Abstract
Purpose
The aim of this retrospective cross-sectional study was to evaluate whether salivary findings of active matrix-metalloproteinase 8 (aMMP-8) chairside (point of care; POC) tests were associated with periodontal risk assessment parameters in patients receiving supportive periodontal therapy (SPT).
Methods
A total of 125 patients receiving regular SPT were included, and their records were examined. The following inclusion criteria were used: a diagnosis of chronic periodontitis, at least 1 non-surgical periodontal treatment (scaling and root planning) with following regular SPT (minimum once a year), at least 6 remaining teeth, and clinical and aMMP-8 findings that were obtained at the same appointment. In addition to anamnestic factors (e.g., smoking and diabetes), oral hygiene indices (modified sulcus bleeding index [mSBI] and approximal plaque index), periodontal probing depth simultaneously with bleeding on probing, and dental findings (number of decayed, missing, and filled teeth) were recorded. Salivary aMMP-8 levels were tested using a commercial POC test system (Periomarker, Hager & Werken, Duisburg, Germany). Statistical analysis was performed using the t-test, Mann-Whitney U test, Fisher's exact test, and χ2 test, as appropriate (P<0.05).
Results
Only the mSBI was significantly associated with positive salivary aMMP-8 findings (aMMP-8 positive: 27.8%±20.9% vs. aMMP-8 negative: 18.0%±14.5%; P=0.017). No significant associations were found between aMMP-8 and smoking, diabetes, periodontal parameters, or parameters related to the maintenance interval (P>0.05).
Conclusions
Salivary aMMP-8 chairside findings were not associated with common parameters used for periodontal risk assessment in patients receiving SPT. The diagnostic benefit of POC salivary aMMP-8 testing in risk assessment and maintenance interval adjustment during SPT remains unclear.
Keywords: Maintenance, Metalloproteinase (aMMP-8), Periodontitis, Risk assessment, Supportive periodontal therapy
Graphical Abstract
INTRODUCTION
The prevalence of gingival and periodontal diseases remains high despite the global decline in dental caries [1]. Although the incidence of severe periodontitis in German adults (35–44 years of age) declined by half from 16% to 8.2% during the last decade, the incidence of moderate periodontitis remains high, as does the need for periodontal treatment, with approximately 60% of German adults requiring periodontal care [2]. Accordingly, periodontal diseases should still be considered a highly relevant and important public health issue.
In addition to the active treatment of periodontitis, including non-surgical (scaling and root planing) and potentially surgical therapy, supportive periodontal therapy (SPT) is vitally important for maintaining stable periodontal conditions [3,4,5]. During SPT, a risk-adapted maintenance interval must be determined, which allows each individual patient to receive appropriate treatment [6]. The classical parameters that serve as a basis for risk assessment are periodontal probing depth (PPD), bleeding on probing (BOP), bone loss in relation to the patient's age, tooth loss, records of pre-existing systemic diseases (e.g., diabetes), and environmental risk factors, such as smoking habits [7]. After the completion of initial treatment, individualized intervals of SPT are determined by evaluating these parameters according to existing definitions [8,9]. However, the evidence supporting specific maintenance intervals for patients receiving SPT is still weak [6]. Accordingly, further diagnostic instruments might be of interest.
A potential tool for determining maintenance intervals in SPT could be the measurement of active matrix-metalloproteinase 8 (aMMP-8) levels. The aMMP-8 enzyme is released in response to bacterial infiltration into the organism and leads to the depletion of collagen types I, II, and III by cleaving the collagen peptide bond. It is expressed by polymorphonuclear leukocytes, macrophages, monocytes, as well as some autochthonic cells. aMMP-8 enhances the destruction of gingival tissue and initiates periodontal soft and hard tissue loss [10,11]. Therefore, aMMP-8 might be highly relevant for periodontal inflammation and may reflect the risk of progressive periodontal destruction at a given point in time [10,11,12,13]. Beneath the gingival and periodontal tissue, aMMP-8 also enters the gingival crevicular fluid, as well as the saliva, serum, and plasma [14,15,16]. A recent meta-analysis showed that salivary aMMP-8 levels were significantly higher in periodontitis patients than in healthy controls [17], suggesting the potential value of detecting aMMP-8 with a non-invasive, saliva-based point of care (POC) diagnostic test. Available POC aMMP-8 chairside tests were found to be promising, considering the possible suitability of aMMP-8 as a biomarker of periodontal diseases. However, previous studies have shown heterogeneous results regarding the test's applicability [18,19,20]. To the best of the authors' knowledge, no study has yet investigated the use of an aMMP-8 chairside test for patients receiving SPT.
