Azithromycin is extensively used in children with community-acquired pneumonia (CAP). Currently, the intravenous azithromycin is used off-label in children partly due to lacking of pharmacokinetic data.
KEYWORDS: azithromycin, pharmacokinetics, children, dosing regimen, community-acquired pneumonia
ABSTRACT
Azithromycin is extensively used in children with community-acquired pneumonia (CAP). Currently, the intravenous azithromycin is used off-label in children partly due to lacking of pharmacokinetic data. Our objective was to evaluate the population pharmacokinetics (PPK) and optimize dose strategy in order to improve treatment in this distinctive population. This was a prospective, multicenter, open-labeled pharmacokinetic study. Blood samples were collected from hospitalized pediatric patients and concentrations were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). PPK analysis was conducted using NONMEM software. The pharmacokinetic data from 95 pediatric patients (age range, 2.1 to 11.7 years) were available for analysis. The PPK was best fitted by a two-compartment model with linear elimination. Covariate analysis verified that body weight and alanine aminotransferase (ALT) had significant effects on azithromycin pharmacokinetics, yielding a 24% decrease of clearance in patients with ALT of >40. Monte Carlo simulation showed that for children with normal liver function, a loading-dose strategy (a loading dose of 15 mg/kg of body weight followed by maintenance doses of 10 mg/kg) would achieve the ratio of the area under free drug plasma concentration-time curve over 24 h (fAUC) to MIC90 (fAUC/MIC) target of 3 h in 53.2% of hypothetical patients, using a normative MIC susceptibility breakpoint of 2 mg/liter. For children with ALT of >40, the proposed dose needed to decrease by 15% to achieve comparable exposure. The corresponding risk of overdose for the recommended dosing regimen was less than 5.8%. In conclusion, the PPK of azithromycin was evaluated in children with CAP and an optimal dosing regimen was constructed based on developmental pharmacokinetic-pharmacodynamic modeling and simulation.
INTRODUCTION
Azithromycin, a macrolide antibiotic containing a 15-member azalactone ring, is frequently prescribed in pediatric patients for the empirical treatment of community-acquired pneumonia (CAP) (1–3). By binding to the 50S ribosomal subunit of susceptible organisms and interfering with protein synthesis, azithromycin exerts high potency against Mycoplasma pneumoniae, Streptococcus pneumoniae, and Chlamydia pneumoniae, yielding low MICs that could inhibit a wide range of pathogens in CAP infection (4, 5).
After intravenous administration, azithromycin undergoes a rapid distribution from blood into phagocytes and then is released into infection sites, with sustained tissue concentrations and a long half-life, which lead to substantial advantages of increased convenience and short treatment regimens (6). The dominant route of elimination is biliary excretion of unchanged drug, accounting for approximately 80 to 90% of the dose (7). On the basis of peculiar pharmacokinetic (PK) and pharmacodynamic (PD) features, as well as favorable safety profiles, azithromycin is particularly appealing for children with CAP (8). PD studies indicate that the efficacy of azithromycin therapy was correlated best with the ratio of the area under free drug plasma concentration-time curve over 24 h (fAUC) to the MIC90 (fAUC/MIC) (9, 10). A target fAUC/MIC value of 3 h was associated with pathogen eradication and good clinical outcome in neonates (11).
Currently, intravenous azithromycin is used off-label in children, partly due to a lack of PK data, which are recognized as indispensable elements for dosing optimization (12). Inadequate dosing in pediatrics may cause treatment failure or increased toxicity (13–15). Given the extensive use of azithromycin in the empirical treatment of CAP in children, the optimal dosage regime needs to be well defined in order to fortify antimicrobial efficacy and minimize drug-related toxicity.
Therefore, we aimed to assess the population PK (PPK) parameters of azithromycin in children and to establish an evidence-based dosage regimen on the basis of developmental pharmacokinetics-pharmacodynamics.
RESULTS
Study population.
After exclusion of 9 patients with incomplete dosing information, a total of 95 pediatric patients from 2015 to 2017 were recruited as an original group for model building. All the patients met the inclusion criteria, and informed consent was obtained. The mean (standard deviation [SD]) age and weight of the 95 patients at sampling time were 6.2 (2.6) (range, 2.1 to 11.7) years and 23.9 (9.8) (range, 11.0 to 51.0) kilograms (kg), respectively. The patient characteristics are summarized in Table 1.
TABLE 1.
