Abstract
This study aimed to identify single nucleotide polymorphisms (SNPs) associated with milk cholesterol (CHL) content via a genome wide association study (GWAS). Milk CHL content was determined by gas chromatography and expressed as mg of CHL in 100 g of fat (CHL_fat) or in 100 mg of milk (CHL_milk). GWAS was performed with 1,183 cows and 40,196 SNPs using a univariate linear mixed model. Two and 20 SNPs were significantly associated with CHL_fat and CHL_milk, respectively. The important regions for CHL_fat and CHL_milk were at 41.9 Mb on chromosome (BTA) 17 and 1.6–3.2 Mb on BTA 14, respectively. DGAT1, PTPN1, INSIG1, HEXIM1, SDS, and HTR5A genes, also known to be associated with human plasma CHL phenotypes, were identified as potential candidate genes for bovine milk CHL. Additional new potential candidate genes for milk CHL were RXFP1, FAM198B, TMEM144, CXXC4, MAML2 and CDH13. Enrichment analyses suggested that identified candidate genes participated in cell-cell signaling processes and are key members in tight junction, focal adhesion, Notch signaling and glycerolipid metabolism pathways. Furthermore, identified transcription factors such as PPARD, LXR, and NOTCH1 might be important in the regulation of bovine milk CHL content. The expression of several positional candidate genes (such as DGAT1, INSIG1 and FAM198B) and their correlation with milk CHL content were further confirmed with RNA sequence data from mammary gland tissues. This is the first GWAS on bovine milk CHL. The identified markers and candidate genes need further validation in a larger cohort for use in the selection of cows with desired milk CHL content.
Introduction
Bovine milk is an important human dietary component, serving as an important delivery medium for proteins, minerals, vitamins and lipids including fatty acids and cholesterol (CHL). Milk fat is one of the principal contributors to daily dietary CHL intake for humans1. Milk CHL content is highly variable between species, breeds and herds and is influenced by many factors including genetics and nutrition2,3. Previously, we demonstrated that genetic factors contributed 10 to 18% of the total phenotypic variation in milk CHL content4.
High concentrations of total or low-density lipoprotein CHL (LDL-CHL) in human blood are linked to risk of cardiovascular diseases (CVD)5–10. Consequently, numerous genome wide association studies (GWAS) have been devoted to mapping genomic regions and variants affecting total CHL, LDL-CHL, high density lipoprotein CHL (HDL-CHL) and triglyceride11–14. In total, 126 GWAS have been performed on CHL related phenotypes in humans and animal model species (https://www.ebi.ac.uk/gwas/search?query=cholesterol, accessed on 09th January, 2018). Although mechanisms regulating CHL have been intensively studied in humans15–18, few studies have been devoted to the genetics of CHL in livestock species. In cows, several gene expression/proteomics studies have reported genes with potential involvement in milk CHL concentration/metabolism19–27 but their actual roles and associated SNPs with CHL content in milk have not been investigated. For instance, Mani et al.28 identified ATP-binding cassette sub-family A member 1 (ABCA1) and ATP-binding cassette sub-family G member 1 (ABCG1) proteins in milk fat globule membranes and suggested their potential involvement in CHL exchange between mammary epithelial cells and alveolar milk. Using cell culture studies, Ontsouka et al.21 indicated that CHL transport in mammary epithelial cells was mediated by APOA-1/ABCA1 and ABCG1/HDL dependent pathways. Studying the response of CHL metabolism to negative energy balance induced by feed restriction, Gross et al.27 observed that CHL metabolism was influenced by nutrient and energy deficiency according to stage of lactation in dairy cows. Together, these studies19–27 suggest modulatory roles of cow’s genetics, physiological stage and diet on the expression of genes involved in CHL synthesis. However, the specific roles of the various genes and their sequence variants in regulating CHL synthesis and content in bovine milk have not been studied and no GWAS has been performed for milk CHL content. This study aimed to identify associated single nucleotide polymorphisms (SNPs), candidate genes and biological pathways involved in the regulation of milk CHL content via GWAS and pathway enrichment. Moreover, mRNA sequence data of mammary gland tissues from 12 cows were used to verify that the candidate genes identified by GWAS are expressed in the mammary gland.
Results
SNPs associated with milk cholesterol
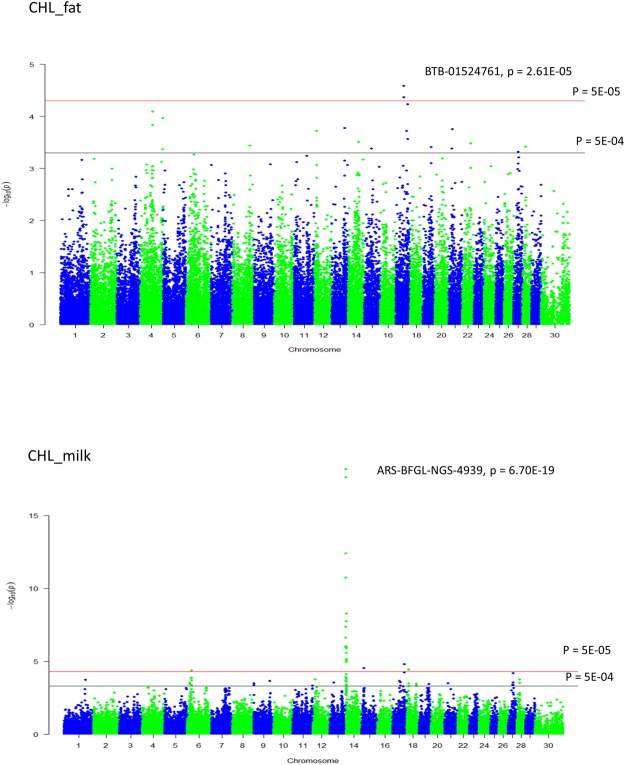
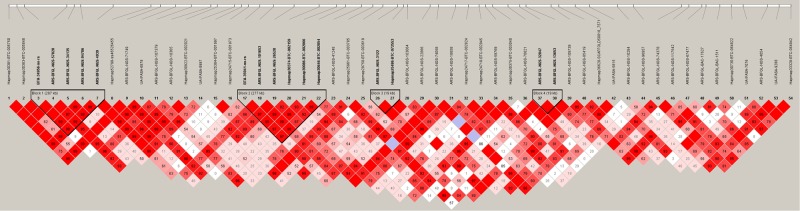
Two and 20 SNPs were significantly associated with CHL_fat and CHL_milk, respectively at the genome wide significant threshold p < 5E-05 (Table 1, Fig. 1); while 19 and 36 SNPs (7 in common) were suggestively associated (p < 5E-04) with CHL_fat and CHL_milk, respectably (Table S1). The quantile-quantile (q-q) plot showed no systematic deviation from the diagonal (Y = X) indicating that the data were corrected for population stratification (Fig. S1). BTB-01524761 (rs42640895) and ARS-BFGL-NGS-4939 (rs109421300) were the most significantly associated SNPs with CHL_fat (p = 2.61E-05) and CHL_milk (p = 6.70E-19), respectively. Two significant SNPs for CHL_fat are located in an intergenic region of bovine chromosome (BTA) 17. The majority of significant SNPs (16 out of 20) for CHL_milk are located within a region of 1.4 to 3.3 Mb of BTA 14. Four LD blocks were detected in this region (Fig. 2) and one of the LD blocks also contained the most significant SNP (ARS-BFGL-NGS-4939 [rs109421300]) for CHL_milk. Other significant SNPs for CHL_milk are located on BTA 6, 15, 17 and 18. Several of the significant SNPs for CHL_milk are located in gene regions (seven within introns and two within exons) (Table 1). Three genes (relaxin–insulin-like family peptide receptor 1 (RXFP1), transmembrane protein 144 (TMEM144) and family with sequence similarity 198, member B (FAM198B)) are located in 0.5 Mb flanking regions to significant SNPs for CHL_fat. Genes including diacylglycerol O-Acyltransferase 1 (DGAT1), rhophilin-1 (RHPN1), cysteine and histidine rich 1 (CYHR1), ENSBTAG00000003606, vacuolar protein sorting 28 (VPS28), two pore segment channel 1 (TPCN1), cadherin 13 (CDH13), ENSBTAG00000045727 and MAF1 homolog, negative regulator of RNA polymerase III (MAF1) contained significant SNPs for CHL_milk (Table 1).
Table 1.
Genome-wide significant SNPs for milk cholesterol content.