Therefore, the aim of this clinical cross-sectional study was to evaluate the possible associations of salivary aMMP-8 chairside test findings with periodontal risk assessment parameters in SPT patients. The associations between aMMP-8 chairside test results and common clinical and anamnestic parameters of periodontal risk assessment should be assessed to investigate whether salivary aMMP-8 could be used to support or even amend available parameters.
MATERIALS AND METHODS
Study design
This retrospective cross-sectional study was reviewed and approved by the ethics committee of the University Medical Center Goettingen, Germany (application No. 4/7/14). All patients were informed verbally and in writing and gave written informed consent. The study was based on the analysis of retrospectively assessed data from the patients' records and documentation.
Patients
All participants were drawn from the patient pool of the Department of Preventive Dentistry, Periodontology and Cariology, University Medical Center Goettingen, Germany. Data were collected from 500 available potential SPT patients between April 2013 and January 2014 during regular SPT appointments in the periodontal student (education) course by supervised dental students. The following inclusion criteria were used as conditions for participation in the study. All patients had to be diagnosed with chronic periodontitis according to the Classification System for Periodontal Diseases and Conditions [21]. Furthermore, the study participants had to attend SPT at least once a year, no earlier than 12 weeks after at least 1 non-surgical periodontal treatment (scaling and root planing). Additionally, the presence of at least 6 remaining teeth was a condition for participating. The presence of peri-implantitis and missing information (medical records) were defined as exclusion criteria.
SPT and risk assessment
SPT was performed equally for all patients, depending on their assessed periodontitis risk. Twelve weeks after non-surgical therapy, patients received a professional tooth cleaning and re-evaluation of their periodontal status, including PPD and BOP. If the PPD was ≤4 mm and BOP was negative, active periodontal therapy was considered to have successfully finished, at which point a patient could start SPT, which included re-instructions, re-motivation, and professional tooth cleaning. If the PPD was ≥5 mm or BOP was positive, selective scaling and root planing were performed at the affected teeth, with a subsequent SPT interval of 3 to 4 months in the first year after scaling and root planing.
Subsequently, a periodontal examination, including measurements of PPD and BOP, clinical attachment loss, tooth mobility, and the degree of furcation defects, was performed once a year during regular SPT appointments (starting 1 year after SPT). According to the periodontal findings, the SPT interval was determined according to a risk assessment based on the functional diagram by Lang and Tonetti [7]. In this process, a patient’s risk of experiencing progression of periodontitis was graded as low, moderate, or high, based upon the periodontal risk assessment parameters of BOP, PPD, tooth loss, age-related bone loss, systemic diseases, and environment/smoking. Depending on their extent, each parameter was individually categorized as low-risk, moderate-risk, or high-risk. The maintenance interval was determined using the following guidelines: once a year, in patients at low risk with a maximum of 1 parameter that received a score of moderate risk; twice a year, in patients at moderate risk, with at least 2 parameters that received a score of moderate risk and a maximum of 1 parameter in the high-risk category; and 3–4 times a year, in patients at high risk with at least 2 parameters that received a score of high risk [7].
Examination parameters
General data
All examinations were performed by supervised dental students (no study-related calibrated examiners) during regular SPT appointments under practical conditions. The data for the current study were retrospectively obtained from patients’ documentation and records. Based on the patients' medical records, several parameters relevant to periodontitis were assessed. Information about patients' smoking behavior, including how many cigarettes they smoked per day, was obtained. Furthermore, it was recorded whether the patients had systemic diseases, with a special focus on evaluating their diabetes status. Moreover, the date of the last non-surgical or surgical periodontitis therapy, the time since the last SPT appointment, and the maintenance interval were obtained.