Baseline characteristics of the 95 children in this studya
| Patient characteristic | Mean (SD) | Median (range) |
|---|---|---|
| Age (yrs) | 6.2 (2.6) | 5.9 (2.1–11.7) |
| Body wt (kg) | 23.9 (9.8) | 21.5 (11.0–51.0) |
| Height (cm) | 117.7 (19.4) | 120.0 (86.0–158.0) |
| Alanine aminotransferase (IU/liter) | 26.8 (40.2) | 20.3 (3.3–374.4) |
| Aspartate aminotransferase (IU/liter) | 33.8 (32.3) | 25.4 (13.8–245.6) |
| Blood urea nitrogen (mmol/liter) | 3.3 (1.0) | 3.1 (1.8–6.7) |
| Serum creatinine concn (μmol/liter) | 36.0 (6.8) | 35.4 (23.5–49.1) |
Subjects included 53 males and 42 females.
Model building.
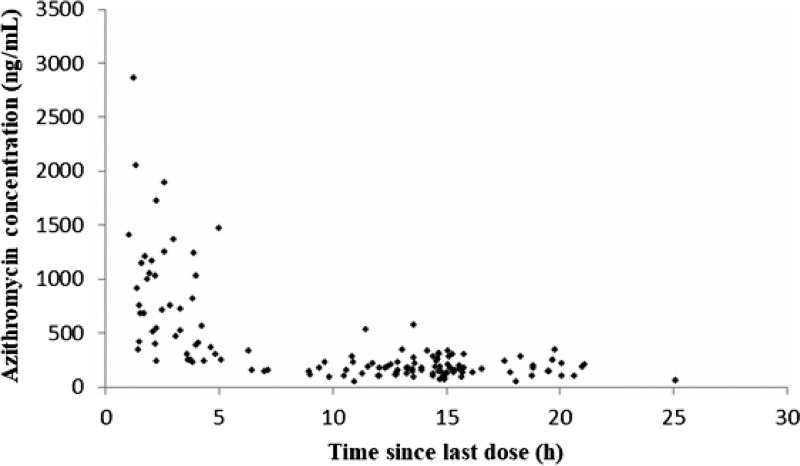
One hundred forty measureable azithromycin concentrations were obtained for PPK modeling. The plasma concentration-time profile is illustrated in Fig. 1.
FIG 1.
Observed concentrations of azithromycin in 95 children.
Initiatory analysis showed that a two-compartment structural model (ADVAN3 TRANS4 NONMEM subroutine) with linear elimination best described the data. The two-compartment model resulted in lower change in objective function value (ΔOFV) value and residual variability than in a one-compartment model. The decrease of the OFV was 144.26. The model-derived pharmacokinetic parameters for azithromycin were clearance (CL), central volume of distribution (V1), intercompartment clearance (Q), and peripheral volume of distribution (V2). For azithromycin, both the interindividual variability and residual variability were best fitted by exponential models. The interindividual variability was further estimated for V1, V2, and CL.
Covariate analysis.
The effect of weight on PPK parameters was evaluated by both fixed exponential allometric scaling and covariate systemic analysis methods. The allometric scaling approach was conducted by incorporating a priori the body weight with an allometric coefficient value of 0.75 for clearances and 1 for volumes into the basic PPK model, which brought a significant OFV drop, of 33.877, and showed better model fit than the covariate systemic analysis method. Therefore, the fixed exponential allometric scaling method was used in our following analysis. In the stepwise forward-inclusion process, alanine aminotransferase (ALT) was verified as the most significant covariate that impacts CL, associated with an OFV drop of 11.119 units. Age on V2 and sex on CL caused significant drops in the OFV of 9.393 and 6.078, respectively. However, only ALT was retained into the model after backward-elimination process. No other predefined covariates were found to be significant. Table 2 presents the detailed process of covariate analysis.
TABLE 2.
