329836. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan VB, Rajamohan G, Pancholi P, Marcon M, Gebreyes WA. 2011. Molecular cloning and functional characterization of two novel membrane fusion proteins in conferring antimicrobial resistance in Acinetobacter baumannii. J Antimicrob Chemother 66:499–504. doi: 10.1093/jac/dkq469. [DOI] [PubMed] [Google Scholar]
- 14.Su X-Z, Chen J, Mizushima T, Kuroda T, Tsuchiya T. 2005. AbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. Antimicrob Agents Chemother 49:4362–4364. doi: 10.1128/AAC.49.10.4362-4364.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan VB, Rajamohan G, Gebreyes WA. 2009. Role of AbeS, a novel efflux pump of the SMR family of transporters, in resistance to antimicrobial agents in Acinetobacter baumannii. Antimicrob Agents Chemother 53:5312–5316. doi: 10.1128/AAC.00748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Hassan KA, Brown MH, Paulsen IT. 2016. Rapid multiplexed phenotypic screening identifies drug resistance functions for three novel efflux pumps in Acinetobacter baumannii. J Antimicrob Chemother 71:1223–1232. doi: 10.1093/jac/dkv460. [DOI] [PubMed] [Google Scholar]
- 17.Hassan KA, Liu Q, Henderson PJF, Paulsen IT. 2015. Homologs of the Acinetobacter baumannii AceI transporter represent a new family of bacterial multidrug efflux systems. mBio 6:e01982-14. doi: 10.1128/mBio.01982-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribera A, Roca I, Ruiz J, Gibert I, Vila J. 2003. Partial characterization of a transposon containing the tet(A) determinant in a clinical isolate of Acinetobacter baumannii. J Antimicrob Chemother 52:477–480. doi: 10.1093/jac/dkg344. [DOI] [PubMed] [Google Scholar]
- 19.Martí S, Fernández-Cuenca F, Pascual A, Ribera A, Rodríguez-Baño J, Bou G, Miguel Cisneros J, Pachón J, Martínez-Martínez L, Vila J, Grupo de Estudio de Infección Hospitalaria (GEIH). 2006. Prevalence of the tetA and tetB genes as mechanisms of resistance to tetracycline and minocycline in Acinetobacter baumannii clinical isolates. Enferm Infecc Microbiol Clin 24:77–80. doi: 10.1157/13085012 (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 20.Roca I, Marti S, Espinal P, Martínez P, Gibert I, Vila J. 2009. CraA, a major facilitator superfamily efflux pump associated with chloramphenicol resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 53:4013–4014. doi: 10.1128/AAC.00584-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamidian M, Nigro SJ, Hartstein RM, Hall RM. 2017. RCH51, a multiply antibiotic-resistant Acinetobacter baumannii ST103IP isolate, carries resistance genes in three plasmids, including a novel potentially conjugative plasmid carrying oxa235 in transposon Tn6252. J Antimicrob Chemother 72:1907–1910. doi: 10.1093/jac/dkx069. [DOI] [PubMed] [Google Scholar]
- 22.Fournier P-E, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, Richet H, Robert C, Mangenot S, Abergel C, Nordmann P, Weissenbach J, Raoult D, Claverie J-M. 2006. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet 2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma A, Sharma R, Bhattacharyya T, Bhando T, Pathania R. 2017. Fosfomycin resistance in Acinetobacter baumannii is mediated by efflux through a major facilitator superfamily (MFS) transporter-AbaF. J Antimicrob Chemother 72:68–74. doi: 10.1093/jac/dkw382. [DOI] [PubMed] [Google Scholar]
- 24.Lin M-F, Lin Y-Y, Lan C-Y. 2017. Contribution of EmrAB efflux pumps to colistin resistance in Acinetobacter baumannii. J Microbiol 55:130–136. doi: 10.1007/s12275-017-6408-5. [DOI] [PubMed] [Google Scholar]
- 25.Hassan KA, Brzoska AJ, Wilson NL, Eijkelkamp BA, Brown MH, Paulsen IT. 2011. Roles of DHA2 family transporters in drug resistance and iron homeostasis in Acinetobacter spp. J Mol Microbiol Biotechnol 20:116–124. doi: 10.1159/000325367. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Li W, Liu S, Xu J. 2016. RaptorX-Property: a web server for protein structure property prediction. Nucleic Acids Res 44:W430–W435. doi: 10.1093/nar/gkw306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrödinger LLC. 2010. The PyMOL molecular graphics system, version 1.3r1 http://www.pymol.org.