Clinical data
The clinical data were retrospectively extracted from the patients' documentation. All patients received the same treatment and diagnostic tests during their SPT appointments. During the clinical examination, the decayed-, missing-, and filled-teeth index (DMF-T) was obtained [22]. Before the periodontal examination was performed, the modified sulcus bleeding index (mSBI) [23] was measured on vestibular surfaces in the first and third quadrants and on oral surfaces in the second and fourth quadrants by slightly probing the gingival margin using a periodontal probe (PCP 15, Hu-Friedy, Chicago, IL, USA). Periodontal status included PPD and BOP at 6 measurement points per tooth using a millimeter-scaled periodontal probe (PCP 15, Hu-Friedy). Subsequently, a modified BOP was assessed, which only considered bleeding in cases where the pocket depth was ≥5 mm. Plaque accumulation was recorded after plaque staining with mira-II-ton® (Hager & Werken GmbH & Co. KG, Duisburg, Germany) and evaluation using the approximal plaque index (API) at the oral sides in the first and third quadrants and the vestibular sides in the second and fourth quadrants [24].
aMMP-8 chairside test
Additionally, the aMMP-8 findings of the included participants were retrospectively recorded from the patients’ records. The aMMP-8 chairside tests were performed using a commercially available test system (Periomarker®, Hager & Werken GmbH & Co. KG) prior to clinical examinations and risk assessment during regular SPT appointments. The test was performed in accordance to the manufacturer's instructions, and has been validated in various studies [18,19,20]. The patients were directed not to eat, drink, or perform oral hygiene on the same day prior to the test performance. Additionally, the gingiva must not have shown spontaneous bleeding. For saliva collection, the participants rinsed with the (test) mouth rinse liquid for 30 seconds and spat it into the provided tube. Three drops of the filtered combination of saliva and solvent were pipetted into the fluid recess of the chairside test. The aMMP-8 chairside test is a qualitative test (positive or negative) based on a lateral flow-immunochromatographic assay, meaning that the solvent flows from the pipetted side to the other side, passing through 3 different series of solvent-soaking capillary beds. In short, the beds work as follows. The first blocks excess solvent. The second contains a dissolved salt-sugar mixture with a bioactive conjugate providing an antibody-immobilizing surface that is transported to the third bed by the solvent. This solvent-conjugate mixture with linked antibody interacts with another molecule in the third area, which is provided on stripes. These stripes accumulate the molecule-interacted conjugate-antibody mixture and take on color. The first stripe is the test stripe, which presents a positive test finding with a blue stain in response to a minimum measured quantity of 25 ng/mL aMMP-8. The second is the control stripe, which is used to verify that the test is working correctly.
Statistical analysis
All parameters obtained during this study were statistically analyzed to identify possible associations with the aMMP-8 test results. Data are presented as the average mean or proportional percentage±standard deviation. The normality of the distribution was tested using the Kolmogorov-Smirnov test. To compare 2 independent samples, the t-test and Mann-Whitney U test were performed. Categorical data were analyzed using the χ2 test or the Fisher's exact test. Statistical significance was determined by P values <0.05.
RESULTS
Patients
A total of 125 patients met all the inclusion criteria and were eligible for inclusion in the present study. Therefore, the study population consisted of 125 patients (52 female, 73 male), who had a mean age of 59.9±11.8 years and on average 4.51±4.41 missing teeth. The study participants had received SPT for an average of 67.97±67.02 months. Table 1 provides an overview of the patients' characteristics.
Table 1. Demographic and clinical characteristics of all included patients.
| Parameter | Study participants (n=125) | |
|---|---|---|
| Gender | ||
| Female | 52 (42) | |
| Male | 73 (58) | |
| Age (yr) | 59.9±11.8 | |
| Smoking habits | ||
| Smoker | 32 (26) | |
| Non-smoker | 93 (74) | |
| Dental status | ||
| DMF-T | 19.3±5.31 | |
| D-T | 0.38±1.26 | |
| M-T | 4.51±4.41 | |
| F-T | 14.41±5.27 | |
| Oral hygiene status (%) | ||
| API | 70.87±23.04 | |
| mSBI | 24.04±19.25 | |
| SPT-specific information | ||
| Years receiving SPT | 8.6±6.67 | |
| SPT appointments per year (No.) | 2.85±0.75 | |
| Time since last SPT appointment (mon) | 6.6±3.5 | |
| Time since last active periodontal treatment (mon) | 67.97±67.02 | |
Values are presented as number (%) or mean±standard deviation.