Covariate analysisa
| Parameter | PK parameter(s) | Objective function value |
|---|---|---|
| Structural model | 1,477.506 | |
| Body wt (allometric model) | CL + V1 + V2 + Q | 1,443.629 |
| Impact of age | CL | 1,443.747 |
| V1 | 1,443.648 | |
| V2 | 1,434.236 | |
| Q | 1,437.701 | |
| V2 + Q | 1,442.732 | |
| Impact of liver function, ALT | CL | 1,432.51 |
| Impact of renal function, serum creatinine | CL | 1,443.622 |
| Impact of sex | CL | 1,437.551 |
| V1 | 1,443.419 | |
| V2 | 1,443.692 | |
| Q | 1,443.692 | |
| Impacts of age and liver function | V2 (age) + CL (AST) | 1,431.775 |
| Impacts of age and sex | V2 (age) + CL (sex) | 1,434.24 |
| Impacts of liver function and sex | CL (AST) + CL (sex) | 1,436.548 |
| Impacts of age, liver function, and sex | V2 (age) + CL (AST) + CL (sex) | 1,434.898 |
CL, clearance; V1, central volume of distribution; V2, peripheral volume of distribution; Q, intercompartment clearance; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
The estimated PPK parameters of azithromycin in children are presented in Table 3. The median (range) for predicted weight-normalized CL and volume of distribution at steady state were 1.23 (0.56 to 2.74) liters/h/kg and 19.36 (17.91 to 26.46) liters/kg, respectively. The AUC at steady state for the evaluated 10-mg/kg dose regimen ranged from 3.65 to 18.50 mg · h/liter, with the median value of 8.14 mg · h/liter. Azithromycin CL decreased allometrically with body weight in children and showed a 24% decrease in patients with ALT of >40. The function between azithromycin weight-normalized CL (liters per kilogram) versus patient ALT is shown as CL = θ1 × (wt/21.5)0.75 × θ5** Fliver, where wt is body weight in kilograms, Fliver = 0 for ALT of ≤40, Fliver = 1 for ALT of >40, and asterisks (**) mean exponential function.
TABLE 3.
Final population pharmacokinetic parameters of azithromycin and bootstrap resultsa
| Parameter | Full data set |
Bootstrap |
||
|---|---|---|---|---|
| Final estimate | RSE (%) | Median | 5th–95th | |
| CL (liters/h) | ||||
| CL = θ1 × (wt/21.5)0.75 × θ5** Fliver | 27.8 | 7.1 | 27.1 | 22.5–30.4 |
| θ1 | ||||
| If ALT ≤ 40, Fliver = 0 | ||||
| If ALT > 40, Fliver = 1 | 0.761 | 14.7 | 0.796 | 0.584–1.05 |
| θ5 | ||||
| V1 (liters) | ||||
| V1 = θ2 × (wt/21.5) | 39.5 | 43.8 | 32.6 | 14.2–62.6 |
| θ2 | ||||
| V2 (liters) | ||||
| V2 = θ3 × (wt/21.5) | 377 | 13.4 | 375 | 221–491 |
| θ3 | ||||
| Q (liters/h) | ||||
| Q = θ4 × (wt/21.5)0.75 | 55.7 | 22.4 | 51.4 | 26.4–73.8 |
| θ4 | ||||
| Interindividual variability (%) | ||||
| CL | 32.1 | 25.2 | 28.9 | 11.3–36.5 |
| V1 | 84.9 | 41.2 | 82.6 | 31.4–123 |
| Q | 51.6 | 29.4 | 46.4 | 9.8–63.2 |
| Residual variability (%) | 5.7 | 161.6 | 7.2 | 2.2–30.5 |
RSE, relative standard error; wt, body weight in kilograms; Fliver, scaling factor applied for patients with impaired hepatic function. In our population, 21.5 kg is the median body weight.
Model qualification. (i) Internal qualification.
The diagnostic plots demonstrated acceptable goodness of fit for the final PPK model of azithromycin. As depicted in Fig. S1A and B in the supplemental material, there is no apparent systematic bias. The diagnostics of conditional weighted residuals (CWRES) versus time as well as population predicted azithromycin plasma concentrations (PRED) are presented in Fig. S1C and D, showing no obvious trends. Additionally, the median parameter estimates derived from the bootstrap approach correlate well with the corresponding values in the final PPK model, implying that the final PPK model is stable and the parameter estimates can be redetermined (Table 3). In Fig. S2, the NPDE histogram and distribution comply well with the theoretical N (0, 1) distribution as well as density, implying that the model fit the individual data well. The mean value and variance of NPDE were 0.0196 (P = 0.798, Wilcoxon signed-rank test) and 1.09 (P = 0.432, Fisher variance test), respectively.
(ii) External qualification.