| Traita | SNP ID | BTAb | Positionc | Alleles | MAFd | rs# | Allele_sube | p-value | Consequencef | Gene (nearby gene)g |
|---|---|---|---|---|---|---|---|---|---|---|
| CHL_fat | Hapmap40322-BTA-100742 | 17 | 41965769 | G/T | 0 .340 | rs41600454 | 11.29 | 4.26E-05 | intergenic | (FAM198B) |
| CHL_fat | BTB-01524761 | 17 | 41939826 | C/T | 0.336 | rs42640895 | −11.66 | 2.61E-05 | intergenic | (FAM198B) |
| CHL_milk | Hapmap30383-BTC-005848 | 14 | 1489496 | A/G | 0.423 | rs109752439 | 0.85 | 1.80E-11 | downstream | ZNF34 |
| CHL_milk | ARS-BFGL-NGS-18858 | 14 | 2909929 | A/G | 0.450 | rs109558046 | 0.71 | 1.76E-08 | intergenic | (ARC) |
| CHL_milk | Hapmap30646-BTC-002054 | 14 | 2553525 | C/T | 0.356 | rs110060785 | 0.66 | 1.24E-06 | intergenic | (LY6H) |
| CHL_milk | ARS-BFGL-NGS-41837 | 6 | 22129886 | C/T | 0.212 | rs110597360 | 0.63 | 4.14E-05 | intergenic | (ENSBTAG00000001751) |
| CHL_milk | ARS-BFGL-NGS-18365 | 14 | 2117455 | C/T | 0.250 | rs110892754 | −0.67 | 2.68E-06 | intergenic | (bta_mir_2309) |
| CHL_milk | Hapmap36620-SCAFFOLD50018_7571 | 14 | 3297177 | C/T | 0.495 | rs29024688 | 0.58 | 8.37E-06 | intergenic | (TSNARE1) |
| CHL_milk | Hapmap38637-BTA-88156 | 15 | 13964124 | G/T | 0.450 | rs41596665 | −0.54 | 2.86E-05 | intergenic | (ENSBTAG00000009511) |
| CHL_milk | ARS-BFGL-NGS-4939 | 14 | 1801116 | A/G | 0.336 | rs109421300 | −1.17 | 6.70E-19 | intron | DGAT1 |
| CHL_milk | Hapmap30374-BTC-002159 | 14 | 2468020 | A/G | 0.490 | rs109529219 | 0.59 | 7.02E-06 | intron | RHPN1 |
| CHL_milk | ARS-BFGL-NGS-34135 | 14 | 1675278 | A/G | 0.491 | rs109968515 | −0.66 | 2.34E-07 | intron | CYHR1 |
| CHL_milk | Hapmap30086-BTC-002066 | 14 | 2524432 | A/G | 0.406 | rs110199901 | 0.77 | 5.14E-09 | intron | ENSBTAG00000003606 |
| CHL_milk | ARS-BFGL-NGS-94706 | 14 | 1696470 | A/C | 0.493 | rs17870736 | −0.70 | 4.27E-08 | intron | VPS28 |
| CHL_milk | Hapmap52830-rs29014800 | 17 | 63541690 | A/G | 0.403 | rs29014800 | −0.57 | 1.58E-05 | intron | TPCN1 |
| CHL_milk | Hapmap39330-BTA-42256 | 18 | 9797478 | A/C | 0.388 | rs41605812 | −0.54 | 3.63E-05 | intron | CDH13 |
| CHL_milk | Hapmap30922-BTC-002021 | 14 | 2138926 | C/T | 0.240 | rs110749653 | −0.64 | 1.12E-05 | non_coding_transcript_exon | ENSBTAG00000045727 |
| CHL_milk | Hapmap52798-ss46526455 | 14 | 1923292 | A/G | 0.396 | rs41256919 | −0.62 | 1.08E-06 | synonymous | MAF1 |
| CHL_milk | ARS-BFGL-NGS-57820 | 14 | 1651311 | C/T | 0.340 | rs109146371 | −1.15 | 2.42E-18 | upstream | FOXH1 |
| CHL_milk | ARS-BFGL-NGS-107379 | 14 | 2054457 | A/G | 0.372 | rs109350371 | −0.94 | 4.06E-13 | upstream | PLEC |
| CHL_milk | BTA-35941-no-rs | 14 | 2276443 | G/T | 0.498 | rs41627764 | −0.64 | 1.03E-06 | upstream | ENSBTAG00000046866 |
| CHL_milk | UA-IFASA-6878 | 14 | 2002873 | C/T | 0.419 | rs41629750 | −0.62 | 9.06E-07 | upstream | SPATC1 |
aCHL_fat: mg of cholesterol in 100 g of fat, CHL_milk: mg of cholesterol in 100 g of milk. bBos taurus autosome. cSNP position on the UMD3.1 assembly in base pairs. dMinor allele frequency. eAllelic substitution effect. fSNP consequence obtained from Variant effect predictor (http://www.ensembl.org/Tools/VEP). gGene or nearest gene to the corresponding SNP (obtained from Ensembl gene database: http://www.ensembl.org/Bos_taurus/Info/Index.
Figure 1.
Manhattan plot of genome-wide significant (p < 5E-05) and suggestive (p < 5E-04) SNP associations for milk cholesterol content in Canadian Holstein cows. The most significant SNPs with their corresponding p-values are indicated. CHL_fat: mg of cholesterol in 100 gram of fat, CHL_milk: mg of cholesterol in 100 gram of milk.
Figure 2.
Linkage disequilibrium (LD) pattern on a 1.4–3.4 Mb region of BTA 14. LD blocks are marked with triangles; values in boxes are LD (squared correlation coefficient, r2) between SNP pairs; red boxes indicate LOD > 2 and D′ = 1 (LOD is the log of the likelihood odds ratio, a measure of confidence in the value of D′, where D′ is the ratio of the linkage disequilibrium coefficient D to its maximum possible).
Gene ontology, pathways and transcription factor enrichments of positional candidate genes
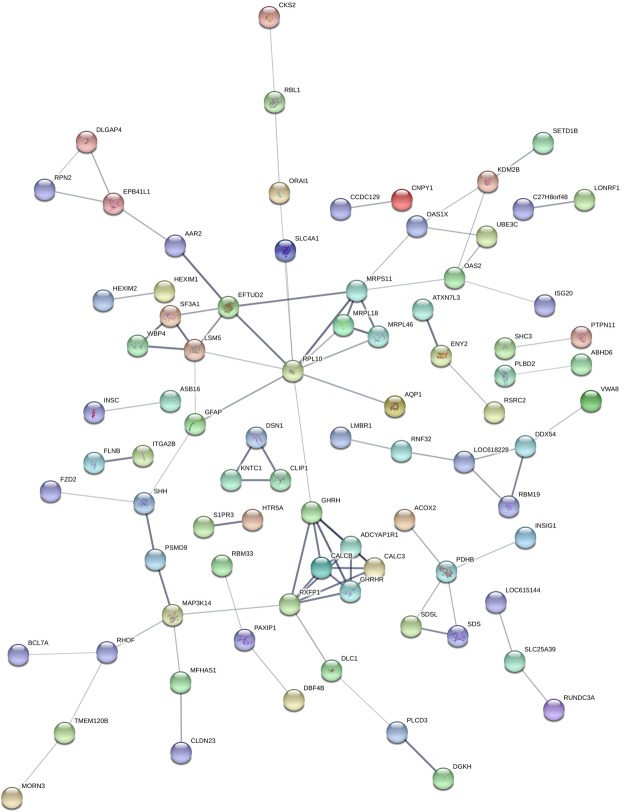
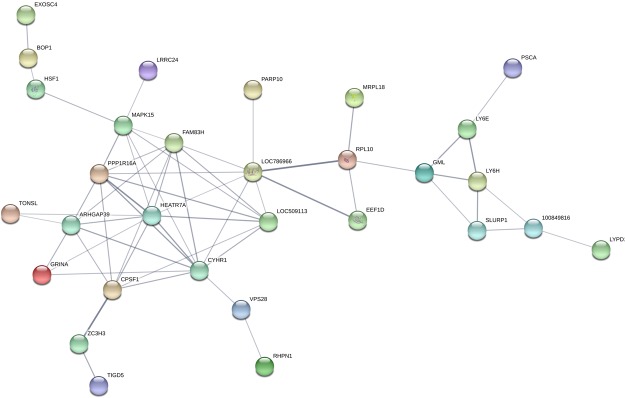
A total of 207 and 320 genes (positional candidate genes) (58 in common, Table S1) annotated at 0.5 Mb flanking regions of 21 and 56 SNPs (significant and suggestive) for CHL_fat and CHL_milk, respectively (Table S1), were used as input for GO and pathways enrichment. A total of 59 and 112 GO terms were enriched for CHL_fat and CHL_milk positional candidate genes, respectively (Table S2). For CHL_fat, negative regulation of cyclin-dependent protein kinase activity (p = 0.001), basolateral plasma membrane (p = 0.007) and cyclin-dependent protein kinase regulator activity (p = 1.10E-04) were the most significant biological processes, cellular component and molecular function GO terms, respectively, enriched for positional candidate genes (Table 2). Meanwhile, cardiac muscle tissue development (p = 1.10E-04), anchored to membrane (p = 0.001) and interleukin-2 receptor binding (p = 8.60E-05) were the most significant biological processes, cellular component and molecular function GO terms, respectively, enriched for CHL_milk positional candidate genes (Table 3). In addition, 5 KEGG pathways (neuroactive ligand-receptor interaction, focal adhesion, leukocyte transendothelial migration, tight junction and basal cell carcinoma) and 2 (glycerolipid metabolism and Notch signaling) were enriched for CHL_fat and CHL_milk positional candidate genes, respectively (Tables 2 and 3). The potential interactions between the positional candidate genes for CHL_fat and CHL_milk are shown in Figs 3 and 4, respectively. PRL10, GHRH, CALCB and RXFP1 interacted highly with other genes for CHL_fat (Fig. 3) while MAPK15, FAM83H, ARHGAP39, HEATR7A, CYHR1 and CPSF1 were among highly interacting genes in the CHL_milk protein interaction network (Fig. 4). Moreover, a total of 20 and 16 transcription factors were enriched for positional candidate genes for CHL_fat and CHL_milk, respectively (Table 4). The most enriched transcription factors for CHL_fat were CREB1 (p = 0.002), PPARD (p = 0.004) and CEBPB (p = 0.005) and for CHL_milk were LXR (p = 1E-11), DACH1 (p = 1E-07) and SMC4 (p = 1.19E-07).
Table 2.
Gene ontology and pathways enriched for positional candidate genes of CHL_fata.
| Categoryb | Names | Number of genes | p-value |
|---|---|---|---|
| GO_BP | Negative regulation of cyclin-dependent protein kinase activity | 2 | 0.001 |
| GO_BP | Cell-cell signaling | 5 | 0.001 |
| GO_BP | Cell communication | 6 | 0.004 |
| GO_BP | Regulation of cyclin-dependent protein kinase activity | 2 | 0.006 |
| GO_BP | Regulation of nervous system development | 3 | 0.007 |
| GO_BP | Organic acid catabolic process | 3 | 0.008 |
| GO_BP | Carboxylic acid catabolic process | 3 | 0.008 |
| GO_BP | Regulation of adenylate cyclase activity | 2 | 0.009 |
| GO_BP | G-protein signaling, coupled to cAMP nucleotide second messenger | 2 | 0.009 |
| GO_BP | G-protein signaling, coupled to cyclic nucleotide second messenger | 2 | 0.009 |
| GO_BP | cAMP-mediated signaling | 2 | 0.010 |
| GO_CC | Basolateral plasma membrane | 3 | 0.007 |
| GO_MF | Cyclin-dependent protein kinase regulator activity | 3 | 1.10E-04 |
| GO_MF | snRNA binding | 2 | 4.80E-04 |
| GO_MF | Cyclin-dependent protein kinase inhibitor activity | 2 | 0.001 |
| GO_MF | Protein serine/threonine kinase inhibitor activity | 2 | 0.003 |
| GO_MF | Protein kinase regulator activity | 3 | 0.003 |
| GO_MF | Kinase regulator activity | 3 | 0.005 |
| GO_MF | Protein kinase inhibitor activity | 2 | 0.006 |
| GO_MF | Kinase inhibitor activity | 2 | 0.008 |
| KEGG | Neuroactive ligand-receptor interaction | 5 | 0.015 |
| KEGG | Focal adhesion | 4 | 0.026 |
| KEGG | Leukocyte transendothelial migration | 3 | 0.032 |
| KEGG | Tight junction | 3 | 0.040 |
| KEGG | Basal cell carcinoma | 2 | 0.043 |
aCHL_fat: mg of cholesterol in 100 g of fat. Only gene ontologies with p-values < 0.01 are shown.
bGO_BP: Biological processes gene ontology term, GO_CC: Cellular component gene ontology term and GO_MF: Molecular function gene ontology term.