DMF-T: number of decayed- (D-T), missing- (M-T), and filled-teeth (F-T), API: approximal plaque index, mSBI: modified sulcus bleeding index, SPT: supportive periodontal therapy.
Association of aMMP-8-findings with risk assessment parameters
A total of 62% (n=78) of the patients showed aMMP-8 positive findings. Although the overall DMF-T value of the cohort was not associated with positive aMMP8 values, the M-T showed a non-significant relationship with aMMP-8 positivity (aMMP-8 positive: 4.65±3.84 vs. aMMP-8 negative: 4.28±5.25; P=0.077). Table 2 shows the relationship of aMMP-8 findings with the periodontal risk parameters and the resulting risk profiles. None of these parameters showed any significant associations with aMMP-8 results (P>0.05).
Table 2. Associations of aMMP-8 findings with common risk assessment parameters for SPT according to Lang and Tonetti [7].
| Parameter | aMMP-8 | P value | Test | |||
|---|---|---|---|---|---|---|
| Total | Positive | Negative | ||||
| BOP (%) | 14.88±10.9 | 16.29±12.76 | 12.52±6.21 | 0.111 | Mann-Whitney U test | |
| PPD ≥5 mm (No.) | 8.0±10.9 | 9.2±12.5 | 6.2±7.2 | 0.295 | ||
| M-T (No.) | 4.51±4.41 | 4.65±3.84 | 4.28±5.25 | 0.077 | ||
| No. of smoked cigarettes | 11.61±8.08 | 12.0±6.29 | 11.14±10.07 | 0.301 | ||
| General diseases | 0.473 | Fisher's exact test | ||||
| Diabetes mellitus | 8 (100) | 4 (50) | 4 (50) | |||
| Other | 84 (100) | 54 (64.3) | 30 (35.7) | |||
| Assessed risk profile according to Lang and Tonetti [7] | 0.369 | χ2 test | ||||
| Low | 15 (100) | 7 (47) | 8 (53) | |||
| Moderate | 79 (100) | 52 (66) | 27 (34) | |||
| High | 31 (100) | 19 (61) | 12 (39) | |||
Values are presented as number (%) or mean±standard deviation. Significance level P<0.05.
aMMP-8: active matrix-metalloproteinase 8, SPT: supportive periodontal therapy, BOP: bleeding on probing, PPD: periodontal pocket depth, M-T: missing-teeth.
aMMP-8 findings in association with maintenance parameters
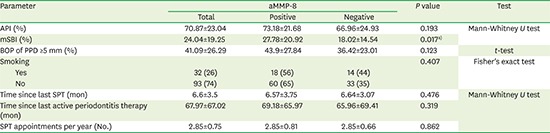
Finally, the aMMP-8 results were analyzed in relation to parameters relevant for extended treatment and compliance with the maintenance system (Table 3). A significant association of positive aMMP-8 values with the mSBI was observed (aMMP-8 positive: 27.78±20.92 vs. 18.02±14.54; P=0.017). Other values, such as the modified BOP, API, smoking habits, period since the last periodontitis therapy, and SPT attendance during the previous 2 years, did not show significant associations with positive aMMP-8 findings (Table 3).
Table 3. Association of aMMP-8 findings with further SPT-specific parameters.
| Parameter | aMMP-8 | P value | Test | |||
|---|---|---|---|---|---|---|
| Total | Positive | Negative | ||||
| API (%) | 70.87±23.04 | 73.18±21.68 | 66.96±24.93 | 0.193 | Mann-Whitney U test | |
| mSBI (%) | 24.04±19.25 | 27.78±20.92 | 18.02±14.54 | 0.017a) | ||
| BOP of PPD ≥5 mm (%) | 41.09±26.29 | 43.9±27.84 | 36.42±23.01 | 0.123 | t-test | |
| Smoking | 0.407 | Fisher's exact test | ||||
| Yes | 32 (26) | 18 (56) | 14 (44) | |||
| No | 93 (74) | 60 (65) | 33 (35) | |||
| Time since last SPT (mon) | 6.6±3.5 | 6.57±3.75 | 6.64±3.07 | 0.476 | Mann-Whitney U test | |
| Time since last active periodontitis therapy (mon) | 67.97±67.02 | 69.18±65.97 | 65.96±69.41 | 0.319 | ||
| SPT appointments per year (No.) | 2.85±0.75 | 2.85±0.81 | 2.85±0.66 | 0.862 | ||
Values are presented as number (%) or mean±standard deviation.
aMMP-8: active matrix-metalloproteinase 8, SPT: supportive periodontal therapy, API: approximal plaque index, mSBI: modified sulcus bleeding index, BOP: bleeding on probing, PPD: periodontal pocket depth.