In the aggregate, 28 samples from 28 patients were collected for the external evaluation. The individual weights of this independent group ranged from 10.0 to 50.0 (median, 18.5; SD, 8.7) kg. The ages ranged from 2.0 to 11.0 (median, 4.1; SD 2.8) years. The mean prediction error (MPE) and mean absolute prediction error (MAE) were 1.37% and 3.84%, respectively, showing good prediction of developed models on new patients.
Simulation and dosing regimen optimization.
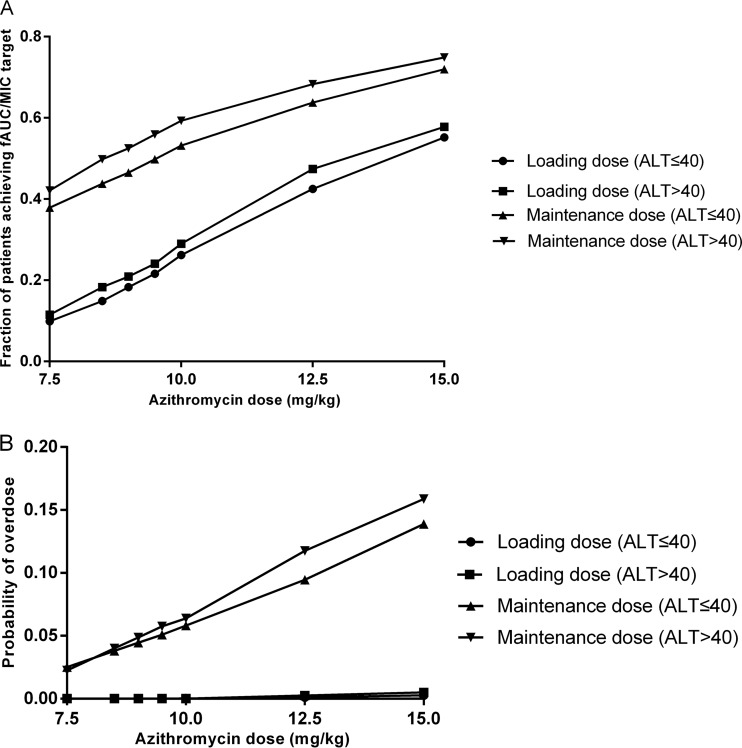
Figure 2 illustrates the target attainment rates as a function of dose and ALT. In children with normal liver function, the probability of attainment of the fAUC/MIC target value of 3 h at the first day for a loading dose of 15 mg/kg was 55.2%, and that at the steady state for the maintenance dose of 10 mg/kg was 53.2%. For children with impaired liver function, simulation showed that a decrease of dose by 15% yielded almost 50% probability of attainment.
FIG 2.
Results of the Monte Carlo simulation with target attainment rates (A) and probability of overdose (B) against a range of doses. The rates of different loading doses were calculated for pediatric patients with ALT ≤40 (●) and ALT >40 (■), and the rates of different maintenance doses were calculated for pediatric patients with ALT of ≤40 (▲) and ALT of >40 (▼).
The related risks of overdose for the proposed dosing regimens were 5.8% and 3.8%, respectively, for pediatric patients with normal and impaired liver function. If the 15% dose reduction is not performed in children with ALT of >40, the probability of overdose increased from 3.8% to 6.3%.
DISCUSSION
To the best of our knowledge, this is the first PPK analysis of intravenous azithromycin performed in a cohort of pediatric patients with CAP. The overall objective was to describe the PK properties of azithromycin in children, assess the effects of demographic, clinical, and biological elements on azithromycin disposition, and establish an optimized regimen. The present results showed that a two-compartment model with linear elimination along with body weight and ALT as significant covariates were optimal for data modeling.
In the present study, the mean CL of azithromycin in children aged 2 to 12 years was 1.288 liters/h/kg. This is in accordance with a previous pharmacokinetic study of intravenous azithromycin that reported average CL values of 1.062 liters/h/kg in 7 children aged 2 to 6 years and 0.960 liter/h/kg in 8 children aged 6 to 12 years (12). After stepwise forward-inclusion and backward-elimination processes, body weight and ALT were identified as significant factors influencing azithromycin PK in children. Indeed, the inclusion of body weight as a covariate significantly improved the model fit. The significant effects of body weight as a surrogate of size on azithromycin PK have been reported for preterm infants (11, 13).