Table 3.
Gene ontology and pathways enriched for potential candidate genes of CHL_milka.
| Categoryb | Names | Number of genes | p-value |
|---|---|---|---|
| GO_BP | Cardiac muscle tissue development | 4 | 1.00E-04 |
| GO_BP | Positive regulation of cell-matrix adhesion | 2 | 4.30E-04 |
| GO_BP | Heart development | 5 | 0.001 |
| GO_BP | Negative regulation of protein ubiquitination | 2 | 0.002 |
| GO_BP | Striated muscle tissue development | 4 | 0.002 |
| GO_BP | Muscle tissue development | 4 | 0.003 |
| GO_BP | Ribosome biogenesis | 4 | 0.003 |
| GO_BP | Ventricular cardiac muscle morphogenesis | 2 | 0.003 |
| GO_BP | Regulation of cell-matrix adhesion | 2 | 0.005 |
| GO_BP | Cardiac muscle cell differentiation | 2 | 0.005 |
| GO_BP | Negative regulation of translation | 2 | 0.005 |
| GO_BP | Cardiac muscle tissue morphogenesis | 2 | 0.005 |
| GO_BP | Muscle tissue morphogenesis | 2 | 0.005 |
| GO_BP | Cardiac cell differentiation | 2 | 0.005 |
| GO_BP | Ribonucleoprotein complex biogenesis | 4 | 0.006 |
| GO_BP | Nuclear-transcribed mRNA catabolic process, nonsense-mediated decay | 2 | 0.006 |
| GO_BP | Muscle organ development | 4 | 0.006 |
| GO_BP | rRNA processing | 3 | 0.008 |
| GO_BP | Negative regulation of cellular protein metabolic process | 3 | 0.008 |
| GO_BP | rRNA metabolic process | 3 | 0.008 |
| GO_BP | Regulation of protein ubiquitination | 2 | 0.008 |
| GO_BP | Negative regulation of protein metabolic process | 3 | 0.009 |
| GO_BP | Regulation of macromolecule metabolic process | 21 | 0.009 |
| GO_BP | Notch signaling pathway | 2 | 0.009 |
| GO_BP | Regulation of cell proliferation | 7 | 0.009 |
| GO_BP | Negative regulation of cellular process | 11 | 0.009 |
| GO_BP | Anatomical structure formation involved in morphogenesis | 5 | 0.010 |
| GO_CC | Anchored to membrane | 5 | 0.001 |
| GO_CC | Intracellular | 66 | 0.006 |
| GO_MF | Interleukin-2 receptor binding | 2 | 8.60E-05 |
| GO_MF | ATP-dependent helicase activity | 5 | 2.50E-04 |
| GO_MF | Purine NTP-dependent helicase activity | 5 | 2.50E-04 |
| GO_MF | Nucleic acid binding | 31 | 0.002 |
| GO_MF | Helicase activity | 5 | 0.002 |
| GO_MF | ATPase activity, coupled | 6 | 0.006 |
| GO_MF | 3′–5′ exonuclease activity | 2 | 0.006 |
| KEGG | Glycerolipid metabolism | 2 | 0.043 |
| KEGG | Notch signaling pathway | 2 | 0.045 |
aCHL_milk: mg of cholesterol in 100 g of milk. Only gene ontologies with p-values < 0.01 are shown.
bGO_BP: Biological processes gene ontology term, GO_CC: Cellular component gene ontology term and GO_MF: Molecular function gene ontology term.
Figure 3.
Protein-protein interaction network created using the STRING database for CHL_fat positional candidate genes. Network analysis was set at medium confidence (STRING score = 0.4). The line widths represent the level of interactions (wider lines represent stronger evidence of interactions). CHL_fat: mg of cholesterol in 100 gram of fat.
Figure 4.
Protein-protein interaction network created using the STRING database for CHL_milk positional candidate genes. Network analysis was set at medium confidence (STRING score = 0.4). Line widths represent the level of interactions (wider lines represent stronger evidence of interactions). CHL_milk: mg of cholesterol in 100 gram of milk.
Table 4.
Significantly enriched transcription factors for positional candidate genes for CHL_fat and CHL_milk.
| Traita | Transcription factor | Overlap | p-value |
|---|---|---|---|
| CHL_fat | CREB1 | 40/3057 | 0.002 |
| CHL_fat | PPARD | 11/516 | 0.004 |
| CHL_fat | CEBPB | 9/382 | 0.005 |
| CHL_fat | MYC | 14/797 | 0.006 |
| CHL_fat | GRHL2 | 16/1000 | 0.009 |
| CHL_fat | CIITA | 9/459 | 0.014 |
| CHL_fat | CLOCK | 8/407 | 0.020 |
| CHL_fat | NANOG | 13/840 | 0.022 |
| CHL_fat | FOXP3 | 19/1404 | 0.023 |
| CHL_fat | E2A | 25/2000 | 0.023 |
| CHL_fat | SMAD4 | 25/2000 | 0.023 |
| CHL_fat | FOXA1 | 25/2000 | 0.023 |
| CHL_fat | TFAP2A | 24/1904 | 0.024 |
| CHL_fat | TAL1 | 23/1875 | 0.035 |
| CHL_fat | MITF | 57/5578 | 0.036 |
| CHL_fat | ATF3 | 26/2189 | 0.036 |
| CHL_fat | EST1 | 14/1001 | 0.038 |
| CHL_fat | CTCF | 24/2000 | 0.039 |
| CHL_fat | EOMES | 13/932 | 0.045 |
| CHL_fat | NFIB | 9/573 | 0.048 |
| CHL_milk | LXR | 60/2000 | 1.00E-11 |
| CHL_milk | DACH1 | 46/1698 | 1.00E-07 |
| CHL_milk | SMC4 | 51/2000 | 1.19E-07 |
| CHL_milk | BCL6 | 39/2000 | 0.001 |
| CHL_milk | P68 | 39/2000 | 0.001 |
| CHL_milk | ZNF274 | 11/327 | 0.002 |
| CHL_milk | P300 | 38/2000 | 0.003 |
| CHL_milk | EZH2 | 20/935 | 0.008 |
| CHL_milk | EGR1 | 91/6207 | 0.010 |
| CHL_milk | KDM2B | 35/2000 | 0.013 |
| CHL_milk | MYCN | 7/234 | 0.022 |
| CHL_milk | NOTCH1 | 7/245 | 0.028 |
| CHL_milk | ERG | 8/321 | 0.039 |
| CHL_milk | PRDM5 | 19/1029 | 0.039 |
| CHL_milk | FOXO3 | 14/695 | 0.039 |
| CHL_milk | EWS-FLI1 | 12/574 | 0.043 |
aCHL_fat: mg of cholesterol in 100 g of fat, CHL_milk: mg of cholesterol in 100 g of milk.
Pearson correlation of candidate gene expression (read counts) in mammary gland tissues with milk cholesterol content
Examination of RNA sequence data (read counts) of mammary gland tissues from 12 cows at mid lactation (day 120–180) indicated that among 207 positional candidate genes for CHL_fat, 35 genes were not expressed, 25 genes were very lowly expressed (each with total read counts <10), while 12 genes (TMEM120B, INSIG1, FLNB, RPN2, RASAL1, ARF4, MYL9, GRN, ORAI1, PLBD2, AQP1 and RSRC2) were highly expressed (each with total read counts >10,000) (Table S4a,b). Out of 320 genes for CHL_milk, 70 genes were not expressed, 36 genes were lowly expressed (each with total read counts <10), while 19 genes were highly expressed (each with total read counts >10,000) (Table S4a,c). LGB, RPL8, RPS19, EEF1D, ITGB1 and HNRNPF were the most highly expressed genes among the CHL_milk positional candidate genes. Moreover, the expression of 45 out of 207 CHL_fat and 72 out of 320 CHL_milk positional candidate genes was significantly correlated with CHL_fat and CHL_milk, respectively (Tables 5 and 6). The expression of genes including EPB41L1, DET1, DTX1, ABHD6, RSRC2, ITGA2B, MLXIP, KCTD6 and DLGAP4 was strongly and significantly correlated (|cor| > 0.8 and p < 0.01) to CHL_fat (Table 5). Moreover, the expressions of 28 genes were strongly and significantly correlated (|cor| > 0.8 and p < 0.01) with CHL_milk (Table 6) including ENSBTAG00000048096 and TONSL, as the two most significantly correlated (|cor| > 0.9 and p < 0.001) to CHL_milk.
Table 5.
Positional candidate genes for milk cholesterol which are expressed in mammary gland tissues and also significantly correlated to cholesterol concentration in milk fat (CHL_fat)a of the same cows.