Significance level P<0.05, a)statistically significant.
DISCUSSION
In the literature, the validated POC test of aMMP8 in saliva has been described as a promising diagnostic approach to differentiate between active and inactive periodontal disease [25,26,27]. Saliva is easy to sample, non-invasive, and pain-free for the patient. Therefore, salivary aMMP-8 detection represents an interesting and clinically relevant possibility that has been investigated by multiple research groups [18,19,20,26]. It has been shown that the chairside test is highly consistent with an enzyme-linked immunosorbent assay analysis, which remains a valid technique for detecting aMMP-8 [26]. A previous study by this working group showed an association between salivary aMMP-8 findings and periodontal inflammation in a large cohort of adolescents, but with limited reliability [20]. Another study investigating adolescents found salivary aMMP-8 to be associated with periodontitis, especially regarding inflammatory signs [19]. Furthermore, a case-control study of adult patients found salivary aMMP-8 to be correlated to chronic periodontitis [18]. This is supported by a recently published review, which recommended the oral fluid-based aMMP-8 chairside test as a good, inexpensive, and practicable diagnostic approach [27]. The current study showed no associations between positive aMMP-8 findings and BOP as a sign of the presence of periodontal inflammation, even when the modified BOP was used (PPD ≥5 mm with positive bleeding). This appears to contradict the current literature [13,14,28]. However, the association of positive aMMP-8 findings with the mSBI, representing a parameter of gingival inflammation, appears to be in line with the literature [13,29]. Therefore, ongoing gingival inflammation, indicated by the mSBI, might cause increased aMMP-8 release in the saliva. SPT aims at continuous control of disease progression in patients with periodontitis. Therefore, the salivary aMMP-8 test might serve as an indicator of early inflammatory changes, which are probably associated with imminent disease progression. However, due to the cross-sectional design of the current study, no longitudinal follow-up data supporting that possibility are available. Furthermore, in this context it is essential to keep in mind that there are no comparative data available for patients undergoing SPT.
The current study aimed at investigating the possible diagnostic benefit of salivary aMMP-8 chairside analysis in periodontal risk assessment in SPT patients. Risk assessment and the subsequent determination of the maintenance interval are controversial topics in the literature [6,30,31,32]. Evidence regarding how to determine the maintenance interval in SPT is still weak [6]. A popular and valid approach is based on the risk assessment approach proposed by Lang and Tonetti [7], who defined several risk parameters, including BOP, PPD, tooth loss, age-related bone loss, systemic diseases, and environment/smoking [8]. However, different approaches have been discussed, considering additional diagnostics as a way to improve the validity of risk-oriented maintenance estimation [6,28,30,31]. One possibility, which was considered in the current study, could be the investigation of inflammation-related cytokines, in addition to the commonly used clinical and anamnestic parameters [31]. As aMMP-8 findings were not associated with common risk factors, the potential diagnostic benefit remains unclear. There are 2 possible explanations for this finding. First, aMMP-8 levels could be independent of periodontal inflammation in patients receiving SPT, and thus provide limited diagnostic benefit in this group of patients. The other explanation could be that salivary aMMP-8 represents a very early inflammatory indicator, which is not reflected in common periodontal parameters. In this case, aMMP-8 levels could show diagnostic benefits for risk assessment and maintenance interval adjustment. The observation of an association between aMMP-8 levels and gingival inflammation (mSBI) in the present study could support the latter possibility. However, the literature shows that aMMP-8 concentrations increase with the severity of periodontitis and that aMMP-8 levels are directly associated with BOP and PPD [31,32,33,34,35]. This conflicts with the findings of the current study. Nevertheless, it is important to consider that these patients were receiving SPT. Of these patients, 62% showed positive aMMP-8 findings in the saliva-based chairside test that was used. In SPT patients, a pre-existing inflammatory burden due to periodontal disease is present, which has been shown to be associated with higher aMMP-8 values than those in healthy individuals [36]. Due to the qualitative nature of the test system that was used, with a cut-off of 25 ng/mL for positive aMMP-8 findings, a large proportion of SPT patients showed positive findings, independently of their current level of periodontal inflammation. This might reduce the potential diagnostic benefit of the chairside test that was used in SPT patients.