As azithromycin is mostly eliminated by the biliary route, hepatic function is expected to have prominent influence on azithromycin clearance and dosing regimen in children (16). ALT, a surrogate of hepatic function, independently influenced azithromycin clearance. Azithromycin CL was significantly lower in patients with ALT of >40 than in those with ALT of ≤40 (0.988 liter/h/kg versus 1.309 liters/h/kg). Impaired hepatic function could reduce biliary secretion of azithromycin into bile, thereby increasing the plasma concentration in the circulation. Therefore, adapted dosing was required in this specific patient group.
According to EUCAST and microbiology data provided in the labeling, most bacterial strains in CAP have a MIC90 of 2 mg/liter or less, so 2 mg/liter was selected in dosing optimization to cover most pathogens in CAP infection (7, 17). Preclinical PD studies indicate that fAUC/MIC90 seems to be the best predictor of antimicroorganism efficacy and clinical outcome (8). It should be noted that MIC and pharmacodynamics for Mycoplasma infection have not been established. A target fAUC/MIC value of 3 h was selected for dosing optimization, as this target has shown positive microbiological and clinical outcome in neonates and infants with mixed infection (11).
The optimal antimicrobial therapy should balance the efficacy and toxicity (18). Thus, in this study, Monto Carlo simulations were conducted using the estimated parameters from the final PPK model, and the dosing regimen was optimized on the basis of achieving the target fAUC/MIC value of 3 h in about 50% of pediatric patients, assuming a considerable safety profile. Accordingly, a loading dose of 15 mg/kg followed by maintenance doses of 10 mg/kg was proposed for azithromycin. In the optimal dosing regimen, the selected maintenance dose is the same as the dose used in the study, and the 15-mg/kg loading dose of azithromycin was proposed for the first time for children, which allows achieving the target concentrations in the first 24 to 48 h. Given the long elimination half-life of azithromycin, a loading dose strategy was required to achieve early target attainment, which is the key factor influencing the outcome of antimicrobial therapy. The relationship between early appropriate therapy and enhanced survival has been well demonstrated (19). The first 24 h is the prime time for the success of azithromycin therapy.
There are limitations in our present study. Owing to small sample volumes, intracellular concentrations of azithromycin were not determined. Azithromycin concentrates in white blood cells, such as neutrophils, macrophages, and monocytes, and then is released at the site of infection, resulting in 100- to 300-fold higher intracellular concentrations than plasma concentrations (6, 20). The concentration in white blood cells might be also related to antibacterial efficacy (21). Future study is required to confirm the clinical value of intracellular azithromycin concentration determination.
Conclusion.
The PPK model of azithromycin was developed in children and elucidated the significant effects of weight and ALT on azithromycin pharmacokinetics. A loading-dose strategy was established in children with CAP based on developmental pharmacokinetics-pharmacodynamics.
MATERIALS AND METHODS
Study design.
This trial was a prospective, multicenter, open-labeled PK study of azithromycin, performed in Beijing Children's Hospital, Children's Hospital of Hebei Province, Shandong Provincial Qianfoshan Hospital, and Xintai People's Hospital. Pediatric patients aged 2 to 12 years with suspected or confirmed CAP receiving intravenous azithromycin as part of their routine antimicrobial therapy were enrolled in the study, which was approved by each institutional ethics committee. The present study was conducted on the basis of ethical principles of the good clinical practice guidelines as well as the Declaration of Helsinki.
Dosing regimen, PK sampling, and data collection.
Azithromycin (Pfizer Ireland Pharmaceuticals) was intravenously administered by syringe pump linked to a microbore valve at an infusion time over 60 min at a dose of 10 mg/kg once daily. Opportunistic sampling design was chosen to collect pharmacokinetic samples (22). Briefly, after routine biochemical and microbiological tests were performed, remaining blood specimens were collected. Only the samples with identified sampling information were included. The form with specific infusion and sample times was filled in to record precise timings. Plasma samples were harvested from blood specimens after 10 min of centrifugation at 2,500 × g at 4°C and stored at −70°C prior to analysis. Clinical and demographic data, including age, sex, body weight, height, blood urea nitrogen (BUN), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and serum creatinine (SCr) were extracted from the medical records of pediatric patients in the study.
Concentration measurement of azithromycin.
The concentration of azithromycin was measured according to a previously published method, with adaption (23). Briefly, an approach using high-performance liquid chromatography coupled with mass spectrometry was developed for azithromycin concentration determination. Positive electrospray ionization tandem mass spectrometry with a multiple-reaction monitoring mode was choose for the detection. Liquid-liquid extraction was applied with acetonitrile as the extraction solvent. The calibration curve was linear over the range of 10 to 2,000 ng/ml. The lower limit of quantification was identical to the lowest calibration levels. The interassay and intra-assay coefficients of variation of controls were less than 7.9% and 4.8%, respectively. The accuracies of controls were 113.5%, 101.6%, and 92.9%, respectively.