| Ensembl Geneb | Gene symbol | Total read counts | cor_CHL_fatc | p_cor_CHL_fat |
|---|---|---|---|---|
| ENSBTAG00000001640 | EPB41L1 | 3115 | −0.893 | 0.001 |
| ENSBTAG00000000967 | DET1 | 974 | −0.892 | 0.001 |
| ENSBTAG00000016738 | DTX1 | 1331 | −0.830 | 0.006 |
| ENSBTAG00000016615 | ABHD6 | 620 | −0.828 | 0.006 |
| ENSBTAG00000006118 | RSRC2 | 11237 | −0.827 | 0.006 |
| ENSBTAG00000008165 | ITGA2B | 1190 | −0.822 | 0.007 |
| ENSBTAG00000004189 | MLXIP | 1609 | −0.815 | 0.007 |
| ENSBTAG00000022656 | KCTD6 | 2746 | −0.804 | 0.009 |
| ENSBTAG00000001741 | DLGAP4 | 2049 | −0.802 | 0.009 |
| ENSBTAG00000017505 | PAXIP1 | 1250 | −0.790 | 0.011 |
| ENSBTAG00000019989 | PXK | 1309 | −0.767 | 0.016 |
| ENSBTAG00000020590 | FZD2 | 242 | −0.767 | 0.016 |
| ENSBTAG00000007387 | ENY2 | 3025 | −0.766 | 0.016 |
| ENSBTAG00000016637 | WBP4 | 2544 | −0.763 | 0.017 |
| ENSBTAG00000008025 | UBE3C | 4692 | −0.763 | 0.017 |
| ENSBTAG00000037527 | OAS1Z | 509 | −0.763 | 0.017 |
| ENSBTAG00000048096 | ENSBTAG00000048096 | 4 | 0.760 | 0.017 |
| ENSBTAG00000006114 | ZCCHC8 | 3333 | −0.758 | 0.018 |
| ENSBTAG00000001133 | VWA8 | 1968 | −0.757 | 0.018 |
| ENSBTAG00000038316 | GPATCH8 | 4564 | −0.753 | 0.019 |
| ENSBTAG00000010694 | BICC1 | 545 | −0.750 | 0.020 |
| ENSBTAG00000047729 | ENSBTAG00000047729 | 20 | 0.749 | 0.020 |
| ENSBTAG00000021669 | SOGA1 | 456 | −0.745 | 0.021 |
| ENSBTAG00000020802 | ENSBTAG00000020802 | 921 | −0.742 | 0.022 |
| ENSBTAG00000011447 | FAM171A2 | 196 | −0.741 | 0.022 |
| ENSBTAG00000007084 | MAP3K14 | 1583 | −0.720 | 0.029 |
| ENSBTAG00000021164 | SLMAP | 6735 | −0.717 | 0.030 |
| ENSBTAG00000016435 | NOM1 | 2232 | −0.714 | 0.031 |
| ENSBTAG00000017069 | FAM198B | 1089 | −0.709 | 0.033 |
| ENSBTAG00000006051 | NMT1 | 4740 | −0.704 | 0.034 |
| ENSBTAG00000030817 | LMBR1 | 3270 | −0.704 | 0.034 |
| ENSBTAG00000013526 | EFTUD2 | 6186 | −0.703 | 0.035 |
| ENSBTAG00000039861 | OAS1Y | 1418 | −0.695 | 0.038 |
| ENSBTAG00000015913 | MFHAS1 | 386 | −0.694 | 0.038 |
| ENSBTAG00000011473 | MYL9 | 20172 | −0.687 | 0.041 |
| ENSBTAG00000004199 | DIABLO | 2763 | −0.683 | 0.042 |
| ENSBTAG00000019463 | SLC25A39 | 9582 | −0.683 | 0.042 |
| ENSBTAG00000000357 | ENSBTAG00000000357 | 4120 | −0.683 | 0.042 |
| ENSBTAG00000019987 | RPP14 | 4821 | −0.681 | 0.043 |
| ENSBTAG00000007051 | CLDN23 | 62 | −0.679 | 0.044 |
| ENSBTAG00000015541 | DLC1 | 2635 | −0.679 | 0.044 |
| ENSBTAG00000018433 | DENND6A | 1496 | −0.679 | 0.044 |
| ENSBTAG00000018823 | GRN | 19107 | −0.677 | 0.045 |
| ENSBTAG00000047599 | GHRHR | 21 | −0.671 | 0.048 |
| ENSBTAG00000022004 | FLNB | 34529 | −0.669 | 0.049 |
aCHL_fat: mg of cholesterol in 100 g of fat, CHL_milk: mg of cholesterol in 100 g of milk.
bGenes in bold face are also positional candidate genes for CHL_milk.
cPearson correlation coefficient.
Table 6.
Positional candidate genes for milk cholesterol which are expressed in mammary gland tissues and also significantly correlated to cholesterol concentration in milk (CHL_milk)a of the same cows.
| Ensembl Geneb | Gene symbol | Total read counts | cor_CHL_milkc | p_cor_CHL_milk |
|---|---|---|---|---|
| ENSBTAG00000048096 | ENSBTAG00000048096 | 4 | 0.933 | 2.39E-04 |
| ENSBTAG00000007749 | TONSL | 634 | −0.923 | 3.84E-04 |
| ENSBTAG00000015910 | ITGB1 | 44254 | −0.897 | 0.001 |
| ENSBTAG00000000967 | DET1 | 974 | −0.897 | 0.001 |
| ENSBTAG00000024889 | HSBP1 | 7826 | −0.893 | 0.001 |
| ENSBTAG00000018456 | ZNF7 | 1524 | −0.892 | 0.001 |
| ENSBTAG00000039328 | PURG | 47 | −0.876 | 0.002 |
| ENSBTAG00000005691 | FGF2 | 2308 | −0.871 | 0.002 |
| ENSBTAG00000013125 | PLAUR | 332 | −0.868 | 0.002 |
| ENSBTAG00000045791 | ZNF623 | 845 | −0.863 | 0.003 |
| ENSBTAG00000018975 | KCNT1 | 555 | −0.857 | 0.003 |
| ENSBTAG00000002883 | RPTOR | 2659 | −0.847 | 0.004 |
| ENSBTAG00000013439 | ARHGEF26 | 2619 | −0.839 | 0.005 |
| ENSBTAG00000006132 | DENND3 | 4706 | −0.835 | 0.005 |
| ENSBTAG00000018912 | ARHGEF1 | 10394 | −0.829 | 0.006 |
| ENSBTAG00000030939 | ZNF575 | 287 | −0.828 | 0.006 |
| ENSBTAG00000014607 | EXOSC4 | 988 | −0.821 | 0.007 |
| ENSBTAG00000001262 | IRGQ | 498 | −0.819 | 0.007 |
| ENSBTAG00000019864 | MAPK15 | 751 | −0.814 | 0.008 |
| ENSBTAG00000039851 | UBAC1 | 6064 | −0.813 | 0.008 |
| ENSBTAG00000012796 | ZNF428 | 465 | −0.811 | 0.008 |
| ENSBTAG00000016268 | XRCC1 | 2290 | −0.809 | 0.008 |
| ENSBTAG00000000312 | GRINA | 6104 | −0.808 | 0.008 |
| ENSBTAG00000021472 | ZC3H3 | 1032 | −0.807 | 0.009 |
| ENSBTAG00000004092 | AK8 | 372 | −0.805 | 0.009 |
| ENSBTAG00000004969 | LRRC14 | 1730 | −0.805 | 0.009 |
| ENSBTAG00000016738 | DTX1 | 1331 | −0.802 | 0.009 |
| ENSBTAG00000011815 | SMG9 | 2101 | −0.801 | 0.009 |
| ENSBTAG00000015267 | SGSH | 2811 | −0.799 | 0.010 |
| ENSBTAG00000031824 | RBM19 | 2179 | −0.799 | 0.010 |
| ENSBTAG00000026356 | DGAT1 | 4493 | −0.794 | 0.011 |
| ENSBTAG00000013283 | PRR19 | 309 | −0.792 | 0.011 |
| ENSBTAG00000020754 | ZNF526 | 1161 | −0.792 | 0.011 |
| ENSBTAG00000004173 | UBXN8 | 2079 | −0.790 | 0.011 |
| ENSBTAG00000008853 | HNRNPF | 35493 | −0.786 | 0.012 |
| ENSBTAG00000011064 | ADCK5 | 3161 | −0.777 | 0.014 |
| ENSBTAG00000003606 | ZNF16 | 1067 | −0.773 | 0.015 |
| ENSBTAG00000006581 | CCDC82 | 1850 | −0.759 | 0.018 |
| ENSBTAG00000016810 | PYCRL | 6075 | −0.757 | 0.018 |
| ENSBTAG00000010606 | PPP1R3B | 607 | −0.757 | 0.018 |
| ENSBTAG00000010947 | PHYHIPL | 6186 | −0.754 | 0.019 |
| ENSBTAG00000020236 | NECAB2 | 163 | −0.753 | 0.019 |
| ENSBTAG00000026320 | VPS28 | 6020 | −0.752 | 0.019 |
| ENSBTAG00000020756 | GSK3A | 5533 | −0.751 | 0.020 |
| ENSBTAG00000038494 | ENSBTAG00000038494 | 330 | −0.743 | 0.022 |
| ENSBTAG00000001826 | SASH1 | 2268 | −0.739 | 0.023 |
| ENSBTAG00000019785 | CIC | 6558 | −0.735 | 0.024 |
| ENSBTAG00000011102 | TPCN1 | 6605 | −0.727 | 0.026 |
| ENSBTAG00000019866 | NRP1 | 7819 | −0.727 | 0.027 |
| ENSBTAG00000018455 | COMMD5 | 2136 | −0.727 | 0.027 |
| ENSBTAG00000002976 | CD177 | 44 | −0.727 | 0.027 |
| ENSBTAG00000011963 | RPS19 | 57636 | −0.724 | 0.028 |
| ENSBTAG00000007115 | GSR | 2239 | −0.724 | 0.028 |
| ENSBTAG00000047729 | ENSBTAG00000047729 | 20 | 0.721 | 0.028 |
| ENSBTAG00000033727 | RBPMS | 1632 | −0.718 | 0.029 |
| ENSBTAG00000003530 | DDX31 | 16551 | −0.711 | 0.032 |
| ENSBTAG00000011937 | RITA1 | 1067 | −0.710 | 0.032 |
| ENSBTAG00000009677 | PARP10 | 3006 | −0.702 | 0.035 |
| ENSBTAG00000014458 | MROH1 | 8527 | −0.701 | 0.035 |
| ENSBTAG00000035254 | CYHR1 | 4420 | −0.697 | 0.037 |
| ENSBTAG00000019040 | PLBD2 | 14432 | −0.697 | 0.037 |
| ENSBTAG00000014610 | GPAA1 | 13022 | −0.696 | 0.037 |
| ENSBTAG00000005761 | DEDD2 | 2653 | −0.695 | 0.038 |
| ENSBTAG00000012691 | GTF2E2 | 4154 | −0.693 | 0.038 |
| ENSBTAG00000007834 | PPP1R16A | 1451 | −0.692 | 0.039 |
| ENSBTAG00000001260 | PINLYP | 7 | −0.686 | 0.041 |
| ENSBTAG00000040086 | SLC38A8 | 7 | −0.686 | 0.041 |
| ENSBTAG00000012235 | SHARPIN | 1729 | −0.686 | 0.042 |
| ENSBTAG00000011103 | SLC8B1 | 4800 | −0.679 | 0.044 |
| ENSBTAG00000006008 | CAMSAP1 | 2406 | −0.675 | 0.046 |
| ENSBTAG00000009245 | PPP2CB | 12515 | −0.674 | 0.047 |
| ENSBTAG00000014642 | NAPRT | 17674 | −0.668 | 0.049 |
aCHL_fat: mg of cholesterol in 100 g of fat, CHL_milk: mg of cholesterol in 100 g of milk.
bGenes in bold face are also positional candidate genes for CHL_fat.
cPearson correlation coefficient.
Discussion
It is known that most cow milk CHL (about 80%) is derived from blood whereas a small portion (about 20%) is derived through local synthesis in the mammary gland29. Therefore, the regulation of milk CHL content may require complex mechanisms and the involvement of many genes and pathways. Recently, we reported heritability estimates for CHL_fat (0.09) and CHL_milk (0.18) suggesting that genetics contributes a proportion of the total phenotypic variances in milk CHL content4.