This is the first study to evaluate associations between the findings of a salivary aMMP-8 POC test with parameters of periodontal risk assessment in patients receiving SPT. Nevertheless, the major limitation of this study is its retrospective cross-sectional design. Moreover, since the study cohort comprised 125 patients who participated in a retrospective cross-sectional study, the results of the current study must be interpreted carefully, and the possibility for strong conclusions to be drawn is limited. The validation of these findings within a longitudinal study design in a larger cohort would appear to be necessary to assess the correlations between aMMP-8 levels and the progression of periodontal inflammation for risk assessment and diagnosis. The periodontal examinations of the patients were performed under realistic and practice-based conditions in a single dental clinic. However, examinations were performed by students without verification by a calibrated examiner, which limits the reliability of the findings. In addition, the aMMP-8 POC test was used in a very stable SPT population (mean BOP: 15%) with mostly good compliance (mean regular SPT interval: 2.85 per year). The absence of a control group is a further limitation; however, the current study aimed to investigate associations within SPT patients with a history of periodontitis. It might be interesting to consider aMMP-8 in periodontally healthy individuals as a potential early indicator of the risk of developing periodontal inflammation in the near future. Furthermore, the test system that was used only differentiates between positive (>25 ng/mL) and negative aMMP-8 findings, without any further categorization. Thus, the quantitative determination of aMMP-8 concentrations might provide additional information, which should be considered in further research. Despite these limitations, the current study might provide new and potentially relevant knowledge in the context of periodontal risk assessment in SPT patients.
In conclusion, within the limitations of this study, salivary aMMP-8 findings were not associated with common parameters considered during periodontal risk assessment of SPT patients. Based on the results of the current study, the potential diagnostic benefit of salivary aMMP-8 measurements for risk assessment and maintenance interval adjustment in SPT patients remains unclear. Prospective studies with a large study cohort are needed for further investigation.
ACKNOWLEDGEMENTS
The authors thank the Department of Preventive Dentistry, Periodontology and Cariology, University Medical Center Goettingen, Germany for providing support to conduct the current study.
Footnotes
Funding: This study was supported by internal funding.
Author Contributions: Conceptualization: Dirk Ziebolz; Data curation: Max Kristian Kummer; Formal analysis; Max Kristian Kummer, Tanja Kottmann, Gerhard Schmalz, Investigation: Max Kristian Kummer, Dirk Ziebolz; Methodology: Dirk Ziebolz, Sven Rinke; Project administration: Dirk Ziebolz; Writing - original draft: Gerhard Schmalz, Max Kristian Kummer, Jana Schmidt; Writing - review & editing: Dirk Ziebolz, Rainer Haak, Felix Krause, Sven Rinke.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T. Global epidemiology of dental caries and severe periodontitis - a comprehensive review. J Clin Periodontol. 2017;44(Suppl 18):S94–S105. doi: 10.1111/jcpe.12677. [DOI] [PubMed] [Google Scholar]
- 2.Jordan RA, Micheelis W. The fifth German oral health study (DMS V) Köln: Institut der Deutschen Zahnärzte; 2016. [in German] [Google Scholar]
- 3.Renvert S, Persson GR. Supportive periodontal therapy. Periodontol 2000. 2004;36:179–195. doi: 10.1111/j.1600-0757.2004.03680.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee CT, Huang HY, Sun TC, Karimbux N. Impact of patient compliance on tooth loss during supportive periodontal therapy: a systematic review and meta-analysis. J Dent Res. 2015;94:777–786. doi: 10.1177/0022034515578910. [DOI] [PubMed] [Google Scholar]