Model development.
PK analysis was conducted by a population compartmental modeling approach using NONMEM V 7.2 software (Icon Development Solutions, USA). The first-order conditional estimation (FOCE) method with interaction was used throughout the modeling procedure to predict PK parameters and their variability.
The interindividual variability of PK parameters was described by the following exponential error model: ϴi = θmean × eηi, where θi is the hypothetical true value of parameter for the ith subject, θmean is the typical population parameter value, and ηi is the proportional difference between subjects, which is supposed to be normally distributed with a mean of zero and a variance of ω2.
The residual errors were evaluated by different residual variability models containing additive, proportional, exponential, and combinational models. Covariate analysis was performed by a stepwise forward-inclusion and backward-elimination procedure. The likelihood ratio test was conducted to evaluate the statistically significant variables on the proposed model parameters. The potential effects of variables including body weight, age, sex, height, BUN, ALT, AST, and SCr on PK parameters were examined. During stepwise forward-inclusion step, a covariate was included in the model if a 0.05 significance of objective function value (OFV) decrease from the base model (OFV reduction > 3.84) was acquired and a reduction in the PK parameter variability was observed. All statistically significant covariates were synchronously added into the basic model to create the “full” model. Subsequently, backward elimination was independently performed in each covariate from the full model on the basis of a 0.01 significance level (OFV increase > 6.635).
Model qualification. (i) Internal model qualification.
Internal model validation was conducted using goodness of fit and a bootstrap approach. First, goodness-of-fit plots using observed concentrations versus population predicted (PRED) as well as individual predicted (IPRED) plasma azithromycin concentrations were examined. Residual plots using conditional weighted residuals (CWRES) versus time as well as PRED were checked (28). Then the nonparametric bootstrap using resampling and replacement methods was conducted to assess the performance and stability of the final PPK model. Resampling was repeated 1,000 times, and the parameter values predicted from the bootstrap method were further contrasted with those derived from the original data set.
A normalized prediction distribution error approach (NPDE) was also conducted to evaluate the final PPK model (24). The NPDE results, derived from a simulated data set of 1,000 using final PPK model parameters, were summarized graphically by the programming language R package (V3.4.1). The NPDE is expected to follow the theoretical N (0, 1) distribution (25).
(ii) External model qualification.
Data based on an independent group of children (n = 28; number of samples = 28) were collected to further evaluate the predictive performance of the constructed model. The predicted concentrations of individuals were obtained by applying Bayesian estimation with NONMEM using the PPK parameters (MAXEVAL = 0′ during the ESTIMATION step) (26). The predictive performance of the PPK model was assessed by mean prediction error (MPE) and mean absolute prediction error (MAE), which were calculated as follows and are expressed as percentages:
where PREDi is the predicted concentration for the ith subject and OBSi is the observed concentration for the ith subject.
Simulation and dosing regimen optimization.
The final PPK model was used to simulate azithromycin concentrations at different time points. Monte Carlo simulations were performed using NONMEM in order to optimize the dose strategy that is able to achieve the target fAUC/MIC value of 3 h in about 50% of children with CAP. To ensure comparable safety conditions, the proportion of pediatric patients exceeding the median of the area drug plasma concentration-time curve over 24 h (AUC) value of 30 mg · h/liter reported in a previous study without drug-related adverse events was taken into account (13). An azithromycin AUC value of 30 mg · h/liter was defined as an overdose. The doses of azithromycin in pediatric patients with CAP were simulated on milligram-per-kilogram bases. Thus, varied milligram-per-kilogram dosing regimens (7.5, 10, 12.5, 15, 17.5, and 20 mg/kg once daily) were simulated in hypothetic clinical trials for children with CAP with normal liver function (ALT ≤ 40) and impaired liver function (ALT > 40), respectively. Simulations were performed 100 times using the original data set, and AUCs at first dose and at steady state were calculated for every simulated patient. The percentage of patients achieving fAUC/MIC target ratio of 3 h were calculated subsequently to optimize the appropriate dose regimen in each subgroup. On the basis of previously published data, an azithromycin plasma protein binding ratio of 30% (13, 27) and MIC value of 2 mg/liter were selected for simulation (7). Thus, the AUC was obtained by extrapolation methods and fAUC was calculated by the following equation: fAUC = 30% × AUC.