More SNPs (20) were significantly associated with CHL_milk as compared to two for CHL_fat at the genome wide significant threshold (p < 5E-05). Furthermore, 36 and 19 SNPs including 7 in common were suggestively associated (p < 5E-04) with CHL_milk and CHL_fat, respectively. In fact, 58 genes are located in 0.5 Mb flanking regions of 7 suggestively (p < 5E-04) associated SNPs (ARS-BFGL-NGS-110646 [rs109154988], ARS-USMARC-Parent-DQ786763-rs29020472 [rs29020472], BTB-01524761 [rs42640895], BTB-01712106 [rs42829960], Hapmap40322-BTA-100742 [rs41600454], Hapmap43002-BTA-63541 [rs41586803], and Hapmap52830-rs29014800 [rs29014800]) for CHL_milk and CHL_fat. Some of the genes have been reported to have potential roles in CHL metabolism such as protein tyrosine phosphatase 1β (PTPN1), diacylglycerol kinase eta (DGKH) and serine dehydratase (SDS). PTPN1 is an important gene for plasma total and HDL-CHL30–33 while DGKH encodes an enzyme responsible for the recycling and degradation of diacylglycerol, known as important for CHL efflux from adipose cells34. SDS gene on the other hand is known to contain a susceptibility loci for low HDL-CHL levels35. The most important QTL region for CHL_fat at 41.9 Mb of BTA 17 contained two significant SNPs (Hapmap40322-BTA-100742 [rs41600454] and BTB-01524761 [rs42640895]) for the trait. Relaxin–insulin-like family peptide receptor 1 (RXFP1), transmembrane protein 144 (TMEM144) and family with sequence similarity 198, member B (FAM198B) genes are positional candidate genes for CHL_fat, however, none of them has been reported to have a direct role in the regulation of CHL metabolism. RXFP1, one of four relaxin receptors, is known to play a role in signal transduction between extracellular/intracellular domains36. The activation of RXFP1 receptor stimulates the phosphorylation of mitogen-activated protein kinases such as ERK1/236. In fact, the phosphorylation of ERK1/2 is important for the regulation of CHL efflux37. RXFP1 is also among genes with more levels of interactions with other CHL-fat candidate genes, as shown by the interaction network (Fig. 3). However, RXFP1 was very lowly expressed in mammary gland tissues (Table S4) so its involvement with CHL_fat concentration might be through its activities in other tissues. The involvement of FAM198B and TMEM144 genes in CHL metabolism might be via their roles in the membrane, since TMEM144 is a carbohydrate transmembrane transporter while FAM198B play roles in golgi membrane functions. In fact, FAM198B was expressed in mammary gland tissues and also significantly correlated to CHL_fat concentration (Tables 5 and S4b), so its role in CHL synthesis in the mammary gland warrants further investigation.
An intergenic region of BTA 17, position 63 Mb, is another interesting region harboring two suggestive SNPs (ARS-BFGL-NGS-64029 [rs110842600] (p = 1.91E-04) and Hapmap52830-rs29014800 [rs29014800] (p = 5.80E-05)) for CHL_fat and CHL_milk, respectively (Table S1a,b). Among many genes (PLBD2, SDS, RITA1, PTPN11, DTX1, RASAL1, LHX5, CFAP73, IQCD, DDX54, OAS2, TPCN1, SLC8B1, SDSL and RPH3A) located within 0.5 Mb flanking regions of these two SNPs, protein tyrosine phosphatase 1β (PTPN1) has been directly linked to CHL metabolism30–33 and it has been identified as a candidate gene for both CHL_fat and CHL_milk in this study. Variants of PTPN11 have been found to associate with serum CHL level in a sex-specific pattern in human30 while Lu et al.32 identified PTPN11 as a candidate gene for human plasma HDL-CHL. In the mammary gland, PTPN11 gene was moderately expressed and had tendency (p = 0.067) of being correlated to CHL_fat concentration (Table S4b), therefore more studies are required to validate its role in CHL metabolism.
The QTL region at 117.7 Mb of BTA 4 harboring suggestive SNP ARS-BFGL-NGS-20980 (rs110814823) (p = 4.26E-04) for CHL_fat also harbors several important genes of CHL metabolism such as 5-hydroxytryptamine (serotonin) receptor 5 A (HTR5A)38,39 and insulin induced gene 1 (INSIG1)40,41. INSIG1 was the second most highly expressed gene among CHL_fat positional candidate genes in the mammary gland (Table S4), whereas HTR5A was not expressed in the mammary gland. However, the expression of INSIG1 gene in the mammary gland was not significantly correlated to CHL_fat concentration. It was shown recently that downregulation of INSIG1 gene in mammary gland tissues of lactating dairy cows following dietary supplementation with 5% linseed oil was predicted by Ingenuity Pathways Analysis software (Invitrogen, Carlsbad, CA, USA) to activate CHL concentration in the mammary gland42. Two flanking genes (disintegrin and metalloproteinase domain-containing protein 11 [ADAM11] and hexamethylene bisacetamide inducible 1 [HEXIM1]) of suggestive SNP ARS-BFGL-NGS-24479 (rs41916457) (p = 3.90E-04) at 45.1 Mb region of Bta 19 (Table S1a) have been reported to be involved in CHL metabolism43–45. However, the expression of both ADAM11 and HEXIM1 genes was not significantly correlated to CHL_fat concentration in this study.
The enrichment analyses identified several GO terms with protein kinase regulator activities including negative regulation of cyclin-dependent protein kinase activity (p = 0.001, most significant biological process GO term) and cyclin-dependent protein kinase regulator activity (p = 1E-04, most significant molecular function GO term). In fact, cyclin-dependent protein kinase has been identified as a key regulator of eukaryotic cell cycle46, and it might be linked to CHL metabolism via its role in the regulation of energy status47,48 or lipid metabolism in the liver49. Regulation of CHL homeostasis and CHL metabolism is associated with plasma membrane activities50,51. Enrichment results suggest a potential role of the (basolateral) plasma membrane in the regulation of CHL_fat. The plasma membrane was the GO term enriched with the largest number of positional candidate genes for CHL_fat while basolateral plasma membrane was the most significantly enriched cell component GO term for CHL_fat candidate genes (Tables 2 and S2a). Meanwhile, cell-cell signaling (p = 0.001) and cell communication (p = 0.004) (Table 2) were among the most significant biological processes GO terms for CHL_fat suggesting that the regulation of CHL_fat probably requires the interaction and shared signaling activities between different cell types. Among the five KEGG pathways significantly enriched for CHL_fat positional candidate genes, the tight junction pathway has important roles in the transportation of milk constituents in mammary gland cells52,53, therefore it might also function in the transportation of CHL from the blood stream into the mammary gland or from mammary gland cells (de novo synthesized) into milk. Focal adhesion is an important pathway for immune functions in bovine mammary cells54, for lactation involution55 and for epigenetic regulation of milk production56. The focal adhesion kinase protein has been found in bovine milk fat globule membrane which is the major store of CHL in milk57, therefore focal adhesion pathway might be important for milk CHL via its role in the milk fat globule. Many significant transcription factors enriched for CHL_fat positional candidate genes have multiple functions. For example, c-Myc (MYC) is essential for the regulation of cell cycle progression, apoptosis and cellular transformation58,59 while peroxisome proliferator activated receptor delta (PPARD) is important for the regulation of the transcription of genes associated with proliferation, metabolism, inflammation, and immunity60. In fact, PPARD is an important transcription factor regulating CHL metabolism since it plays important roles in the reverse CHL transport61.
For CHL_milk, the most significant SNP (ARS-BFGL-NGS-4939 [rs109421300]) is located in an intronic region of diacylglycerol O-acyltransferase 1 (DGAT1) gene at 1,801,116 bp on BTA 14. This SNP has been reported to be in complete linkage disequilibrium with the K232A substitution within the DGAT1 gene in German cows62. This SNP is also important for milk fat62 and fatty acid components63. Moreover, we also reported high LD among SNPs within and around the DGAT1 gene region (Fig. 2). Another significantly associated SNP for CHL_milk (ARS-BFGL-NGS-18365 or rs110892754) has been found to be important for 305 day milk fat yield64. The DGAT1 gene and the centromeric region of BTA 14 is important for the regulation of milk traits (milk fat yield, fat%, protein yield and protein%)62,64–69. DGAT1 is a key enzyme in triacylglycerol biosynthesis and also play important roles in the regulation of CHL metabolism70–72. In ApoE gene knock-out mice, DGAT1 deficiency decreases CHL uptake and absorption71. Therefore, the significant SNPs detected for CHL content in this study suggests that the DGAT1 gene and the centromeric region of BTA 14 might be important in the regulation of milk CHL content. In fact, the expression of DGAT1 gene in mammary gland tissues was also significantly correlated to CHL_milk concentration (p = 0.011) (Table 6), suggesting that DAGT1 might contribute to the regulation of CHL_milk metabolism in the mammary gland.
A significant SNP (ARS-BFGL-NGS-41837 or rs110597360) for CHL_milk on BTA 6 is located in an intergenic region and the nearest gene to this SNP is ENSBTAG00000001751, an orthologue of human CXXC finger protein 4 (CXXC4) gene. CXXC4 encodes a CXXC-type zinc finger domain-containing protein that functions as an antagonist of the canonical wingless/integrated signaling pathway73,74. The role of this novel gene in CHL_milk is unknown. On BTA 15, Hapmap38637-BTA-88156 (rs41596665) was significantly associated with CHL_milk and its flanking gene, mastermind like transcriptional coactivator 2 (MAML2) encodes for a member of the mastermind-like family of proteins which play important roles in the Notch signaling pathway75. In fact, the Notch signaling pathway was one of the pathways enriched for CHL_milk positional candidate genes in this study and it has been shown to have important roles in mammary gland development76. The Notch signaling pathway is important in the regulation of cell fate, cell proliferation and cell death in development77; however, there is no report of its direct role in milk CHL metabolism. On BTA 17, Hapmap52830-rs29014800 (rs29014800) was significantly associated with CHL_milk (p = 1.58E-05) and also suggestively associated with CHL_fat (Tables 1 and S1a), therefore this SNP might be important in the regulation of milk CHL content. On BTA 18, Hapmap39330-BTA-42256 (rs41605812), located in an intronic region of cadherin 13 (CDH13) gene (Table 1), is important for CHL_milk. A SNP within CDH13 has been reported to be associated with plasma adiponectin levels in Japanese population78 and with triglyceride/high density lipoprotein ratio in Korean cardiovascular patients79. This gene is moderately expressed in the bovine mammary gland and also showed a trend (p = 0.075) to correlate to CHL_milk concentration (Table S4c). However, the role of this gene in milk CHL metabolism remains to be characterized.