- 5.Armitage GC, Xenoudi P. Post-treatment supportive care for the natural dentition and dental implants. Periodontol 2000. 2016;71:164–184. doi: 10.1111/prd.12122. [DOI] [PubMed] [Google Scholar]
- 6.Farooqi OA, Wehler CJ, Gibson G, Jurasic MM, Jones JA. Appropriate recall interval for periodontal maintenance: a systematic review. J Evid Based Dent Pract. 2015;15:171–181. doi: 10.1016/j.jebdp.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang NP, Tonetti MS. Periodontal risk assessment (PRA) for patients in supportive periodontal therapy (SPT) Oral Health Prev Dent. 2003;1:7–16. [PubMed] [Google Scholar]
- 8.Costa FO, Cota LO, Lages EJ, Lima Oliveira AP, Cortelli SC, Cortelli JR, et al. Periodontal risk assessment model in a sample of regular and irregular compliers under maintenance therapy: a 3-year prospective study. J Periodontol. 2012;83:292–300. doi: 10.1902/jop.2011.110187. [DOI] [PubMed] [Google Scholar]
- 9.Lang NP, Tonetti MS. Periodontal diagnosis in treated periodontitis. Why, when and how to use clinical parameters. J Clin Periodontol. 1996;23:240–250. doi: 10.1111/j.1600-051x.1996.tb02083.x. [DOI] [PubMed] [Google Scholar]
- 10.Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38:306–321. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- 11.Sorsa T, Tjäderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004;10:311–318. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 12.Golub LM, Payne JB, Reinhardt RA, Nieman G. Can systemic diseases co-induce (not just exacerbate) periodontitis? A hypothetical “two-hit” model. J Dent Res. 2006;85:102–105. doi: 10.1177/154405910608500201. [DOI] [PubMed] [Google Scholar]
- 13.Kraft-Neumärker M, Lorenz K, Koch R, Hoffmann T, Mäntylä P, Sorsa T, et al. Full-mouth profile of active MMP-8 in periodontitis patients. J Periodontal Res. 2012;47:121–128. doi: 10.1111/j.1600-0765.2011.01416.x. [DOI] [PubMed] [Google Scholar]
- 14.Söder B, Jin LJ, Wickholm S. Granulocyte elastase, matrix metalloproteinase-8 and prostaglandin E2 in gingival crevicular fluid in matched clinical sites in smokers and non-smokers with persistent periodontitis. J Clin Periodontol. 2002;29:384–391. doi: 10.1034/j.1600-051x.2002.290502.x. [DOI] [PubMed] [Google Scholar]
- 15.Mäntylä P, Stenman M, Kinane D, Salo T, Suomalainen K, Tikanoja S, et al. Monitoring periodontal disease status in smokers and nonsmokers using a gingival crevicular fluid matrix metalloproteinase-8-specific chair-side test. J Periodontal Res. 2006;41:503–512. doi: 10.1111/j.1600-0765.2006.00897.x. [DOI] [PubMed] [Google Scholar]
- 16.Sorsa T, Tervahartiala T, Leppilahti J, Hernandez M, Gamonal J, Tuomainen AM, et al. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacol Res. 2011;63:108–113. doi: 10.1016/j.phrs.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Li X, Yan H, Huang L. Salivary matrix metalloproteinase (MMP)-8 as a biomarker for periodontitis: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e9642. doi: 10.1097/MD.0000000000009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izadi Borujeni S, Mayer M, Eickholz P. Activated matrix metalloproteinase-8 in saliva as diagnostic test for periodontal disease? A case-control study. Med Microbiol Immunol (Berl) 2015;204:665–672. doi: 10.1007/s00430-015-0413-2. [DOI] [PubMed] [Google Scholar]