A concentration of 2 mg/liter was selected in dosing optimization to cover most pathogens in CAP infection.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported by the National Science Foundation of China (81703603), China Postdoctoral Science Foundation (2015M582102, 2017M620831), National Science and Technology Major Projects for Major New Drugs Innovation and Development (2017ZX09304029-002 and 2017ZX09304029-005), Young Taishan Scholars Program, Fundamental Research Fund (2016GN029), Capital’s Funds for Health Improvement and Research (2016-1-2092), and Young Scholars Program of Shandong University (2015WLJH49).
We declare no conflicts of interest related to this work.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00686-18.
REFERENCES
- 1.Smith C, Egunsola O, Choonara I, Kotecha S, Jacqz-Aigrain E, Sammons H. 2015. Use and safety of azithromycin in neonates: a systematic review. BMJ Open 5(12):e008194. doi: 10.1136/bmjopen-2015-008194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langtry HD, Balfour JA. 1998. Azithromycin: a review of its use in paediatric infectious diseases. Drugs 56:273–297. doi: 10.2165/00003495-199856020-00014. [DOI] [PubMed] [Google Scholar]
- 3.Lee H, Yun KW, Lee HJ, Choi EH. 2018. Antimicrobial therapy of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Expert Rev Anti Infect Ther 16:23–34. doi: 10.1080/14787210.2018.1414599. [DOI] [PubMed] [Google Scholar]
- 4.Retsema J, Girard A, Schelky W, Manousos M, Anderson M, Bright G, Borovoy R, Brennan L, Mason R. 1987. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother 31:1939–1947. doi: 10.1128/AAC.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. 2014. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 143:225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Matzneller P, Krasniqi S, Kinzig M, Sörgel F, Hüttner S, Lackner E, Müller M, Zeitlinger M. 2013. Blood, tissue, and intracellular concentrations of azithromycin during and after end of therapy. Antimicrob Agents Chemother 57:1736–1742. doi: 10.1128/AAC.02011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfizer Laboratories. 1997. Zithromax (azithromycin capsules; azithromycin tablets; azithromycin for oral suspension; and azithromycin for injection). Package insert. Pfizer Laboratories, New York, NY. [Google Scholar]
- 8.Rapp RP. 1998. Pharmacokinetics and pharmacodynamics of intravenous and oral azithromycin: enhanced tissue activity and minimal drug interactions. Ann Pharmacother 32:785–793. [DOI] [PubMed] [Google Scholar]
- 9.Drusano GL, Craig WA. 1997. Relevance of pharmacokinetics and pharmacodynamics in the selection of antibiotics for respiratory tract infections. J Chemother 9(Suppl 3):S38–S44. [PubMed] [Google Scholar]
- 10.Noreddin AM, El-Khatib WF, Aolie J, Salem AH, Zhanel GG. 2009. Pharmacodynamic target attainment potential of azithromycin, clarithromycin, and telithromycin in serum and epithelial lining fluid of community-acquired pneumonia patients with penicillin-susceptible, intermediate, and resistant Streptococcus pneumoniae. Int J Infect Dis 13:483–487. doi: 10.1016/j.ijid.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Hassan HE, Othman AA, Eddington ND, Duffy L, Xiao L, Waites KB, Kaufman DA, Fairchild KD, Terrin ML, Viscardi RM. 2011. Pharmacokinetics, safety, and biologic effects of azithromycin in extremely preterm infants at risk for ureaplasma colonization and bronchopulmonary dysplasia. J Clin Pharmacol 51:1264–1275. doi: 10.1177/0091270010382021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs RF, Maples HD, Aranda JV, Espinoza GM, Knirsch C, Chandra R, Fisher JM, Kearns GL. 2005. Pharmacokinetics of intravenously administered azithromycin in pediatric patients. Pediatr Infect Dis J 24:34–39. doi: 10.1097/01.inf.0000148927.48680.fc. [DOI] [PubMed] [Google Scholar]