The enrichment results for positional candidate genes showed several GO terms related to heart development (Table 3) which might reflect the fact that many candidate genes for CHL also play roles in cardiovascular disease development or heart diseases. An interesting molecular function GO term enriched was interleukin-2 receptor binding. It is known that interleukin-2 gene plays important roles in the activation of STAT5a gene in mammary gland development80. Glycerolipid metabolism, another enriched pathway has been implicated in the biosynthesis of CHL81,82. Therefore, interleukin-2 receptor binding (GO term) and glycerolipid metabolism pathway might also play important roles in bovine milk CHL metabolism. Interestingly, the most important transcription factor enriched for CHL_milk candidate genes was liver X receptor (LXR) (p = 1.00E-11) which is an important regulator of CHL, fatty acid, and glucose homeostasis83–85. There are two LXR subtypes (LXRα and LXRβ) and LXRα, the dominant subtype is highly expressed in the liver and other tissues (intestine, adipose, kidney, and adrenals)86 while LXRβ is widely expressed in different tissues86. In our mammary gland RNA expression data, LXRβ (or NR1H2 gene) was also expressed at a higher level when compared to LXRα (or NR1H3 gene). In the liver, LXRα expression was not significantly correlated to CHL_milk during transition and early lactation20. Another notable transcription factor enriched for CHL_milk positional candidate genes was notch homolog 1 (NOTCH1) (p = 0.028) (Table 4), which indicates the importance of NOCTH signaling pathway in milk CHL regulation. The functions of highly interacted genes (MAPK15, FAM83H, ARHGAP39, HEATR7A, CYHR1 and CPSF1) in CHL_milk protein interaction network (Fig. 4), as well as highly significantly correlated genes (ENSBTAG00000048096, TONSL and ITGB1) (Table 6) in CHL metabolism are unknown and warrant further investigation.
The genetic variants identified in this study may facilitate selection in commercial breeding schemes either by incorporation in marker-enhanced selection or via implementation of genomic prediction including these identified genetic variants in a customized SNP panel. However, it is also important to consider potential limitations of our study including the limited size of resource population for GWAS, the relaxed p-value threshold used to select SNPs for gene set enrichments, potential for false discovery errors for certain enriched gene ontologies and pathways with few enriched genes in the gene list. The results should be interpreted with caution since both the results of associations (GWAS) and correlations derived from RNA sequence data may not reflect actual causative relationships. As already mentioned above, most CHL in milk is derived from the diet (which is partly reflected as CHL concentration in the blood) while only a small proportion, about 20%, is synthesized de novo in the mammary gland. Therefore, association analysis considering data on both blood and milk CHL concentrations might enhance knowledge of the implicated candidate genes in the regulatory pathways of milk CHL concentration such as dietary CHL transport from blood to the mammary gland and de novo synthesis in the mammary gland. Moreover, integration of gene expression data from the mammary gland and other tissues like the liver could identify the link between the mechanisms regulating CHL in the mammary gland and other tissues, and how these connections influence de novo synthesis of CHL in the mammary gland and milk CHL concentration.
To the best of our knowledge, this is the first GWAS on bovine milk CHL. The strongest SNP associations with milk CHL were detected on BTA14 and BTA17. This study identified several candidate genes (DGAT1, PTPN1, INSIG1, HEXIM1, SDS, and HTR5A), also important for human plasma CHL and related traits, that might be important for bovine milk CHL. Novel candidate genes (RXFP1, FAM198B, TMEM144, CXXC4, MAML2 and CDH13) for milk CHL content were identified. Enrichment analyses suggested the involvement of important gene ontology terms ((basolateral) plasma membrane and cell-cell signaling processes), pathways (tight junction, focal adhesion, Notch signaling and glycerolipid metabolism pathways), and several transcription factors (PPARD, LXR and NOTCH1) in the regulation of bovine milk CHL content. The expression of some positional candidate genes in the mammary gland and their correlation with milk CHL content was supported with RNA sequencing data and milk CHL concentrations from the same animals. This study has therefore provided an insight into the genomics of bovine milk CHL and identified potential candidate genes and pathways that might be further studied to identify/confirm casual mutations that might help in the selection of cows with desired milk CHL content.
Materials and Methods
Animal Resource and Cholesterol Measure
Animal selection and milk sampling has been described in our previous study4. In brief, 100 ml of milk from each of 1,848 cows from 29 herds (minimum: 33 cows/herd and maximum: 172 cows/herd) were used. The concentration of CHL in milk fat was determined by direct saponification and capillary gas chromatography according to Fletouris et al.87. About 0.2 mg milk fat was saponified in capped tubes with 0.5 M methanolic KOH solution by heating for 15 minutes and the unsaponifiable fraction was extracted with toluene and analyzed by capillary gas chromatography using Agilent HP 6890 Series Gas Chromatography (GC) System (Agilent Technologies, California, USA). The concentration C (mg/100 g of fat) of CHL (CHL_fat) in analyzed samples was calculated based on computed mass (nanograms) of the analyte in the injected extract. The concentration of CHL was expressed in mg/100 g of fat (CHL_fat) or mg/100 g of milk (CHL_milk). After editing data for cow registration number, dam and sire information, test date, parity and age at calving, a total of 1,793 cows with complete records were retained for further analysis.
Genotyping and Genotype Quality Control
DNA was isolated from hair follicles of 1,200 (out of 1,848) cows and genotyped using the Illumina BovineSNP50K BeadChip following manufacturer’s instructions (Illumina Inc., San Diego, CA). Genotype quality control was implemented by discarding animals and SNPs with call rate <0.95 and SNPs deviating from Hardy Weinberg equilibrium (p < 0.0001). Missing genotypes were imputed with FImpute 2 software88 and subsequently SNPs with MAF <0.05 were excluded. After quality control, 40,196 SNPs and 1,183 animals were retained for the association analyses.
Association Analyses
The association analyses were performed using a univariate single SNP mixed linear model implemented in DMU package89. In summary, the model for each SNP (analyzed individually) was as follows (model 1):
| 1 |
where y is the vector of phenotype (CHL_fat, CHL_milk), 1 is a vector of 1s with length equal to number of observations, μ is the general mean, X is an incidence matrix relating phenotypes to the corresponding fixed effects, and B is the vector for fixed effects which includes interaction between herd and parity and days in milk (DIM), Z is an incidence matrix relating phenotypes to the corresponding random polygenic effect, a is a vector of the random polygenic effect ∼N(0, Aσu2) (where A is the additive relationship matrix and σu2 is the polygenic variance), m is a vector with genotypic indicators 2, 1, or 0 for genotypes AA, AB and BB, respectively associating records to the marker effect, g is a scalar of the associated additive effect of the SNP, and e is a vector of random environmental deviates: N(0, σe2) (where σe2 is the general error variance). The parameters of the model σ2u and σ2e were estimated using restricted maximum likelihood (REML) for each SNP. To determine the significantly associated SNPs, an F-test was used to test the null hypothesis H0: β = 0. Distribution of test statistics was assessed by quantile-quantile (q-q) plot generated from association tests and the deviation from the null hypothesis of no SNP association with the trait. The markers with p nominal < 5E-05 were considered genome wide significant90 and markers with p nominal from 5E-05 to 5E-04 were considered suggestively genome wide significant to avoid many false negative results caused by stringent Bonferroni correction.
Detection of Linkage Disequilibrium Blocks
Since several significant SNPs may be clustered in the same region (QTL region), we performed Linkage Disequilibrium (LD) analysis to characterize Linkage Disequilibrium patterns (LD block) for these regions. The LD block was defined according to Gabriel et al.91 and was detected and visualized with Haploview software92. Gabriel et al.91 defined a LD block as a region within which 95% of SNP pairs show strong LD (strong LD is defined if the one-sided upper 95% confidence bound on D′ is >0.98 and the lower bound is above 0.7). Before constructing LD block, we excluded SNPs with call rate <0.95, SNPs deviating from Hardy Weinberg equilibrium (p < 0.0001) and SNPs with MAF <0.05 and Mendelian inheritance errors >1. During LD construction, pairwise comparisons of markers >500 kb apart were ignored according to default settings in the Haploview software.
Gene Mapping, Pathways and Transcription Factor Enrichment
We selected both significant and suggestive SNPs for pathway analyses because assignment of genes using only genome wise significant SNPs may ignore potentially important SNPs with lower significant levels, consequently missing out on key putative candidates and associated pathways. Nearby genes within a flanking distance of 0.5 Mb from significant and suggestive SNPs were queried from Ensemble database (Ensembl 83, Bos taurus UMD3.1), using bedtools93. Genes were submitted to the Database for Annotation, Visualization, and Integrated Discovery (DAVID, http://david.abcc.ncifcrf.gov/) for KEGG pathways and Gene Ontology (GO) enrichment analyses94 while STRING v10.595 database was used to assess protein-protein interactions. The human genome was selected as background for enrichment instead of the bovine genome in order to take advantage of a richer database of information on the genomics of human CHL. Annotated pathways and GO terms were tested for enrichment using Fisher exact test. Pathways/GO terms were declared significantly enriched if they did not appear by chance with p < 0.0594. For STRING95 enrichment, the default options were used with the network edge selected based on confidence level. The minimum confidence threshold was set-up at the medium level with score of 0.4. In addition, a comprehensive gene set enrichment analysis for transcriptional machinery using ChIP-X enrichment analysis (ChEA2015)96 was performed with Enrichr (http://amp.pharm.mssm.edu/Enrichr/)97. The transcription factors were declared significantly enriched at p < 0.05.
Evaluation of Expression of Positional Candidate Genes Using Mammary Gland RNA-Seq Data
The RNA-Seq expression data of 12 cows used is a subset of the data from our previous study42. Cows were in mid lactation (day 120–180) and fed the control ration (Table S4a). The expression of positional candidate genes for milk CHL as read count (reads per kilo base per million mapped reads (RPKM)) is shown in Table S4b. The CHL content in milk obtained from the 12 cows on the same day that mammary gland biopsies where obtained for RNA-Seq was determined using the same methods described above87. The Pearson correlations of CHL content with the RPKM values of positional candidate genes were calculated using cor() function in R program98. The candidate genes were considered significantly correlated to milk CHL content at p < 0.05.
The care of animals and use procedures were according to the Canadian Council on Animal Care99 and were approved by the Animal Care and Ethics Committee of Agriculture and Agri-Food Canada.
Electronic supplementary material
Acknowledgements
Authors thank participating farmers for animal management and Anne-Marie Christen of Valacta for coordinating milk sampling by Valacta (www.valacta.com). This research was supported by the DairyGen Council of the Canadian Dairy Network and the Natural Sciences and Engineering Research Council of Canada (NSERC).