- 19.Heikkinen AM, Nwhator SO, Rathnayake N, Mäntylä P, Vatanen P, Sorsa T. Pilot study on oral health status as assessed by an active matrix metalloproteinase-8 chairside mouthrinse test in adolescents. J Periodontol. 2016;87:36–40. doi: 10.1902/jop.2015.150377. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt J, Guder U, Kreuz M, Löffler M, Kiess W, Hirsch C, et al. aMMP-8 in correlation to caries and periodontal condition in adolescents-results of the epidemiologic LIFE child study. Clin Oral Investig. 2018;22:449–460. doi: 10.1007/s00784-017-2132-0. [DOI] [PubMed] [Google Scholar]
- 21.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Oral health surveys: basic methods. 4th ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 23.Mühlemann HR, Son S. Gingival sulcus bleeding--a leading symptom in initial gingivitis. Helv Odontol Acta. 1971;15:107–113. [PubMed] [Google Scholar]
- 24.Lange DE, Plagmann HC, Eenboom A, Promesberger A. Clinical methods for the objective evaluation of oral hygiene. Dtsch Zahnarztl Z. 1977;32:44–47. [PubMed] [Google Scholar]
- 25.Sorsa T, Gursoy UK, Nwhator S, Hernandez M, Tervahartiala T, Leppilahti J, et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol 2000. 2016;70:142–163. doi: 10.1111/prd.12101. [DOI] [PubMed] [Google Scholar]
- 26.Lorenz K, Keller T, Noack B, Freitag A, Netuschil L, Hoffmann T. Evaluation of a novel point-of-care test for active matrix metalloproteinase-8: agreement between qualitative and quantitative measurements and relation to periodontal inflammation. J Periodontal Res. 2017;52:277–284. doi: 10.1111/jre.12392. [DOI] [PubMed] [Google Scholar]
- 27.Rathnayake N, Gieselmann DR, Heikkinen AM, Tervahartiala T, Sorsa T. Salivary diagnostics-point-of-care diagnostics of MMP-8 in dentistry and medicine. Diagnostics (Basel) 2017;7:7. doi: 10.3390/diagnostics7010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramseier CA, Kinney JS, Herr AE, Braun T, Sugai JV, Shelburne CA, et al. Identification of pathogen and host-response markers correlated with periodontal disease. J Periodontol. 2009;80:436–446. doi: 10.1902/jop.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mäntylä P, Stenman M, Kinane DF, Tikanoja S, Luoto H, Salo T, et al. Gingival crevicular fluid collagenase-2 (matrix metalloproteinase-8) test stick for chair-side monitoring of periodontitis. J Periodontal Res. 2003;38:436–439. doi: 10.1034/j.1600-0765.2003.00677.x. [DOI] [PubMed] [Google Scholar]
- 30.Mullins JM, Even JB, White JM. Periodontal management by risk assessment: a pragmatic approach. J Evid Based Dent Pract. 2016;16(Suppl):91–98. doi: 10.1016/j.jebdp.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Lyle DM. Risk assessment a key to periodontal health promotion and disease prevention. Compend Contin Educ Dent. 2014;35:392–396. [PubMed] [Google Scholar]
- 32.Lang NP, Suvan JE, Tonetti MS. Risk factor assessment tools for the prevention of periodontitis progression a systematic review. J Clin Periodontol. 2015;42(Suppl 16):S59–S70. doi: 10.1111/jcpe.12350. [DOI] [PubMed] [Google Scholar]
- 33.Ito H, Numabe Y, Sekino S, Murakashi E, Iguchi H, Hashimoto S, et al. Evaluation of bleeding on probing and gingival crevicular fluid enzyme activity for detection of periodontally active sites during supportive periodontal therapy. Odontology. 2014;102:50–56. doi: 10.1007/s10266-012-0090-1. [DOI] [PubMed] [Google Scholar]
- 34.Sorsa T, Mantyla P, Tervahartiala T, Pussinen PJ, Gamonal J, Hernandez M. Matrix metalloproteinase activation in diagnostics of periodontitis and systemic inflammation. J Clin Periodontol. 2011;38:817–819. doi: 10.1111/j.1600-051X.2011.01753.x. [DOI] [PubMed] [Google Scholar]
- 35.Hernández M, Dutzan N, García-Sesnich J, Abusleme L, Dezerega A, Silva N, et al. Host-pathogen interactions in progressive chronic periodontitis. J Dent Res. 2011;90:1164–1170. doi: 10.1177/0022034511401405. [DOI] [PubMed] [Google Scholar]
- 36.Rai B, Kharb S, Jain R, Anand SC. Biomarkers of periodontitis in oral fluids. J Oral Sci. 2008;50:53–56. doi: 10.2334/josnusd.50.53. [DOI] [PubMed] [Google Scholar]