- 13.Viscardi RM, Othman AA, Hassan HE, Eddington ND, Abebe E, Terrin ML, Kaufman DA, Waites KB. 2013. Azithromycin to prevent bronchopulmonary dysplasia in Ureaplasma-infected preterm infants: pharmacokinetics, safety, microbial response, and clinical outcomes with a 20-milligram-per-kilogram single intravenous dose. Antimicrob Agents Chemother 57:2127–2133. doi: 10.1128/AAC.02183-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pineda LC, Watt KM. 2015. New antibiotic dosing in infants. Clin Perinatol 42(1):167–176. doi: 10.1016/j.clp.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sammons HM, Choonara I. 2016. Learning lessons from adverse drug reactions in children. Children (Basel) 3(1):E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan DJ, McLean AJ. 1995. Clinical pharmacokinetic and pharmacodynamic considerations in patients with liver disease. An update. Clin Pharmacokinet 29:370–391. [DOI] [PubMed] [Google Scholar]
- 17.EUCAST. 2018. Breakpoint tables for interpretation of MICs and zone diameters, version 8.1. EUCAST, Växjö, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.1_Breakpoint_Tables.pdf. [Google Scholar]
- 18.Deshpande D, Pasipanodya JG, Gumbo T. 2016. Azithromycin dose to maximize efficacy and suppress acquired drug resistance in pulmonary Mycobacterium avium disease. Antimicrob Agents Chemother 60:2157–2163. doi: 10.1128/AAC.02854-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, Jackson DJ, Baxi SN, Benson M, Burnham CD, Cabana M, Castro M, Chmiel JF, Covar R, Daines M, Gaffin JM, Gentile DA, Holguin F, Israel E, Kelly HW, Lazarus SC, Lemanske RF Jr, Ly N, Meade K, Morgan W, Moy J, Olin T, Peters SP, Phipatanakul W, Pongracic JA, Raissy HH, Ross K, Sheehan WJ, Sorkness C, Szefler SJ, Teague WG, Thyne S, Martinez FD. 2015. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA 314:2034–2044. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legrand T, Elie V, Kotecha S, Junot C, Jacqz-Aigrain E, Pruvost A. 2014. An optimal LC-MS/MS method for determination of azithromycin in white blood cells: application to pediatric samples. Bioanalysis 6:2317–2328. doi: 10.4155/bio.14.81. [DOI] [PubMed] [Google Scholar]
- 21.Pea F. 2018. Intracellular pharmacokinetics of antibacterials and their clinical implications. Clin Pharmacokinet 57:177–189. doi: 10.1007/s40262-017-0572-y. [DOI] [PubMed] [Google Scholar]
- 22.Leroux S, Turner MA, Barin-Le Guellec C, Hill H, van den Anker JN, Kearns GL, Jacqz-Aigrain E, Zhao W. 2015. Pharmacokinetic studies in neonates: the utility of an opportunistic sampling design. Clin Pharmacokinet 54:1273–1285. doi: 10.1007/s40262-015-0291-1. [DOI] [PubMed] [Google Scholar]
- 23.Filist M, Buś-Kwaśnik K, Ksycińska H, Rudzki PJ. 2014. Simplified LC-MS/MS method enabling the determination of azithromycin in human plasma after a low 100 mg dose administration. J Pharm Biomed Anal 100:184–189. doi: 10.1016/j.jpba.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Brendel K, Comets E, Laffont C, Laveille C, Mentré F. 2006. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 23:2036–2049. doi: 10.1007/s11095-006-9067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comets E, Brendel K, Mentré F. 2008. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed 90:154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, Kaguelidou F, Biran V, Zhang D, Allegaert K, Capparelli EV, Holford N, Kimura T, Lo YL, Peris JE, Thomson A, van den Anker JN, Fakhoury M, Jacqz-Aigrain E. 2013. External evaluation of population pharmacokinetic models of vancomycin in neonates: the transferability of published models to different clinical settings. Br J Clin Pharmacol 75:1068–1080. doi: 10.1111/j.1365-2125.2012.04406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merchan LM, Hassan HE, Terrin ML, Waites KB, Kaufman DA, Ambalavanan N, Donohue P, Dulkerian SJ, Schelonka R, Magder LS, Shukla S, Eddington ND, Viscardi RM. 2015. Pharmacokinetics, microbial response, and pulmonary outcomes of multidose intravenous azithromycin in preterm infants at risk for Ureaplasma respiratory colonization. Antimicrob Agents Chemother 59:570–578. doi: 10.1128/AAC.03951-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooker AC, Staatz CE, Karlsson MO. 2007. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res 24:2187–2197. doi: 10.1007/s11095-007-9361-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.