Author Contributions
E.M.I.-A. conceived and designed the study, and revised the manuscript; X.Z. participated in the study design, and revised the manuscript; F.S. and F.M. participated in the experimental and statistical designs of the study; E.M.I.-A. and X.Z. provided materials and reagents; D.N.D. performed the experiments and analyzed the data with inputs from E.M.I.-A., F.S. and F.M.; D.N.D., E.M.I.-A., X.Z., F.S. and F.M. interpreted the data. D.N.D. drafted the manuscript. All authors revised and approved the final manuscript.
Availability of Data
The RNA sequence data has been submitted to the BioProject data base (BioProject ID: PRJNA301774) and it is available through this link: http://www.ncbi.nlm.nih.gov/bioproject/PRJNA301774).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xin Zhao, Email: xin.zhao@mcgill.ca.
Eveline M. Ibeagha-Awemu, Email: Eveline.ibeagha-awemu@agr.gc.ca
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31427-0.
References
- 1.Royo-Bordonada M, et al. Food sources of nutrients in the diet of Spanish children: the Four Provinces Study. Br J. Nutr. 2003;89:105–114. doi: 10.1079/BJN2002754. [DOI] [PubMed] [Google Scholar]
- 2.Altenhofer C, et al. Effects of rapeseed and soybean oil dietary supplementation on bovine fat metabolism, fatty acid composition and cholesterol levels in milk. J Dairy Res. 2014;81:120–128. doi: 10.1017/S002202991300071X. [DOI] [PubMed] [Google Scholar]
- 3.Jensen RG. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002;85:295–350. doi: 10.3168/jds.S0022-0302(02)74079-4. [DOI] [PubMed] [Google Scholar]
- 4.Do, D. N. et al. Genetic parameters of milk cholesterol content in Holstein cattle. Canadian J. Anim. Sci., 10.1139/CJAS-2018-0010 (Published on the web on 27 April 2018) (2018).
- 5.Barter P, et al. HDLcholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J. Med. 2007;357:1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 6.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a metaanalysis of population-based prospective studies. J. Cardiovasc. Risk. 1996;3:213–219. doi: 10.1097/00043798-199604000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM. LDL cholesterol: controversies and future therapeutic directions. Lancet. 2014;384:607–617. doi: 10.1016/S0140-6736(14)61009-6. [DOI] [PubMed] [Google Scholar]
- 8.Saleheen D, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siervo M, et al. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J. Nutr. 2015;113:1–15. doi: 10.1017/S0007114514003341. [DOI] [PubMed] [Google Scholar]
- 10.Peters SA, Singhateh Y, Mackay D, Huxley RR, Woodward M. Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: A systematic review and meta-analysis. Atherosclerosis. 2016;248:123–131. doi: 10.1016/j.atherosclerosis.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Kurano M, et al. Genome-wide association study of serum lipids confirms previously reported associations as well as new associations of common SNPs within PCSK7 gene with triglyceride. J. Hum. Genet. 2016;61:427–433. doi: 10.1038/jhg.2015.170. [DOI] [PubMed] [Google Scholar]
- 12.Sandhu MS, et al. LDL-cholesterol concentrations: a genome-wide association study. Lancet. 2008;371:483–491. doi: 10.1016/S0140-6736(08)60208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumitrescu L, et al. Genetic determinants of lipid traits in diverse populations from the population architecture using genomics and epidemiology (PAGE) study. PLoS Genet. 2011;7:e1002138. doi: 10.1371/journal.pgen.1002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aulchenko YS, et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat. Genet. 2009;41:47–55. doi: 10.1038/ng.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan A, Mooney KM, Wilkinson SJ, Pickles N, Mc Auley MT. Cholesterol metabolism: A review of how ageing disrupts the biological mechanisms responsible for its regulation. Ageing Res. Rev. 2016;27:108–124. doi: 10.1016/j.arr.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Hampton, R. Y. Cholesterol Regulation. Annu. Rev. Cell. Dev. Biol. 33 (2017). [DOI] [PMC free article] [PubMed]
- 17.Strzyz P. Lipid Metabolism: Cholesterol feeds into cell growth control. Nat. Rev. Mol. Cell. Biol. 2017;18:277–277. doi: 10.1038/nrm.2017.41. [DOI] [PubMed] [Google Scholar]
- 18.Howe V, et al. Cholesterol homeostasis: How do cells sense sterol excess? Chem. Phys. Lipids. 2016;199:170–178. doi: 10.1016/j.chemphyslip.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Viturro E, et al. Cholesterol synthesis in the lactating cow: Induced expression of candidate genes. J. Steroid Biochem. Mol. Biol. 2009;115:62–67. doi: 10.1016/j.jsbmb.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Kessler E, Gross J, Bruckmaier R, Albrecht C. Cholesterol metabolism, transport, and hepatic regulation in dairy cows during transition and early lactation. J. Dairy Sci. 2014;97:5481–5490. doi: 10.3168/jds.2014-7926. [DOI] [PubMed] [Google Scholar]
- 21.Ontsouka CE, Huang X, Aliyev E, Albrecht C. In vitro characterization and endocrine regulation of cholesterol and phospholipid transport in the mammary gland. Mol. Cell. Endocrinol. 2017;439:35–45. doi: 10.1016/j.mce.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Weber C, et al. Hepatic gene expression involved in glucose and lipid metabolism in transition cows: Effects of fat mobilization during early lactation in relation to milk performance and metabolic changes. J. Dairy Sci. 2013;96:5670–5681. doi: 10.3168/jds.2012-6277. [DOI] [PubMed] [Google Scholar]
- 23.Schlegel G, Ringseis R, Keller J, Schwarz F, Eder K. Changes in the expression of hepatic genes involved in cholesterol homeostasis in dairy cows in the transition period and at different stages of lactation. J. Dairy Sci. e. 2012;95:3826–3836. doi: 10.3168/jds.2011-5221. [DOI] [PubMed] [Google Scholar]
- 24.Altenhofer C, et al. Temporal variation of milk fat globule diameter, fat and cholesterol content and milk epithelial cell gene expression in dairy cows. Int. J. Dairy Technol. 2015;68:519–526. doi: 10.1111/1471-0307.12220. [DOI] [Google Scholar]
- 25.Ontsouka EC, Huang X, Stieger B, Albrecht C. Characteristics and Functional Relevance of Apolipoprotein-A1 and Cholesterol Binding in Mammary Gland Tissues and Epithelial Cells. PLoS One. 2013;8:e70407. doi: 10.1371/journal.pone.0070407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mani O, et al. Identification of ABCA1 and ABCG1 in milk fat globules and mammary cells—Implications for milk cholesterol secretion. J. Dairy Sci. 2011;94:1265–1276. doi: 10.3168/jds.2010-3521. [DOI] [PubMed] [Google Scholar]
- 27.Gross JJ, Kessler EC, Albrecht C, Bruckmaier RM. Response of the cholesterol metabolism to a negative energy balance in dairy cows depends on the lactational stage. PLoS One. 2015;10:e0121956. doi: 10.1371/journal.pone.0121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mani O, et al. Identification of ABCA1 and ABCG1 in milk fat globules and mammary cells–implications for milk cholesterol secretion. J. Dairy Sci. 2011;94:1265–1276. doi: 10.3168/jds.2010-3521. [DOI] [PubMed] [Google Scholar]
- 29.Long CA, Patton S, McCarthy RD. Origins of the cholesterol in milk. Lipids. 1980;15:853–857. doi: 10.1007/BF02534376. [DOI] [PubMed] [Google Scholar]
- 30.Jia Z-F, et al. Polymorphisms of PTPN11 gene could influence serum lipid levels in a sex-specific pattern. Lipids Health Dis. 2013;12:72. doi: 10.1186/1476-511X-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Y, et al. Multiple genetic variants along candidate pathways influence plasma high-density lipoprotein cholesterol concentrations. J. Lipid Res. 2008;49:2582–2589. doi: 10.1194/jlr.M800232-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Lu Y, et al. Exploring genetic determinants of plasma total cholesterol levels and their predictive value in a longitudinal study. Atherosclerosis. 2010;213:200–205. doi: 10.1016/j.atherosclerosis.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 33.Jamshidi Y, et al. SHP-2 and PI3-kinase genes PTPN11 and PIK3R1 may influence serum apoB and LDL cholesterol levels in normal women. Atherosclerosis. 2007;194:e26–e33. doi: 10.1016/j.atherosclerosis.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theret N, et al. Cholesterol efflux from adipose cells is coupled to diacylglycerol production and protein kinase C activation. Biochem Biophys Res Commun. 1990;173:1361–1368. doi: 10.1016/S0006-291X(05)80938-6. [DOI] [PubMed] [Google Scholar]
- 35.Wakil S, et al. A common variant association study reveals novel susceptibility loci for low HDL‐cholesterol levels in ethnic Arabs. Clin.Genet. 2016;90:518–525. doi: 10.1111/cge.12761. [DOI] [PubMed] [Google Scholar]
- 36.Bathgate RAD, et al. Relaxin Family Peptides and Their Receptors. Physiol. Rev. 2013;93:405–480. doi: 10.1152/physrev.00001.2012. [DOI] [PubMed] [Google Scholar]
- 37.Zhou X, Yin Z, Guo X, Hajjar DP, Han J. Inhibition of ERK1/2 and activation of liver X receptor synergistically induce macrophage ABCA1 expression and cholesterol efflux. J. Biol. Chem. 2010;285:6316–6326. doi: 10.1074/jbc.M109.073601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, et al. Serotonin (5-HT) receptor 5A sequence variants affect human plasma triglyceride levels. Physiol. Genomics. 2010;42:168–176. doi: 10.1152/physiolgenomics.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin LJ, Kissebah AH, Olivier M. Accounting for a quantitative trait locus for plasma triglyceride levels: utilization of variants in multiple genes. PLoS One. 2012;7:e34614. doi: 10.1371/journal.pone.0034614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang T, et al. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 2002;110:489–500. doi: 10.1016/S0092-8674(02)00872-3. [DOI] [PubMed] [Google Scholar]
- 41.Janowski BA. The hypocholesterolemic agent LY295427 up-regulates INSIG-1, identifying the INSIG-1 protein as a mediator of cholesterol homeostasis through SREBP. Proc. Nat. Acad. Sci. USA. 2002;99:12675–12680. doi: 10.1073/pnas.202471599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibeagha-Awemu EM, et al. Transcriptome adaptation of the bovine mammary gland to diets rich in unsaturated fatty acids shows greater impact of linseed oil over safflower oil on gene expression and metabolic pathways. BMC Genomics. 2016;17:104. doi: 10.1186/s12864-016-2423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maxwell KN, Soccio RE, Duncan EM, Sehayek E, Breslow JL. Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J. Lipid Res. 2003;44:2109–2119. doi: 10.1194/jlr.M300203-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Boone LR, Brooks PA, Niesen MI, Ness GC. Mechanism of resistance to dietary cholesterol. J. Lipid. 2011;2011:101242. doi: 10.1155/2011/101242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhar-Mascareno M, et al. Hexim1 heterozygosity stabilizes atherosclerotic plaque and decreased steatosis in ApoE null mice fed atherogenic diet. Int. J. Biochem. Cell. Biol. 2017;83:56–64. doi: 10.1016/j.biocel.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Nigg EA. Cyclin-dependent protein kinases: Key regulators of the eukaryotic cell cycle. BioEssays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 47.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 48.Hardie DG, Carling D, Sim AT. The AMP-activated protein kinase: a multisubstrate regulator of lipid metabolism. Trends Biochem Sci. 1989;14:20–23. doi: 10.1016/0968-0004(89)90084-4. [DOI] [Google Scholar]
- 49.Hou X, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeagle PL. Cholesterol and the cell membrane. B Biochim. Biophys. Acta, Rev. Biomembr. 1985;822:267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 51.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 52.Shennan D, Peaker M. Transport of milk constituents by the mammary gland. Physiol. Rev. 2000;80:925–951. doi: 10.1152/physrev.2000.80.3.925. [DOI] [PubMed] [Google Scholar]
- 53.Stelwagen K, Singh K. The role of tight junctions in mammary gland function. J Mammary Gland Biol. Neoplasia. 2014;19:131–138. doi: 10.1007/s10911-013-9309-1. [DOI] [PubMed] [Google Scholar]
- 54.Katz TA, Huang Y, Davidson NE, Jankowitz RC. Epigenetic reprogramming in breast cancer: From new targets to new therapies. Ann. Med. 2014;46:397–408. doi: 10.3109/07853890.2014.923740. [DOI] [PubMed] [Google Scholar]
- 55.McMahon CD, Farr VC, Singh K, Wheeler TT, Davis SR. Decreased expression of β1‐integrin and focal adhesion kinase in epithelial cells may initiate involution of mammary glands. J. Cell Physiol. 2004;200:318–325. doi: 10.1002/jcp.20011. [DOI] [PubMed] [Google Scholar]
- 56.Singh K, et al. Epigenetic regulation of milk production in dairy cows. J Mammary Gland Biol Neoplasia. 2010;15:101–112. doi: 10.1007/s10911-010-9164-2. [DOI] [PubMed] [Google Scholar]
- 57.Bracco U, Hidalgo J, Bohren H. Lipid composition of the fat globule membrane of human and bovine milk. J. Dairy Sci. 1972;55:165–172. doi: 10.3168/jds.S0022-0302(72)85454-7. [DOI] [PubMed] [Google Scholar]
- 58.Kelly K, Cochran BH, Stiles CD, Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983;35:603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- 59.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat. Rev. Mol. Cell Biol. 2005;6:635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 60.Shi Y, Hon M, Evans RM. The peroxisome proliferator-activated receptor δ, an integrator of transcriptional repression and nuclear receptor signaling. Proc. Nat. Acad. Sci. USA. 2002;99:2613–2618. doi: 10.1073/pnas.052707099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oliver WR, et al. A selective peroxisome proliferator-activated receptor δ agonist promotes reverse cholesterol transport. Proc. Nat. Acad. Sci. USA. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, et al. Identification and Dissection of Four Major QTL Affecting Milk Fat Content in the German Holstein-Friesian Population. PLoS One. 2012;7:e40711. doi: 10.1371/journal.pone.0040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li C, et al. Genome wide association study identifies 20 novel promising genes associated with milk fatty acid traits in Chinese Holstein. PLoS One. 2014;9:e96186. doi: 10.1371/journal.pone.0096186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ibeagha-Awemu, E. M., Peters, S. O., Akwanji, K. A., Imumorin, I. G. & Zhao, X. High density genome wide genotyping-by-sequencing and association identifies common and low frequency SNPs, and novel candidate genes influencing cow milk traits. Sci. Rep. 6, 31109, 10.1038/srep31109(2016). [DOI] [PMC free article] [PubMed]
- 65.Grisart B, et al. Genetic and functional confirmation of the causality of the DGAT1 K232A quantitative trait nucleotide in affecting milk yield and composition. Proc. Nat. Acad. Sci. USA. 2004;101:2398–2403. doi: 10.1073/pnas.0308518100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang L, et al. Genome Wide Association Studies for Milk Production Traits in Chinese Holstein Population. PLoS One. 2010;5:e13661. doi: 10.1371/journal.pone.0013661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winter A, Alzinger A, Fries R. Assessment of the gene content of the chromosomal regions flanking bovine DGAT1. Genomics. 2004;83:172–180. doi: 10.1016/S0888-7543(03)00238-6. [DOI] [PubMed] [Google Scholar]
- 68.Bennewitz J, et al. The DGAT1 K232A Mutation Is Not Solely Responsible for the Milk Production Quantitative Trait Locus on the Bovine Chromosome 14. J. Dairy Sci. 2004;87:431–442. doi: 10.3168/jds.S0022-0302(04)73182-3. [DOI] [PubMed] [Google Scholar]
- 69.Boichard D, et al. Detection of genes influencing economic traits in three French dairy cattle breeds. Genet. Sel. Evol. 2003;35:77–101. doi: 10.1186/1297-9686-35-1-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chandak PG, et al. Lack of acyl-CoA: diacylglycerol acyltransferase 1 reduces intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E knockout mice. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids. 2011;1811:1011–1020. doi: 10.1016/j.bbalip.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamazaki T, et al. Increased very low density lipoprotein secretion and gonadal fat mass in mice overexpressing liver DGAT1. J Biol, Chem. 2005;280:21506–21514. doi: 10.1074/jbc.M412989200. [DOI] [PubMed] [Google Scholar]
- 72.Sachdev V, et al. Novel role of a triglyceride-synthesizing enzyme: DGAT1 at the crossroad between triglyceride and cholesterol metabolism. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids. 2016;1861:1132–1141. doi: 10.1016/j.bbalip.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kojima T, et al. Decreased expression of CXXC4 promotes a malignant phenotype in renal cell carcinoma by activating Wnt signaling. Oncogene. 2009;28:297. doi: 10.1038/onc.2008.391. [DOI] [PubMed] [Google Scholar]
- 74.Lu H, et al. Enhancer of zeste homolog 2 activates wnt signaling through downregulating CXXC finger protein 4. Cell Death Dis. 2013;4:e776. doi: 10.1038/cddis.2013.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Enlund F, et al. Altered Notch signaling resulting from expression of a WAMTP1-MAML2 gene fusion in mucoepidermoid carcinomas and benign Warthin’s tumors. Exp. Cell Res. 2004;292:21–28. doi: 10.1016/j.yexcr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 76.Politi, K., Feirt, N. & Kitajewski, J. In Seminars in cancer biology. 341–347 (Elsevier). [DOI] [PubMed]
- 77.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006;7:678. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 78.Morisaki H, et al. CDH13 gene coding t‐cadherin influences variations in plasma adiponectin levels in the Japanese population. Hum. Mutat. 2012;33:402–410. doi: 10.1002/humu.21652. [DOI] [PubMed] [Google Scholar]
- 79.Choi JR, et al. The Impact of CDH13 Polymorphism and Statin Administration on TG/HDL Ratio in Cardiovascular Patients. Yonsei Med J. 2015;56:1604–1612. doi: 10.3349/ymj.2015.56.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujii H, et al. Activation of Stat5 by interleukin 2 requires a carboxyl-terminal region of the interleukin 2 receptor beta chain but is not essential for the proliferative signal transmission. Proc. Nat. Acad. Sci. USA. 1995;92:5482–5486. doi: 10.1073/pnas.92.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng HC, Yang CM, Shiao MS. Zonation of cholesterol and glycerolipid synthesis in regenerating rat livers. Hepatology. 1993;17:280–286. doi: 10.1002/hep.1840170219. [DOI] [PubMed] [Google Scholar]
- 82.Khalil MB, Blais A, Figeys D, Yao Z. Lipin the bridge between hepatic glycerolipid biosynthesis and lipoprotein metabolism. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids. 2010;1801:1249–1259. doi: 10.1016/j.bbalip.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Repa JJ, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoshikawa T, et al. Cross-talk between peroxisome proliferator-activated receptor (PPAR) alpha and liver X receptor (LXR) in nutritional regulation of fatty acid metabolism. I. PPARs suppress sterol regulatory element binding protein-1c promoter through inhibition of LXR signaling. Mol. Endocrinol. 2003;17:1240–1254. doi: 10.1210/me.2002-0190. [DOI] [PubMed] [Google Scholar]
- 85.Peet DJ, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/S0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 86.Schulman IGL. X receptors link lipid metabolism and inflammation. FEBS Letters. 2017;591:2978–2991. doi: 10.1002/1873-3468.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fletouris D, Botsoglou N, Psomas I, Mantis A. Rapid determination of cholesterol in milk and milk products by direct saponification and capillary gas chromatography. J. Dairy Sci. 1998;81:2833–2840. doi: 10.3168/jds.S0022-0302(98)75842-4. [DOI] [PubMed] [Google Scholar]
- 88.Sargolzaei M, Chesnais JP, Schenkel FS. A new approach for efficient genotype imputation using information from relatives. BMC Genomics. 2014;15:478. doi: 10.1186/1471-2164-15-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Madsen, P. et al. DMU–A package for analyzing multivariate mixed models. Proceedings of the 9th World Congress on Genetics Applied to Livestock Production. Leipzig, Germany (2010).
- 90.Burton PR, et al. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gabriel SB, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 92.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 93.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2008;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 95.Szklarczyk D, et al. STRINGv10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2014;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lachmann A, et al. ChEA: transcription factor regulation inferred from integrating genome-wide ChIP-X experiments. Bioinformatics. 2010;26:2438–2444. doi: 10.1093/bioinformatics/btq466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen EY, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC bioinformatics. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Team, R. Core. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2013. (2014).
- 99.CCAC. Guidelines on the care and use of farm animals in research, teaching and testing. Canadian Council on Animal Care 2009. Documents/Standards/Guidelines/Farm_Animals.pdf) (2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA sequence data has been submitted to the BioProject data base (BioProject ID: PRJNA301774) and it is available through this link: http://www.ncbi.nlm.nih.gov/bioproject/PRJNA301774).